Original Article - DOI:10.33594/000000596
Accepted 6 October 2022 - Published online 20 December 2022
The Role of Matrix Metalloproteinase-13 (MMP13) in TGFβ/BMP Pathway Regulation of Fibro-Adipogenic Progenitor (FAP) Differentiation
bDepartment of Orthopaedic Surgery, University of California, San Francisco, CA, USA,
cCalifornia Northstate University, College of Medicine, Elk Grove, CA, USA
Keywords
Abstract
Background/Aims:
Muscle fibrosis and fatty infiltration (FI) are common complications seen in various muscle disease states. Recent studies indicate that muscle residential fibro/adipogenic progenitors (FAPs) are the major cellular source for muscle fibrosis and FI. We previously showed that MMP13 knockout (KO) mice have significantly increased FI, suggesting an important role of MMP13 in muscle FI. However, how MMP13 affects the differentiation of FAPs remains unknown.Methods:
In order to assess the role of MMP-13 on FAP differentiation, we isolated FAPs from wildtype C57BL/6 and MMP13 knock out mice with FACS using CD31-, CD45-, Integrin α7- and Sca-1+ markers. FAPs were cultured in 24 well plate after FACS.in standard media till 80% confluent and then switched to adipogenic medium. In order to study the role of TGFβ and BMP in their differentiation, FAPs from both wildtype and MMP13 KO mice were treated with TGFβ1 (5 ng/ml). For MMP13 inhibitor treatment, FAPs from wildtype mice were incubated in adipogenic medium containing 10 µM MMP13 inhibitor (or vehicle) for 2 weeks. Immunofluorescence and gene expression analysis were used to assess FAP adipogenic and fibrogenic differentiation. FAPs were stained with Perilipin A (FITC, adipogenesis marker) and αSMA (Red, fibroblast marker), and DAPI. Real time PCR was performed for gene expression evaluation. A two-tailed Anova was used for statistical comparisons between groups, with p ≤ 0.05. Data are presented as mean ± standard deviation.Results:
In this study, we isolated FAPs from wildtype C57BL/6 and MMP13 KO mice and evaluated their adipogenic and fibrogenic differentiation in vitro . MMP13 KO FAPs demonstrated enhanced adipogenesis but reduced fibrogenesis compared to wildtype FAPs. Treating wildtype FAPs with an MMP13 inhibitor simulated phenotypes seen in MMP13 KO FAPs. In order to assess the role of MMP13 on TGFβ/BMP signaling in regulating FAP differentiation, we treated wildtype and MMP13 KO FAPs with TGFβ1, BMP7, TGFβ inhibitor, and BMP inhibitor. TGFβ1 treatment significantly enhanced fibrogenesis, but inhibited adipogenesis of wildtype FAPs. However, treatment with BMP7 showed the opposite effect. Interestingly, the effect of TGFβ1/BMP7 was voided in MMP13 KO FAPs. Treating wildtype FAPs with MMP13 inhibitor also abolished the effect of TGFβ1/BMP7 in FAP differentiation.Conclusion:
Results from this study showed that TGFβ1 inhibits FAP adipogenesis but stimulates FAP fibrogenesis. BMP7 was shown to promote FAP adipogenesis but reduce its fibrogenesis. The role of the TGFβ/BMP signaling pathway regulating FAP differentiation was found to be MMP13 dependent. This study suggests that MMP13 is a critical downstream effector in TGFβ/BMP pathway which may serve as a new therapeutic target for muscle fibrosis and FI.Introduction
Muscle fibrosis and fatty infiltration (FI) are common sequalae of various muscle diseases and disorders. Recent studies indicate that muscle residential fibro/adipogenic progenitors (FAPs), a group of intramuscular interstitial non-myogenic progenitors, are the major cellular source for fibrotic and fat tissue seen in muscle fibrosis and FI [1-3]. In our previous study, we demonstrated that FAPs are the origin of adipocytes and fibroblasts in rotator cuff muscles after rotator cuff tears [4]. However, the signaling pathway regulating FAP adipogenesis during muscle FI has not been clearly identified.
Extracellular matrix (ECM) forms a complex architecture that supports blood vessels and nerves as well as connects myofibers in muscle [5-7]. ECM also participates in fiber force transduction, maintains normal muscle function, stimulates muscle progenitor cell differentiation, and affects a muscle’s ability to adapt to disease and injury [8]. Matrix metalloproteinases (MMPs) are the major enzymes responsible for ECM remodeling in muscle [9]. MMPs are other ECM proteases known to affect a variety of cellular functions including cell proliferation, migration, and differentiation [10].
MMP13 (collagenase 3) is an important member of the MMP family that possesses the ability to digest collagen [11]. MMP13 has been shown to affect cellular migration, thus serving as a marker for metastatic cancers [12]. Upregulation of MMP13 activity enhances myoblast migration [13]. Satellite cells secrete MMP13 to facilitate their migration during muscle regeneration after acute injury [14]. In our previous work, we have observed significantly increased expression of MMP13 in rotator cuff muscle after tendon injury in a sheep model [15]. Follow-up work showed that MMP13 knockout (KO) mice have significantly increased muscle fatty infiltration after massive rotator cuff tears [16]. Though its overall expression level is relatively low, MMP13 expression is highly cell specific in muscle. Among all the cells in muscle, FAPs expressed the highest level of MMP13 in contrast to myoblast and muscle satellite cells during muscle regeneration [17].
The transforming Growth Factor Beta (TGFβ) pathway is involved in various cellular processes including cell growth, differentiation, and apoptosis. Particularly in skeletal muscle, the TGFβ pathway regulates fibroblast differentiation and fibrosis in regeneration [18]. Bone morphogenetic protein 7 (BMP7) is a member of the TGFβ superfamily, which regulates adipogenesis in skeletal muscle [19, 20]. However, the functional role of MMP13 in muscle TGFβ/BMP pathways in remains unknown. In this study, we sought to define the role of MMP13 in TGFβ/BMP signaling and in regulating FAP fibrogenesis and adipogenesis. We hypothesize that MMP13 regulates FAP differentiation that MMP13 is a downstream effector of the TGFβ/BMP pathway.
Materials and Methods
FAP isolation
Quadriceps muscles were harvested from 3-month-old male and female MMP13- knockout mice (a kind gift from Dr. Stephen M. Krane, Harvard Medical School) and age matched colony-control wildtype C57BL/6J mice (3 males and 3 females). Muscles were minced into 1 mm pieces with sterile scissors. The mixture was incubated with 0.2% Collagenase II (Sigma-Aldrich, MO, USA) for 90 minutes at 37°C. Forty milliliters of washing buffer (Ham’s F-10 Nutrient Mix with HEPES, containing 10 % Horse Serum, ThermoFisher, MA, USA) was added into the mixture followed by centrifugation at 1500 rpm for 5 min at room temperature. The supernatant was then transferred to a new 50 mL centrifuge tube. The cell pellet was rinsed again with washing buffer and spun down at 1500 rpm for 5 min. The supernatant was collected and combined with that from the last round. Nine milliliters of D2 solution (0.06% Collagenase II, 0.15% Dispase (1U/ml) with washing buffer) was added in and the sample was incubated for 30 minutes at 370 C. The solutions were then passed through a 70 μm cell strainer (VWR International) followed by a 40 μm cell strainer (VWR International). The filtered samples were washed with 40 mL FACS buffer (2.5%FBS, 20 mM EDTA, 1xPBS) and cells were spun down at 1500 rpm for 5 minutes. The supernatant was discarded and the cell pellets were resuspended with 500 µL of FACS buffer and incubated with anti-CD31-FITC (BD bioscience, CA, USA), anti-CD45-FITIC (BD bioscience, CA, USA), anti-integrin α7-APC (R&D systems, MO, USA), APC-Cy7-PDGFRα (BD bioscience, CA, USA), and PE-Cy7-Sca1(BD bioscience, CA, USA) for 30 minutes before sorted with FACSAriaTM II (BD bioscience, CA, USA). FAPs were collected from the CD31-/CD45-/ITGA7-/Sca1+/PDGFRα+ population (2).
Cell culture
After sorting, 5,000 cells per well were directly seeded into pre-coated Mstrigel 24 well plates. Cells were cultured for 1 week with standard cell culture medium (SM) (Ham’s F-10 medium with 10% fetal bovine serum and 10 ng/ml bFGF, Thermo Fisher Scientific) and 1% antibiotic-antimycotic solution to allow for spontaneous fibro/adipo differentiation. To facilitate FAP adipogenic differentiation, additional cells were cultured with adipogenic differentiation (500 μM IBMX, 0.25 μM Dexamethasone, 100 μM Indomethacin, 10 μg/ml Insulin).
