Unprecedentedly High Level of Intracellular Vitamin C and DNA Epigenetic Marks in Prostate: Relevant for Male Fertility?
Keywords
Abstract
Background/Aims:
Seminal plasma composition is affected by the physiological state of the prostate, the major male reproductive gland. Semen components, like vitamin C, can modulate sperm function. Vitamin C is an effective scavenger of free radicals and is an essential component of enzymes such as TET proteins involved in the DNA demethylation process. In the present study, a broad range of parameters which may influence the metabolic state of the prostate gland were analysed including blood and prostate tissue vitamin C, epigenetic DNA modifications and 8-oxo-7,8-dihydro-2’-deoxyguanosine in DNA of leukocytes and prostate tissues.Methods:
The experimental material were tissue samples from patients with benign prostatic hyperplasia (BPH), normal/marginal prostate tissues from prostate cancer patients, leukocytes from healthy donors, and blood plasma from BPH patients and healthy donors. We applied ultra-performance liquid chromatography methods with mass spectrometry and/or UV detection.Results:
We found an unprecedentedly high level of intracellular vitamin C in all analysed prostatic tissues (benign prostatic hyperplasia and normal, marginal ones), a value much higher than in leukocytes and most human tissues. DNA epigenetic patterns in prostate cells are similar to other soft tissues like the colon, however, its uniqueness is the unprecedentedly high level of 5-(hydroxymethyl)-2’-deoxyuridine and a significant increase in 5-formyl-2’-deoxycytidine value compared to aforementioned tissues. Moreover, the level of 8-oxo-7,8-dihydro-2’-deoxyguanosine, an established marker of oxidative stress, is significantly higher in prostate tissues than in leukocytes and many previously studied soft tissues.Conclusion:
Our results pointed out that prostatic vitamin C (regarded as the main supplier of the vitamin C to seminal plasma) and the DNA modifications (which may be linked to the regeneration of prostate epithelium) may play important role to maintain the prostate health.Introduction
Vitamin C (VC) is an essential component of numerous enzymes, among them TET (ten-eleven translocation) proteins involved in the DNA demethylation process. Moreover, VC is effective free radicals/reactive oxygen species (ROS) scavenger; therefore, it can protect cellular biomolecules such as DNA from degradation/modifications [1]. Thus, these properties make VC essential for the proper functioning of our bodies. Furthermore, it has been widely recognised that VC has a beneficial effect on the process of spermatogenesis to attain fertility [2], (reviewed in [3]). Seminal plasma (SP) components, like VC, modulates sperm function, sperm cell count, motility, morphology, and oxidation-reduction metabolism (reviewed in [3]).
The methylation of cytosine, a key epigenetic DNA modification, is closely linked to gene repression, a process that exerts a profound effect on cellular identity [4]. Active DNA demethylation, in turn, is a process which results in the activation of previously silenced genes. The molecular background of active DNA demethylation involves TET proteins that catalyse the oxidation of 5-methylcytosine (5-mCyt) to 5-hydroxymethylcytosine (5-hmCyt), and then to 5-formylcytosine (5-fCyt) which is eventually converted to 5-carboxycytosine (5-caCyt) [5, 6]. The results from experimental studies demonstrated that TETs are also involved in the synthesis of 5-hydroxymethyluracil (5-hmUra), a compound with epigenetic function [7].
Our study with cell cultures demonstrated that supplementation with ascorbate in physiological concentrations in medium (about 100 µM) and inside the cell (about 1 mM), resulted in a remarkable increase in the level of epigenetic DNA modifications [8].
Seminal plasma composition is affected by the physiological state of the different organs/glands associated with fertility, and the prostate is the major male reproductive gland. Therefore, in the present study, for the first time, a broad range of parameters which may influence the metabolic state of the prostate gland were analysed in benign prostatic hyperplasia (BPH) patients and normal, marginal tissue of prostate cancer (PC) patients. These parameters include epigenetic DNA modifications: 5-methyl-2’-deoxycytidine (5-mdC), 5-(hydroxymethyl)-2’-deoxycytidine (5-hmdC), 5-formyl-2’-deoxycytidine (5-fdC) and 5-(hydroxymethyl)-2’-deoxyuridine (5-hmdU) in DNA isolated from leukocytes and prostate tissues. Aside from the epigenetic DNA modifications, we also analysed in DNA the level of 8-oxo-7, 8-dihydro-2’-deoxyguanosine (8-oxodG), as an established marker of oxidative stress [9]. Moreover, we quantified vitamin C in the blood plasma and within cells (leukocytes and prostate tissues).
Materials and Methods
Subjects
The material for the study consisted of: (1) tissue samples from patients with benign prostatic hyperplasia (n = 28, median age 67 years), (2) normal/marginal prostate tissues, free of neoplastic features from prostate cancer patients (the only source of normal prostatic tissue, n = 43, median age 68 years), (3) peripheral blood leukocytes from healthy donors (n = 31, median age 51 years), (4) blood plasma from patients with benign prostatic hyperplasia and healthy donors. The biological specimens were collected between May 2020 and July 2022. The control group, consisting of healthy individuals, was recruited from the participants in national cancer screening programs. All participants of the study, including the healthy donors, were recruited in a hospital setting (Jan Biziel University Hospital No. 2 in Bydgoszcz, Poland). None of the study subjects were related with one another. None of the patients had received any anticancer therapy before the sample collection. Prostate tissues samples were obtained from prostate biopsy specimens or after surgical resection from patients. All the clinical investigations were conducted according to the principles of the Declaration of Helsinki.
Determination of vitamin C in blood plasma by UPLC-UV
Determination of vitamin C in blood plasma by UPLC-UV was established in [10] with some modifications. To stabilise vitamin C and to precipitate proteins, 200 µL aliquots of freshly prepared or partially thawed plasma were mixed with 200 µL of precooled 10 % (w/v) meta-phosphoric acid (MPA, Merck KGaA, Germany) containing allopurinol (75 µM, Merck KGaA, Germany) as an internal standard. The samples were kept on ice for 40 min and then diluted with 200 µL of MilliQ-grade deionised water (Merck Millipore, Germany), vortexed and centrifuged at 25155 × g for 20 min at 4 °C. The supernatants (200 µL) were purified by ultrafiltration using AcroPrep Advance 96-Well Filter Plates 10 K (Pall Corporation, USA) at 1355 × g for a minimum of 30 min at 4 °C and injected into the Waters Acquity ultra-performance liquid chromatographic (UPLC) system. The method was validated with the reference material from Chromsystems.
