Biochemical and Biophysical in Vitro Studies and Systematic Literature Review on the Antioxidant and Antiglycation Activities of Trazodone
bDepartment of Hygiene, Epidemiology and Ergonomics, Medical University of Bialystok, Białystok, Poland;
c1st Department of General and Endocrine Surgery, Medical University of Bialystok, Białystok, Poland;
dIndependent Laboratory of Experimental Dentistry, Medical University of Bialystok, Białystok, Poland
Keywords
Abstract
Background/Aims:
Trazodone is a selective serotonin reuptake inhibitor; however, other mechanisms of the drug’s anti-depressive properties have also been postulated. Hence, the aim of the study was to perform a systematic review and assess antiglycoxidative properties of trazodone in in vitro models.Methods:
Trazodone’s scavenging and chelating properties were measured with spectrophotometric method. The impact of the drug on carbonyl/oxidative stress was marked in the bovine serum albumin (BSA) model where sugars (glucose, fructose, galactose, ribose) and aldehydes (glyoxal and methylglyoxal) were used as glycation agents. Aminoguanidine and N-acetylcysteine (NAC) were applied as reference glycation/free radical inhibitors. Glycation biomarkers (kynurenine, N-formylkynurenine, dityrosine as well as advanced glycation end products contents) were assessed spectrofluorometrically. Concentrations of oxidation parameters (total thiols (TTs), protein carbonyls (PCs) and also advanced oxidation protein products (AOPPs) levels) were determined spectrophotometrically.Results:
We demonstrated that trazodone poorly scavenged radicals (hydroxyl radical, nitric oxide, hydrogen peroxide and 2,2-diphenyl-1-picrylhydrazyl radical) and showed low ferrous ion chelating, unlike aminoguanidine and NAC. Sugars/aldehydes caused enhancement of glycation parameters, as well as a decrease of TTs and an increase of PCs and AOPPs levels compared to BSA incubated alone. Trazodone did not reduce oxidation parameters to the baseline (BSA) and significantly exacerbated glycation markers in comparison with both BSA and BSA+glycators. The content of glycation products was markedly lower in aminoguanidine and NAC than in trazodone. The molecular docking of trazodone to BSA revealed its very low affinity, which may indicate non-specific binding of trazodone, facilitating the attachment of glycation factors.Conclusion:
According to our findings, it may be concluded that trazodone poorly counteracts oxidation and intensifies glycation in vitro . A possible mechanism for antiglycoxidative effect of trazodone in vivo may be the enhancement of the body’s adaptive response, as indicated by the results of our systematic review.Introduction
According to the report of the World Health Organization (WHO) of 2015, about 4.4% of the world’s population, that is 322 million people, suffer from depression [1]. This is an increase by 18.4% since 2005 [2]. The prevalence of depression is the lowest among men of the Western Pacific Region (2.6%) and the highest among African and American women (5.9%) [1]. Since depression worsens patients’ daily functioning, it often prevents them from working. According to the WHO’s predictions, depression will be the leading cause of disability worldwide by 2030 [1, 2].
Depression is a multifactorial disorder. The main biological factor in depression is disrupted interplay between neurotransmitters in the central nervous system – serotonin, norepinephrine, or dopamine [3]. Moreover, imbalance between neuroprotective (e.g., brain-derived neurotrophic factor [BDNF], progranulin, cystatin C) and neuroprogressive (e.g., nuclear factor kappa-light-chain-enhancer of activated B cells [NF-κB] and nitric oxide [NO]) factors in favor of the latter bring negative effects [3, 4]. Another known cause of depression is telomerase shortening and inflammation, which increases oxidative damage at both the central (brain) and systemic (blood) level [5, 6]. Polymorphisms of various genes, including those encoding enzymes of the tryptophan catabolite (TRYCAT) pathway, are also related to overproduction of reactive oxygen species (ROS) [6]. Indeed, oxidative stress and the associated protein glycoxidation play a key role in the pathogenesis of depression [6–8]. Although proteins, lipids and nucleic acids undergo glycoxidative modifications, proteins are the primary target of an ROS attack [6]. Oxidative and carbonyl stress products aggregate and accumulate in the nerve cells, thus disrupting neurotransmitter synthesis/secretion and inducing neuronal apoptosis [9]. Therefore, it is not surprising that the content of glycoxidant protein products correlates with the severity of depression or suicidal tendencies [10]. Compounds that could inhibit oxidation/glycation of brain biomolecules are being intensively searched for as a new therapeutical target in depression [11–13].
Trazodone (C19H22ClN5O; 2-{3-[4-(3-chlorophenyl)piperazin-1-yl]propyl}[1, 2,4]triazolo [4, 3-a]pyridin-3(2H)-one; Fig. 1) is a substance used for depression of various etiologies, including depression accompanied by anxiety [14, 15]. Trazodone is a serotonin reuptake inhibitor – it blocks serotonin transport from the synaptic space and directs it back into the nerve cell. Trazodone, exhibiting strong affinity for 5-HT2A receptors, antagonizes their action by increasing the binding of serotonin to the 5-HT1A receptor. On the other hand, trazodone blocks the inhibitory effect of 5-HT2A on the 5-HT1A receptor [16, 17]. However, not all mechanisms of trazodone action are well known. The drug administered orally is absorbed well and the maximum serum concentration is reached after four hours. Trazodone is metabolized in the liver, excreted in the urine, and the biological half-life of the drug is about 12 h [15, 18].
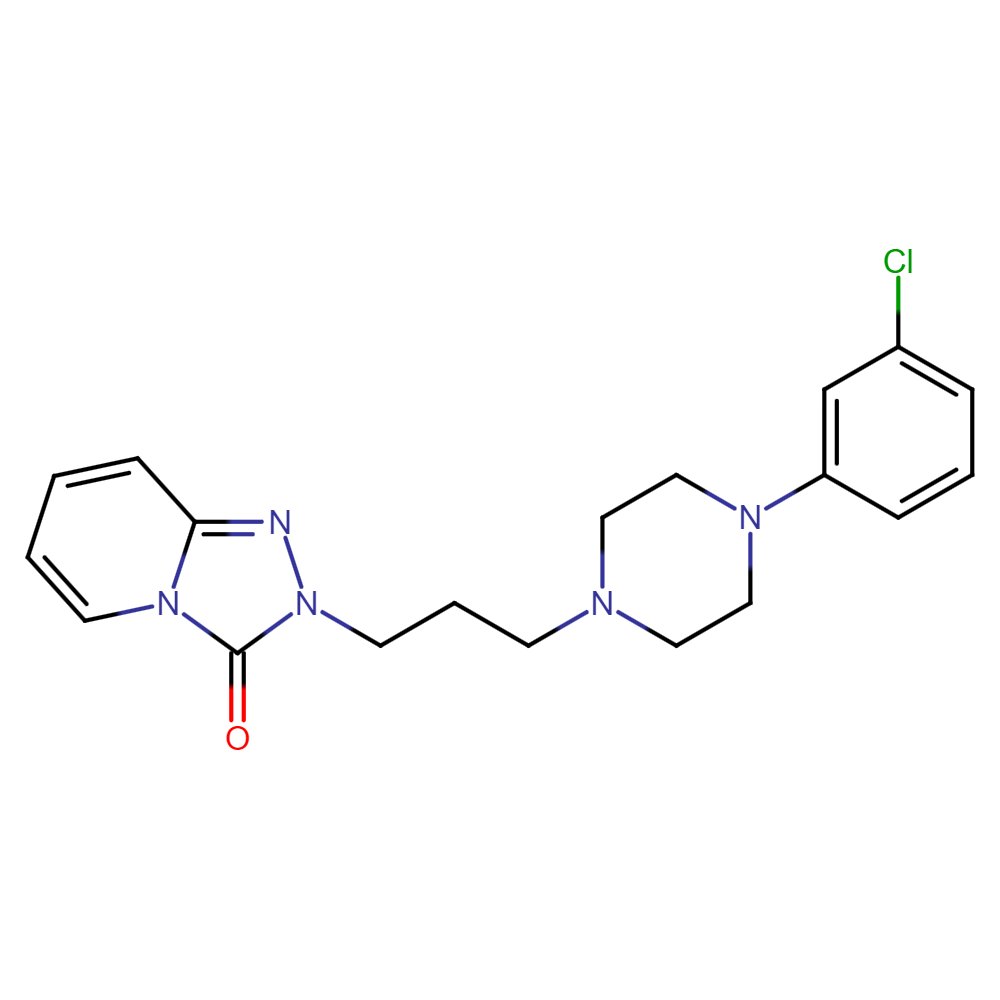
Fig. 1: Trazodone chemical formula.
Literature data on the effects of trazodone on oxidative stress are scarce, the results remain inconclusive, and only one paper addresses the issue of the effect of trazodone on protein glycation. The authors demonstrated the inhibitory effect of trazodone on the ROS-mediated generation of tau fibrils in a laboratory assay. Moreover, this drug prevents the formation of tau oligomers in a cell culture, thus reducing the mortality of cells [19]. Since carbonyl stress plays a crucial role in developing psychiatric disorders [20–22], trazodone may also exhibit effective antidepressant results by preventing glycoxidation. Therefore, we decided to investigate this mechanism holistically.
Materials and Methods
The scheme of the study is shown in the schematic workflow (Fig. 2).
Fig. 2: Schematic workflow of the study.
Systematic Review
The literature review was performed from 1995 to September 2022 on Medline (PubMed) database. The available references were trawled through based on the following keywords: [trazodone and antioxidant], [trazodone and oxidative stress], [trazodone and ROS], [trazodone and glycation], [trazodone and glycemia], [trazodone and hyperglycemia], as well as [trazodone and amyloid]. Inclusion and exclusion criteria are presented in Table 1.
Two researchers explored the provisional data by independently assessing the titles of articles and abstracts. Then, all the previously selected publications were reviewed by two other investigators. Next, manuscripts meeting the set criteria were selected for the final analysis. The Cohen’s kappa coefficient (κ) of the researchers’ reliability level was 0.92. The methodology of all papers was evaluated. The following variables were assessed: authors, publication year, design of research, experiment population size, inclusion and exclusion criteria as well as experiment duration and results.
Chemicals and Equipment
The analytical grade reagents used were acquired from Sigma-Aldrich Co. (St. Louis, MO, USA). In order to sterilize the chemical solutions, they were filtrated with 0.2-mm membrane filters immediately prior to utilization. Tecan Infinite 200 Pro Microplate reader was employed to calculate absorbance and fluorescence.
Antiradical and Antioxidant Activity
Scavenging of Hydroxyl Radical (OH).
OH scavenging activity of trazodone was determined based on the interaction of sodium salicylate with the residual radicals. OH was produced by the FeSO4-hydrogen peroxide (H2O2) system in the Fenton reaction (Fe2+ + H2O2 g Fe3+ + OH + OH−). Initially, the reaction mixture containing 50 μL of FeSO4 (8 mM), 80 μL of H2O2 (6 mM), 50 μL of distilled water, 100 μL of the tested solutions (terminal strength of 1 mM) as well as 20 μL of sodium salicylate (20 mM) was prepared and then incubated at 37°C for one hour. Mixture absorbance was assessed spectrophotometrically at a wavelength of 562 nm. The inhibition rate of OH percentage value was determined using the following formula: [1 − {(A 1 − A 2)/A 0}] × 100% (A 0 – control absorbance [without additives], A 1 – absorbance after the drugs are mixed, A 2 – absorbance without sodium salicylate) [23].
Scavenging of Nitric Oxide (NO). To 50 μL of samples, 100 μL of phosphate buffered saline, including 5 mM sodium nitroprusside was added. Then, 150-minute incubation of this mixture was conducted at 25°C. After that, 150 μL of Griess reagent (1% sulfanilamide, 2% H3PO4 as well as 0.1% N-(1-naphthyl)ethylenediamine) was added to the reaction mixture. As a consequence of nitrite diazotization by sulfanilamide and its conjugation with N-(1-naphthyl)ethylenediamine, chromophore was released. The absorbance of this product was marked by means of a spectrophotometer at 546 nm. The scavenging activity was calculated according to the formula: [1 − (A 1/A 2)] × 100% (A 0 – absorbance of the control [without drugs], A 1 – sample absorbance after reaction) [24].
