Corresponding Author: Zhao-Hui Song
Department of Pharmacology and Toxicology, University of Louisville School of Medicine, Louisville, Kentucky, 40292 (USA)
Tel. +1-502-852-5160, Fax +1-502-852-7868, E-Mail zhsong@louisville.edu
Cannabidiol Signaling in the Eye and Its Potential as an Ocular Therapeutic Agent
Alyssa Aebersold Max Duff Lucy Sloan Zhao-Hui Song
Department of Pharmacology and Toxicology, University of Louisville School of Medicine, Louisville, Kentucky, USA
Introduction
A brief history of cannabis
Cannabis sativa is a plant species that includes both cannabis and hemp. It first appeared in Chinese medical texts around 2000 years ago [1]. Records from Britain indicate that cannabis was brought from Egypt by Napoleon’s troops in the early 1800s [2]. Shortly thereafter, hemp was introduced to Western medicine when in 1840, a hemp tincture from ground plant matter was reported to be an effective treatment for convulsive disorders and tetanus [3]. By 1851, a cannabis extract was included in the 3rd edition of the Unites States Pharmacopoeia and readily available in American pharmacies [4, 5].
In 1913, however, cannabis was made illegal in California due to a wide-spread anti-narcotics campaign [5]. Cannabis became federally illegal when Harry Anslinger from California introduced the Marijuana Tax Act of 1937 banning the sale and use of cannabis nationally [5, 6]. A negative stigma continued to develop in the US around cannabis, then associated with narcotics, that culminated with the Controlled Substances Act (CSA) of 1970, which classified cannabis and cannabinoids as Schedule I with no recognized medical use [7]. Recently, America is witnessing a revival in the popularity of cannabis, both medically and recreationally. In 1996, California was the first state to legalize cannabis for medical use and more states have followed California in recent years [8]. To date, 16 states and Washington D.C. have legalized both medical and recreational cannabis with an additional 26 states legalizing medical cannabis at varying degrees. Moreover, the Agricultural Acts of 2014 and 2018 removed hemp from the list of controlled substances and redefined industrial hemp as cannabis containing less than 0.3% THC [9, 10]. As a result of the recent wave of recreational and medical cannabis legalization, in conjunction with the end to the prohibition of hemp, cannabis research is quickly expanding.
Cannabidiol
Cannabidiol (CBD) is one of over 120 chemicals produced by the Cannabis sativa plant termed phytocannabinoids [11, 12]. There are potentially more, as 21 previously unknown cannabinoids were recently identified [13]. The two most abundant phytocannabinoids in cannabis are psychoactive and intoxicating D9-tetrahydrocannabinol (THC) and non-intoxicating CBD.
CBD was first isolated in the 1940 and its structure and stereochemistry fully determined in 1963 [14, 15]. CBD and THC are both derived from cannabigerolic acid [16]. Although the structure of CBD was discovered before THC [15, 17], THC had been the major focus of research related to cannabis and cannabinoids. This focus is driven, in part, by the activity of THC at the canonical cannabinoid receptors, CB1 and CB2. However, there are many targets for cannabinoids other than CB1 and CB2. For example, CBD has upwards of 65 known targets consisting of receptors, enzymes, ion channels and transient receptor potential (TRP) channels [18].
Cannabinoids in pharmaceuticals
Cannabinoid containing drugs are approved for medical use in the USA and other countries. The drugs differ in their formulation and indicated uses. Dronabinol (Marinol) was the first cannabinoid-containing medicine approved by the FDA in 1985. It is a soft gel capsule containing synthetic THC [19]. Syndros is an oral solution of dronabinol [20]. Cesamet (nabilone) is the third synthetic cannabinoid drug approved by the FDA in May of 2006 [21]. All three are prescribed for anorexia associated with weight loss in AIDS patients and nausea/vomiting in cancer patients [19-22]. While plant-derived THC is a Schedule I substance, Marinol is listed under Schedule III and Cesamet and Syndros are controlled under Schedule II [19-21].