The UPLC system consisted of a binary solvent manager, sample manager, column manager and photodiode array detector, all from Waters. The samples were separated on Waters CORTECS® UPLC T3 1.6 µm (3 x 150 mm) with CORTECS® UPLC T3 1.6 µm Van GuardTM Pre-column, 2.1 mm x 5 mm at a flow rate 0.3 mL/min and 2 µL injection volume. Methanol and 0.01 % (v/v) acetic acid were used as solvents A and B, respectively. The following program was used for VC elution: 0 - 0.3 min 0.1 % A, 99.9 % B, 0.3 - 1.2 min 15 % A, 85 % B, 1.2 - 2.5 min 20% A, 80% B, 2.5 - 3.0 min 40 % A, 60 % B, 3.0 - 3.1 min 40 % A, 60 % B, 3.1 - 3.2 min 0.1% A, 99, 9 % B, 3.2 - 6.0 min - linear gradient, 6.0 - 7.8 min - 0.1 % A flow 0.4 mL/min. At 7.8 min, the 0.3 mL/min flow was established. The column thermostat was set at 15 °C. The chromatographic peak of VC was monitored with a photodiode array detector at 245 nm and analysed with Empower software.
Leukocytes and tissue preparation
Leukocytes were isolated from heparinised blood samples with Histopaque 1119 (Merck KGaA, Germany), according to the manufacturer’s instructions, and stored at -80 °C until analysis.
Frozen tissues obtained from patients with BPH and prostate cancer (normal, marginal tissue) were transferred to tubes containing ceramic beads (1.4 mm), and 200 µL of PBS (Biomed Lublin S.A., Poland) were added to each tube. Samples were homogenised using a Bead Ruptor Elite Homogenizer (OMNI International, USA). After homogenisation, samples were placed on ice, and 25 µL were aspirated for intracellular VC determination and thymine concentration and diluted of 75 µL Milli-Q grade deionised water for this purpose. The rest of the homogenate (125 µL) was transferred to the new tubes and used for isolation and determination of DNA modifications.
Determination of intracellular vitamin C in tissues and leukocytes by UPLC-MS
Determination of intracellular vitamin C was described in [11]. The samples were homogenised using an ultrasonic homogeniser (SONOPULS UW 2070, BANDELIN electronic GmbH & Co. KG), twice for 10 s on ice. Forty-five microliters of the sample were mixed with 50 µL of 10 % (m/v) trichloroacetic acid (Merck KGaA, Germany) and 5 µL of 10 µM stable isotope-labeled internal standard solution ([13C6] L-ascorbic acid, Toronto Research Chemicals) and incubated for 20 min on ice. Then, the samples were vortexed and centrifuged at 24400 × g for 20 min at 4 °C. The supernatants were filtrated using AcroPrep Advance 96-Well Filter Plates 10 K MWCO (Pall Corporation, USA). One microliter of the aliquots was chromatographically separated on a CORTECS® UPLC T3 1.6 µm (3 mm × 150 mm) column with a Waters Xevo TQ-XS tandem mass spectrometer. The column (20 °C) was eluted at a flow rate of 0.3 mL/min with 5 µM ammonium formate in 0.05 % acetic acid (solvent A) and methanol (solvent B). The electrospray ionisation was set to negative ion mode. The desolvation gas (nitrogen) flow rate was 1200 L/h, the nitrogen cone gas flow was 200 L/h, the desolvation temperature was 500 °C, and the nebuliser gas pressure was 7 bar. Collision-induced dissociation was obtained with argon (3 × 10-6 bar pressure) as the collision gas. Transition patterns that were selected as quantitative (175>115 and 181>119 for L-ascorbic acid and [13C6]-L-ascorbic acid, respectively) were acquired using MassLynx 4.2 software from Waters. Quantitative analyses were performed using the Target Lynx application. All the samples were analysed in three to five technical replicates. The content of intracellular vitamin C (fmol/cell) was also recalculated to mmol/L, assuming prostate and leukocyte cell volumes of 221 fL and 679 fL, respectively.
DNA isolation and enzymatic hydrolysis to deoxyribonucleosides
DNA isolation procedure was described earlier by Skalska-Bugala et al [13]. with following modifications. Leukocytes and tissue homogenates were diluted in ice-cold buffer B (10 mM Tris-HCl (Merck KGaA, Germany), 5 mM Na2EDTA (Merck KGaA, Germany) and 0.15 mM deferoxamine mesylate (Merck KGaA, Germany), pH 8.0) in a 1:1 ratio. SDS (Merck KGaA, Germany) solution was added (to a final concentration of 0.5 %), and the mixture was gently mixed using a polypropylene Pasteur pipette. The samples were incubated at 37 °C for 30 min. Proteinase K (Merck KGaA, Germany) was added to a final concentration of 4 mg/mL and incubated at 37 °C for 1.5 h. The mixture was cooled to 4 °C and transferred to Phase Lock Gel Light tubes (QuantaBio, USA). Phenol: chloroform: isoamyl alcohol (25:24:1) was added in a 1:1 ratio and vortexed vigorously. After extraction, the aqueous phase was treated with a chloroform: isoamyl alcohol mixture (24:1). The supernatant was treated with three volumes of cold 96 % (v/v) ethanol to precipitate high molecular weight nucleic acids. The precipitate was removed with a plastic spatula, washed with ethanol and dissolved in Milli-Q grade deionised water. The samples were mixed with 200 mM ammonium acetate containing 0.2 mM ZnCl2, pH 4.6 (1:1). Nuclease P1 (100 U, New England Biolabs) and tetrahydrouridine (Merck KGaA, Germany), 10 μg/sample was added to the mixture and incubated at 37 °C for 3 h. Subsequently, 10 % (v/v) NH4OH and 6 U of shrimp alkaline phosphatase (rSAP, New England Biolabs) was added to each sample and incubated for 1.5 h at 37 °C. Finally, all the hydrolysates were ultrafiltered prior to injection to eliminate macromolecular compounds, using AcroPrep Advance 96-Well Filter Plates 10 K MWCO (Pall Corporation, USA) and centrifugation at 2000 × g for 60 min at 4 °C.