Scavenging of Hydrogen Peroxide (H2O2)
At first, in order to produce ferrous ion oxidation-xylenol orange (FOX), 87.3 mg of butylated hydroxytoluene (BHT), 10 μL of H2SO4, 7.6 mg of xylenol orange and 10 mg of ferrous ammonium sulphate were mixed in 100 mL of 90% methanol-water solution. Next, 30-minute incubation of a mixture (1:1, v/v) of H2O2 (50 mM) with the samples (at terminal strength of 1 mM) was performed at room temperature. After that, 10 μL of high-performance liquid chromatography (HPLC)-grade methanol was introduced to 90 μL of the above-mentioned solution. Then, 0.9 mL of the FOX was added to this mixture, whereupon it was vortexed and incubated at room temperature for 30 minutes. The absorbance of ferric-xylenol orange complex generated in the reaction was measured at 560 nm wavelength via the spectrophotometer method. The percentage of H2O2 inhibition rate was calculated based on the following formula: [1 − {(A 1 − A 2)/A 0}] × 100% (A 0 – control absorbance [without drugs], A 1 – absorbance after the introduction of additives, A 2 – absorbance without the FOX reagent) [23].
Scavenging of 2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical. The assessment of DPPH radical (DPPH) scavenging activity was estimated based on decolorization of this radical. The amount of 180 μL of 0.13 mg/mL DPPH was mixed with 30 μL of diluted samples, after which the solution was supplemented with methanol to the volume of 210 µL. The DPPH solution was served as a control. Next, 20-minute incubation was performed at room temperature. After that, the absorbance was evaluated at 517 nm wavelength by a spectrophotometer. The following formula was applied to estimate DPPH• Elimination: [(A blank − A sample)/A blank] × 100%, where: A blank – the solution of blank DPPH absorbance, A sample – DPPH absorbance in the sample mixture [23].
Ferrous Ion Chelating (FIC)
FIC was determined by examining the decrease in the generation of Fe2+-ferrozine complex. To 90 μL of samples (at the terminal strength of 0.5 mg/mL) or BHT (used as a control), 18 μL of 0.6 mM FeCl2 solution and 162 μL of methanol were added. Then, the reaction mixture was incubated for 10 minutes at room temperature. After that, 18 μL of 5 mM solution of ferrozine was added. Next, the mixture was incubated for 5 more minutes at room temperature. The absorbance was assayed spectrophotometrically at a wavelength of 562 nm. FIC was calculated as a percentage of absorbance decrease of the control [23].
Bovine Serum Albumin (BSA) Model
The glycation of BSA was performed based on the previously applied methods [25–28]. Promptly, the solution of 96% BSA was added to 0.1 M sodium phosphate buffer of pH 7.4, where 0.02% sodium azide served as a preservative. The terminal concentration of BSA in incubated mixtures was 0.09 mM. Sugars (glucose [Glc], fructose [Fru], galactose [Gal], and ribose [Rib]), as well as aldehydes (glyoxal [GO] and methylglyoxal [MGO]) were used as glycation agents. BSA incubation was conducted in the presence of 1 mM trazodone with 0.5 M Glc, Fru, Gal, and Rib for 6 days, or with 2.5 mM GO and also MGO for 12 h, in order to evaluate the impact of the drugs on protein glycation [25–28]. GO as well as MGO were utilized within a period of a month after delivery. The solutions of these aldehydes were prepared shortly before the experiment [26].
According to earlier kinetic studies, glycation agent concentrations and the most optimal incubation conditions were measured and verified in order to examine the modulation of the glycoxidation rate by additives [25, 26]. The levels of glycation agents were significantly enhanced in comparison with their concentrations found in the human body. However, they were nonetheless beneficial for simulating the physiological processes in a relatively short time, which usually lasts for weeks or even months [25, 26, 29]. The antiglycation characteristics of novel compounds are frequently evaluated under experimental conditions [25–28, 30].
To compare the results obtained for trazodone, we used aminoguanidine – protein glycation inhibitor, as well as N-acetylcysteine (NAC) – free radical scavenger [25, 26]. All additives were employed at a concentration of 1 mM which was calculated proportionally to the high levels of glycation agents based on the previous in vitro experiments [25–28]. The study was conducted in three series, and each of them was repeated twice.
Products of Protein Glycation
The levels of kynurenine (KN), N-formylkynurenine (NFK) and dityrosine (DT) were measured spectrofluorimetrically at 365/480, 325/434 and 330/415 nm wavelengths of emission and excitation, respectively. Before the reading, 0.1 M H2SO4 (1:5, v/v) was used to dilute the samples. Results were standardized according to the fluorescence of 0.1 mg/mL quinine sulfate solution in 0.1 M H2SO4 [31, 32].
Advanced Glycation End Products (AGEs)
The content of AGEs was evaluated by means of a spectrofluorometer. The fluorescence of AGEs was calculated at 440/370 nm wavelength. Before the assessment, the assayed solutions were diluted with PBS (1:5, v/v). Evaluation of AGEs was also performed via the commercial method of enzyme-linked immunosorbent assay (ELISA) (USCN, Life Science, Wuhan, China), in compliance with the manufacturer’s instructions [33].
Products of Protein Oxidation
Total Thiols (TTs)
The concentration of TTs was measured with a spectrophotometer at 412 nm using Ellman’s reagent. The level of TTs was determined based on the reduced glutathione (GSH) standard curve [34].
Protein Carbonyls (PCs) level
The level of PCs was assayed based on the reaction of 2, 4-dinitrophenylhydrazine (DNPH) and carbonyls in oxidation-damaged proteins. The absorbance of the color reaction products was evaluated spectrophotometrically at 355 nm wavelength. The 2, 4-DNPH absorption coefficient of 22, 000 M−1 cm−1 was used as a standard [35].
Advanced Oxidation Protein Products (AOPPs)
The concentration of AOPPs was determined via the spectrophotometric method. 200 μL of the tested solutions were diluted by PBS at a ratio of 1:5 (v/v). Thus prepared samples as well as standard solutions at concentrations of 0 to 100 μM and 200 μL of blank solution of PBS were placed on a 96-well microplate. After that, 10 μL of KI (1.16 M) and 20 μL of CH3COOH were added. The absorbance of the samples was assayed promptly at 340 nm wavelength against the blank solution of: 200 μL PBS, 10 μL KI and 20 μL CH3COOH [36].
Molecular Docking
Molecular docking is recognized in the in silico technique which aims to anticipate the best suitable mode of ligand binding with a macromolecule (usually protein) [37]. Our study investigated the interaction between trazodone hydrochloride and BSA particles. The 3D crystal structure of BSA (ID: 4F5S) sourced from the Protein Data Bank (PDB) was obtained in a form of a .pdb file. The protein structure was determined using the method of X-ray diffraction (resolution value: 2.47 Å). The 3D trazodone hydrochloride (ID: 62935) was downloaded from the National Library of Medicine website in an .sdf format. Firstly, all water molecules were removed using AutoDock MGL Tools, and polar hydrogens and Kollman’s partial charges were introduced. After that, processed protein particle was saved as a .pdbqt file. The docking was studied in a grid box of 40 × 40 × 40 with 0.375 Å spacing located at 34.885, 23.976 as well as 98.792, respectively. It was determined that the value of exhaustiveness was 8. Simulation of molecular docking was obtained with the use of AutoDock Vina software, and the docking was visualized using PyMOL 2.5 [27, 28].
Statistical Analysis
The statistical analysis was obtained using GraphPad Prism 9.0.0 (GraphPad Software, San Diego, California, USA). The results were presented as a percentage of relative values of the controls (BSA with glycation agents). A one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons were applied to assess differences between the groups. The value of p < 0.05 was found to be statistically significant. A multiplicity adjusted p -value was marked as well.
Results
Systematic Review
The systematic bibliography review led to the selection of 81 articles in the Medline (PubMed) database, 53 of which were rejected due to the title. A total of 28 abstracts were read, and 19 of them met the inclusion and exclusion criteria. Out of the remaining works, 7 were not associated with the topic of our study. Ultimately, 12 manuscripts were included in the research (Fig. 3). Table 2 below presents the results of our systematic review.
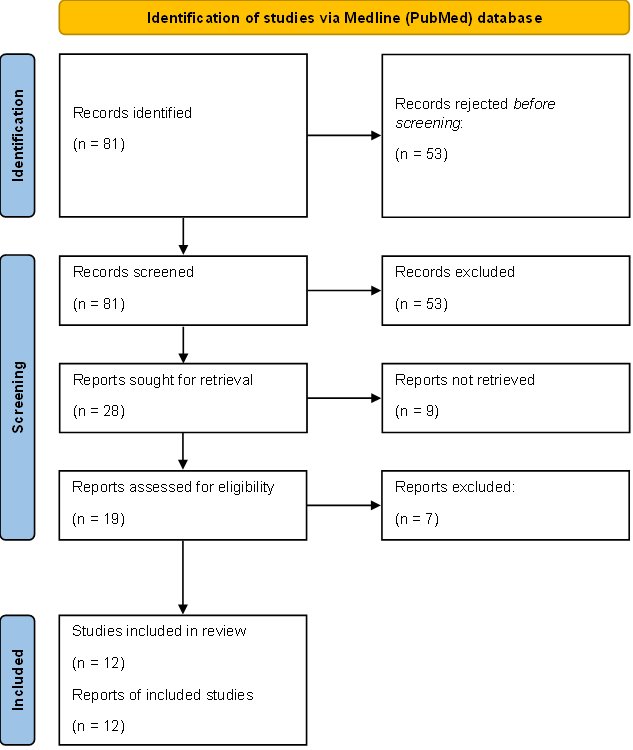
Fig. 3: Systematic review methodology flow diagram (Prisma).
Antiradical and Antioxidant Activity
Excess ROS and reactive nitrogen species (RNS) lead to oxidative stress. Oxidative stress is exacerbated by accumulation of redox-active metals (such as iron). Thus, antioxidant properties of a substance depend not only on the scavenging capacity of free radicals (e.g., OH and DPPH) and other ROS/RNS (NO or H2O2) but also on its ability to chelate metals (like FIC) [6, 38, 39].
Scavenging of Hydroxyl Radical (OH)
OH inhibition rate of trazodone was 41%. Aminoguanidine and NAC scavenged OH more efficiently compared to trazodone (+34% and +53%, respectively) (Fig. 4A).
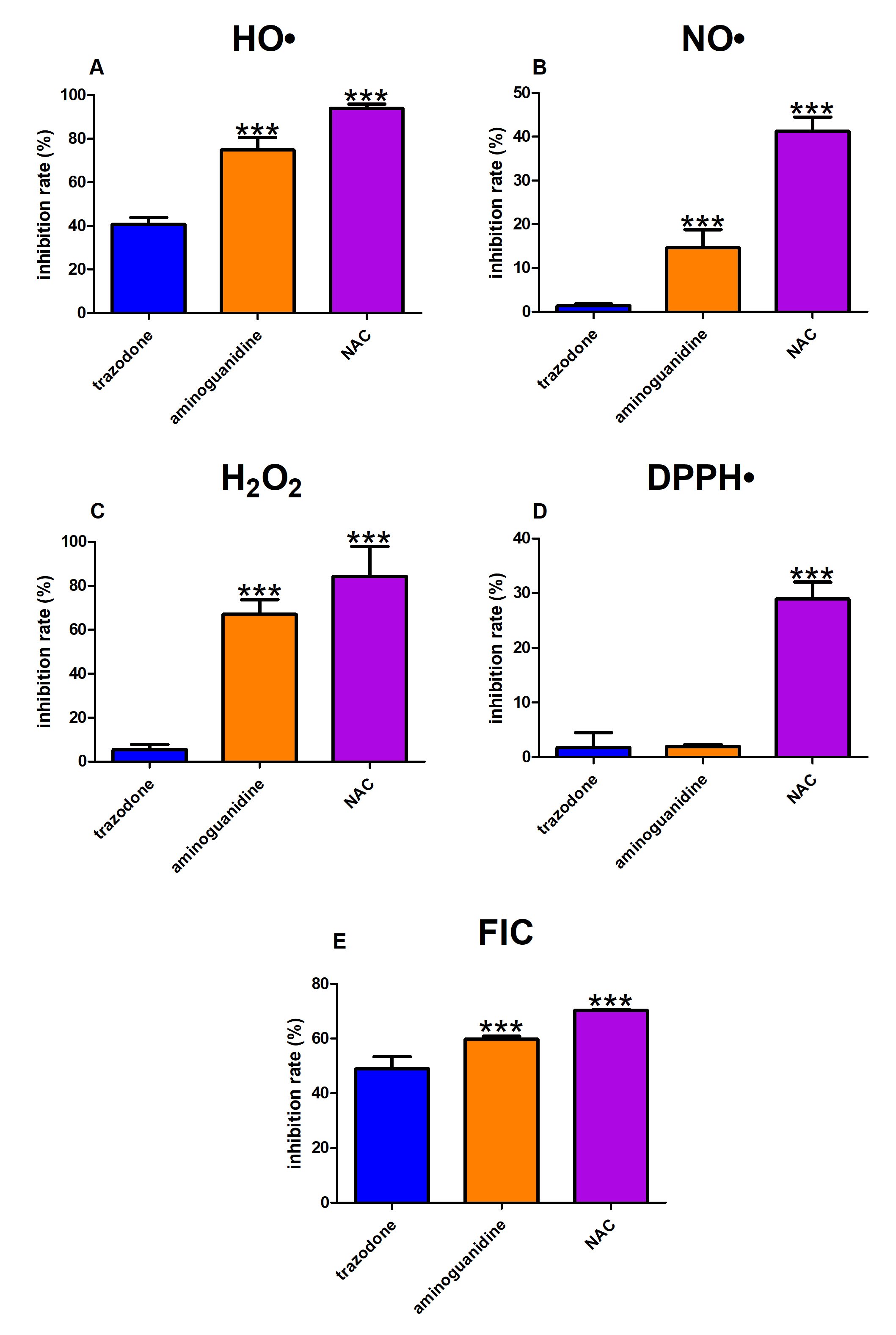
Fig. 4: The effect of trazodone, protein glycation (aminoguanidine) and free radical (N-acetylcysteine, NAC) inhibitors on hydroxyl radical (OH, Fig. 4A), nitric oxide (NO, Fig. 4B), hydrogen peroxide (H2O2, Fig. 4C) and 2,2-diphenyl-1-picrylhydrazyl radical (DPPH , Fig. 4D) scavenging as well as ferrous iron chelating (FIC, Fig. 4E). ∗∗∗p<0.001 vs. control (trazodone).