MMP13 treatment
Recombinant Human MMP13 (R&D systems, 511-MM-010, Minneapolis, MN, USA) were first activated with 1 μM APMA p-Aminophenylmercuric acetate (APMA), (Sigma, Catalog # A-9563, MO, USA) at 37°C for 2 hours. The activated MMP13 was then diluted with 0.1% DMSO and administered to the FAP culture medium at a final concentration of 100 ng/ml. Medium was then changed with freshly activated MMP13 every other day.
MMP13 inhibition
In order to inhibit intrinsic MMP13 activity from FAPs, MMP13 inhibitor (Cayman Chemical, Item #19540, CAS #544678-85-5, Michigan, USA) [21] was added to the FAP culture medium with a final concentration of 10 μM. Medium was then changed with fresh MMP13 inhibitor every other day.
TGFβ-1/BMP7 treatment
In order to test the role of TGFβ and BMP signaling in FAP adipogenesis, 5 ng/mL of TGFβ-1 (R&D systems, USA) or 100 ng/mL of BMP-7 (R&D systems, MO, USA) was added into FAPs’ standard cell culture medium (SM) (Ham’s F-10, 10% fetal bovine serum, 10 ng/ml bFGF and 1% antibiotic-antimycotic). The Culture media was changed every other day with fresh TGFβ-1 or BMP-7. In order to inhibit TGFβ and BMP signaling, 1 μM TGFβ inhibitor (SB431542, Cat# 1614/10, R&D systems, MO, USA) or 10 μM BMP inhibitor (LDN193189, Cat#72147, Stem Cell technology, BC, Canada) was added to the standard culture medium. Ten micromole of MMP13 inhibitor was also added in combination with TGFβ1, BMP-7, SB431542 (TGFβ inhibitor) and LDN193189 (BMP inhibitor) in separated groups. Two weeks after treatment, cells were either fixed followed by staining for perilipin A (adipogenic marker) and αSMA (fibrogenic marker) (N=6) or dissolved in Trizol reagent for RNA extraction (N=6).
Immunofluorescent staining
The FAPs were stained with goat-anti-mouse perilipin A (1:1000, ab3526, Abcam, CA, USA) or pre-conjugated mouse-anti-mouse Cy 3 alpha smooth muscle actin (αSMA, 1:500, c6198. Sigma-Aldrich, MO, USA) and then stained with Donkey-anti-Goat FITC (1:2000, ab6717, Abcam, CA, USA). DAPI was added to the mounting medium to stain the nuclei. The adipogenesis index was determined by the percentage of cells expressing perilipin over the total number of nuclei in each image. The fibrogenesis index was determined by the percentage of cells expressing αSMA over the total number of nuclei in each image.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total cell RNA was isolated using Trizol reagent (Invitrogen Inc., CA, USA) from cells according to manufacturer’s instructions. cDNA was synthesized using the Maxima First Strand cDNA Synthesis Kit (ThermoFisher, MA, USA) Real-time PCR was performed to quantify the expression of adipogenesis markers of Adiponectin (ACRP30), PPARγ, C/EBPA and fibrogenesis markers of ACTA2 and Col1a using SYBR with an Applied Biosystems Prism 7900HT Real-Time PCR system (Applied Biosystems Inc., Foster City, CA). Adiponectin is an adipose tissue- specific adipocytokine [22]. Peroxisome proliferator-activated receptor gamma (PPARγ) is a lipid-activated nuclear hormone receptor with roles in adipocyte differentiation [23]. CCAAT/enhancer-binding protein alpha (C/EBPA) induces adipogenesis through PPARγ [24]. Actin alpha 2 smooth muscle (ACTA2) provides instructions for making the protein - smooth muscle alpha a-2 actin, which is a marker of fibroblasts [25]. Collagen 1a (COL1A) is responsible for producing collagen, and shown to be a marker for fibrosis [26]. Sequences of primers are listed in Table 1. The gene expression level was normalized to the internal control of GAPDH. Fold changes were calculated using ΔΔCT.
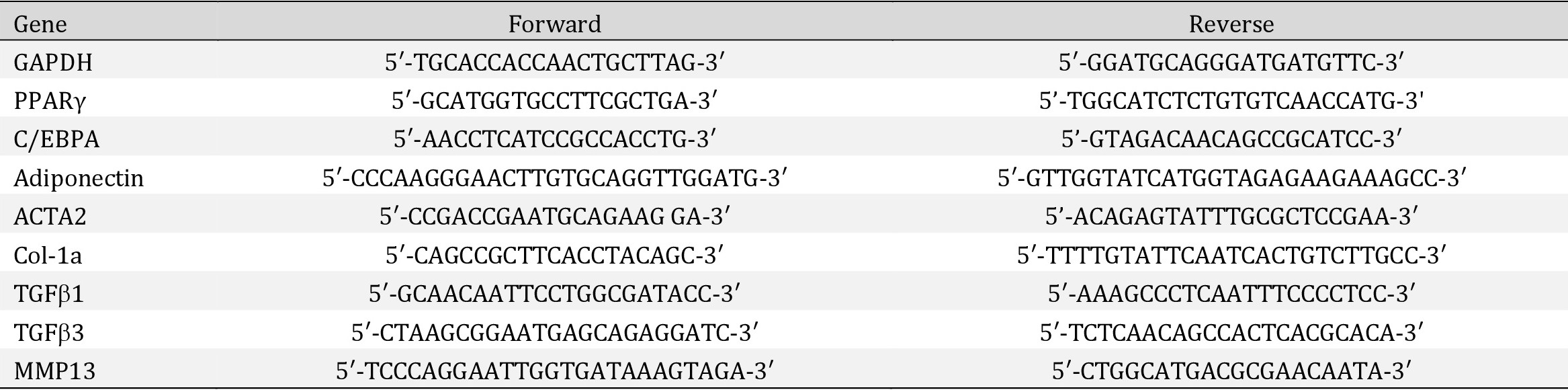
Table 1: RT-PCR primer sequences
Statistical Analysis
T-test was used for data analysis between comparison of wild type and MMP13 KO FAP, as well as between MMP13 (or MMP13 inhibitor) and DMSO-treated wildtype FAPs. One-way ANOVA analysis with Tukey post-hoc multiple comparison was used for TGFβ/BMP treatment experiments. A Significant difference was considered when p<0.05.
Results
MMP13 KO FAPs have increased adipogenesis and reduced fibrogenesis potency compared to wildtype FAPs
After culturing for 14 days in standard medium, 48.3%±8.5% of MMP13 KO FAPs was stained positive for perilipin A. However, 17.9%±6.4% of wildtype FAPs stained positive for perilipin A. In MMP13 KO FAPs, 36.1%±4.5% stained positive for αSMA while 65.4%±14.21% of wildtype FAPs stained positive for αSMA. The MMP13 KO FAPs had significantly increased perilipin (+) and decreased αSMA (+) stained cells compared to wildtype FAPs (p<0.01 for both) (Fig. 1A-C). The RT-PCR results showed significantly increased expression of adiponectin and decreased expression of collagen Type 1a in MMP13 KO FAPs compared to wildtype FAPs (Fig. 1D). The same trend was observed in wildtype and MMP13 KO FAPs cultured in adipogenic medium (Supplementary Fig. 1 – for all supplementary material see www.cellphysiolbiochem.com).