Determination of epigenetic modifications and 8-oxodG in DNA isolated from leukocytes and prostate tissues
The analyses were performed using a method described earlier by Gackowski et al. and Starczak et al. [14, 15]. The DNA hydrolysates were spiked with a mixture of internal standards at a volumetric ratio of 4:1 to a final concentration of 50 fmol/µL: [D3]-5-(hydroxymethyl-2’-deoxycytidine, [13C10, 15N2]-5-formyl-2’-deoxycytidine, [13C10, 15N2]-5-carboxy-2’-deoxycytidnie, [13C10, 15N2]-5-(hydroxymethyl)-2’-deoxyuridine, and [15N5]-8-oxo7, 8-dihydro-2’-deoxyguanosine. Chromatographic separation was performed with a Waters ACQUITY 2D-UPLC system with a photodiode array detector for the first dimension of the 2D-chromatography (used for quantification of the unmodified deoxyribonucleosides and 5-methyl-2’-deoxycytidine) and a Xevo TQ-XS tandem quadrupole mass spectrometer (used for the second dimension of the 2D-chromatography, and to analyse 5-hmdC from the first dimension in the positive mode, to assure better ionisation at higher acetic acid concentrations). At-column-dilution technique was used between the first and second dimensions to improve the retention of the trap/transfer column. The following columns were used: a Waters CORTECS® T3 column (150 mm × 3 mm, 1.6 µm) with a precolumn for the first dimension, a Waters XSelect C18 CSH (100 mm × 2.1 mm, 1.7 µm) for the second dimension and a Waters XSelect C18 CSH (20 mm × 3 mm, 3.5 µm) column as a trap/transfer column. The chromatographic system was operated in heart-cutting mode, indicating that selected fractions of the effluent from the first dimension were loaded onto the trap/transfer column by 6-port valve switching, which served as the “injector” for the second dimension of the 2D-chromatography process. The flow rate for the first dimension was 0.5 mL/min, and the injection volume was 2 µL. Separation was performed with a gradient elution for 10 min using a mobile phase of 0.05 % acetate (A) and acetonitrile (B) (0.7 – 5 % B for 5 min, column washing with 30 % acetonitrile and re-equilibration with 99 % A for 3.6 min). The flow rate for the second dimension was 0.3 mL/min. The separation was performed with a gradient elution for 10 min using a mobile phase of 0.01 % acetate (A) and methanol (B) (1 – 50 % B for 4 min, an isocratic flow of 50 % B for 1.5 min, and re-equilibration with 99 % A until the next injection). Collision-induced dissociation was obtained using argon 6.0 at 3 × 10-6 bar pressure as the collision gas. Transition patterns for all the analysed compounds and the specific detector settings were determined using the MassLynx 4.2 IntelliStart feature set in a quantitative mode to ensure the best signal-to-noise ratio and a resolution of 1 at MS1 and 0.75 at MS2. Transition patterns-specific detector settings and sources of standards for the analysed compounds are presented in the Supplementary table 1. All samples were analysed with three to five technical replicates, of which the technical mean was used for further calculation. The quantities of canonical deoxynucleosides were determined by UV detection at 260 nm for 2’-deoxythymidine (dT), and at 280 nm for 2’-deoxyguanosine (dG) and 5-mdC. The total deoxynucleosides amount (dN) calculated as the doubled sum of dT and dG was used as a reference for the quantitative expression of the modified ones.
Statistical analyses
The results are presented as median values, interquartile ranges, and non-outlier ranges. Statistical analyses were carried out with Statistica 13.3 PL software [TIBCO Software Inc. (2017). Statistica (data analysis software system), version 13. http://statistica.io]. Normal distribution of the study variables was verified with the Kolmogorov–Smirnov test with Lilliefors correction. The variables with non-normal distributions were analyzed with nonparametric Mann–Whitney U test. The correlations were assessed using the Spearman’s correlation analysis. The results were considered statistically significant at p<0.05.
Results
In prostate tissue samples obtained from BPH patients, as well as in normal, marginal prostate tissues taken from prostate cancer patients, we observed a significantly higher level of intracellular VC, than in leukocytes of healthy donors’ (Fig. 1A, Table 1). We did not find a significant difference in blood vitamin C levels between BPH patients and healthy subjects (the median values are 67.920 and 78.581 mmol/L, respectively, Fig. 1B). Furthermore, in the present study, we did not find any correlation between plasma concentrations of vitamin C and respectively its the intracellular level in all aforementioned tissues.
No significant differences were found in the levels of all analysed DNA epigenetic modifications (5-mdC, 5-hmdC, 5-fdC, and 5-hmdU) between hyperplastic and normal/marginal prostatic tissues (Fig. 2). To compare status of the modifications in patients tissues we also analysed leukocytes of healthy donors. The level of 5-mdC in DNA from healthy donors’ leukocytes was determined to be a median value of 8556.55/106dN and is significantly higher than in DNA isolated from analysed prostate tissues (Fig. 2A). However, significantly higher levels of 5-hmdC, 5-fdC and 5-hmdU are found in prostatic tissues compared with DNA isolated from control leukocytes (Fig. 2B-D). The levels of 5-hmdU and 5-fdC in the DNA of prostate tissues are unprecedentedly high compared to other soft tissues like the colon determined in our recent work [16].
Determination of 8-oxodG revealed a significantly higher level of this modification in DNA of prostate tissue DNA in comparison with DNA isolated from healthy subjects’ leukocytes. Moreover, the levels of 8-oxodG gradually decrease in the sequence: BPH tissues (3.991/106dN), normal, marginal prostate tissues (3.444/106dN) and control leukocytes (1.502/106dN) (Fig. 2E).
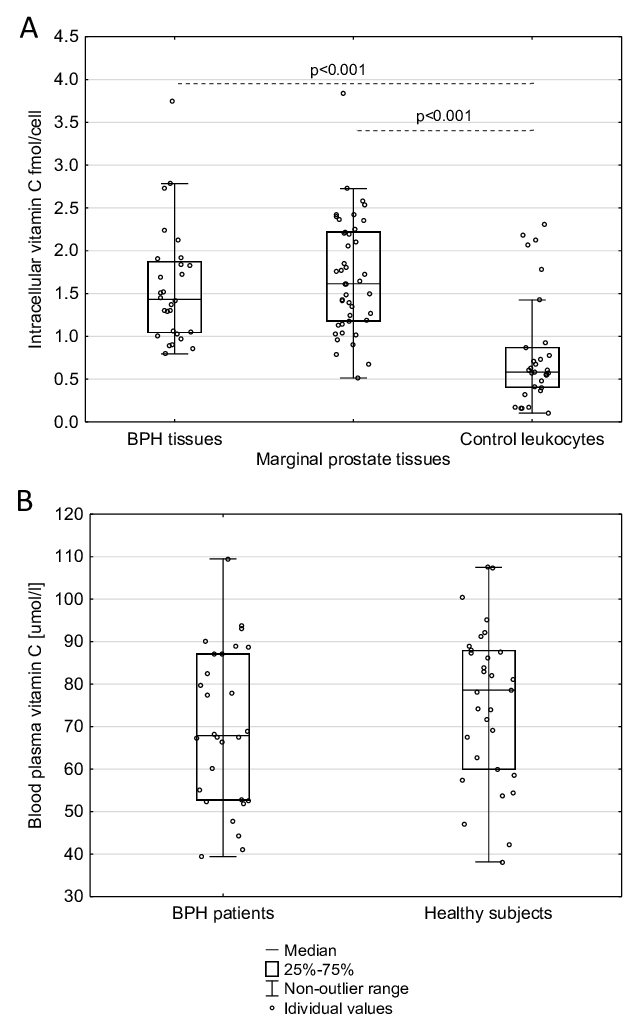
Fig. 1: Comparison of (A) the level of vitamin C in prostatic tissues and leukocytes (B) vitamin C concentration in blood plasma from patients with benign prostatic hyperplasia (BPH) and healthy subjects. Statistically significant differences were determined with the Mann-Whitney U test.