Scavenging of Nitric Oxide (NO)
Trazodone scavenged NO at the level of 1%. Both aminoguanidine (+13%) and NAC (+40%) showed substantially higher inhibition rates than trazodone (Fig. 4B).
Scavenging of Hydrogen Peroxide (H2O2)
H2O2 scavenging capacity of aminoguanidine (+61%) as well as NAC (+79%) was significantly elevated compared to trazodone (6%) (Fig. 4C).
Scavenging of 2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical
Trazodone presented a DPPH• Inhibition rate of 2%. Only DPPH Scavenging of NAC was markedly increased in comparison with the study drug (+27%) (Fig. 4D).
Ferrous Ion Chelating (FIC)
The FIC of trazodone was 49%. This parameter was effectively increased in aminoguanidine and NAC compared to trazodone (+11% and +21%, respectively) (Fig. 4E).
Products of Protein Glycation
Protein glycation leads to modifications of amino acids, including tyrosine (Tyr) which is particularly susceptible to the glycation process generating DT. Moreover, in the Maillard reaction, glycoxidation factors react with amino acids of proteins to form Schiff bases. These, in turn, are transformed into Amadori products (APs) from which AGEs are ultimately formed [40, 41].
Kynurenine (KN), N-formylkynurenine (NFK) and dityrosine (DT)
The content of KN was significantly potentiated in Glc+trazodone (+244%) compared to Glc. The marker was relevantly decreased in Glc+aminoguanidine as well as Glc+NAC versus Glc alone (−88% and −82%, respectively). The fluorescence of KN was considerably elevated in Glc (+1371%), Glc+trazodone (+4963%), Glc+aminoguanidine (+84%) and Glc+NAC (+166%) in comparison with BSA (Fig. 5A). This parameter was substantially higher in Fru+trazodone (+185%) but markedly lower in Fru+aminoguanidine and Fru+NAC (−87% and −86%, respectively) than in Fru. KN fluorescence was significantly augmented in Fru (+858%), Fru+trazodone (+2632%) as well as Fru+NAC (+34%) versus BSA (Fig. 5B). The biomarker was significantly enhanced in Gal+trazodone (+279%) compared to Gal alone. The content of KN was pointedly attenuated in both Gal+aminoguanidine and Gal+NAC versus Gal (−81% and −85%, respectively). The parameter in question was meaningfully increased in Gal (+879%), Gal+trazodone (+3609%), Gal+aminoguanidine (+82%) and Gal+NAC (+49%) compared to BSA (Fig. 5C). The level of KN was relevantly potentiated in Rib+trazodone (+84%); however, the marker was markedly reduced in Rib+aminoguanidine (−39%) as well as Rib+NAC (−43%) versus Rib alone. The fluorescence of KN was substantially increased in Rib, Rib+trazodone, Rib+aminoguanidine and Rib+NAC versus BSA (+328%, +687%, +162% and +146%, respectively) (Fig. 5D). This parameter was considerably elevated in GO+trazodone (+277%) in comparison with GO. Nevertheless, KN fluorescence was effectively decreased in GO+aminoguanidine (−31%) as well as GO+NAC (−59%) over against GO alone. The biomarker was significantly elevated in GO (+693%), GO+trazodone (+2890%), GO+aminoguanidine (+450%) and GO+NAC (+226%) versus BSA (Fig. 5E). The content of KN was meaningfully higher in MGO+trazodone (+37%) but relevantly lower in MGO+NAC (−29%) than in MGO. This parameter was considerably improved in MGO (+1192%), MGO+trazodone (+1670%), MGO+aminoguanidine (+1007%) as well as MGO+NAC (+822%) in comparison with BSA (Fig. 5F).
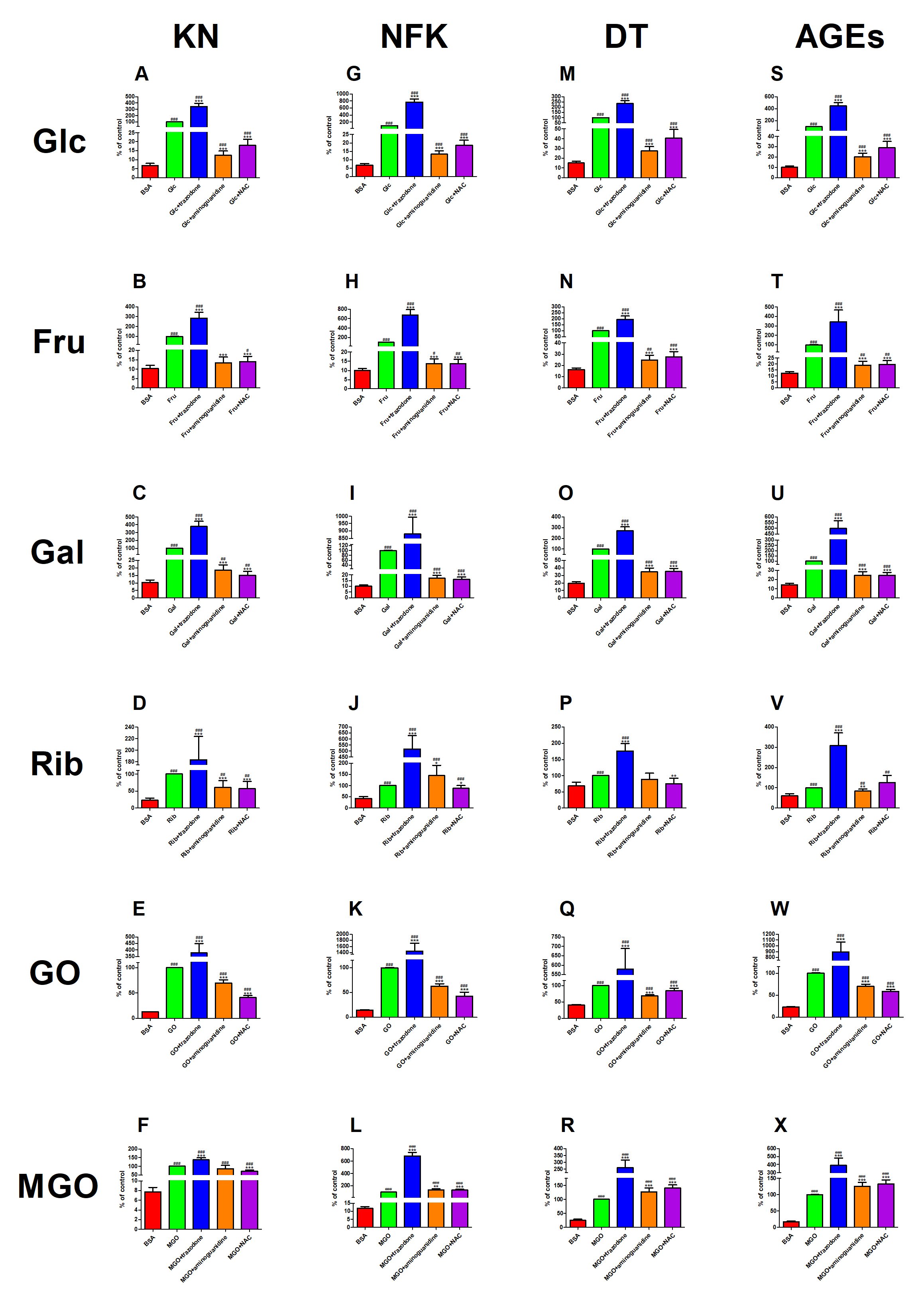
Fig. 5: The effect of trazodone, protein glycation (aminoguanidine) and free radical (N-acetylcysteine, NAC) inhibitors on kynurenine (KN, Fig. 5A–F), N-formylkynurenine (NFK, Fig. 5G–L), dityrosine (DT, Fig. 5M–R) as well as advanced glycation end products (AGEs, Fig. 5S–X) fluorescence in bovine serum albumin (BSA) glycated with glucose (Glc), fructose (Fru), galactose (Gal), ribose (Rib), glyoxal (GO) and methylglyoxal (MGO). ∗p<0.05 vs. positive control (glycoxidation agent); ∗∗p<0.01 vs. positive control (glycoxidation agent); ∗∗∗p<0.001 vs. positive control (glycoxidation agent); #p<0.05 vs. negative control (BSA); ##p<0.01 vs. negative control (BSA); ###p<0.001 vs. negative control (BSA).
The fluorescence of NFK was significantly higher in Glc+trazodone (+669%) but markedly lower in Glc+aminoguanidine (−87%) and Glc+NAC (−81%) than in Glc alone. The content of NFK was considerably augmented in Glc, Glc+trazodone, Glc+aminoguanidine as well as Glc+NAC compared to BSA (+1356%, +11089%, +93%, and +171%, respectively) (Fig. 5G). The biomarker was elevated in Fru+trazodone (+579%) versus Fru. However, NFK fluorescence in Fru+aminoguanidine as well as Fru+NAC was substantially diminished versus Fru alone (−86% in both). This parameter was significantly enhanced in Fru (+909%), Fru+trazodone (+6748%), Fru+aminoguanidine (+38%) and Fru+NAC (+38%) in comparison with BSA (Fig. 5H). The content of NFK was relevantly higher in Gal+trazodone (+780%), but significantly lower in Gal+aminoguanidine as well as Gal+NAC than in Gal (−83% and −84%, respectively). The content of the marker was increased in Gal, Gal+trazodone, Gal+aminoguanidine and Gal+NAC versus BSA (+898%, +8679%, +71% and +60%, respectively) (Fig. 5I). The fluorescence of NFK was markedly improved in Rib+trazodone (+418%) and Rib+aminoguanidine (+44%) compared to Rib alone. This parameter was substantially lowered in Rib+NAC (−12%) over against Rib. The concentration of NFK was markedly potentiated in Rib (+137%), Rib+trazodone (+1125%), Rib+aminoguanidine (+242%) as well as Rib+NAC (+108%) versus BSA (Fig. 5J). The biomarker was substantially higher in GO+trazodone (+1352%) but significantly lower in GO+aminoguanidine and GO+NAC than in GO alone (−37% and −57%, respectively). The fluorescence of NFK was largely enhanced in GO (+586%), GO+trazodone (+9859%), GO+aminoguanidine (+331%), GO+NAC (+192%) versus BSA (Fig. 5K). This parameter was significantly increased in MGO+trazodone (+583%), MGO+aminoguanidine (+33%) and MGO+NAC (+35%) than in MGO. The content of NFK parameter was substantially elevated in MGO, MGO+trazodone, MGO+aminoguanidine and MGO+NAC compared to BSA (+753%, +5726%, +1035% and +1048%, respectively) (Fig. 5L).