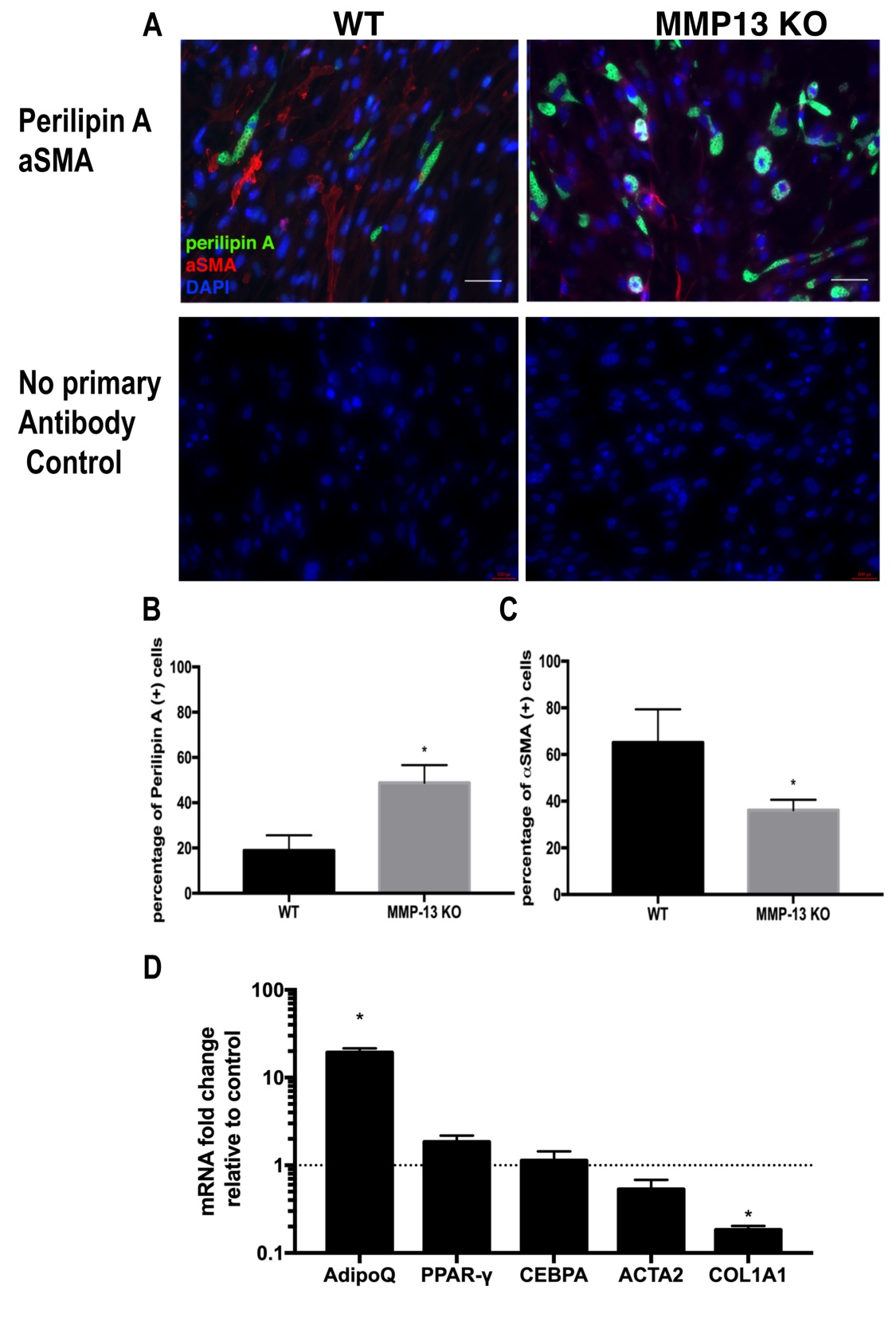
Fig. 1: FAPs from MMP13 KO mice have increased spontaneous adipogenesis and decreased spontaneous fibrogenesis compared to FAPs from wildtype mice cultured in a standard medium. A) A typical image of immunostaining for perilipin A and αSMA for FAPs from WT and MMP13 KO mice after 2 weeks of culture in standard medium Bottom: negative control of perilipin A immunofluorescence staining (without primary antibody), negative control of αSMA immunofluorescence staining (without primary αSMA antibody). B) FAPs from MMP13 KO mice had a significantly higher percentage of perilipin A positive cells when compared to FAPs from WT mice. C) FAPs from MMP13 KO mice had significantly lower αSMA positive cells when compared to FAPs from WT mice. D) Real time PCR showed that FAPs from MMP13 KO mice had significantly higher expression of Adiponectin, but a lower expression of Collagen I compared to FAPs from wildtype mice (* p<0.05).
MMP13 inhibition promotes adipogenesis and decreases fibrogenesis in wildtype FAPs
Two weeks of treatment with the MMP13 inhibitor on wildtype FAPs in standard medium resulted in a significantly increased percentage of perilipin (+) adipocytes compared to the control group treated with DMSO (56.4%±4.3% in the MMP13 inhibitor treatment group vs. 24.2%±10.1% in the DMSO control group). The MMP13 inhibitor also significantly reduced αSMA (+) fibroblast percentage when compared to the DMSO-treated control group (38.2%±7.3% in the MMP13 inhibitor treatment group vs 61.6%±6.5% in the DMSO control group, p<0.01 for both). In support of the immunofluorescence data, RT-PCR results demonstrated significantly increased expression of adipogenesis-related genes and decreased fibrogenesis-related genes in FAPs treated with MMP13 inhibitor when compared to DMSO (Fig. 2). The same trend was observed in MMP13 inhibitor treatment of FAPs cultured in the adipogenic medium (Supplementary Fig. 2).
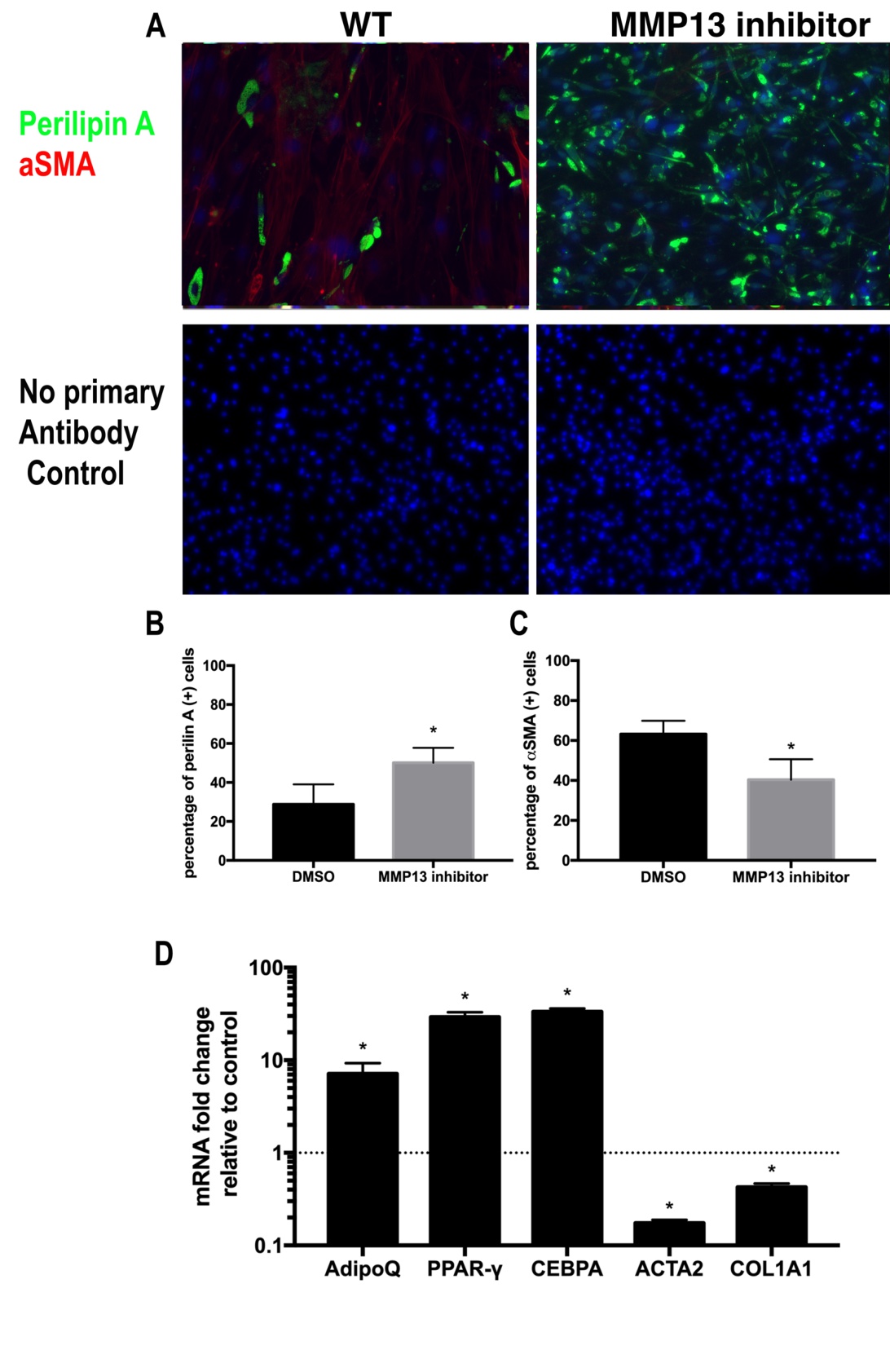
Fig. 2: Wildtype FAPs treated with MMP13 inhibitor had significantly increased adipogenesis. A) A Typical image of immunostaining for FAPs treated with 10μM MMP13 inhibitor and 0.1% DMSO in standard medium for 2 weeks. B) Wildtype FAPs treated with MMP13 inhibitor had a significantly higher percentage of perilipin A(+) cells compared to those treated with DMSO. C) FAPs treated with MMP13 inhibitor had a significantly reduced number of αSMA(+) cells compared to those treated with DMSO. D) Real time PCR showed that FAPs treated with the MMP13 inhibitor had a significantly increased expression of Adiponectin, PPARγ, C/EBPA and deceased expression of αSMA and Collagen 1a (* p<0.05).