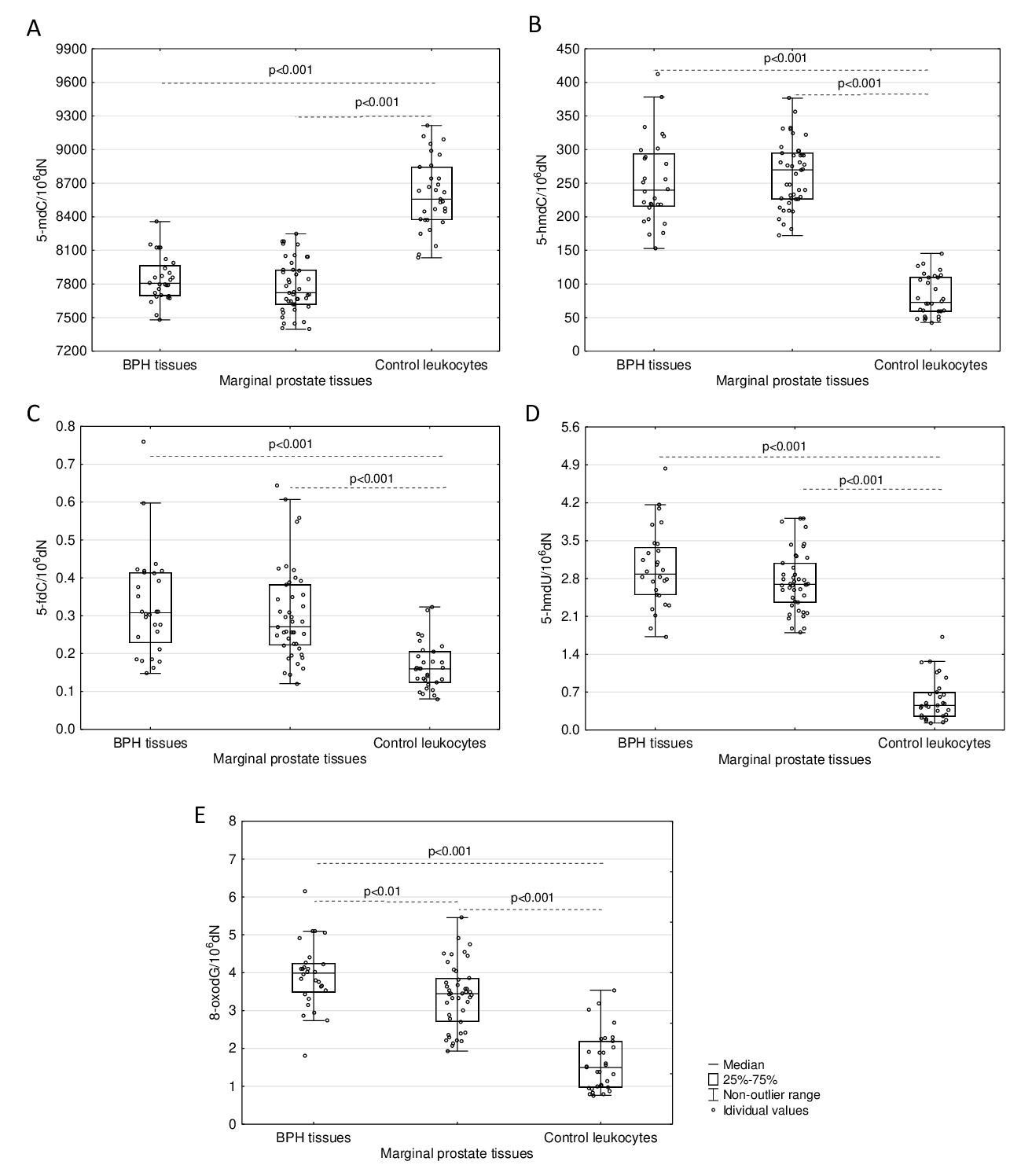
Fig. 2: Comparison of the levels of analysed DNA modifications in prostatic tissues (BPH and normal/marginal ones) and leukocytes from healthy subjects. (A) Level of 5-mdC. (B) Level of 5-hmdC. (C) Level of 5-fdC. (D) Level of 5-hmdU. (E) Level of 8-oxodG. Statistically significant differences were determined with the Mann-Whitney U test.
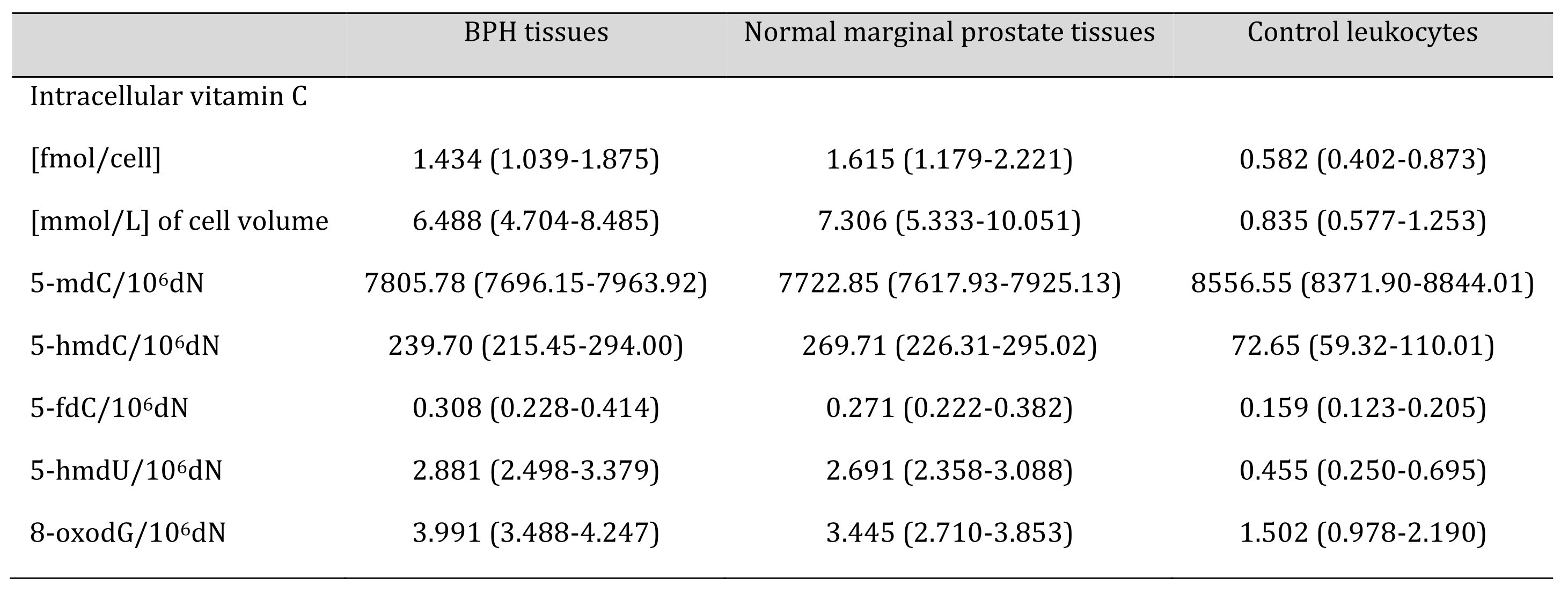
Table 1: Levels of the analysed parameters in BPH tissues, normal marginal prostate tissues and control leukocytes from healthy subjects. Values are expressed as median and interquartile range
Discussion
Benign prostatic hyperplasia appears in almost all males as they age. Histologically, BPH is defined as the excessive growth of epithelial and stromal prostate cells [17]. Accumulation of citrate, which is a result of the Zn-dependent short circuit of the Krebs cycle, is characteristic of normal prostate and BPH [18]. Moreover, no evidence of neoplastic features in BPH is found: no evidence of driver genomic alterations, including a low number of coding mutations, minimal copy number alterations, and no genomic rearrangements [17]. Thus, the metabolism of BPH cells resembles normal prostatic tissue. Therefore, in our study, we used BPH tissues and normal, marginal tissue taken from prostate cancer patients. Obtaining altered tissues from patients may be challenging. However, some studies demonstrated that the analysis of non-affected tissues can provide equally informative results (reviewed in [19]). Leukocytes are often used as easily accessible cells carrying information about environmentally-induced DNA modifications in other tissues [20]. Moreover, there are few studies concerning analyses of intracellular VC and the most reliable values were mostly restricted to leukocytes. Therefore, in our work, we also used leukocytes as reference tissue and the only one available from healthy donors.