The fluorescence of DT was relevantly improved in Glc+trazodone (+136%) compared to Glc alone. The biomarker was considerably decreased in Glc+aminoguanidine as well as Glc+NAC (−73% and −59%, respectively) versus Glc. The content of DT was substantially elevated in Glc (+564%), Glc+trazodone (+1465%), Glc+aminoguanidine (+82%) and Glc+NAC (+170%) compared to BSA (Fig. 5M). The parameter was effectively higher in Fru+trazodone (+95%) but markedly lower in Fru+aminoguanidine and Fru+NAC (−75% and −73%, respectively) than in Fru alone. The level of DT was significantly augmented in Fru (+518%), Fru+trazodone (+1105%), Fru+aminoguanidine (+52%) as well as Fru+NAC (+70%) versus BSA (Fig. 5N). The marker was significantly enhanced in Gal+trazodone (+172%) in comparison with Gal. The fluorescence of DT was markedly decreased both in Gal+aminoguanidine and Gal+NAC (both −65%) versus Gal alone. This parameter was substantially increased in Gal (+413%), Gal+trazodone (+1294%), Gal+aminoguanidine (+78%) and Gal+NAC (+81%) compared to BSA (Fig. 5O). The fluorescence of DT was relevantly elevated in Rib+trazodone (+76%); however, the biomarker was notedly reduced in Rib+NAC (−25%) versus Rib. The content of DT was markedly enhanced in Rib and Rib+trazodone versus BSA (+47% and +159%, respectively) (Fig. 5P). The parameter was considerably increased in GO+trazodone (+478%) versus GO alone. Nevertheless, DT content was significantly lowered in GO+aminoguanidine (−32%) as well as GO+NAC (−15%) in comparison with GO. The marker was considerably elevated in GO (+147%), GO+trazodone (+1329%), GO+aminoguanidine (+69%) and GO+NAC (+110%) versus BSA (Fig. 5Q). The fluorescence of DT was markedly higher in MGO+trazodone, MGO+aminoguanidine and MGO+NAC (+161%, +27% and +40%, respectively) than in MGO alone. This parameter was significantly increased in MGO (+289%), MGO+trazodone (+915%), MGO+aminoguanidine (+394%) as well as MGO+NAC (+447%) compared to BSA (Fig. 5R).
Advanced Glycation End Products (AGEs)
The content of AGEs was considerably higher in Glc+trazodone (+348%) but much lower in Glc+aminoguanidine (−80%) and Glc+NAC (−71%) than in Glc. AGEs fluorescence was significantly elevated in Glc, Glc+trazodone, Glc+aminoguanidine as well as Glc+NAC in comparison with BSA (+893%, +4344%, +101% and +188%, respectively) (Fig. 5S). The biomarker was markedly enhanced in Fru+trazodone (+242%) over against Fru alone. Nevertheless, the fluorescence of AGEs in Fru+aminoguanidine (−81%) as well as Fru+NAC (−80%) was relevantly diminished versus Fru. This parameter was relevantly elevated in Fru (+710%), Fru+trazodone (+2668%), Fru+aminoguanidine (+53%) and Fru+NAC (+59%) compared to BSA (Fig. 5T). The content of AGEs was substantially higher in Gal+trazodone (+401%) but significantly lower in Gal+aminoguanidine as well as Gal+NAC than in Gal alone (−75% in both). The marker was considerably increased in Gal, Gal+trazodone, Gal+aminoguanidine and Gal+NAC versus BSA (+604%, +3425%, +75% and +74%, respectively) (Fig. 5U). The content of AGEs was markedly boosted in Rib+trazodone (+210%) when compared to Rib. This parameter was significantly lowered in Rib+NAC (−16%) versus Rib alone. The fluorescence of AGEs was relevantly improved in Rib (+67%), Rib+trazodone (+418%), Rib+aminoguanidine (+40%) as well as Rib+NAC (+111%) versus BSA (Fig. 5V). The biomarker was substantially higher in GO+trazodone (+796%) mut significantly lower in GO+aminoguanidine and GO+NAC than in GO (−30% and −41%, respectively). The level of AGEs was considerably enhanced in GO (+336%), GO+trazodone (+3802%), GO+aminoguanidine (+206%) and GO+NAC (+156%) versus BSA (Fig. 5W). This parameter was markedly increased in MGO+trazodone (+292%), MGO+aminoguanidine (+26%) as well as MGO+NAC (+33%) versus MGO alone. The content of AGEs was substantially elevated in MGO, MGO+trazodone, MGO+aminoguanidine and MGO+NAC compared to BSA (+479%, +2171%, +628%, and +671%, respectively) (Fig. 5X).
Validation of Results by the Enzyme-Linked Immunosorbent Assay (ELISA)
The additives used may have an impact on the glycoxidation process of BSA assessed fluorometrically. Therefore, the content of AGEs was also marked by means of the ELISA test. It was demonstrated that fluorometrically-assayed AGEs content was equivalent to the data obtained via the reference method, i.e., ELISA (Fig. S1) [25].
Products of Protein Oxidation
The BSA molecule contains 35 thiol groups, only one of which does not form a disulfide bridge. This thiol group is involved in the oxidation of the protein as well as attachment of various ligands to it. Thus, oxidative damage was measured based on the degree of decline in TTs. Oxidative stress also leads to an increase in the concentration of PCs which are formed from the oxidation of amino acids containing free hydroxyl amine as well as amide groups. End products of these transformations are AOPPs [42–44].
Total thiols (TTs)
The concentration of TTs was considerably lower in Glc+aminoguanidine (−33%) but significantly higher in Glc+NAC (+744%) than in Glc alone. The marker was notedly reduced in Glc+aminoguanidine (−39%) versus BSA. TTs level was markedly increased in Glc+NAC (+664%) in comparison with BSA (Fig. 6A). The parameter was significantly diminished in Fru+aminoguanidine (−27%) but relevantly enhanced in Fru+NAC (+272%) versus Fru. The concentration of TTs was meaningfully suppressed in Fru, Fru+trazodone as well as Fru+aminoguanidine (−59%, −59% and −70%, respectively) compared to BSA. This biomarker was pointedly augmented in Fru+NAC (+53%) over against BSA (Fig. 6B). The level of TTs was substantially attenuated in Gal+trazodone and Gal+aminoguanidine (−22% and −35%, respectively) versus Gal alone. This parameter was relevantly potentiated in Gal+NAC (+549%) in comparison with Gal. TTs concentration was markedly lowered in Gal+trazodone (−47%) as well as Gal+aminoguanidine (−55%) but considerably increased in Gal+NAC (+344%) versus BSA (Fig. 6C). The marker was significantly improved in Rib+aminoguanidine (+142%) and Rib+NAC (+290%) compared to Rib. Nevertheless, the level of TTs was notedly reduced in Rib as well as Rib+trazodone (−58% and −50%, respectively) versus BSA. This parameter was significantly enhanced in Rib+NAC when compared to BSA (+63%) (Fig. 6D). The content of TTs was considerably improved in GO+trazodone (+16%) and GO+NAC (+569%) in comparison with GO. This biomarker was markedly elevated in GO (+27%), GO+trazodone (+46%), GO+aminoguanidine (+38%) and GO+NAC (+747%) in comparison with BSA (Fig. 6E). The concentration of TTs was substantially higher in MGO+NAC (+624%) than in MGO alone. This parameter was significantly inhibited in MGO, MGO+trazodone as well as MGO+NAC versus BSA (−45%, −43% and −43%, respectively). TTs level was relevantly enhanced in MGO+NAC compared to BSA (+295%) (Fig. 6F).
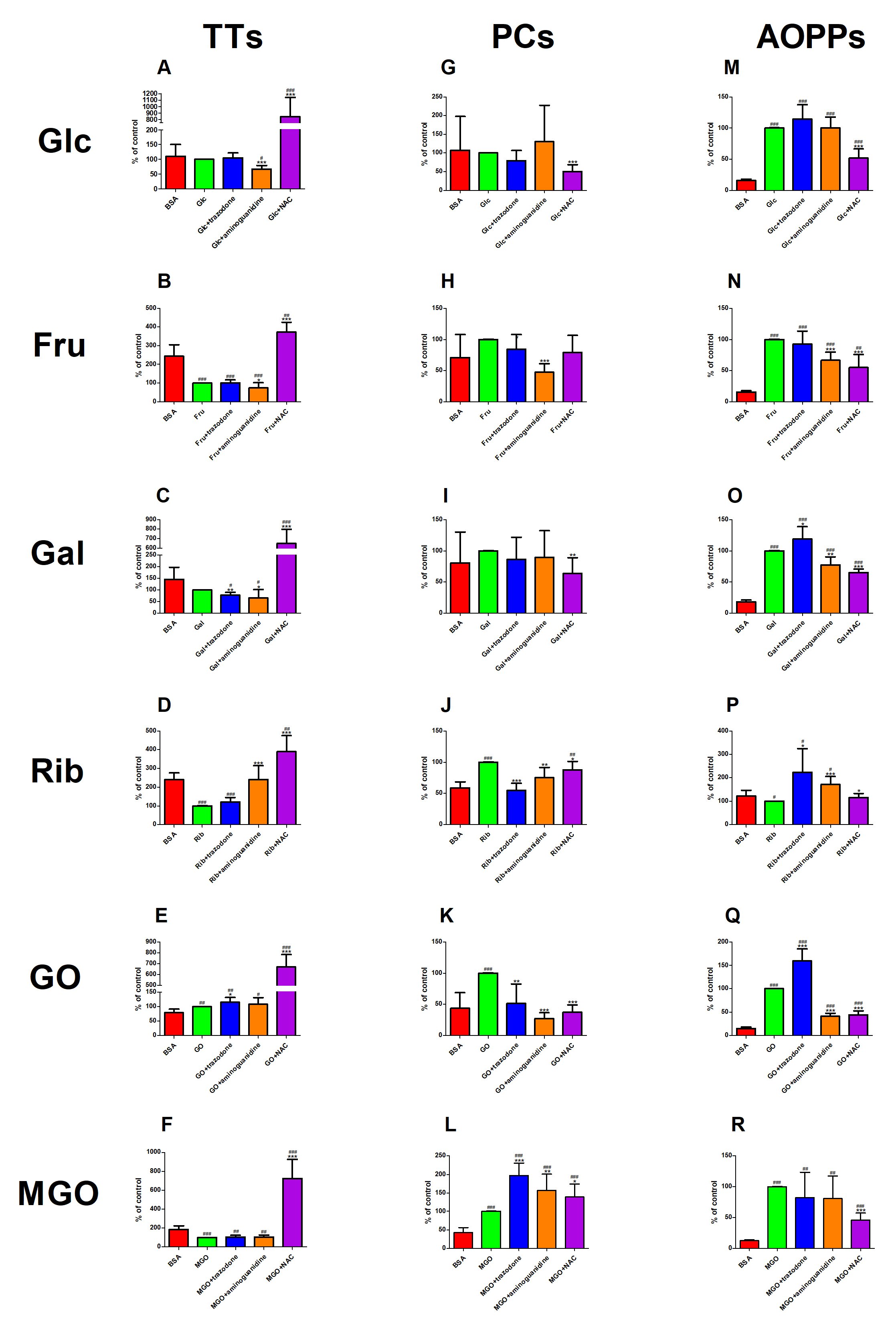
Fig. 6: The effect of trazodone, protein glycation (aminoguanidine) and free radical (N-acetylcysteine, NAC) inhibitors on total thiols (TTs, Fig. 6A–F), protein carbonyls (PCs, Fig. 6G–L) as well as advanced oxidation protein products (AOPPs, Fig. 6M–R) concentration in bovine serum albumin (BSA) glycated with glucose (Glc), fructose (Fru), galactose (Gal), ribose (Rib), glyoxal (GO) and methylglyoxal (MGO). ∗p<0.05 vs. positive control (glycoxidation agent); ∗∗p<0.01 vs. positive control (glycoxidation agent); ∗∗∗p<0.001 vs. positive control (glycoxidation agent); #p<0.05 vs. negative control (BSA); ##p<0.01 vs. negative control (BSA); ###p<0.001 vs. negative control (BSA).