Epidiolex is an oil formulation of CBD approved by the FDA in June of 2018 for treatment of Lennox-Gastaut syndrome and Dravet syndrome, two rare and severe forms of pediatric epilepsy [23]. In July of 2020, it was approved for treating seizures in a rare genetic disease, tuberous sclerosis complex (TSC) [24]. Epidiolex is the only FDA approved drug containing a compound directly derived from cannabis. It was originally classified as schedule V, but is no longer a controlled substance as the FDA deemed it safe and effective for treatment of the aforementioned conditions [25]. Sativex is a 1:1 alcohol solution of THC and CBD administered as an oromucosal spray that is approved in 25 countries for the treatment of pain and spasticity in multiple sclerosis patients [26]. Despite its approval in other countries, Sativex is not yet approved by the FDA in the US.
Research on cannabidiol
CBD, through a variety of mechanisms and targets, has numerous potential therapeutic uses for a plethora of conditions. The assertion of potential therapeutic actions of CBD is based on pre-clinical data, limited clinical data and ongoing human clinical trials. Pre-clinical studies show that CBD has antioxidant [27, 28] anti-inflammatory [27], anti-
convulsant [29, 30], neuroprotective [31], and anti-cancer properties [32]. CBD also shows potential as a therapeutic agent in cardiovascular [33], neurological, and neuropsychiatric disorders [26]. The completed clinical trials involve CBD use in epilepsy and seizures disorders (21 trials), general pain and pain associated disorders (19 trials), drug abuse and use disorders (14 trials), other neurologic conditions (4 trials) and psychiatric conditions (11 trials). In addition, there are currently 85 active clinical trials in the United States containing CBD (including Epidiolex and Sativex) on clinicaltrials.gov.
Over the past two decades, multiple studies have investigated the therapeutic potentials of CBD in the eye. There are several published reviews of cannabinoids for treatment of glaucoma [34, 35], and retinal disorders [36, 37]. Nevertheless, there are currently no reviews that focus solely on CBD for ocular conditions. In this review, we aim to fill the gap in literature with a focus on CBD ocular pharmacology. We will discuss therapeutic potentials of CBD for ocular conditions, ocular molecular targets for CBD, and mechanisms of actions of CBD in the eye.
Results