Up to 70 % of fertility problems have a beginning in the male partner, and successful male potency depends on the cooperation of a variety of male organs, i.e., testes, epididymis, and the male accessory glands (prostate) [3, 21].
Since the prostate is an essential part of the male genital tract and the major contributor of secretion into SP, we decided to analyse a broad range of factors in above mentioned prostate tissues, which may have a key influence on the process of sperm production to attain fertility. These parameters include a broad spectrum of DNA modifications and vitamin C levels in prostate cells and in the blood.
Calculations of intracellular VC in human tissues are often based on inaccurate VC analyses and using post-mortem samples [22]. Most of the reliable assays accurately measured VC concentration by HPLC-based techniques, which were mainly restricted to blood cells [22]. Proper choice of normalisation method is also an important step and issue. Therefore, in our study, the intracellular concentration of vitamin C was determined with the UPLC-MS technique, and normalisation and standardisation of the results was based on thymine amount [12]. Using this methodology, we found an unprecedentedly high level of intracellular VC in all analysed prostatic tissues (independent of their origin), a value much higher than in leukocytes (see Table 1). It should be remembered that VC concentration is much higher in leukocytes than in most human soft tissues, including testes [22]. The level of blood plasma VC in BPH patients was similar to that characteristic of healthy subjects (Fig. 1B). Since the intracellular level of VC in the testes, the other reproductive gland, is very low (200 µM) [22] when compared with our value for the prostate, we hypothesise that this high level is necessary to supply seminal plasma. Interestingly, we and others demonstrated that the concentration of VC in SP is much higher than in blood [2, 23].
Several works have demonstrated that vitamin C might increase the generation of epigenetic DNA modifications in cultured cells, likely acting as a cofactor of TETs, specifically as a regulator of DNA demethylation [8, 24-26]. Moreover, we demonstrated that VC in cell cultures is involved in a striking increase in 5-hmdU content [8]. Although DNA epigenetic patterns in prostate cells are similar to other soft tissues like the colon [16], its uniqueness is underlined by the unprecedentedly high level of 5-hmdU, not seen in other tissues (Fig. 2D) and a significant increase in 5-fdC value (Fig. 2C).
Evidence from experimental studies supports the hypothesis that TET enzymes may be involved in the synthesis of 5-hmUra, a molecule with epigenetic function [27]. Moreover, 5‑hmUra present in DNA was shown to recruit proteins involved in chromatin remodelling, as well as transcription factors [7]. It has also been suggested that 5-hmUra itself has a regulatory function [7] and may be a regulatory element of ”ready to go’’ (poised) genes, as it was recently observed in the case of another intermediate of the active demethylation process, 5-fCyt [28, 29]. Interestingly, also the 5-fdC level was significantly higher in prostate tissues than in leukocytes (Table 1, Fig. 2C) and normal colon tissues [16]. Notably, both of these modifications were shown to act as transcription regulators [28-30]. Interestingly, 5-fCyt and 5-hmUra are recognised by thymidine DNA glycosylase (TDG) [31], and in recruiting this enzyme, it may regulate transcription independently of its repair activity (for review, see [32]). Intriguingly, the levels of 5-hmdU and 5-fdC in various somatic tissues are relatively stable and resemble that observed in leukocytes, about 0.5/106dN for the former and about 0.15/106dN for the latter in human colorectal cancer [10] as well as various rat and porcine tissues [33]. 5-FCyt is DNA a modified base that is generated by the direct oxidation of 5-hmCyt by TET enzymes. However, it has been shown that the presence of a single 5-fCyt moiety is sufficient to change the flexibility of the DNA strand [34], which in turn, might locally impact chromatin structure [35]. In the context of the above-described features of 5-fCyt and 5-hmUra, it is likely that their profound increase in prostate cells may be directly linked with chromatin reorganisation characteristic for a regenerative capacity of the prostate epithelium [36, 37]. This feature, in turn, may be linked with androgen cycles and therefore play a role in male fertility status. It was hypothesised that the prostate epithelium might also harbour stem cells responsible for tissue renewal [36, 37]. Intriguingly, hematopoietic stem cells had an unusually high level of intracellular VC [38], similar to our finding with prostate tissue.
Of note, in work published in Biology of Reproduction [39], we have demonstrated that absolute values of 5‑hmdU in sperm were much higher than those observed in leukocytes’ DNA, and similar to this characteristic for the prostate. Likely, both kinds of cells, i.e. prostatic and sperm, share the same mechanism(s) to strengthen TETs activity necessary to generate an unusually high level of 5-hmUra, i.e. high concentration of VC and citrate-Fe (II) complexes (the prostate is a main supplier of SP with citrate [21] (for explanation see below).
Summing up this part, the key contribution of the high level of prostatic VC is linked to its role as the main supplier of vitamin C to seminal plasma. Separately from this, the unique level of VC is linked to its role as the trigger of TETs activity to generate the epigenetic DNA modifications and may be linked to the regeneration of prostate epithelium.