Protein Carbonyls (PCs) level
The level of PCs was significantly decreased in Glc+NAC versus Glc (−50%) (Fig. 6G). This parameter was markedly lowered in Fru+aminoguanidine (−53%) compared to Fru alone (Fig. 6H). The concentration of PCs in Gal+NAC was substantially mitigated (−37%) versus Gal (Fig. 6I). PCs level was significantly reduced in Rib+trazodone, Rib+aminoguanidine as well as Rib+NAC over against Rib alone (−45%, −25% and −12%, respectively). The marker was considerably increased in Rib (+70%) and Rib+NAC (+50%) versus BSA (Fig. 6J). The concentration of PCs was relevantly lower in GO+trazodone, GO+aminoguanidine as well as GO+NAC (−49%, −73%, and −63%, respectively) than in GO. This parameter was markedly elevated only in GO compared to BSA (+128%) (Fig. 6K). The concentration of PCs was substantially enhanced in MGO+trazodone, MGO+aminoguanidine and MGO+NAC versus MGO alone (+97%, +57% and +39%, respectively). The marker was significantly improved in MGO (+133%), MGO+trazodone (+358%), MGO+aminoguanidine (+266%) and MGO+NAC (+223%) versus BSA (Fig. 6L).
Advanced Oxidation Protein Products (AOPPs)
The level of AOPPs was substantially attenuated in Glc+NAC (−48%) versus Glc alone. The biomarker was effectively augmented in Glc, Glc+trazodone, Glc+aminoguanidine as well as Glc+NAC compared to BSA (+535%, +627%, +536% and +228%, respectively) over against BSA (Fig. 6M). The concentration of AOPPs was relevantly reduced in Fru+aminoguanidine (−33%) and Fru+NAC (−45%) versus Fru alone. This parameter was considerably elevated in Fru (+552%), Fru+trazodone (+504%), Fru+aminoguanidine (+334%) and Fru+aminoguanidine (+259%) in comparison with BSA (Fig. 6N). The content of AOPPs was significantly higher in Gal+trazodone (+19%), but markedly lower in Gal+aminoguanidine (−22%) and Gal+NAC (−35%) than in Gal. The marker was considerably increased in Gal, Gal+trazodone, Gal+aminoguanidine and Gal+NAC (+453%, +558%, +329% and +260%, respectively) versus BSA (Fig. 6O). The level of AOPPs was markedly potentiated in Rib+trazodone (+124%), Rib+aminoguanidine (+71%) and Rib+NAC (+16%) in comparison with Rib alone. The parameter was substantially diminished in Rib versus BSA (−18%). The level of AOPPs was largely enhanced in Rib+trazodone as well as Rib+aminoguanidine (+83% and +40%, respectively) compared to BSA (Fig. 6P). The biomarker was significantly higher in GO+trazodone (+60%) but substantially lower in GO+aminoguanidine (−59%) and GO+NAC (−56%) than in GO. The concentration of AOPPs was considerably elevated in GO (+552%), GO+trazodone (+940%), GO+aminoguanidine (+168%) as well as GO+NAC (+188%) over against BSA (Fig. 6Q). This parameter was relevantly decreased in MGO+NAC (−54%) versus MGO alone. The concentration of AOPPs was markedly raised in MGO, MGO+trazodone, MGO+aminoguanidine and MGO+NAC in comparison with BSA (+699%, +557%, +544% and +267%, respectively) (Fig. 6R).
Molecular Docking Analysis
The molecular docking simulation revealed a very weak (≥ −10 kcal/mol) affinity of trazodone hydrochloride to a particle of BSA [45], amounting to −9.3 kcal/mol. Merely three modes of docking sites presented root-mean-square deviations of atomic positions (RMSD) below 3 (Table 3) [46]. Two of them exhibited a polar contact with a side chain of a BSA particle, and in both the ligand interfered with lysine (Lys) residue in the position of 136. Fig. 7 presents mode 1.
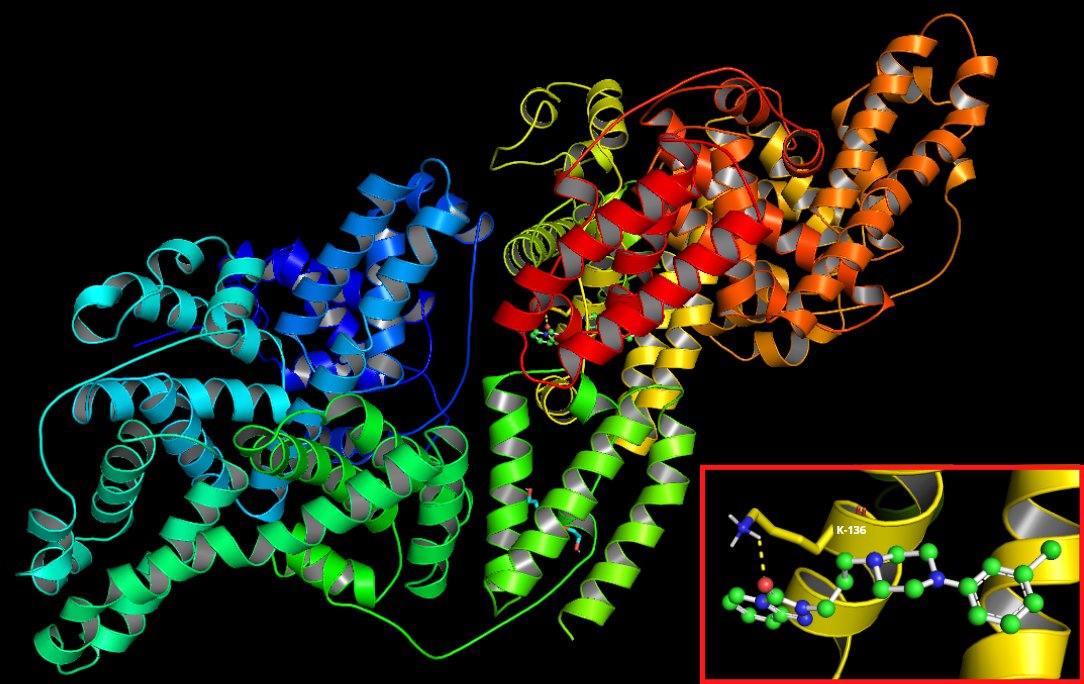
Fig. 7: Visualization of a docking site (mode 1) of trazodone hydrochloride in a BSA particle.
Discussion
The association of psychiatric disorders with abnormalities of the KN pathway has been thoroughly investigated [47]. Tryptophan (Try) is an exogenous amino acid that serves as a major source of neurotransmitters such as serotonin and melatonin [48]. In the KN pathway, enzyme indoleamine-2, 3-dioxygenase (IDO) converts Try to NFK, which is then converted to KN [49]. Excessive IDO activation (e.g., due to mental stressors, inflammation or oxidative stress) reduces Try availability, resulting in insufficient neurotransmitter synthesis [49, 50]. In addition, KYN may be converted to 3-hydroxyquinurenine that plays a special role in the pathogenesis of depression. 3-hydroxykynurenine is responsible for overproduction of ROS, which contribute to neuronal apoptosis and the dysfunction of serotonergic and noradrenergic receptors [9, 50, 51]. Interestingly, disturbances in the KN pathway are also accompanied by abnormal carbohydrate metabolism [52]. Increased glycation of proteins, particularly those containing arginine (Arg) or Lys, has been noted in patients with depression [6, 22, 53]. Another amino acid prone to protein glycation is Tyr [40]. Increased levels of plasma DT and AGEs were found in adults with depression, which indicates increased glycation of brain proteins in the course of the disease [22, 54]. Given the important role of protein glycation in the pathogenesis of depression, it is not surprising that antidepressants with additional antiglycation activity are being sought [55, 56]. In this study, we comprehensively evaluated the antiglycation properties of trazodone in various in vitro and in silico models. Trazodone is an N-arylpiperazine in which one nitrogen is substituted by 3-chlorophenyl and the other one – by 3-(3-oxo [1, 2,4]triazolo [4, 3-a]pyridin-2(3H)-yl)propyl [57]. Trazodone is used for treatment of depression, especially with accompanying insomnia, anxiety or sexual dysfunction [14, 15, 58]. Trazodone exerts an antidepressant effect by inhibiting the reuptake of serotonin into synapses. Acting as an antagonist of the excitatory 5-HT2 receptors, it enhances the binding of serotonin to the inhibitory 5-HT1A receptor. Trazodone also counteracts the suppressive effect of the 5-HT2A receptor on the 5-HT1A receptor. Nevertheless, other mechanisms of the drug’s antidepressant action have also been postulated [14, 16, 17].
To evaluate the antiglycation activity of trazodone, we used BSA treated with various glycation agents [25, 26, 59]. The use of albumin should come as no surprise, since it constitutes 50–70% of all plasma proteins [60]. Albumin is fundamental in maintaining the oncotic pressure of the blood, acts as a pH buffer, is involved in CO2 transport as well as transporting some drugs, hormones, fatty acids and bile pigments [60, 61]. The BSA molecule contains 583 amino acids, while human serum albumin (HSA) contains 585 of them. Both proteins show 76% homology of polypeptide chains, which is about 67% in the α-helix form. The spatial structure of BSA/HSA is heart-shaped, stabilized by 17 disulfide bridges forming 9 loops. Binding sites I and II have a similar location and the same functions for both albumins. BSA and HSA have one free thiol group located at position 34. Despite the slight difference in albumin structure (BSA contains threonine in the place of alanine), the N-terminus of both albumins has the same function – it binds transition metal ions (Cu2+, Co2+, Ni2+ or Zn2+) [60, 62–65]. Thus, BSA is a frequently used model protein in in vitro studies. This is also evidenced by its wide availability, low cost, good stability, binding properties and structural similarity to HSA. Kinetic studies comparing diabetic-derived HSA with BSA confirm the analogous glycoxidation mechanism of both. The resemblance demonstrates the usefulness of the glycated albumin model for assessing antiglycation and antioxidant properties [25, 26, 66–68]. Furthermore, with regard to brain cells, proteins are the most sensitive to oxidation and glycation. The brain, compared to other organs, is characterized by very high oxygen consumption, unfavorable surface-to-volume ratio, lower activity of antioxidant enzymes and higher content of prooxidant metal ions [69–71].
In our study, BSA was effectively glycated by all sugars (Glc, Fru, Gal, Rib) and aldehydes (GO and MGO). This is evidenced by a significant increase in protein glycoxidation products in BSA+sugar/aldehyde samples compared to the controls (BSA). Glycation is a multiphase process of post-translational modification of proteins called the Maillard reaction. It begins with the non-enzymatic reaction of carbonyl groups of the glycation agent with amino groups of the protein, forming Schiff bases. During the Amadori reaction, the Schiff base is regrouped to APs. Schiff bases and APs are the early products of glycation. Their degradation leads to the formation of intermediate products of the Maillard reaction – dicarbonyl compounds characterized by significantly higher reactivity, like GO or MGO. The final stages of protein glycation are oxidation, polymerization, dehydration and condensation with other amino groups. These irreversible reactions result in the formation of persistent AGEs [40, 41, 67]. Thus, it is not surprising to find an increase in early (hKN, hNFK, hDT) and late (hAGEs) glycation protein products under the influence of all sugars and aldehydes. The content of glycation products was assessed by the fluorimetric method, as some of the modified amino acids (KN, NFK and DT) and AGEs demonstrate fluorescent properties [32, 33]. AGEs were also assessed via the reference ELISA method, as additives can hinder determinations made via spectrofluorometry [33]. However, there were no statistical differences between both techniques. The process of protein glycation is inextricably linked to protein oxidation referred to as glycoxidation [72]. In our study, the concentration of protein oxidation markers was also statistically different compared to the controls (iTTs, hPCs, hAOPPs).