MMP13 inhibits adipogenesis but promotes fibrogenesis in wildtype FAPs
Active MMP13 molecules were added to a standard medium of wildtype FAPs for 14 days (Fig. 3A). MMP13 treatment significantly decreased perilipin A (+) cell percentage in FAPs (4.1%±2.9% in MMP13 treated group vs. 30.9%±8.4% in DMSO treated control group, N=6, p<0.05). MMP13 treatment also resulted in an increased percentage of αSMA (+) in FAPs when compared to the DMSO-treated control group (95.3%±3.9% in the MMP13 treatment group vs. 61.3%±5.7% in DMSO treatment control group, N=6, p<0.05). MMP13 significantly decreased expression level of adipogenesis-related genes of Adiponectin and PPARγ and increased expression level of fibrogenesis-related genes of αSMA and Collagen 1a in FAPs (Fig. 3). The same trend was also observed in the adipogenic medium (Supplementary Fig. 3).
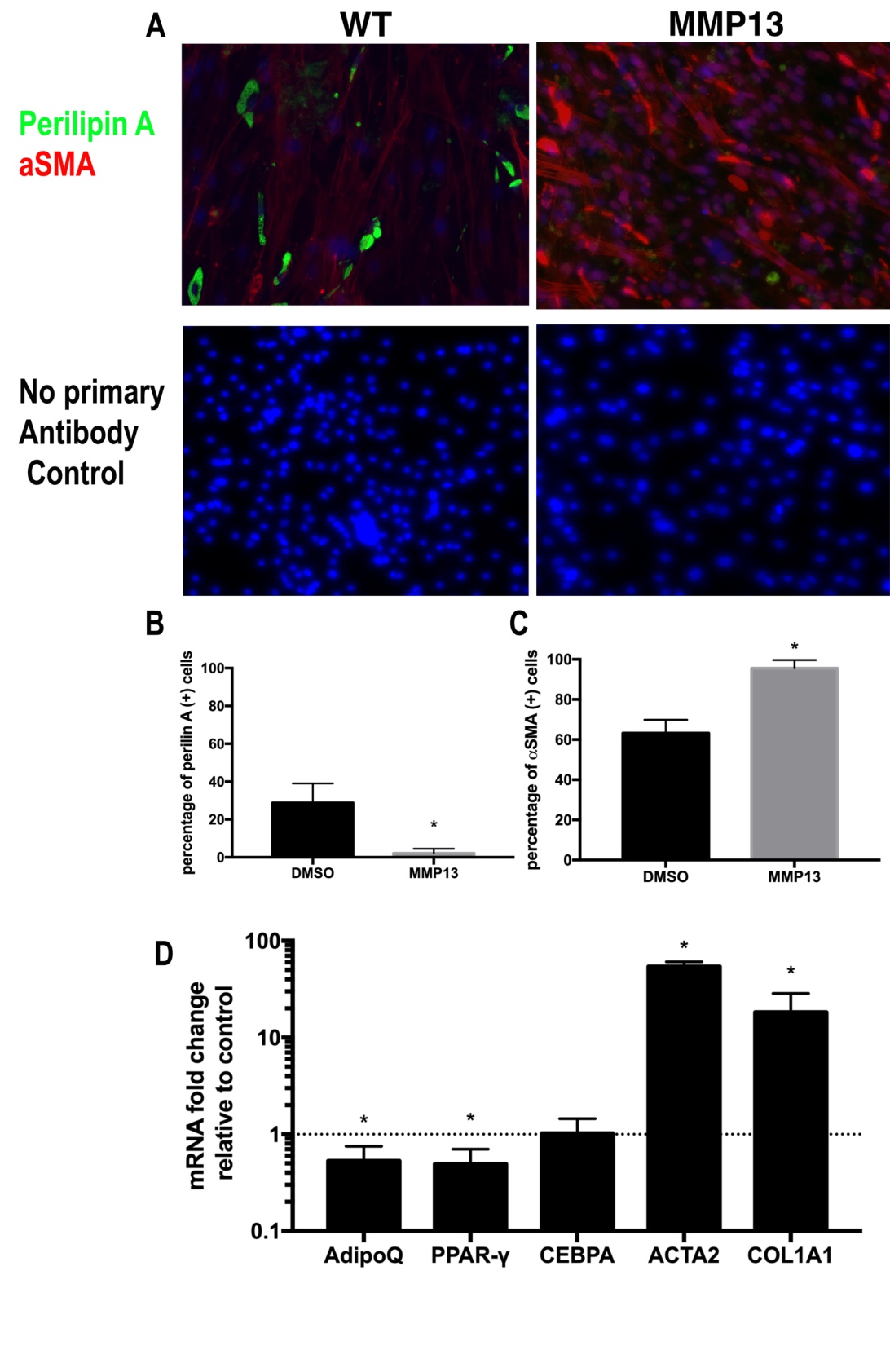
Fig. 3: MMP13 treatment inhibits FAP adipogenesis and promotes FAP fibrogenesis. A) A typical image of immunostaining for FAPs treated with 100 ng/ml MMP13 and 0.1% DMSO in standard medium for 2 weeks. B) FAPs treated with MMP13 have a significantly reduced the number of perilipin (+) cells compared to DMSO. C) FAPs treated with MMP13 have a significantly increased the number of αSMA (+) cells compared to DMSO. D) Real time PCR results of FAPs treated with MMP13 have a significantly higher expression of αSMA and collagen I and decreased expression of Adiponectin and PPARγ (* p<0.05).
TGFβ promotes fibrogenesis and inhibits adipogenesis in wildtype FAPs but not in MMP13 KO FAPs
To study the role of the TGFβ pathway in regulating FAP adipogenesis with the MMP13 deficient condition, FAPs from the MMP13 KO and wildtype mice were both treated with TGFβ-1 and TGFβ inhibitor (SB431542). After 14 days of treatment, TGFβ-1 significantly reduced perilipin (+) cell percentage but increased αSMA (+) cell percentage in wildtype FAPs (perilipin (+) 2.52%±1.36% in TGFβ vs. 23.1%±2.14% in DMSO). However, there was no significant difference between the TGFβ-1 treatment group and the DMSO control group on MMP13 KO FAPs (perilipin (+) 40.14%±4.36% in TGFβ vs. 43.21%±6.96% in DMSO N=6, p<0.05). SB431542 significantly increased the perilipin (+) cell percentage and decreased the αSMA (+) cell percentage in wildtype FAPs but had no effect on MMP13 KO FAPs (Fig. 4A-C). TGFβ-1 and SB431542 significantly changed the gene expression level of fibrogenesis and adipogenesis related genes, results inconsistent with our immunofluorescence findings. However, neither TGFβ-1 nor SB431542 had an effect on the expression of those genes in MMP13 KO FAPs (Fig. 4D-E).