Aside from the epigenetic DNA modifications, we also analysed the level of 8-oxodG as an established marker of oxidative stress. In this study, the level of 8-oxodG was significantly higher in prostate tissues than in leukocytes (Fig. 2E) and many soft tissues studied by us [16]. The most likely reason for this increase may be the unique metabolism of iron in prostate tissues or the high VC levels acting as a prooxidant (perhaps in conjunction with the iron metabolism). Iron plays an essential role in basic biological processes linked with the utilisation of oxygen and DNA synthesis. The most important complexes of cellular iron are iron-binding proteins, namely transferrin (TF) and ferritin.
Before iron is transported from the endosomal TF to the cytoplasm [40, 41], iron (III) should be released from TF and reduced to iron (II). One of the best-characterised iron reductases is the six-transmembrane epithelial antigen of the prostate 1-4 family (STEAP 1-4), (reviewed in [42]).
Although iron is present as a part of many enzymes and proteins, it appears that the presence of free iron (the so-called labile iron pool (LIP)), when it is complexed with low molecular weight compounds like citrate in cells [43], can result in the production of reactive oxygen species (ROS), such as hydroxyl radicals. ROS are involved in the formation of 8-oxodG in cellular DNA. Moreover, a good correlation has been demonstrated between LIP and 8-oxodG in lymphocytes [44]. This, in turn, points out the possibility that in prostate cells there is, as mentioned above, citrate availability for catalysing Fenton-type reactions in close proximity to cellular DNA [44]. As mentioned above, citrate may chelate Fe (II), and the concentration of citrate differs significantly in different prostatic tissues. The highest concentration was found in BPH tissue – 12000 and then gradually decreased, reaching 4000 - 6000 in normal tissue, and finally dropped to the lowest values in all other human tissues 100 - 400 nmoles/g (all values are expressed in nmoles/g wet weight) [45].
Of note, the values of 8-oxodG in BPH, normal, marginal prostatic tissues and leukocytes found in our work imitate the aforementioned range of citrate.
It is worth mentioning that although 8-oxodG is widely recognised as a good biomarker of oxidatively damaged DNA [46], a growing number of experimental data suggest that its presence in specific DNA sequences may function as an epigenetic mark and may be used for transcription regulation [47, 48]. Although it is difficult to decisively categorise the role of this modification, it is possible that a fraction of the 8-oxodG pool detected in our study may play a regulatory role. Anyway, both properties of 8-oxodG, such as the damage and the epigenetic mark, may potentially be linked to the regenerative properties of prostate cells (see above). In this context - just recently, it was demonstrated that elevated 8-oxodG level in human airway epithelial cells is central for tissue homeostasis and regeneration [49].
It is also possible that citrate-Fe (II) complexes, similarly to VC, may enhance the activity of TET enzymes by the renewal of a reduced iron pool.
Importantly, the values of all the analysed parameters, except for 8-oxodG, were similar, almost identical, in BPH and normal tissues. (This reinforces an argument that the metabolism of BPH cells resembles that of normal prostatic tissue).
Conclusion
In conclusion, the prostate is a pivotal male reproductive gland involved in fertility, and this feature essentially relies upon the ingredients of the prostatic fluid secreted by the gland to seminal plasma and is affected by the physiological state of accessory glands, i.e. prostate [21].
In relation to the aforementioned features of the prostate gland, our data suggest that:
- the prostate may be the main supplier of VC in seminal plasma;
- a regenerative capacity of the prostate epithelium may be linked with the uniquely high level of 5-hmdU as well as 5-fdC (possibly also by an elevated level of 8-oxodG).
Acknowledgements
Author Contributions
R.O, E.Z and J.G conceived and designed the study. P.M, A.W, E.Z, J.S, D.G, B.B and P.J performed the research and acquired the data, E.Z and J.G analyzed and interpreted the data, R.O, E.Z, J.G and D.G drafted the article, R.O, E.Z, J.G and M.F performed review and editing the article, R.O provided the final approval of the completed article. All authors have read and agreed to the published version of the manuscript.
Funding Sources
The research was supported by the National Science Center, Poland (Grant No. 2017/27/B/NZ7/01487).
Statement of Ethics
The protocol of the study was approved by the Bioethics Committee, Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Toruń (No. KB 315/2018). All enrolled subjects signed an informed consent form.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
| 1 | Kazmierczak-Baranska J, Boguszewska K, Adamus-Grabicka A, Karwowski BT: Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020;12: 1501.
https://doi.org/10.3390/nu12051501 |
| 2 | Colagar AH, Marzony ET: Ascorbic Acid in human seminal plasma: determination and its relationship to sperm quality. J Clin Biochem Nutr 2009;45:144-149.
https://doi.org/10.3164/jcbn.08-251 |
| 3 | Pascoal GFL, Geraldi MV, Marostica MR, Jr., Ong TP: Effect of Paternal Diet on Spermatogenesis and Offspring Health: Focus on Epigenetics and Interventions with Food Bioactive Compounds. Nutrients 2022;14:2150.
https://doi.org/10.3390/nu14102150 |
| 4 | Feng S, Jacobsen SE, Reik W: Epigenetic reprogramming in plant and animal development. Science 2010;330:622-627.
https://doi.org/10.1126/science.1190614 |
| 5 | Bhutani N, Burns DM, Blau HM: DNA demethylation dynamics. Cell 2011;146:866-872.
https://doi.org/10.1016/j.cell.2011.08.042 |
| 6 | Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A: Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009;324:930-935.
https://doi.org/10.1126/science.1170116 |
| 7 | Pfaffeneder T, Spada F, Wagner M, Brandmayr C, Laube SK, Eisen D, Truss M, Steinbacher J, Hackner B, Kotljarova O, Schuermann D, Michalakis S, Kosmatchev O, Schiesser S, Steigenberger B, Raddaoui N, Kashiwazaki G, Muller U, Spruijt CG, Vermeulen M, Leonhardt H, Schar P, Muller M, Carell T: Tet oxidizes thymine to 5-hydroxymethyluracil in mouse embryonic stem cell DNA. Nat Chem Biol 2014;10:574-581.
https://doi.org/10.1038/nchembio.1532 |
| 8 | Modrzejewska M, Gawronski M, Skonieczna M, Zarakowska E, Starczak M, Foksinski M, Rzeszowska-Wolny J, Gackowski D, Olinski R: Vitamin C enhances substantially formation of 5-hydroxymethyluracil in cellular DNA. Free Radic Biol Med 2016;101:378-383.
https://doi.org/10.1016/j.freeradbiomed.2016.10.535 |
| 9 | Chao MR, Evans MD, Hu CW, Ji Y, Moller P, Rossner P, Cooke MS: Biomarkers of nucleic acid oxidation - A summary state-of-the-art. Redox Biol 2021;42:101872.