The mechanisms of protein glycation vary, depending on the agent used [59, 73, 74]. Thus, we applied different in vitro models to objectively assess the effect of trazodone on BSA glycation. Trazodone caused a significant increase (p < 0.001) in all the assessed glycation products (hKN, hNFK, hDT and hAGEs) in the presence of both sugars (Glc, Fru, Gal, Rib) and aldehydes (GO and MGO). Lys, Arg and cysteine (Cys) are susceptible to glycation due to their strong nucleophilicity [75]. Of the 29 albumin Glc-binding sites, 18 are Lys residues. The main site is Lys-525, responsible for 33% of glycation with Glc. Other Lys residues important in the Glc attachment are located at positions 199 (5%), 281 and 439. Smaller contributions to glycation are made by Lys-12, Lys-51, Lys-205, Lys-233, Lys-276, Lys-317 and Lys-538 [76, 77]. On the other hand, MGO glycates mainly Arg residues of albumin. The most important binding site of MGO is Arg-410 (characterized by 89% reactivity), while Arg-114, Arg-186, Arg-218 and Arg-428 demonstrate lower contribution in this regard. MGO can react with Cys residue at position 34 (the only one that does not form a disulfide bridge) and the site exhibits reactivity as high as 80% [78, 79]. Interestingly, the synthesis of early glycation protein products boosts the formation of free radicals [80–82]. This occurs by increasing the affinity of modified Lys for prooxidants such as Cu2+ and Fe2+ ions [83–86]. However, the formation of ROS does not necessarily occur in the Fenton or Haber-Weiss reactions [79]. In high concentrations, Glc and other sugars undergo autooxidation which also leads to ROS overproduction [87]. In addition, albumin exposed to oxidative stress gains prooxidant activity itself by cross-reacting with other proteins [88–90]. In our study, trazodone increased the production of PCs and AOPPs, which are major post-translational protein modifications through oxidation [44]. We also observed depletion of TTs in BSA samples incubated with glycation agents and trazodone. This explains the reduced antioxidant activity of albumin treated with trazodone. The thiol group of Cys-34 is a major free radical scavenger due to its ability to specifically bind ROS [91]. The weak antiradical and antioxidant properties of trazodone were also confirmed in other studies [92–96]. Indeed, we showed that trazodone poorly scavenges OH, NO and H2O2, and has low antioxidant activity in the DPPH assay. The drug also poorly chelates transition metal ions in the FIC test.
The effects of trazodone were compared to substances with documented antiglycation (aminoguanidine) and antioxidant (NAC) properties [25, 26]. Aminoguanidine counteracts carbonyl stress due to the guanidinium group in its structure. The drug displaces glycation factors from binding sites on the protein, traps α-dicarbonyls via nucleophilic transformation and acts as an antioxidant [97]. On the other hand, NAC is an acetylated precursor of L-cysteine. As a source of a free thiol group, NAC reduces ROS by donating one electron or acts as a nucleophile by donating one or two electrons [98]. In our study, the concentrations of early and late glycation products as well as protein oxidation products were significantly higher in BSA samples incubated with trazodone compared to aminoguanidine and NAC. In BSA treated with trazodone, we also showed enhanced carbonyl and oxidative stress compared to BSA+glycation agent. This indicates the proglycation properties of trazodone. Concentrations of trazodone and other additives were selected in kinetic studies of BSA glycation, in a manner proportional to high concentrations of glycation agents [25, 26, 59]. Nevertheless, it should be remembered that in a different range of concentrations, trazodone may exhibit different activity.
Why does trazodone demonstrate proglycation properties? This may be explained by the molecular docking analysis, which, in our study, revealed a very weak affinity of trazodone to BSA of above −10 kcal/mol. Merely three modes of docking sites presented RMSD below 3. Additionally, two of them exhibited a polar contact with a side chain of the BSA particle [27, 37, 45, 46]. However, ligand binding by albumin can alter its spatial conformation, preventing the attachment of other substances or strengthening the binding already formed. Ligand attachment at one binding site can change the structure of other binding sites or even their number [99, 100]. A two-stage course of ligand-protein interaction is postulated. In the first phase, the substance molecule approaches the hydrophobic cavity of albumin due to the hydrophobic effect. Then it binds to the protein through short-range interactions (hydrogen bonds, van der Waals forces as well as spherical or electrostatic interactions). Specific binding is characterized by very high affinity and low binding capacity, while non-specific binding presents low affinity and unlimited ligand binding capacity [101–103]. Trazodone (which demonstrated very low affinity to the albumin molecule) may bind to this protein non-specifically. A possible effect could be facilitating BSA glycation by sugars or aldehydes. Nonetheless, this hypothesis requires further research in both in silico and in vitro models.
Protein glycation together with oxidative stress mutually induce their adverse effects on the body [72]. In the course of a literature review, we found only one paper on the effects of trazodone on carbonyl stress [19]. Trazodone counteracted the formation of ROS-mediated tau fibrils in SHSY5Y cells. The authors reported that trazodone had inhibited tau protein oligomerization, which increased cell survival [19]. However, no study to date has evaluated the effects of trazodone on typical biomarkers of protein glycation and glycoxidation. Additionally, all the other in vitro studies showed prooxidant action of trazodone [92–94]. The drug increased ROS fluorescence, enhanced MDA formation and lowered mitochondrial membrane potential. Trazodone increased GSSG and decreased GSH levels. In addition, trazodone was demonstrated to be involved in oxidative stress-induced hepatocytotoxicity [92–94]. On the other hand, significant number of animal studies indicate that trazodone inhibits oxidative and nitrosative stress [14, 104–109]. The drug boosted antioxidant enzyme activity (catalase [CAT], superoxide dismutase [SOD], as well as glutathione-S-transferase [GST]) in the brain. Trazodone had positive effects on cerebral GSH level, glutathione redox ratio (GSH/GSSG) and mitochondrial function. It also presented alleviating effect on MDA and nitrite concentrations in the brain. The study drug reduced NO concentration in plasma and inducible nitric oxide synthase (iNOS) activity in lymphocytes [14, 104–109]. Therefore, trazodone can induce an adaptive response of the body. Moreover, the drug can stimulate defense mechanisms and strengthen the antioxidant barrier against carbonyl stress [110]. Therefore, further studies are needed to evaluate the effects of trazodone on protein glycation in vivo . Also, it cannot be ruled out that additional effects of trazodone are demonstrated by its metabolites. In the liver, trazodone undergoes biotransformation processes involving N-oxidation and hydroxylation reactions by the CYP3A4 isoenzyme of cytochrome P450. The metabolite m-chlorophenylpiperazine exhibits antidepressant activity (similar to trazodone), but might also present additional effects [111]. Since a systematic review of the literature indicates potential antioxidant properties of trazodone in vivo , further investigations are required to assess the biological activity of the drug’s metabolites as well.
Conclusion
In conclusion, we demonstrated weak antiradical and antioxidant activity of trazodone in in vitro and in silico studies. We were the first to demonstrate that the drug has strong proglycation effects, making further studies in animal models as well as in humans necessary. A systematic review of the literature indicates a prooxidant nature of trazodone in vitro , in contrast to its protective effect against oxidative stress in animal models. Thus, trazodone may induce an adaptive response by stimulating antioxidant mechanisms at the tissue/organ level. Further studies, both molecular and clinical, are required.
Acknowledgements
Author Contributions
M.N. performed laboratory determinations, interpreted the data, prepared the graphic part of the manuscript, wrote the manuscript and granted final approval of the version to be published. M.Z.P. interpreted the data. J.R.L. interpreted the data. A.Z. conceptualized and reviewed the article, and gave final approval of the version to be published. M.M. conceptualized the article, performed laboratory determinations, interpreted the data, prepared the graphic part of the manuscript, wrote the manuscript, reviewed the article and granted final approval of the version to be published.
Funding Sources
This work was supported by the Medical University of Bialystok, Poland (Grants No. SUB/1/DN/22/002/3330; SUB/1/DN/20/001/3330; SUB/1/DN/21/001/3330).
Statement of Ethics
The authors have no conflicts of interest to declare.
Disclosure Statement
The authors report no conflicts of interest in this work.
References
| 1 | Malhi GS, Mann JJ. Depression. Lancet 2018;392:2299-3212.
https://doi.org/10.1016/S0140-6736(18)31948-2 |
| 2 | Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007;370:851-858.
https://doi.org/10.1016/S0140-6736(07)61415-9 |
| 3 | Nutt DJ. Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry 2008;69 Suppl E:4-7. |
| 4 | Caviedes A, Lafourcade C, Soto C, Wyneken U. BDNF/NF-κB Signaling in the Neurobiology of Depression. Curr Pharm Des 2017;23. DOI: 10.2174/1381612823666170111141915
https://doi.org/10.2174/1381612823666170111141915 |
| 5 | Schroder JD, de Araújo JB, de Oliveira T, de Moura AB, Fries GR, Quevedo J, et al. Telomeres: the role of shortening and senescence in major depressive disorder and its therapeutic implications. Rev Neurosci 2022;33:227-255.
https://doi.org/10.1515/revneuro-2021-0070 |
| 6 | Anderson G, Maes M. Oxidative/Nitrosative Stress and Immuno-inflammatory Pathways in Depression: Treatment Implications. Curr Pharm Des 2014;20:3812-3847.
https://doi.org/10.2174/13816128113196660738 |
| 7 | Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017;76:197-205.
https://doi.org/10.1016/j.psyneuen.2016.11.031 |
| 8 | Eriksson MD, Eriksson JG, Kautiainen H, Salonen MK, Mikkola TM, Kajantie E, et al. Advanced glycation end products measured by skin autofluorescence are associated with melancholic depressive symptoms - Findings from Helsinki Birth Cohort Study. J Psychosom Res 2021;145:110488.
https://doi.org/10.1016/j.jpsychores.2021.110488 |
| 9 | Deng S, Liu S, Jin P, Feng S, Tian M, Wei P, et al. Albumin Reduces Oxidative Stress and Neuronal Apoptosis via the ERK/Nrf2/HO-1 Pathway after Intracerebral Hemorrhage in Rats. Oxid Med Cell Longev 2021;2021:1-14.
https://doi.org/10.1155/2021/8891373 |
| 10 | Martin-Subero M, Anderson G, Kanchanatawan B, Berk M, Maes M. Comorbidity between depression and inflammatory bowel disease explained by immune-inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut-brain pathways. CNS Spectr 2016;21:184-198.
https://doi.org/10.1017/S1092852915000449 |
| 11 | Zhang L, Previn R, Lu L, Liao R-F, Jin Y, Wang R-K. Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathway. Brain Res Bull 2018;142:352-359.
https://doi.org/10.1016/j.brainresbull.2018.08.021 |
| 12 | Li K, Yan L, Zhang Y, Yang Z, Zhang C, Li Y, et al. Seahorse treatment improves depression-like behavior in mice exposed to CUMS through reducing inflammation/oxidants and restoring neurotransmitter and neurotrophin function. J Ethnopharmacol 2020;250:112487.
https://doi.org/10.1016/j.jep.2019.112487 |
| 13 | Majumdar S, Gupta S, Prajapati SK, Krishnamurthy S. Neuro-nutraceutical potential of Asparagus racemosus: A review. Neurochem Int 2021;145:105013.
https://doi.org/10.1016/j.neuint.2021.105013 |
| 14 | Khouzam HR. A review of trazodone use in psychiatric and medical conditions. Postgrad Med 2017;129:140-148.
https://doi.org/10.1080/00325481.2017.1249265 |
| 15 | Cuomo A, Ballerini A, Bruni AC, Decina P, Di Sciascio G, Fiorentini A, et al. Clinical guidance for the use of trazodone in major depressive disorder and concomitant conditions: pharmacology and clinical practice. Riv Psichiatr 2019;54:137-149. |
| 16 | Feighner JP. Mechanism of action of antidepressant medications. J Clin Psychiatry 1999;60 Suppl 4:4-11; discussion 12-13.
https://doi.org/10.4088/JCP.v60n1204 |
| 17 | Haria M, Fitton A, McTavish D. Trazodone. Drugs Aging 1994;4:331-355.
https://doi.org/10.2165/00002512-199404040-00006 |
| 18 | Shin JJ, Saadabadi A. Trazodone. The Essence of Analgesia and Analgesics. StatPearls Publishing 2022:351-353. |
| 19 | Akbari V, Ghobadi S, Mohammadi S, Khodarahmi R. The antidepressant drug; trazodone inhibits Tau amyloidogenesis: Prospects for prophylaxis and treatment of AD. Arch Biochem Biophys 2020;679:108218.
https://doi.org/10.1016/j.abb.2019.108218 |
| 20 | Arai M, Miyashita M, Kobori A, Toriumi K, Horiuchi Y, Itokawa M. Carbonyl stress and schizophrenia. Psychiatry Clin Neurosci 2014;68:655-665.
https://doi.org/10.1111/pcn.12216 |
| 21 | Luca M, Guzik T, Luca A. Oxidative Stress as a Link between Cerebrocardiovascular and Psychiatric Disorders. Oxid Med Cell Longev. 2020:5685317.