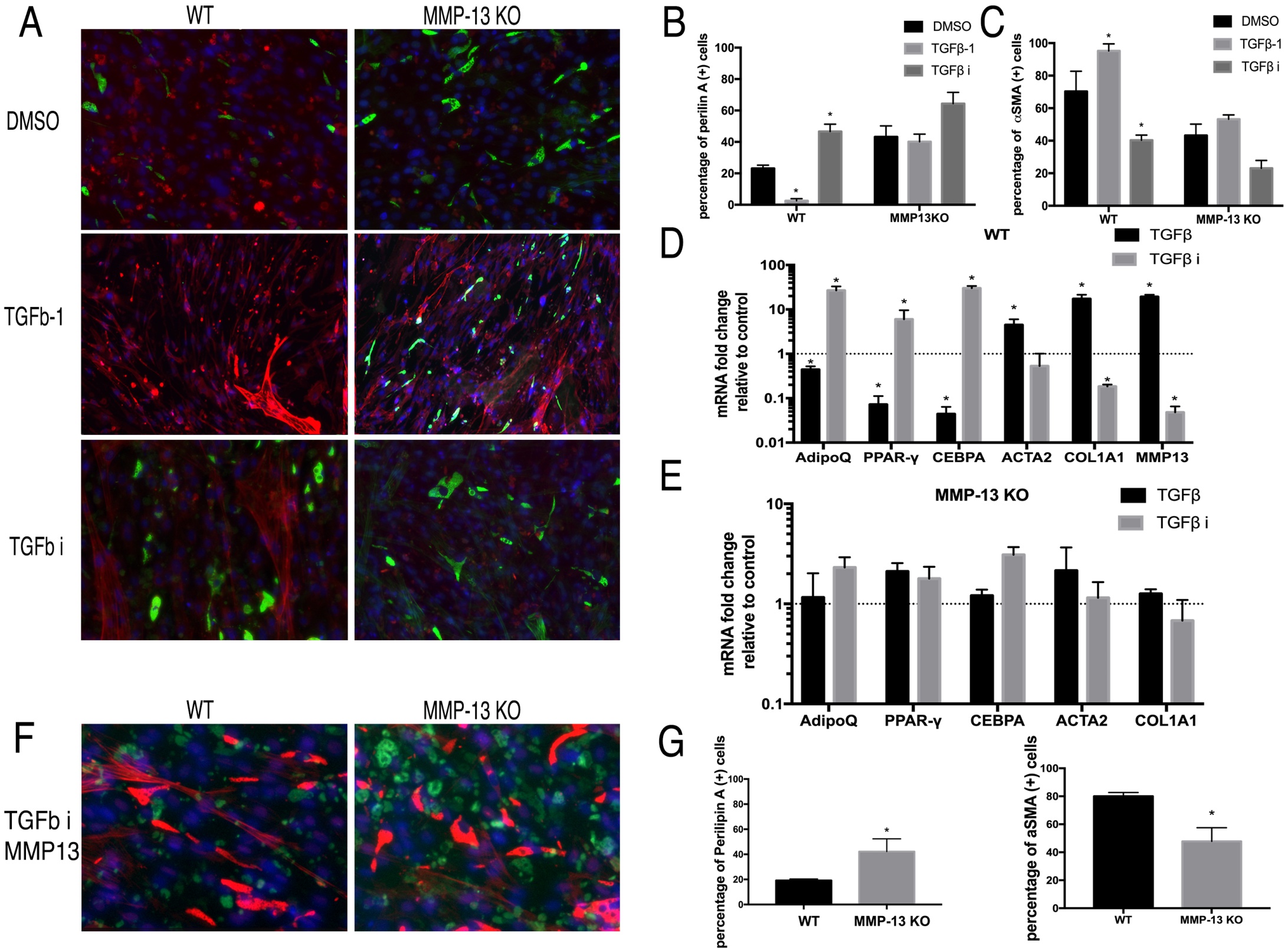
Fig. 4: A) The typical images of FAPs from wildtype and MMP13 KO mice treated with TGFβ-1, TGFβ inhibitor, or 0.1% DMSO in a standard medium for 2 weeks. B) TGFβ-1 significantly decreased the number of perilipin (+) cells and the TGFβ inhibitor significantly increased the number of perilipin (+) cells in FAP WT mice, but not in FAPs from MMP13 KO mice. C) TGFβ-1 significantly increased the number of αSMA (+) cells and the TGFβ inhibitor significantly decreased the number of αSMA (+) cells in FAPs from WT mice, but not in FAPs from MMP13 KO mice. D) & E) Real time PCR results showed that TGFβ-1 significantly decreased the expression of adipogenesis-related genes and increased fibrogenesis-related gene expression of FAPs in WT mice, while the TGFβ inhibitor had an opposite effect. However, the effect of the TGFβ-1 and TGFβ inhibitors had no effect on their expression in FAPs from MMP13 KO mice ( * p<0.05). F) Exogenous MMP13 added to the TGFβ inhibitor treatment group in both WT mice and MMP13 KO mice. Exogenous MMP13 added to the MMP-13 KO mice as a control. G) Quantification of the percentage of the number of Perilipin A(+) cells and αSMA (+) cells out of total number of cells.
TGFβ showed the same effects as in Wildtype FAPs after addition of exogenous MMP13 molecule into MMP13 KO FAPs
Next, exogenous active MMP13 was added to the MMP13 KO FAPs. With exogenous MMP13, TGFβ inhibitor significantly increased the percentage of perilipin A (+) cells within MMP13 KO FAPs compared to the DMSO control group (42.2%±10.21% in the MMP13 treated group, vs 19.2%±1.19% in DMSO control group). This data suggested that the exogenous MMP13 could compensate for the intrinsic loss of MMP13 in FAPs and resume the role of TGFβ signaling in regulating MMP13 KO FAPs adipogenesis (Fig. 4F-G).
BMP7 promotes adipogenesis and inhibits fibrosis in wildtype FAPs but not MMP13 KO FAPs
Contrary to TGFβ-1, BMP7 significantly increased perilipin (+) cell percentage but decreased αSMA (+) cell percentage in wildtype FAPs. However, BMP-7 had no effect on MMP13 KO FAPs differentiation. LDN193189 (BMP-7 inhibitor) significantly decreased perilipin (+) cell percentage and increased αSMA (+) cell percentage in wildtype FAPs. LDN193189 showed no effect on the expression of those genes in MMP13 KO FAPs. (Fig. 5A-C). RT-PCR results supported the immunofluorescence findings in wildtype and MMP13 KO FAPs (Fig. 5D-E).
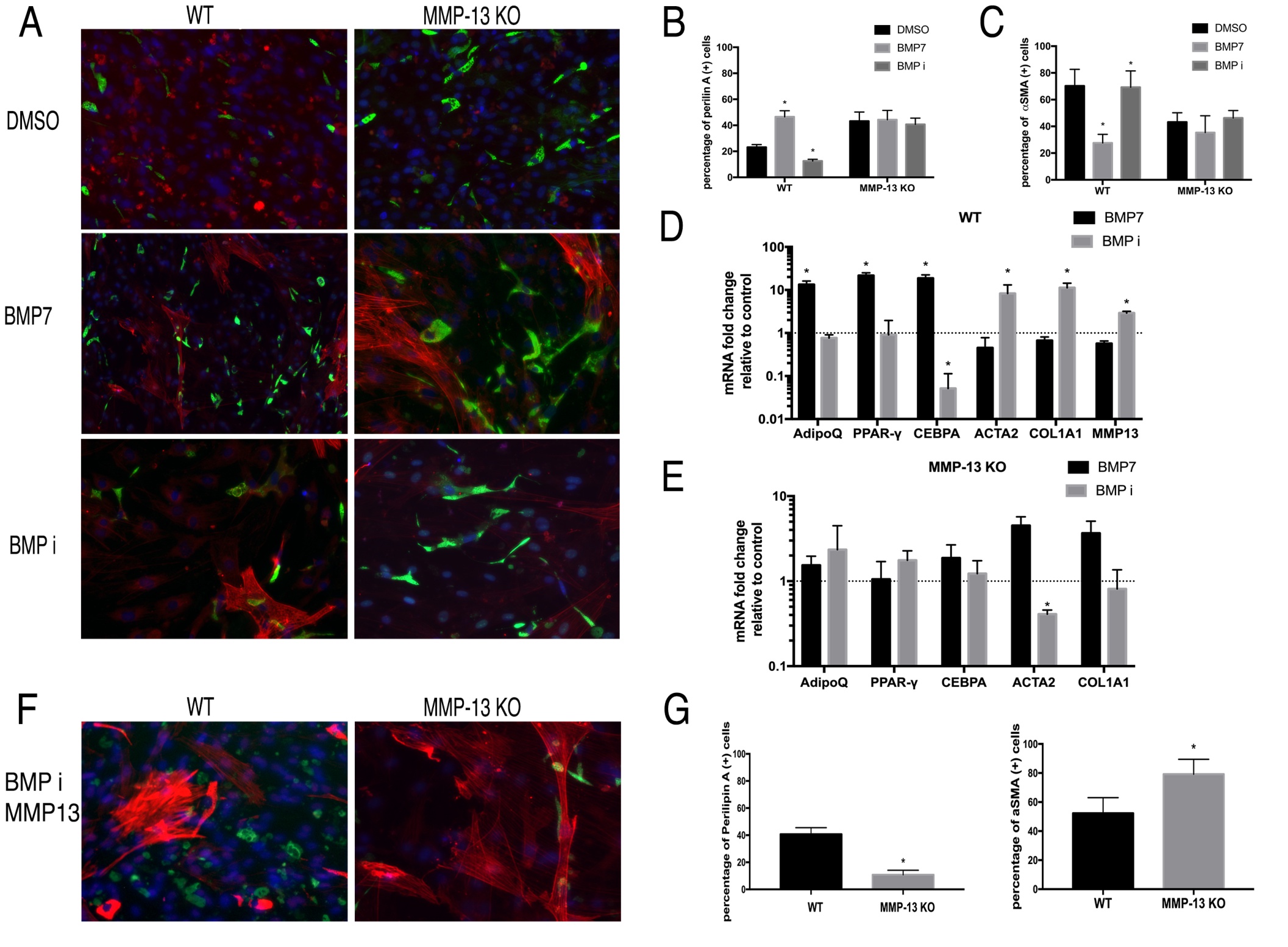
Fig. 5: A) Typical images of FAPs from WT and MMP13 KO mice treated with BMP-7, BMP inhibitor, or 0.1% DMSO in standard medium for 2 weeks. B) BMP-7 significantly increased the number of perilipin (+) cells and the BMP inhibitor significantly decreased the number of perilipin (+) cells in FAPs in WT mice, but not in FAPs from MMP13 KO mice. C) BMP-7 significantly decreased αSMA (+) cells and the BMP inhibitor significantly increased the number of αSMA (+) cells in FAPs in WT mice, but not in FAPs from MMP13 KO mice. D) & E) Real time PCR results showed that BMP-7 significantly increased the expression of adipogenesis-related genes and decreased fibrogenesis-related gene expression of FAPs in WT mice, while BMP inhibitor had an opposite effect. Neither BMP-7 nor BMP inhibitor showed an effect on adipogenesis-related and fibrogenesis-related gene expression in FAPs from MMP13 KO mice ( * p<0.05). F) Exogenous MMP13 added to the BMP inhibitor treatment group in both WT mice and MMP13 KO mice. Exogenous MMP13 was added to the MMP13 KO mice as a control. G) Quantification of Perilipin A and αSMA.