Therapeutic potentials of cannabidiol for ocular conditions
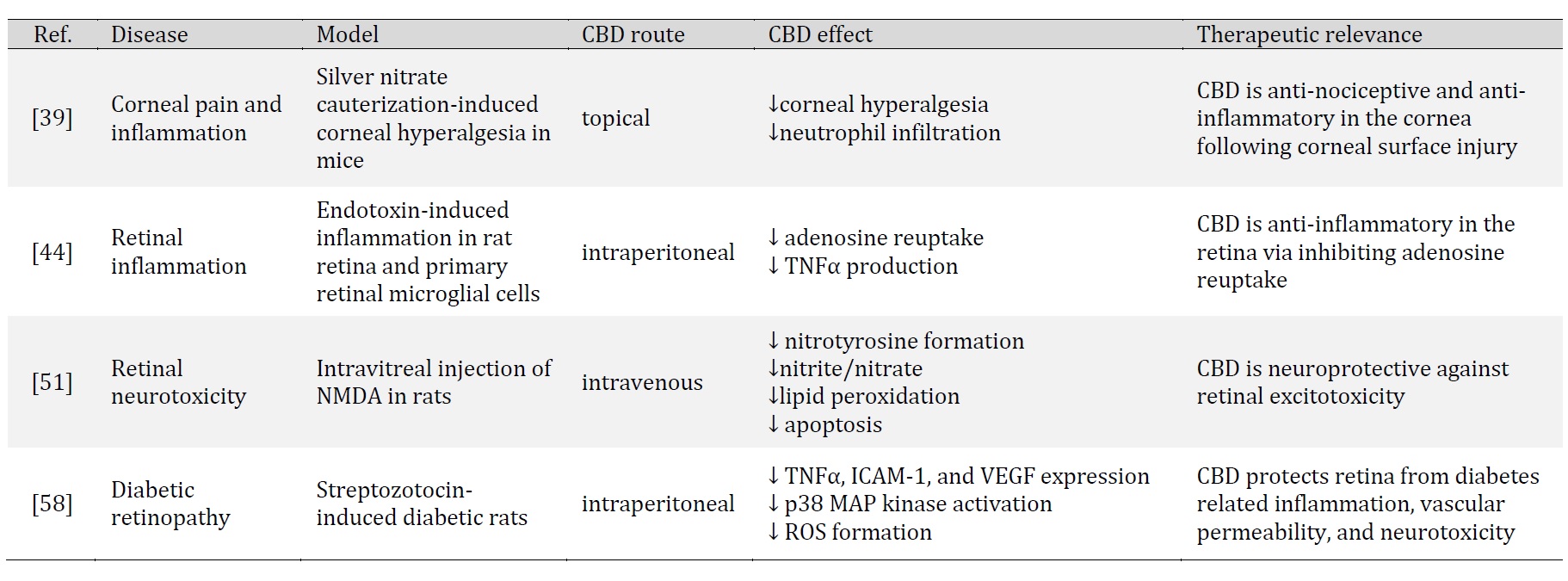
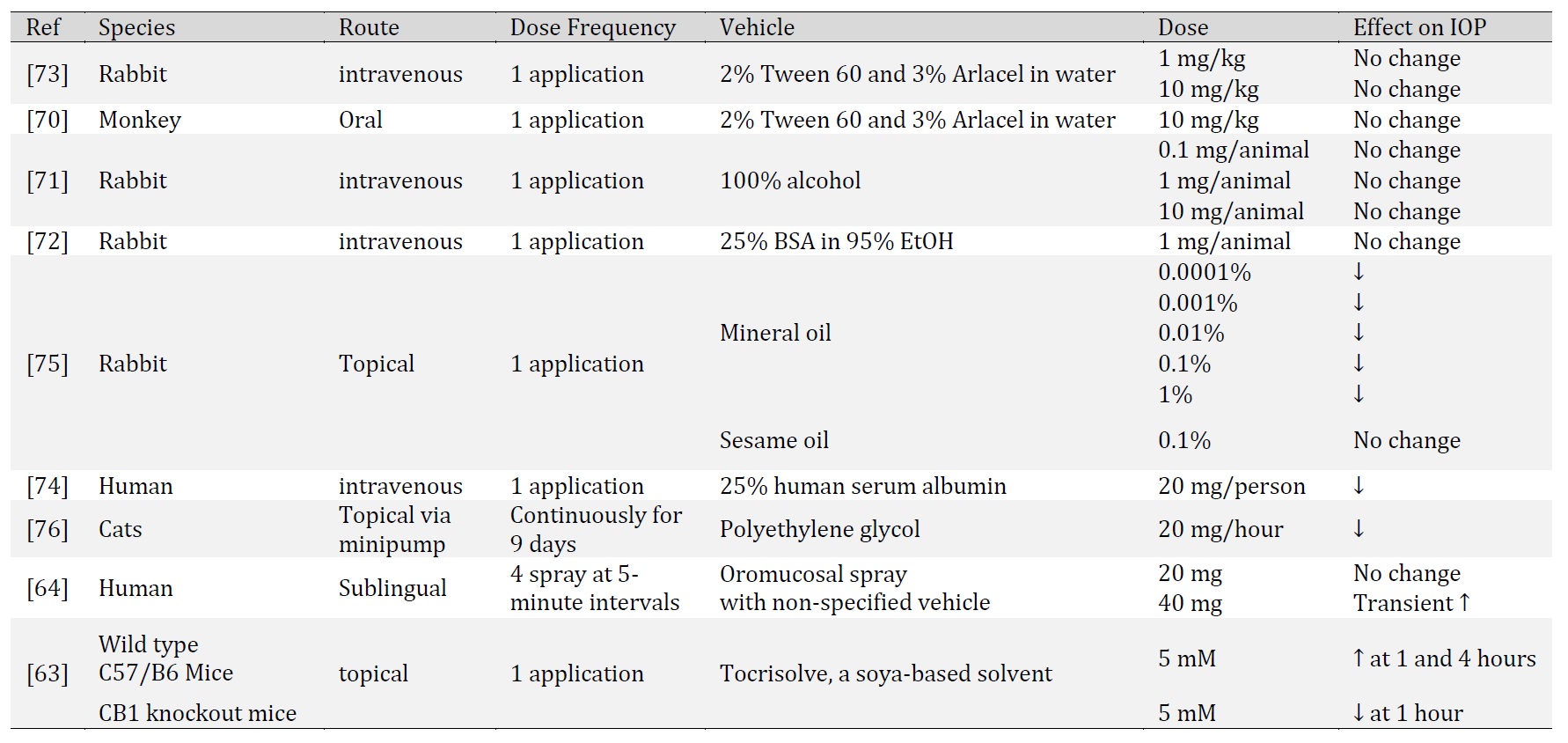
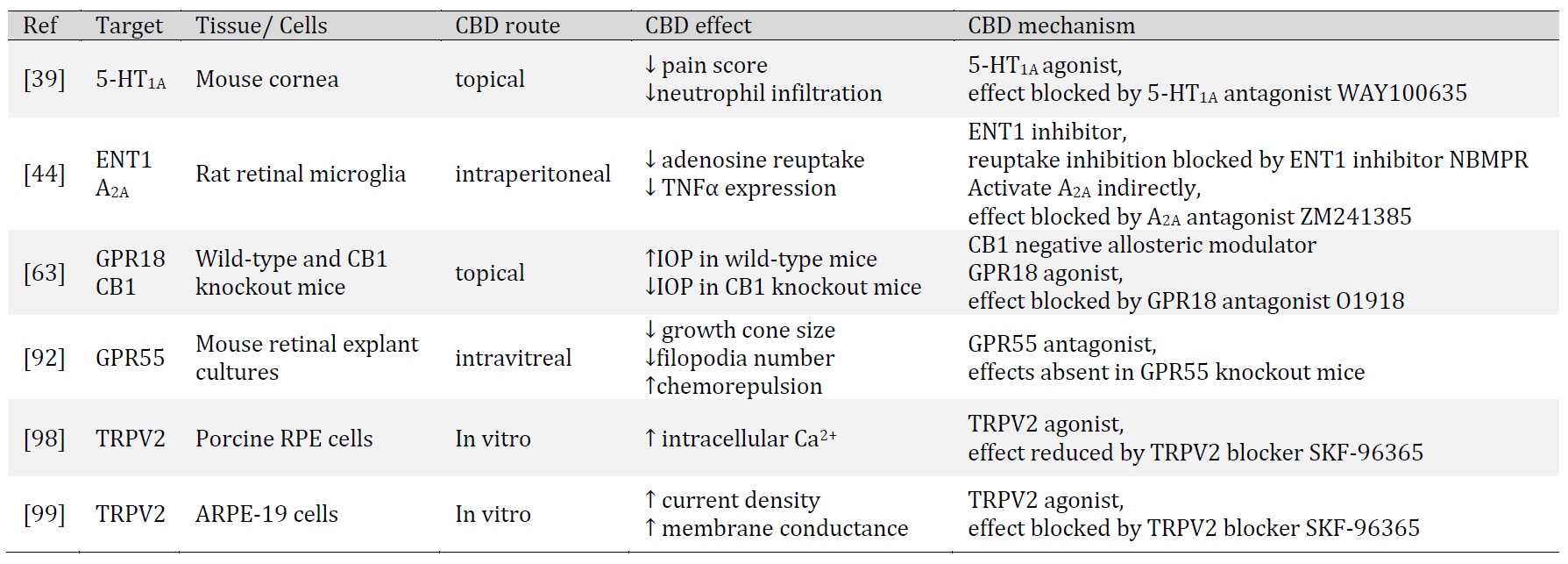
CBD is recognized for its antioxidant, anti-inflammatory and neuroprotective properties. In this section, we discuss the observed effects of CBD in ocular tissues and its indication for ocular disorders. Specifically, we will discuss studies of CBD in corneal inflammation and pain, endotoxin-induced inflammation, excitotoxicity, diabetic retinopathy, and intraocular pressure (Table 1 and Table 2).
The authors acknowledge the support of Department of Pharmacology and Toxicology, University of Louisville School of Medicine.
Author Contributions
AA and MD wrote the initial versions of the review. LS and ZHS edited and finalized the manuscript.
Funding Sources
While writing this manuscript, AA is supported in part by NIH grant T32 ES011564; MD is supported in part by NIH grant R25 CA134283; LS is supported in part by University of Louisville Integrated Programs in Biomedical Sciences (IPIBS) Fellowship; and ZHS is supported in part by NIH grant EY030186.
Statement of Ethics
The authors have no ethical conflicts to disclose.