https://doi.org/10.1155/2020/5685317 |
| 22 | Krivosova M, Gondas E, Murin R, Dohal M, Ondrejka I, Tonhajzerova I, et al. The Plasma Levels of 3-Hydroxybutyrate, Dityrosine, and Other Markers of Oxidative Stress and Energy Metabolism in Major Depressive Disorder. Diagnostics 2022;12:813.
https://doi.org/10.3390/diagnostics12040813 |
| 23 | Kwon SH, Wang Z, Hwang SH, Kang Y-H, Lee J-Y, Lim SS. Comprehensive evaluation of the antioxidant capacity of Perilla frutescens leaves extract and isolation of free radical scavengers using step-wise HSCCC guided by DPPH-HPLC. Int J Food Prop 2017;20(sup1):921-934.
https://doi.org/10.1080/10942912.2017.1318289 |
| 24 | Nitha B, De S, Adhikari SK, Devasagayam TPA, Janardhanan KK. Evaluation of free radical scavenging activity of morel mushroom, Morchella esculenta mycelia: A potential source of therapeutically useful antioxidants. Pharm Biol 2010;48:453-460.
https://doi.org/10.3109/13880200903170789 |
| 25 | Sadowska-Bartosz I, Galiniak S, Bartosz G. Kinetics of Glycoxidation of Bovine Serum Albumin by Glucose, Fructose and Ribose and Its Prevention by Food Components. Molecules 2014;19:18828-18849.
https://doi.org/10.3390/molecules191118828 |
| 26 | Sadowska-Bartosz I, Galiniak S, Bartosz G. Kinetics of Glycoxidation of Bovine Serum Albumin by Methylglyoxal and Glyoxal and its Prevention by Various Compounds. Molecules 2014;19:4880-4896.
https://doi.org/10.3390/molecules19044880 |
| 27 | Drygalski K, Fereniec E, Zalewska A, Krętowski A, Żendzian-Piotrowska M, Maciejczyk M. Phloroglucinol prevents albumin glycation as well as diminishes ROS production, glycooxidative damage, nitrosative stress and inflammation in hepatocytes treated with high glucose. Biomed Pharmacother 2021;142:111958.
https://doi.org/10.1016/j.biopha.2021.111958 |
| 28 | Nesterowicz M, Żendzian-Piotrowska M, Ładny JR, Zalewska A, Maciejczyk M. Antiglycoxidative properties of amantadine - a systematic review and comprehensive in vitro study. J Enzyme Inhib Med Chem 2023;38:138-155.
https://doi.org/10.1080/14756366.2022.2137161 |
| 29 | Sadowska-Bartosz I, Stefaniuk I, Galiniak S, Bartosz G. Glycation of bovine serum albumin by ascorbate in vitro: Possible contribution of the ascorbyl radical? Redox Biol 2015;6:93-99.
https://doi.org/10.1016/j.redox.2015.06.017 |
| 30 | Galiniak S, Bartosz G, Sadowska-Bartosz I. Is Iron Chelation Important in Preventing Glycation of Bovine Serum Albumin in vitro? Cell Mol Biol Lett 2015. DOI: 10.1515/cmble-2015-0033
https://doi.org/10.1515/cmble-2015-0033 |
| 31 | Bisby R. Techniques in free radical research: (Laboratory techniques in biochemistry and molecular biology, volume 22). FEBS Lett 1992;308:107-107.
https://doi.org/10.1016/0014-5793(92)81064-S |
| 32 | Hawkins CL, Morgan PE, Davies MJ. Quantification of protein modification by oxidants. Free Radic Biol Med 2009;46:965-988.
https://doi.org/10.1016/j.freeradbiomed.2009.01.007 |
| 33 | Münch G, Keis R, Weßels A, Riederer P, Bahner U, Heidland A, et al. Determination of Advanced Glycation End Products in Serum by Fluorescence Spectroscopy and Competitive ELISA. Clin Chem Lab Med. 1997;35. DOI: 10.1515/cclm.1997.35.9.669
https://doi.org/10.1515/cclm.1997.35.9.669 |
| 34 | Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70-77.
https://doi.org/10.1016/0003-9861(59)90090-6 |
| 35 | Reznick AZ, Packer L [38]. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. 1994; pp 357-363.
https://doi.org/10.1016/S0076-6879(94)33041-7 |
| 36 | Škrha J, Prázný M, Hilgertová J, Kvasnička J, Kalousová M, Zima T. Oxidative stress and endothelium influenced by metformin in type 2 diabetes mellitus. Eur J Clin Pharmacol 2007;63:1107-1114.
https://doi.org/10.1007/s00228-007-0378-1 |
| 37 | Salmaso V, Moro S. Bridging Molecular Docking to Molecular Dynamics in Exploring Ligand-Protein Recognition Process: An Overview. Front Pharmacol. 2018;DOI: 10.3389/fphar.2018.00923
https://doi.org/10.3389/fphar.2018.00923 |
| 38 | Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 2006;160:1-40.
https://doi.org/10.1016/j.cbi.2005.12.009 |
| 39 | Gulcin İ. Antioxidants and antioxidant methods: an updated overview. Arch Toxicol. 2020;94:651-715.
https://doi.org/10.1007/s00204-020-02689-3 |
| 40 | Thornalley PJ. Quantitative Screening of Protein Glycation, Oxidation, and Nitration Adducts by LC-MS/MS: Protein Damage in Diabetes, Uremia, Cirrhosis, and Alzheimer's Disease. Redox Proteomics. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2006; pp 681-727.
https://doi.org/10.1002/0471973122.ch22 |
| 41 | Rabbani N, Xue M, Thornalley PJ. Dicarbonyl stress, protein glycation and the unfolded protein response. Glycoconj J 2021;38:331-340.
https://doi.org/10.1007/s10719-021-09980-0 |
| 42 | Bujacz A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr Sect D Biol Crystallogr. 2012;68:1278-1289.
https://doi.org/10.1107/S0907444912027047 |
| 43 | Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70-77.
https://doi.org/10.1016/0003-9861(59)90090-6 |
| 44 | Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci 2000;899:191-208.
https://doi.org/10.1111/j.1749-6632.2000.tb06187.x |
| 45 | Wang J, Guo Z, Fu Y, Wu Z, Huang C, Zheng C, et al. Weak-binding molecules are not drugs?-toward a systematic strategy for finding effective weak-binding drugs. Brief Bioinform. 2016;bbw018.
https://doi.org/10.1093/bib/bbw018 |
| 46 | Reva BA, Finkelstein A V, Skolnick J. What is the probability of a chance prediction of a protein structure with an rmsd of 6 å? Fold Des 1998;3:141-147.
https://doi.org/10.1016/S1359-0278(98)00019-4 |
| 47 | Savitz J. The kynurenine pathway: a finger in every pie. Mol Psychiatry. 2020;25:131-147.
https://doi.org/10.1038/s41380-019-0414-4 |
| 48 | Davidson M, Rashidi N, Nurgali K, Apostolopoulos V. The Role of Tryptophan Metabolites in Neuropsychiatric Disorders. Int J Mol Sci 2022;23.
https://doi.org/10.3390/ijms23179968 |
| 49 | Salminen A. Role of indoleamine 2, 3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res Rev 2022;75:101573.
https://doi.org/10.1016/j.arr.2022.101573 |
| 50 | Dell'Osso L, Carmassi C, Mucci F, Marazziti D. Depression, Serotonin and Tryptophan. Curr Pharm Des 2016;22:949-954.
https://doi.org/10.2174/1381612822666151214104826 |
| 51 | Kocki T, Wnuk S, Kloc R, Kocki J, Owe-Larsson B, Urbanska EM. New insight into the antidepressants action: modulation of kynurenine pathway by increasing the kynurenic acid/3-hydroxykynurenine ratio. J Neural Transm 2012;119:235-243.
https://doi.org/10.1007/s00702-011-0668-8 |
| 52 | Kiluk M, Lewkowicz J, Pawlak D, Tankiewicz-Kwedlo A. Crosstalk between Tryptophan Metabolism via Kynurenine Pathway and Carbohydrate Metabolism in the Context of Cardio-Metabolic Risk-Review. J Clin Med 2021;10:2484.
https://doi.org/10.3390/jcm10112484 |
| 53 | Vaváková M, Ďuračková Z, Trebatická J. Markers of Oxidative Stress and Neuroprogression in Depression Disorder. Oxid Med Cell Longev 2015;2015:1-12.
https://doi.org/10.1155/2015/898393 |
| 54 | van Dooren FEP, Pouwer F, Schalkwijk CG, Sep SJS, Stehouwer CDA, Henry RMA, et al. Advanced Glycation End Product (AGE) Accumulation in the Skin is Associated with Depression: The Maastricht Study. Depress Anxiety 2017;34:59-67.
https://doi.org/10.1002/da.22527 |
| 55 | Babizhayev M. Biochemical, Biomedical and Metabolic Aspects of Imidazole-Containing Dipeptides with the Inherent Complexity to Neurodegenerative Diseases and Various States of Mental Well-Being: A Challenging Correction and Neurotherapeutic Pharmaceutical Biotechnology. Curr Pharm Biotechnol 2014;15:738-778.
https://doi.org/10.2174/1389201015666140827104918 |
| 56 | Menon K, Cameron JD, de Courten M, de Courten B. Use of carnosine in the prevention of cardiometabolic risk factors in overweight and obese individuals: study protocol for a randomised, double-blind placebo-controlled trial. BMJ Open 2021;11:e043680.
https://doi.org/10.1136/bmjopen-2020-043680 |
| 57 | Jaśkowska J, Zaręba P, Śliwa P, Pindelska E, Satała G, Majka Z. Microwave-Assisted Synthesis of Trazodone and Its Derivatives as New 5-HT1A Ligands: Binding and Docking Studies. Molecules 2019 ;24:1609.
https://doi.org/10.3390/molecules24081609 |
| 58 | Wichniak A, Wierzbicka AE, Jarema M. Treatment of insomnia - effect of trazodone and hypnotics on sleep. Psychiatr Pol 2021 ;55:743-755.
https://doi.org/10.12740/PP/125650 |
| 59 | Ledesma-Osuna AI, Ramos-Clamont G, Vázquez-Moreno L. Characterization of bovine serum albumin glycated with glucose, galactose and lactose. Acta Biochim Pol 2008;55:491-497.
https://doi.org/10.18388/abp.2008_3054 |
| 60 | M H, Azzazy E, Christenson RH. All About Albumin: Biochemistry, Genetics, and Medical Applications. Theodore Peters, Jr. San Diego, CA: Academic Press, 1996, 432 pp. ISBN 0-12-552110-3. 1997. DOI: 10.1093/clinchem/43.10.2014a
https://doi.org/10.1093/clinchem/43.10.2014a |
| 61 | Doweiko JP, Nompleggi DJ. Reviews: Role of Albumin in Human Physiology and Pathophysiology. J Parenter Enter Nutr 1991;15:207-211.
https://doi.org/10.1177/0148607191015002207 |
| 62 | Nakamura K, Era S, Ozaki Y, Sogami M, Hayashi T, Murakami M. Conformational changes in seventeen cystine disulfide bridges of bovine serum albumin proved by Raman spectroscopy. FEBS Lett 1997;417:375-378.
https://doi.org/10.1016/S0014-5793(97)01326-4 |
| 63 | Sevilla P, Rivas JM, García-Blanco F, García-Ramos J V., Sánchez-Cortés S. Identification of the antitumoral drug emodin binding sites in bovine serum albumin by spectroscopic methods. Biochim Biophys Acta - Proteins Proteomics 2007;1774:1359-1369.
https://doi.org/10.1016/j.bbapap.2007.07.022 |
| 64 | Quagraine E., Kraatz H-B, Reid R. Peptides mimicking the N-terminal Cu(II)-binding site of bovine serum albumin: synthesis, characterization and coordination with Cu(II) ions. J Inorg Biochem 2001;85:23-32.
https://doi.org/10.1016/S0162-0134(00)00227-0 |
| 65 | Zhang Y, Wilcox DE. Thermodynamic and spectroscopic study of Cu(II) and Ni(II) binding to bovine serum albumin. JBIC J Biol Inorg Chem 2002;7:327-337.
https://doi.org/10.1007/s00775-001-0302-6 |
| 66 | Westwood ME, Thornalley PJ. Molecular characteristics of methylglyoxal-modified bovine and human serum albumins. Comparison with glucose-derived advanced glycation endproduct-modified serum albumins. J Protein Chem 1995;14:359-372.