https://doi.org/10.1016/j.redox.2021.101872 |
| 10 | Starczak M, Zarakowska E, Modrzejewska M, Dziaman T, Szpila A, Linowiecka K, Guz J, Szpotan J, Gawronski M, Labejszo A, Liebert A, Banaszkiewicz Z, Klopocka M, Foksinski M, Gackowski D, Olinski R: In vivo evidence of ascorbate involvement in the generation of epigenetic DNA modifications in leukocytes from patients with colorectal carcinoma, benign adenoma and inflammatory bowel disease. J Transl Med 2018;16:204.
https://doi.org/10.1186/s12967-018-1581-9 |
| 11 | Starczak M, Gawronski M, Wasilow A, Mijewski P, Olinski R, Gackowski D: Dynamic changes in genomic 5-hydroxymethyluracil and N6-methyladenine levels in the Drosophila melanogaster life cycle and in response to different temperature conditions. Sci Rep 2022;12:17552.
https://doi.org/10.1038/s41598-022-22490-9 |
| 12 | Modrzejewska M, Gawronski M, Gackowski D: Normalization of metabolic data to total thymine content and its application to determination of 2-hydroxyglutarate. Anal Biochem 2021;618:114129.
https://doi.org/10.1016/j.ab.2021.114129 |
| 13 | Skalska-Bugala A, Starczak M, Szukalski L, Gawronski M, Siomek-Gorecka A, Szpotan J, Labejszo A, Zarakowska E, Szpila A, Jachalska A, Szukalska A, Kruszewski M, Sadowska A, Wasilow A, Baginska P, Czyz J, Olinski R, Rozalski R, Gackowski D: Diagnostic and Prognostic Power of Active DNA Demethylation Pathway Intermediates in Acute Myelogenous Leukemia and Myelodysplastic Syndromes. Cells 2022;11
https://doi.org/10.3390/cells11050888 |
| 14 | Gackowski D, Starczak M, Zarakowska E, Modrzejewska M, Szpila A, Banaszkiewicz Z, Olinski R: Accurate, Direct, and High-Throughput Analyses of a Broad Spectrum of Endogenously Generated DNA Base Modifications with Isotope-Dilution Two-Dimensional Ultraperformance Liquid Chromatography with Tandem Mass Spectrometry: Possible Clinical Implication. Anal Chem 2016;88:12128-12136.
https://doi.org/10.1021/acs.analchem.6b02900 |
| 15 | Starczak M, Gawronski M, Olinski R, Gackowski D: Quantification of DNA Modifications Using Two-Dimensional Ultraperformance Liquid Chromatography Tandem Mass Spectrometry (2D-UPLC-MS/MS); in Ruzov A, Gering M (eds): DNA Modifications: Methods and Protocols. New York, NY, Springer US, 2021, pp 91-108.
https://doi.org/10.1007/978-1-0716-0876-0_8 |
| 16 | Dziaman T, Gackowski D, Guz J, Linowiecka K, Bodnar M, Starczak M, Zarakowska E, Modrzejewska M, Szpila A, Szpotan J, Gawronski M, Labejszo A, Liebert A, Banaszkiewicz Z, Klopocka M, Foksinski M, Marszalek A, Olinski R: Characteristic profiles of DNA epigenetic modifications in colon cancer and its predisposing conditions-benign adenomas and inflammatory bowel disease. Clin Epigenetics 2018;10:72.
https://doi.org/10.1186/s13148-018-0505-0 |
| 17 | Liu D, Shoag JE, Poliak D, Goueli RS, Ravikumar V, Redmond D, Vosoughi A, Fontugne J, Pan H, Lee D, Thomas D, Salari K, Wang Z, Romanel A, Te A, Lee R, Chughtai B, Olumi AF, Mosquera JM, Demichelis F, Elemento O, Rubin MA, Sboner A, Barbieri CE: Integrative multiplatform molecular profiling of benign prostatic hyperplasia identifies distinct subtypes. Nat Commun 2020;11:1987.
https://doi.org/10.1038/s41467-020-15913-6 |
| 18 | Singh KK, Desouki MM, Franklin RB, Costello LC: Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol Cancer 2006;5:14.
https://doi.org/10.1186/1476-4598-5-14 |
| 19 | Schubeler D: Function and information content of DNA methylation. Nature 2015;517:321-326.
https://doi.org/10.1038/nature14192 |
| 20 | Collins AR, Gedik CM, Olmedilla B, Southon S, Bellizzi M: Oxidative DNA damage measured in human lymphocytes: large differences between sexes and between countries, and correlations with heart disease mortality rates. The FASEB Journal 1998;12:1397-1400.
https://doi.org/10.1096/fasebj.12.13.1397 |
| 21 | Verze P, Cai T, Lorenzetti S: The role of the prostate in male fertility, health and disease. Nat Rev Urol 2016;13:379-386.
https://doi.org/10.1038/nrurol.2016.89 |
| 22 | Padayatty SJ, Levine M: Vitamin C: the known and the unknown and Goldilocks. Oral Dis 2016;22:463-493.
https://doi.org/10.1111/odi.12446 |
| 23 | Guz J, Gackowski D, Foksinski M, Rozalski R, Zarakowska E, Siomek A, Szpila A, Kotzbach M, Kotzbach R, Olinski R: Comparison of oxidative stress/DNA damage in semen and blood of fertile and infertile men. PLoS One 2013;8:e68490.
https://doi.org/10.1371/journal.pone.0068490 |
| 24 | Minor EA, Court BL, Young JI, Wang G: Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Biol Chem 2013;288:13669-13674.
https://doi.org/10.1074/jbc.C113.464800 |
| 25 | Blaschke K, Ebata KT, Karimi MM, Zepeda-Martinez JA, Goyal P, Mahapatra S, Tam A, Laird DJ, Hirst M, Rao A, Lorincz MC, Ramalho-Santos M: Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013;500:222-226.
https://doi.org/10.1038/nature12362 |
| 26 | Yin R, Mao SQ, Zhao B, Chong Z, Yang Y, Zhao C, Zhang D, Huang H, Gao J, Li Z, Jiao Y, Li C, Liu S, Wu D, Gu W, Yang YG, Xu GL, Wang H: Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc 2013;135:10396-10403.
https://doi.org/10.1021/ja4028346 |
| 27 | Olinski R, Starczak M, Gackowski D: Enigmatic 5-hydroxymethyluracil: Oxidatively modified base, epigenetic mark or both? Mutat Res Rev Mutat Res 2016;767:59-66.
https://doi.org/10.1016/j.mrrev.2016.02.001 |
| 28 | Song CX, He C: Potential functional roles of DNA demethylation intermediates. Trends Biochem Sci 2013;38:480-484.