BMP7 showed same effects as in Wildtype FAPs after addition of exogenous MMP13 molecule into the MMP13 KO FAPs
In addition, exogenous active MMP13 was added to the MMP13 KO FAPs. That allowed BMP inhibitor LDN193189 to significantly reduce the percentage of perilipin A (+) cells in MMP13 KO FAPs when compared to the DMSO treated control group (10.8%±3.33% in the BMP inhibitor group, vs 40.7%±4.81% in DMSO control group). This data suggested that the exogenous MMP13 could compensate for the intrinsic loss of MMP13 in FAPs and resume the role of BMP signaling in regulating MMP13 KO FAPs adipogenesis (Fig. 5F-G).
MMP13 inhibition blocked the effect of TGF β-1 and SB431542 on wildtype FAP differentiation
In order to confirm that the phenotype seen in MMP13 KO FAPs was due to the loss of MMP13 enzymatic activity, we treated wildtype FAPs with TGFβ-1 or SB431542 in combination with an MMP13 inhibitor. TGFβ-1 significantly reduced the perilipin (+) cell percentage and increased αSMA (+) cell percentage in wildtype FAPs, but had no effect on MMP13 inhibitor-treated FAPs. SB431542 significantly increased the perilipin (+) cell percentage and decreased thhw perilipin (+) cell percentage in wildtype FAPs but had no effect on MMP13 inhibitor treated FAPs (Fig. 6A-C).
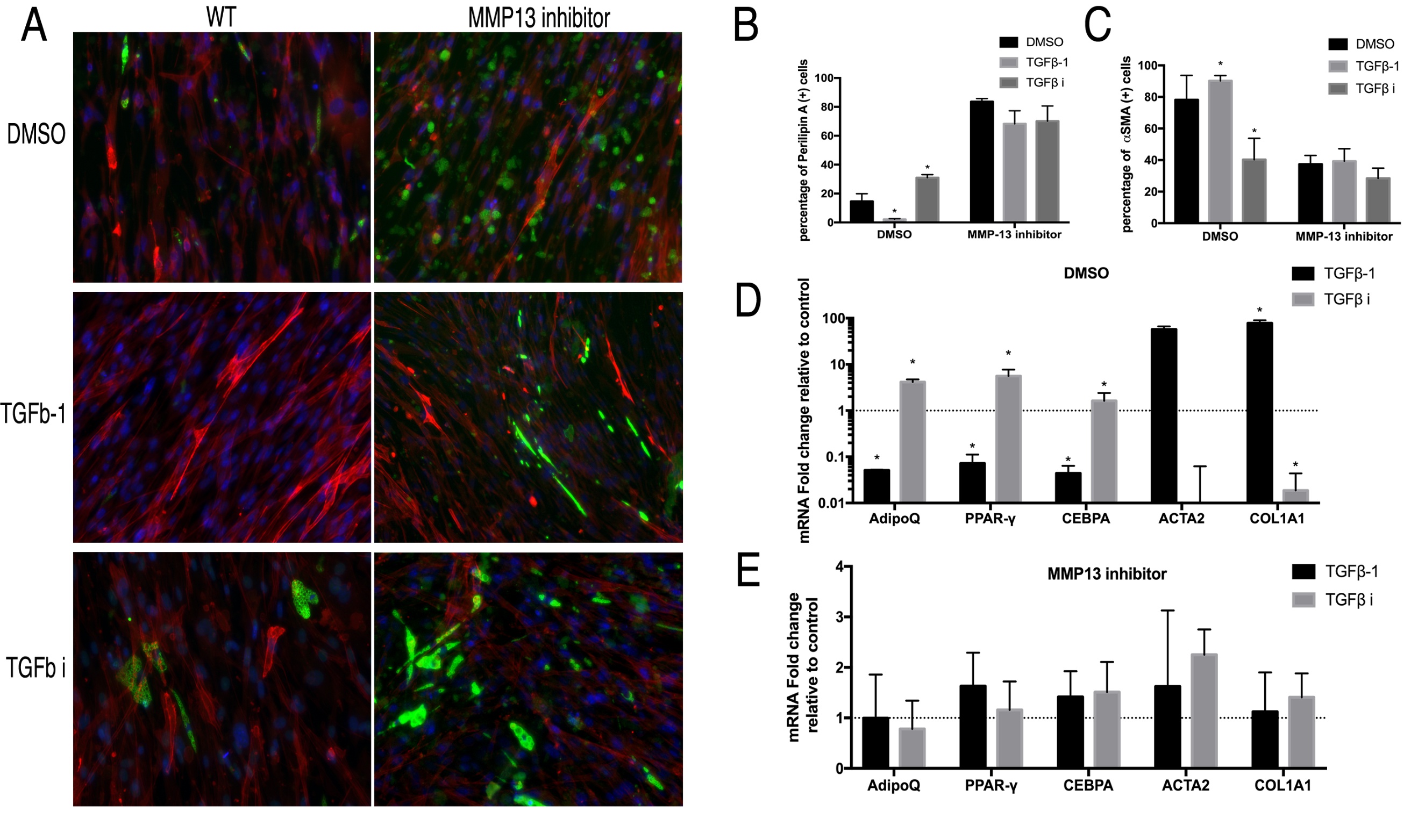
Fig. 6: A) Typical images of FAPs from WT mice treated with the MMP13 inhibitor (or DMSO) in combination of TGFβ-1 and TGFβ inhibitor in standard medium for 2 weeks. B) TGFβ-1 significantly decreased the number of perilipin A(+) cells and TGFβ inhibitor significantly increased the number of perilipin A (+) cells in FAPs without the MMP13 inhibitor. However, MMP13 inhibitor blocked the effect of TGFβ-1 and TGFβ inhibitors. C) TGFβ-1 significantly increased the numbers of αSMA (+) cells and the TGFβ inhibitor significantly decreased the number of αSMA (+) cells in FAPs without the MMP13 inhibitor. However, the MMP13 inhibitor blocked the effects of the TGFβ-1 and TGFβ inhibitor. D) & E) Real time PCR results showed that TGFβ-1 significantly decreased adipogenesis-related gene expression and increased fibrogenesis-related gene expression of FAPs without the MMP13 inhibitor, but not in combination with the MMP13 inhibitor (* p<0.05).
MMP13 inhibitor blocked the effect of BMP-7 and LDN193189 on wildtype FAP differentiation
MMP13 inhibition in combination with BMP-7 or LDN193189 simulated the phenotype of MMP13 KO FAPs on wildtype FAPs. The effect of BMP-7 and LDN193189 on FAP fibrogenesis and adipogenesis was completely abolished with the use of MMP13 inhibitor. Both immunofluorescence and RT-PCR showed that BMP-7 and LDN193189 had no effect on FAP differentiation in combination with the MMP13 inhibitor (Fig. 7).