https://doi.org/10.1007/BF01886793 |
| 67 | Anguizola J, Matsuda R, Barnaby OS, Hoy KS, Wa C, DeBolt E, et al. Review: Glycation of human serum albumin. Clin Chim Acta 2013;425:64-76.
https://doi.org/10.1016/j.cca.2013.07.013 |
| 68 | Ding F, Huang J, Lin J, Li Z, Liu F, Jiang Z, et al. A study of the binding of C.I. Mordant Red 3 with bovine serum albumin using fluorescence spectroscopy. Dye Pigment 2009;82:65-70.
https://doi.org/10.1016/j.dyepig.2008.11.003 |
| 69 | Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 2018;15:490-503.
https://doi.org/10.1016/j.redox.2018.01.008 |
| 70 | Fedorova M, Bollineni RC, Hoffmann R. Protein carbonylation as a major hallmark of oxidative damage: update of analytical strategies. Mass Spectrom Rev 33:79-97.
https://doi.org/10.1002/mas.21381 |
| 71 | Chakravarti B, Chakravarti DN. Oxidative Modification of Proteins: Age-Related Changes. Gerontology 2007;53:128-139.
https://doi.org/10.1159/000097865 |
| 72 | Ahmad S, Khan MY, Rafi Z, Khan H, Siddiqui Z, Rehman S, et al. Oxidation, glycation and glycoxidation-The vicious cycle and lung cancer. Semin Cancer Biol 2018;49:29-36.
https://doi.org/10.1016/j.semcancer.2017.10.005 |
| 73 | Luers L, Rysiewski K, Dumpitak C, Birkmann E. Kinetics of Advanced Glycation End Products Formation on Bovine Serum Albumin with Various Reducing Sugars and Dicarbonyl Compounds in Equimolar Ratios. Rejuvenation Res 2012;15:201-205.
https://doi.org/10.1089/rej.2011.1284 |
| 74 | Liu Y-H, Lee T-L, Han C-H, Lee Y-S, Hou W-C. Anti-glycation, anti-hemolysis, and ORAC activities of demethylcurcumin and tetrahydroxycurcumin in vitro and reductions of oxidative stress in d-galactose-induced BALB/c mice in vivo. Bot Stud 2019;60:9.
https://doi.org/10.1186/s40529-019-0258-x |
| 75 | Rondeau P, Bourdon E. The glycation of albumin: Structural and functional impacts. Biochimie 2011;93:645-658.
https://doi.org/10.1016/j.biochi.2010.12.003 |
| 76 | Spiller S, Li Y, Blüher M, Welch L, Hoffmann R. Diagnostic Accuracy of Protein Glycation Sites in Long-Term Controlled Patients with Type 2 Diabetes Mellitus and Their Prognostic Potential for Early Diagnosis. Pharmaceuticals 2018;11:38.
https://doi.org/10.3390/ph11020038 |
| 77 | Iberg N, Flückiger R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J Biol Chem 1986;261:13542-1355.
https://doi.org/10.1016/S0021-9258(18)67052-8 |
| 78 | Ahmed N, Thornalley PJ. Peptide mapping of human serum albumin modified minimally by methylglyoxal in vitro and in vivo. Ann N Y Acad Sci 2005;1043:260-266.
https://doi.org/10.1196/annals.1333.031 |
| 79 | Blokhina O, Virolainen E, Fagerstedt K V. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 2003;91 Spec No:179-194.
https://doi.org/10.1093/aob/mcf118 |
| 80 | Mullarkey CJ, Edelstein D, Brownlee M. Free radical generation by early glycation products: A mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun 1990;173:932-939.
https://doi.org/10.1016/S0006-291X(05)80875-7 |
| 81 | Oak J-H, Nakagawa K, Miyazawa T. Synthetically prepared Amadori-glycated phosphatidylethanolamine can trigger lipid peroxidation via free radical reactions. FEBS Lett 2000;481:26-30.
https://doi.org/10.1016/S0014-5793(00)01966-9 |
| 82 | Yim MB, Yim HS, Lee C, Kang SO, Chock PB. Protein glycation: creation of catalytic sites for free radical generation. Ann N Y Acad Sci 2001;928:48-53.
https://doi.org/10.1111/j.1749-6632.2001.tb05634.x |
| 83 | Eaton JW, Qian M. Interactions of copper with glycated proteins: possible involvement in the etiology of diabetic neuropathy. Mol Cell Biochem. 234-235:135-142.
https://doi.org/10.1007/978-1-4615-1087-1_15 |
| 84 | Argirova MD, Ortwerth BJ. Activation of protein-bound copper ions during early glycation: study on two proteins. Arch Biochem Biophys 2003;420:176-184.
https://doi.org/10.1016/j.abb.2003.09.005 |
| 85 | Tkachev S V., Ushkov AA. Role of Cu2+ in Free Radical Oxidation of Human Serum Albumin and L-Tyrosine Dipeptide with Multicomponent Metal-Containing Xenobiotic. Bull Exp Biol Med 2005;140:309-311.
https://doi.org/10.1007/s10517-005-0475-z |
| 86 | Kagan VE, Tyurin VA, Borisenko GG, Fabisiak JP, Hubel CA, Ness RB, et al. Mishandling of copper by albumin: role in redox-cycling and oxidative stress in preeclampsia plasma. Hypertens pregnancy 2001;20:221-241.
https://doi.org/10.1081/PRG-100107826 |
| 87 | Thornalley PJ. Monosaccharide Autoxidation in Health and Disease. Environ Health Perspect 1985;64:297.
https://doi.org/10.1289/ehp.8564297 |
| 88 | Martinez Fernandez A, Regazzoni L, Brioschi M, Gianazza E, Agostoni P, Aldini G, et al. Pro-oxidant and pro-inflammatory effects of glycated albumin on cardiomyocytes. Free Radic Biol Med 2019;144:245-255.
https://doi.org/10.1016/j.freeradbiomed.2019.06.023 |
| 89 | Das S, Maras JS, Hussain MS, Sharma S, David P, Sukriti S, et al. Hyperoxidized albumin modulates neutrophils to induce oxidative stress and inflammation in severe alcoholic hepatitis. Hepatology 2017;65:631-646.
https://doi.org/10.1002/hep.28897 |
| 90 | Stolzing A, Widmer R, Jung T, Voss P, Grune T. Degradation of glycated bovine serum albumin in microglial cells. Free Radic Biol Med 2006;40:1017-1027.
https://doi.org/10.1016/j.freeradbiomed.2005.10.061 |
| 91 | Piarulli F, Banfi C, Brioschi M, Altomare A, Ragazzi E, Cosma C, et al. The Burden of Impaired Serum Albumin Antioxidant Properties and Glyco-Oxidation in Coronary Heart Disease Patients with and without Type 2 Diabetes Mellitus. Antioxidants (Basel, Switzerland) 2022;11.
https://doi.org/10.3390/antiox11081501 |
| 92 | Yoshikawa Y, Hosomi H, Fukami T, Nakajima M, Yokoi T. Establishment of knockdown of superoxide dismutase 2 and expression of CYP3A4 cell system to evaluate drug-induced cytotoxicity. Toxicol Vitr 2009;23:1179-1187.
https://doi.org/10.1016/j.tiv.2009.05.024 |
| 93 | Taziki S, Sattari MR, Eghbal MA. Mechanisms of Trazodone-Induced Cytotoxicity and the Protective Effects of Melatonin and/or Taurine toward Freshly Isolated Rat Hepatocytes. J Biochem Mol Toxicol 2013;27:457-462.
https://doi.org/10.1002/jbt.21509 |
| 94 | Najibi A, Heidari R, Zarifi J, Jamshidzadeh A, Firoozabadi N, Niknahad H. Evaluating the Role of Drug Metabolism and Reactive Intermediates in Trazodone-Induced Cytotoxicity toward Freshly-Isolated Rat Hepatocytes. Drug Res 2016;66:592-596.
https://doi.org/10.1055/s-0042-109536 |
| 95 | Khedr NF, El-Feky OA, Werida RH. L-Carnitine Mitigates Trazadone Induced Rat Cardiotoxicity Mediated via Modulation of Autophagy and Oxidative Stress. Cardiovasc Toxicol 2022;22:831-841.
https://doi.org/10.1007/s12012-022-09759-1 |
| 96 | Khedr NF, Werida RH. l-carnitine modulates autophagy, oxidative stress and inflammation in trazodone induced testicular toxicity. Life Sci 2022;290:120025.
https://doi.org/10.1016/j.lfs.2021.120025 |
| 97 | S. Rahbar BSP, J.L. Figarola BSP. Inhibitors and Breakers of Advanced Glycation Endproducts (AGEs): A Review. Curr Med Chem Endocr Metab Agents 2002;2:135-161.
https://doi.org/10.2174/1568013023358889 |
| 98 | Pedre B, Barayeu U, Ezeriņa D, Dick TP. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species. Pharmacol Ther 2021;228:107916.
https://doi.org/10.1016/j.pharmthera.2021.107916 |
| 99 | Azman N 'Ain, Thanh NX, Yong Kah JC. Sequestration of Cetyltrimethylammonium Bromide on Gold Nanorods by Human Serum Albumin Causes Its Conformation Change. Langmuir 2020;36:388-396.
https://doi.org/10.1021/acs.langmuir.9b03187 |
| 100 | Rabbani G, Ahn SN. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo. Int J Biol Macromol 2019;123:979-990.
https://doi.org/10.1016/j.ijbiomac.2018.11.053 |
| 101 | Peyrin E, Guillaume YC, Morin N, Guinchard C. Sucrose Dependence of Solute Retention on Human Serum Albumin Stationary Phase: Hydrophobic Effect and Surface Tension Considerations. Anal Chem 1998;70:2812-2818.
https://doi.org/10.1021/ac980039a |
| 102 | Zenei T, Hiroshi T. Specific and non-specific ligand binding to serum albumin. Biochem Pharmacol 1985;34:1999-2005.
https://doi.org/10.1016/0006-2952(85)90322-3 |
| 103 | Bertucci C, Domenici E. Reversible and Covalent Binding of Drugs to Human Serum Albumin: Methodological Approaches and Physiological Relevance. Curr Med Chem 2002;9:1463-1481.
https://doi.org/10.2174/0929867023369673 |
| 104 | Kumar A, Garg R, Kumar P. Nitric oxide modulation mediates the protective effect of trazodone in a mouse model of chronic fatigue syndrome. Pharmacol Rep 2008;60:664-672. |
| 105 | Kumar A, Garg R, Gaur V, Kumar P. Possible role of NO modulators in protective effect of trazodone and citalopram (antidepressants) in acute immobilization stress in mice. Indian J Exp Biol 2010;48:1131-1135. |
| 106 | Shen K-P, Lo Y-C, Yang R-C, Liu H-W, Chen I-J, Wu B-N. Antioxidant eugenosedin-A protects against lipopolysaccharide-induced hypotension, hyperglycaemia and cytokine immunoreactivity in rats and mice. J Pharm Pharmacol 2005;57:117-125.
https://doi.org/10.1211/0022357055137 |
| 107 | Shen K-P, Liou S-F, Hsieh S-L, Chen I-J, Wu B-N. Eugenosedin-A amelioration of lipopolysaccharide-induced up-regulation of p38 MAPK, inducible nitric oxide synthase and cyclooxygenase-2. J Pharm Pharmacol 2007;59:879-889.
https://doi.org/10.1211/jpp.59.6.0015 |
| 108 | Kumar P, Kalonia H, Kumar A. Nitric oxide mechanism in the protective effect of antidepressants against 3-nitropropionic acid-induced cognitive deficit, glutathione and mitochondrial alterations in animal model of Huntington's disease. Behav Pharmacol 2010;21:217-230.
https://doi.org/10.1097/FBP.0b013e32833a5bf4 |
| 109 | Kumar P, Kalonia H, Kumar A. Novel protective mechanisms of antidepressants against 3-nitropropionic acid induced Huntington's-like symptoms: a comparative study. J Psychopharmacol 2011;25:1399-1411.
https://doi.org/10.1177/0269881110364269 |
| 110 | Crawford DR, Davies KJ. Adaptive response and oxidative stress. Environ Health Perspect. 1994 Dec;102 Suppl:25-8.
https://doi.org/10.1289/ehp.94102s1025 |
| 111 | Rotzinger S, Fang J, Baker GB. Trazodone is metabolized to m-chlorophenylpiperazine by CYP3A4 from human sources. Drug Metab Dispos 1998;26:572-575. |