https://doi.org/10.1016/j.tibs.2013.07.003 |
| 29 | Song CX, Szulwach KE, Dai Q, Fu Y, Mao SQ, Lin L, Street C, Li Y, Poidevin M, Wu H, Gao J, Liu P, Li L, Xu GL, Jin P, He C: Genome-wide profiling of 5-formylcytosine reveals its roles in epigenetic priming. Cell 2013;153:678-691.
https://doi.org/10.1016/j.cell.2013.04.001 |
| 30 | Kellinger MW, Song CX, Chong J, Lu XY, He C, Wang D: 5-formylcytosine and 5-carboxylcytosine reduce the rate and substrate specificity of RNA polymerase II transcription. Nat Struct Mol Biol 2012;19:831-833.
https://doi.org/10.1038/nsmb.2346 |
| 31 | Hashimoto H, Hong S, Bhagwat AS, Zhang X, Cheng X: Excision of 5-hydroxymethyluracil and 5-carboxylcytosine by the thymine DNA glycosylase domain: its structural basis and implications for active DNA demethylation. Nucleic Acids Res 2012;40:10203-10214.
https://doi.org/10.1093/nar/gks845 |
| 32 | Huang Y, Rao A: New functions for DNA modifications by TET-JBP. Nat Struct Mol Biol 2012;19:1061-1064.
https://doi.org/10.1038/nsmb.2437 |
| 33 | Gackowski D, Zarakowska E, Starczak M, Modrzejewska M, Olinski R: Tissue-Specific Differences in DNA Modifications (5-Hydroxymethylcytosine, 5-Formylcytosine, 5-Carboxylcytosine and 5-Hydroxymethyluracil) and Their Interrelationships. PLoS One 2015;10:e0144859.
https://doi.org/10.1371/journal.pone.0144859 |
| 34 | Ngo TT, Yoo J, Dai Q, Zhang Q, He C, Aksimentiev A, Ha T: Effects of cytosine modifications on DNA flexibility and nucleosome mechanical stability. Nat Commun 2016;7:10813.
https://doi.org/10.1038/ncomms10813 |
| 35 | Raiber EA, Portella G, Martinez Cuesta S, Hardisty R, Murat P, Li Z, Iurlaro M, Dean W, Spindel J, Beraldi D, Liu Z, Dawson MA, Reik W, Balasubramanian S: 5-Formylcytosine organizes nucleosomes and forms Schiff base interactions with histones in mouse embryonic stem cells. Nat Chem 2018;10:1258-1266.
https://doi.org/10.1038/s41557-018-0149-x |
| 36 | Maitland NJ, Collins AT: Prostate cancer stem cells: a new target for therapy. J Clin Oncol 2008;26:2862-2870.
https://doi.org/10.1200/JCO.2007.15.1472 |
| 37 | Kukkonen K, Taavitsainen S, Huhtala L, Uusi-Makela J, Granberg KJ, Nykter M, Urbanucci A: Chromatin and Epigenetic Dysregulation of Prostate Cancer Development, Progression, and Therapeutic Response. Cancers (Basel) 2021;13
https://doi.org/10.3390/cancers13133325 |
| 38 | Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, Crane GM, Cowin BL, Bruner E, Murphy MM, Chen W, Spangrude GJ, Hu Z, DeBerardinis RJ, Morrison SJ: Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 2017;549:476-481.
https://doi.org/10.1038/nature23876 |
| 39 | Guz J, Gackowski D, Foksinski M, Rozalski R, Olinski R: Comparison of the absolute level of epigenetic marks 5-methylcytosine, 5-hydroxymethylcytosine, and 5-hydroxymethyluracil between human leukocytes and sperm. Biol Reprod 2014;91:55.
https://doi.org/10.1095/biolreprod.114.121541 |
| 40 | Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA: Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997;388:482-488.
https://doi.org/10.1038/41343 |
| 41 | Su MA, Trenor CC, Fleming JC, Fleming MD, Andrews NC: The G185R Mutation Disrupts Function of the Iron Transporter Nramp2. Blood 1998;92:2157-2163.
https://doi.org/10.1182/blood.V92.6.2157.418k16_2157_2163 |
| 42 | Xu M, Evans L, Bizzaro CL, Quaglia F, Verrillo CE, Li L, Stieglmaier J, Schiewer MJ, Languino LR, Kelly WK: STEAP1-4 (Six-Transmembrane Epithelial Antigen of the Prostate 1-4) and Their Clinical Implications for Prostate Cancer. Cancers (Basel) 2022;14:4034.
https://doi.org/10.3390/cancers14164034 |
| 43 | Lane DJ, Richardson DR: The active role of vitamin C in mammalian iron metabolism: much more than just enhanced iron absorption! Free Radic Biol Med 2014;75:69-83.
https://doi.org/10.1016/j.freeradbiomed.2014.07.007 |
| 44 | Gackowski D, Kruszewski M, Bartlomiejczyk T, Jawien A, Ciecierski M, Olinski R: The level of 8-oxo-7, 8-dihydro-2'-deoxyguanosine is positively correlated with the size of the labile iron pool in human lymphocytes. J Biol Inorg Chem 2002;7:548-550.
https://doi.org/10.1007/s00775-001-0335-x |
| 45 | Costello LC, Franklin RB: Concepts of Citrate Production and Secretion by Prostate .2. Hormonal Relationships in Normal and Neoplastic Prostate. Prostate 1991;19:181-205.
https://doi.org/10.1002/pros.2990190302 |
| 46 | Cooke MS, Loft S, Olinski R, Evans MD, Bialkowski K, Wagner JR, Dedon PC, Moller P, Greenberg MM, Cadet J: Recommendations for standardized description of and nomenclature concerning oxidatively damaged nucleobases in DNA. Chem Res Toxicol 2010;23:705-707.
https://doi.org/10.1021/tx1000706 |
| 47 | Hahm JY, Park J, Jang ES, Chi SW: 8-Oxoguanine: from oxidative damage to epigenetic and epitranscriptional modification. Exp Mol Med 2022;54:1626-1642.
https://doi.org/10.1038/s12276-022-00822-z |
| 48 | Zarakowska E, Gackowski D, Foksinski M, Olinski R: Are 8-oxoguanine (8-oxoGua) and 5-hydroxymethyluracil (5-hmUra) oxidatively damaged DNA bases or transcription (epigenetic) marks? Mutat Res Genet Toxicol Environ Mutagen 2014;764-765:58-63.
https://doi.org/10.1016/j.mrgentox.2013.09.002 |
| 49 | Pan L, Hao W, Xue Y, Wang K, Zheng X, Luo J, Ba X, Xiang Y, Qin X, Bergwik J, Tanner L, Egesten A, Brasier AR, Boldogh I: 8-Oxoguanine targeted by 8-oxoguanine DNA glycosylase 1 (OGG1) is central to fibrogenic gene activation upon lung injury. Nucleic Acids Res 2023;51: 1087-1102.
https://doi.org/10.1093/nar/gkac1241 |