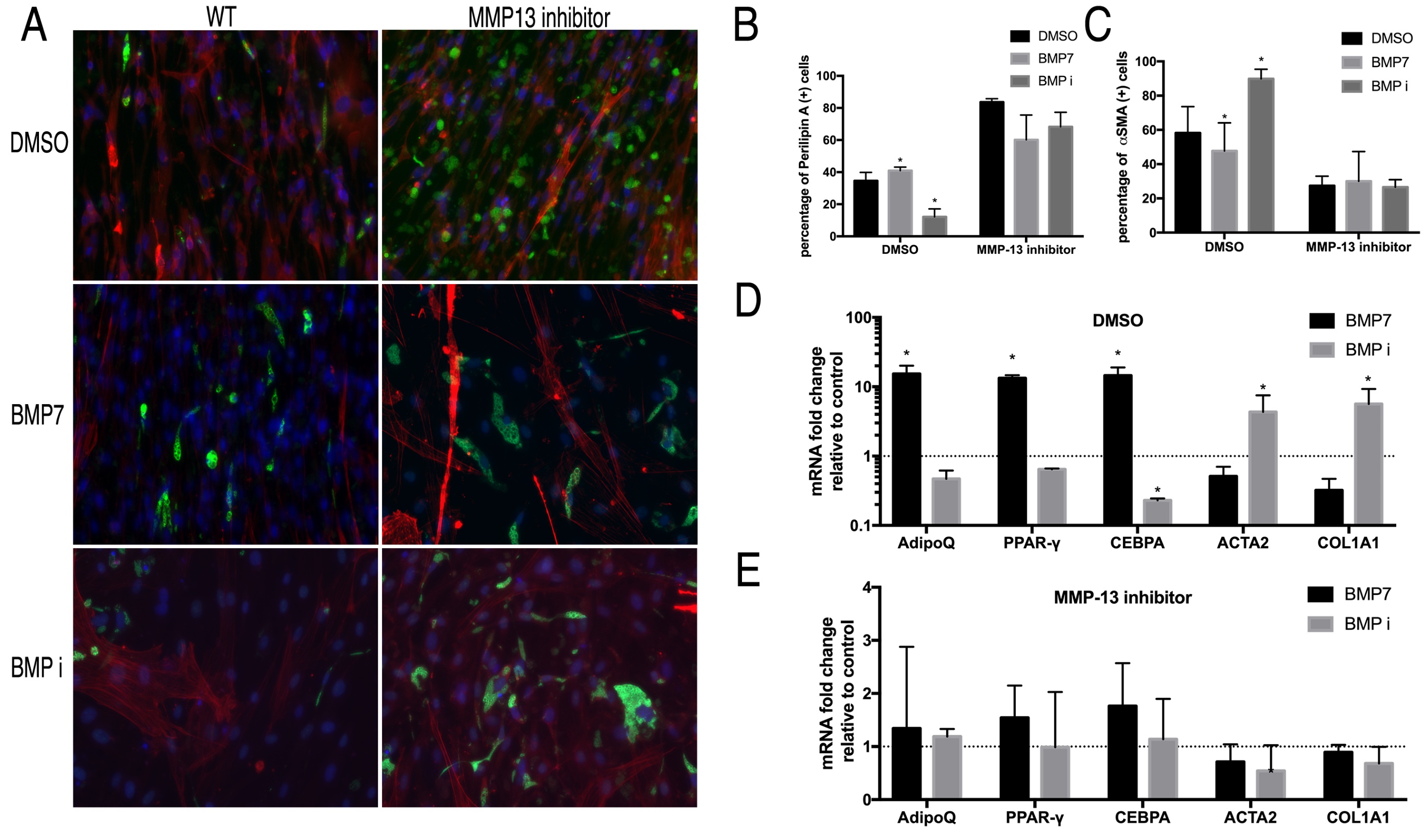
Fig. 7: A) Typical images of FAPs from WT mice treated with an MMP13 inhibitor (or DMSO) in combination with BMP-7 and the BMP inhibitor in standard medium for 2 weeks. B) BMP-7 significantly increased the number of perilipin (+) cells and BMP inhibitor significantly decreased the number of perilipin (+) cells in FAPs without the MMP13 inhibitor, but not in combination with the MMP13 inhibitor. C) BMP-7 significantly decreased the number of αSMA (+) cells and the BMP inhibitor significantly increased the number of αSMA (+) cells in FAPs from WT mice, but not in FAPs from MMP13 KO mice. D) & E) Real time PCR results showed that BMP significantly increased the expression of adipogenesis-related genes and decreased the expression of fibrogenesis-related in FAPs from WT mice, while the BMP inhibitor had an opposite effect. However, the effect of BMP-7 and BMP inhibitor was abolished in FAPs from MMP13 KO mice (* p<0.05).
Discussion
The TGFβ family is a common signal transduction pathway that regulates stem cell differentiation [27]. TGFβ is considered the master regulator of tissue fibrosis [28, 29]. TGF-β has also been shown to have a role in stem/progenitor cell adipogenesis. It has been reported that TGF-β1 inhibits preadipocyte 3T3-L1 differentiation into mature adipocytes [30]. BMP, another subgroup of the TGFβ superfamily, also has a role in stem cell adipogenesis. Both TGFβ and BMP engage serine/threonine kinase receptors that phosphorylate Smads, which are subsequently transported to the nuclei and governs gene expression in a cell through a context dependent manner. Previous studies have demonstrated the key role of BMP signaling, especially BMP-7, in playing an important role in promoting stem cell adipogenesis [19, 31]. The results from this study demonstrated that TGFβ1 and BMP7 play important but opposing roles in the regulation of FAP differentiation: BMP7 promotes FAP adipogenesis, while TGFβ1 inhibits FAP adipogenesis. While TGFβ1 promotes FAP fibrogenesis, BMP-7 inhibits this process.
MMP13 (collagenase 3) is an important member of MMP family, that possesses the ability to digest collagen. The MMP13 gene is comprised of 10 exons and 9 introns spanning a length of 12.5kb on chromosome 11 [32]. There is a TIE (TGFb inhibitory element) site in the MMP13 promoter. There are also two other sites that resemble consensus sequences for the activin-response element and Smad binding element [32]. Previous studies have reported that TGFb regulates MMP13 expression in various tissues. A recent study showed that BMP-14 inhibits MMP13 expression in chondrocytes [33]. However, the role of BMP-7 in regulating MMP13 expression has not reported. Knocking-out of the MMP13 gene abolished the role of TGFβ/BMP in FAP fibro/adipogenesis. These data suggest that TGFβ/BMP is upstream of signals that regulate MMP13 gene expression during FAP differentiation. Lei et al. [13] reported that MMP13 had an influence on the migration of muscle progenitor cells. However, the role of MMP13 on FAP proliferation, migration and differentiation, especially how MMP13 involved in the TGFβ/BMP signaling pathway in FAP adipogenesis, remains unknown. In this study, we found that MMP13 also has a role in affecting the TGFβ/BMP gene expression in FAPs. This data suggests TGFβ/BMP–MMP13 maintains a positive feedback loop in FAP differentiation.
The detailed mechanism of how MMP13 is involved in TGFβ/BMP signaling pathway in FAP adipogenesis remains unknown at this time. The ECM forms a complex architecture that not only integrates mechanical signals, but also stores various growth factors including TGFβ [34]. It has been reported that TGFβ is secreted and stored as a latent form in the ECM [31] and the first stage of TGFβ activation – release from the extracellular matrix – is mediated by MMPs [35]. Evidence indicates that BMP signaling is also regulated by the ECM. It has been reported that cartilage oligomeric matrix protein (COMP), an ECM glycoprotein, sustains BMP signaling by acting as a co-receptor that bridges BMPs with other ECM components like collagen [36]. A recent study showed that BMP-7 activity is regulated by an ECM component, named fibrillin-1. Upon pro-domain binding to fibrillin-1, the BMP-7 complex undergoes a conformational change, which denies access of BMP receptors to the growth factor, thus inactivating BMP-7 [37]. Thus, it is possible that MMP13 mediated ECM digestion is required for TGFβ/BMP-7 activation in regulating FAP adipogenesis after FAPs secretion of the TGFβ and BMP-7 proteins into the extracellular matrix. Future work is needed to demonstrate the detailed mechanisms.
There are some limitations in this study that ought to be addressed. First of all, this was an in vitro study with primary cultured murine FAPs. Conclusions from study will be validated in human FAPs in vitro and in vivo studies in the near future. Secondly, TGFβ and BMP inhibitors that were used in this study are not extremely selective to TGFβ1 and BMP-7. SB431542 inhibits the activin receptor-like kinase (ALK) receptors 4, 5 and 7, which consequently can be inhibit the activin signaling pathway. LDN193189 inhibits ALK 2 and 3, thus inhibiting all BMP pathways. Thirdly, though the MMP13 inhibitor we used in this study is relatively selective, it also inhibits MMP-1 at higher concentrations. However, results from the MMP13 inhibitor experiments are consistent with those from MMP13 KO FAPs. Thus, we believe our results are reliable. Lastly, only a single dose of TGFβ1, BMP-7, MMP13 and their inhibitors were used in this study. The working dose for each reagent was decided based on previous experiments or literature. Future work may include a dose range to define the most effective dose for each reagent.
Conclusion
In conclusion, our study clearly demonstrates that MMP13 plays a role in regulating FAP adipogenic differentiation. Data from this study further suggests that MMP13 is the downstream effector of TGFβ/BMP signaling in regulating FAP adipogenesis.Abbreviations
BMP (Bone Morphogenetic Protein); FAPs (fibro/adipogenic progenitors); FI (Fatty infiltration); KO (knockout); MMP13 (Matrix metalloproteinase-13); TGFb (Transforming Growth Factor beta); WT (Wild Type); αSMA (Alpha Smooth Muscle Actin).Acknowledgements
We thank Ms. Colby Choi for her assistance in cell culture and Ms. Olivia Wu for her assistance in editing.
Author Contributions
Mengyao Liu: Conceptualization, Investigation, Writing-original draft; Brian Feeley: Supervision; Hubert Kim: Supervision, Funding acquisition, Xuhui Liu: Conceptualization, Writing - Review Editing, Supervision, Funding acquisition.
Funding
This material is based upon work supported by U.S. Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Merit Review Grant (1I01BX002680) and NIH/NIAMS Research Grant (1R01AR072669-01A1).
Statement of Ethics
This article does not contain any studies with human participants or animals performed by any of the authors.
Disclosure Statement
The authors declare that no conflict of interests exists.
References
| 1 | Natarajan A, Lemos DR, Rossi FM: Fibro/adipogenic progenitors: a double-edged sword in skeletal muscle regeneration. Cell Cycle 2010;9:2045-2046. https://doi.org/10.4161/cc.9.11.11854 |
| 2 | Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM: Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 2010;12:153-163. https://doi.org/10.1038/ncb2015 |
| 3 | Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K: Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 2010;12:143-152. https://doi.org/10.1038/ncb2014 |
| 4 | Liu X, Ning AY, Chang NC, Kim H, Nissenson R, Wang L, Feeley BT: Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J 2016;6:6-15. https://doi.org/10.11138/mltj/2016.6.1.006 |
| 5 | Cukierman E, Pankov R, Stevens DR, Yamada KM: Taking cell-matrix adhesions to the third dimension. Science 2001;294:1708-1712. https://doi.org/10.1126/science.1064829 |
| 6 | Kjaer M: Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 2004;84:649-698. https://doi.org/10.1152/physrev.00031.2003 |
| 7 | Maas H, Huijing PA: Myofascial force transmission between transferred rat flexor carpi ulnaris muscle and former synergistic palmaris longus muscle. Muscles Ligaments Tendons J 2011;1:127-133. |
| 8 | Ohlendieck K: Proteomic profiling of skeletal muscle plasticity. Muscles Ligaments Tendons J 2011;1:119-126. https://doi.org/10.4061/2011/908035 |
| 9 | Chen X, Li Y: Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adh Migr 2009;3:337-341. https://doi.org/10.4161/cam.3.4.9338 |
| 10 | Bonnans C, Chou J, Werb Z: Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014;15:786-801. https://doi.org/10.1038/nrm3904 |
| 11 | Nagase H, Woessner JF, Jr.: Matrix metalloproteinases. J Biol Chem 1999;274:21491-21494. https://doi.org/10.1074/jbc.274.31.21491 |
| 12 | Vihinen P, Kahari VM: Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer 2002;99:157-166. https://doi.org/10.1002/ijc.10329 |
| 13 | Lei H, Leong D, Smith LR, Barton ER: Matrix metalloproteinase 13 is a new contributor to skeletal muscle regeneration and critical for myoblast migration. Am J Physiol Cell Physiol 2013;305:C529-538. https://doi.org/10.1152/ajpcell.00051.2013 |
| 14 | Smith LR, Kok HJ, Zhang B, Chung D, Spradlin RA, Rakoczy KD, Lei H, Boesze-Battaglia K, Barton ER: Matrix Metalloproteinase 13 from Satellite Cells is Required for Efficient Muscle Growth and Regeneration. Cell Physiol Biochem 2020;54:333-353. https://doi.org/10.33594/000000223 |
| 15 | Luan T, Liu X, Easley JT, Ravishankar B, Puttlitz C, Feeley BT: Muscle atrophy and fatty infiltration after an acute rotator cuff repair in a sheep model. Muscles Ligaments Tendons J 2015;5:106-112. https://doi.org/10.11138/mltj/2015.5.2.106 |
| 16 | Liu X, Ravishankar B, Ning A, Liu M, Kim HT, Feeley BT: Knocking-out matrix metalloproteinase-13 exacerbates rotator cuff muscle fatty infiltration. Muscles Ligaments Tendons J 2017;7:202-207. https://doi.org/10.11138/mltj/2017.7.2.202 |
| 17 | Kok HJ, Barton ER: Actions and interactions of IGF-I and MMPs during muscle regeneration. Semin Cell Dev Biol 2021;119:11-22. https://doi.org/10.1016/j.semcdb.2021.04.018 |
| 18 | Ismaeel A, Kim JS, Kirk JS, Smith RS, Bohannon WT, Koutakis P: Role of Transforming Growth Factor-beta in Skeletal Muscle Fibrosis: A Review. Int J Mol Sci 2019;20:2446. https://doi.org/10.3390/ijms20102446 |
| 19 | Winbanks CE, Chen JL, Qian H, Liu Y, Bernardo BC, Beyer C, Watt KI, Thomson RE, Connor T, Turner BJ, McMullen JR, Larsson L, McGee SL, Harrison CA, Gregorevic P: The bone morphogenetic protein axis is a positive regulator of skeletal muscle mass. J Cell Biol 2013;203:345-357. https://doi.org/10.1083/jcb.201211134 |
| 20 | Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR: New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008;454:1000-1004. https://doi.org/10.1038/nature07221 |
| 21 | Nishimura R, Wakabayashi M, Hata K, Matsubara T, Honma S, Wakisaka S, Kiyonari H, Shioi G, Yamaguchi A, Tsumaki N, Akiyama H, Yoneda T: Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J Biol Chem 2012;287:33179-33190. https://doi.org/10.1074/jbc.M111.337063 |
| 22 | Fu Y, Luo N, Klein RL, Garvey WT: Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res 2005;46:1369-1379. https://doi.org/10.1194/jlr.M400373-JLR200 |
| 23 | Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA: Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 1994;135:798-800. https://doi.org/10.1210/endo.135.2.8033830 |
| 24 | Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM: C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev 2002;16:22-26. https://doi.org/10.1101/gad.948702 |
| 25 | Rockey DC, Weymouth N, Shi Z: Smooth muscle alpha actin (Acta2) and myofibroblast function during hepatic wound healing. PLoS One 2013;8:e77166. https://doi.org/10.1371/journal.pone.0077166 |
| 26 | Akamatsu T, Arai Y, Kosugi I, Kawasaki H, Meguro S, Sakao M, Shibata K, Suda T, Chida K, Iwashita T: Direct isolation of myofibroblasts and fibroblasts from bleomycin-injured lungs reveals their functional similarities and differences. Fibrogenesis Tissue Repair 2013;6:15. https://doi.org/10.1186/1755-1536-6-15 |
| 27 | Lee MJ: Transforming growth factor beta superfamily regulation of adipose tissue biology in obesity. Biochim Biophys Acta Mol Basis Dis 2018;1864:1160-1171. https://doi.org/10.1016/j.bbadis.2018.01.025 |
| 28 | Higgins SP, Tang Y, Higgins CE, Mian B, Zhang W, Czekay RP, Samarakoon R, Conti DJ, Higgins PJ: TGF-beta1/p53 signaling in renal fibrogenesis. Cell Signal 2018;43:1-10. https://doi.org/10.1016/j.cellsig.2017.11.005 |
| 29 | Stewart AG, Thomas B, Koff J: TGF-beta: Master regulator of inflammation and fibrosis. Respirology 2018;23:1096-1097. https://doi.org/10.1111/resp.13415 |
| 30 | Zamani N, Brown CW: Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocr Rev 2011;32:387-403. https://doi.org/10.1210/er.2010-0018 |
| 31 | Zhang X, Zhang J, Bauer A, Zhang L, Selinger DW, Lu CX, Ten Dijke P: Fine-tuning BMP7 signalling in adipogenesis by UBE2O/E2-230K-mediated monoubiquitination of SMAD6. EMBO J 2013;32:996-1007. https://doi.org/10.1038/emboj.2013.38 |
| 32 | Pendas AM, Balbin M, Llano E, Jimenez MG, Lopez-Otin C: Structural analysis and promoter characterization of the human collagenase-3 gene (MMP13). Genomics 1997;40:222-233. https://doi.org/10.1006/geno.1996.4554 |
| 33 | Enochson L, Stenberg J, Brittberg M, Lindahl A: GDF5 reduces MMP13 expression in human chondrocytes via DKK1 mediated canonical Wnt signaling inhibition. Osteoarthritis Cartilage 2014;22:566-577. https://doi.org/10.1016/j.joca.2014.02.004 |
| 34 | Taipale J, Keski-Oja J: Growth factors in the extracellular matrix. FASEB J 1997;11:51-59. https://doi.org/10.1096/fasebj.11.1.9034166 |
| 35 | Maeda S, Dean DD, Gomez R, Schwartz Z, Boyan BD: The first stage of transforming growth factor beta1 activation is release of the large latent complex from the extracellular matrix of growth plate chondrocytes by matrix vesicle stromelysin-1 (MMP-3). Calcif Tissue Int 2002;70:54-65. https://doi.org/10.1007/s002230010032 |
| 36 | Ishida K, Acharya C, Christiansen BA, Yik JH, DiCesare PE, Haudenschild DR: Cartilage oligomeric matrix protein enhances osteogenesis by directly binding and activating bone morphogenetic protein-2. Bone 2013;55:23-35. https://doi.org/10.1016/j.bone.2013.03.007 |
| 37 | Wohl AP, Troilo H, Collins RF, Baldock C, Sengle G: Extracellular Regulation of Bone Morphogenetic Protein Activity by the Microfibril Component Fibrillin-1. J Biol Chem 2016;291:12732-12746. https://doi.org/10.1074/jbc.M115.704734 |