The authors have no conflicts of interest to declare.
| 1 Brand EJ, Zhao Z: Cannabis in Chinese Medicine: Are Some Traditional Indications Referenced in Ancient Literature Related to Cannabinoids? Front Pharmacol 2017;8:108. https://doi.org/10.3389/fphar.2017.00108 |
||||
| 2 Piomelli D: The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 2003;4:873-884. https://doi.org/10.1038/nrn1247 |
||||
| 3 O'Shaughnessy WB, Calcutta MD: New remedy for tetanus and other convulsive disorders. Boston Med Surg J 1840:153-155. https://doi.org/10.1056/NEJM184010140231001 |
||||
| 4 Convention USP: Pharmacopoeia of the United States, ed 3. Philadelphia, PA, Lippincott, Grambo & Company, 1851. | ||||
| 5 Gieringer DH: The Origins of Cannabis Prohibition in California. Contemp Drug Probl1999;26:237-288. https://doi.org/10.1177/009145099902600204 |
||||
| 6 Marijuana Tax Act of 1937. URL: https://www.druglibrary.org/schaffer/hemp/taxact/mjtaxact.htm. | ||||
| 7 Controlled Substances Act, 1970. URL: https://www.govinfo.gov/content/pkg/STATUTE-84/pdf/STATUTE-84-Pg1236.pdf. | ||||
| 8 Compassionate Use Act of 1996. URL: https://leginfo.legislature.ca.gov/faces/codes_displaySection.xhtml?lawCode=HSC§ionNum=11362.5. | ||||
| 9 Agricultural Act of 2014. URL: https://www.congress.gov/bill/113th-congress/house-bill/2642. | ||||
| 10 Agriculture Improvement Act of 2018. URL: https://www.congress.gov/bill/115th-congress/house-bill/2. | ||||
| 11 Turner CE, Elsohly MA, Boeren EG: Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod 1980;43:169-234. https://doi.org/10.1021/np50008a001 |
||||
| 12 ElSohly AM, Gul W: Constituents of Cannabis Sativa; in Pertwee R (ed): Handbook of Cannabis. Oxford, UK, Oxford University Press, 2014. https://doi.org/10.1093/acprof:oso/9780199662685.003.0001 |
||||
| 13 Mudge EM, Murch SJ, Brown PN: Chemometric Analysis of Cannabinoids: Chemotaxonomy and Domestication Syndrome. Sci Rep 2018;8:13090. https://doi.org/10.1038/s41598-018-31120-2 |
||||
| 14 Adams R, Hunt M, Clark JH: Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp. I. J Am Chem Soc 1940;62:196-200. https://doi.org/10.1021/ja01858a058 |
||||
| 15 Mechoulam R, Shvo Y: Hashish. I. The structure of cannabidiol. Tetrahedron 1963;19:2073-2078. https://doi.org/10.1016/0040-4020(63)85022-X |
||||
| 16 Taura F, Morimoto S, Shoyama Y: Purification and characterization of cannabidiolic-acid synthase from Cannabis sativa L.. Biochemical analysis of a novel enzyme that catalyzes the oxidocyclization of cannabigerolic acid to cannabidiolic acid. J Biol Chem 1996;271:17411-17416. https://doi.org/10.1074/jbc.271.29.17411 |
||||
| 17 Gaoni Y, Mechoulam R: Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J Am Chem Soc 1964;86:1646-1647. https://doi.org/10.1021/ja01062a046 |
||||
| 18 Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ: Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015;12:699-730. https://doi.org/10.1007/s13311-015-0377-3 |
||||
| 19 FDA: MARINOL (dronabinol): Highlights of Prescribing Information, 2017. URL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf. | ||||
| 20 FDA: Syndros: Highlights of Prescribing Information, 2017. URL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205525s003lbl.pdf. | ||||
| 21 FDA: Cesamet (nabilone) Capsules For Oral Administration, 2006. URL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/018677s011lbl.pdf. | ||||
| 22 Badowski ME, Yanful PK: Dronabinol oral solution in the management of anorexia and weight loss in AIDS and cancer. Ther Clin Risk Manag 2018;14:643. https://doi.org/10.2147/TCRM.S126849 |
||||
| 23 FDA: Epidiolex: Highlights of Prescribing Information, 2018. URL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf. | ||||
| 24 FDA: FDA Approves New Indication for Drug Containing an Active Ingredient Derived from Cannabis to Treat Seizures in Rare Genetic Disease, 2020. URL: https://www.fda.gov/news-events/press-announcements/fda-approves-new-indication-drug-containing-active-ingredient-derived-cannabis-treat-seizures-rare#:~:text=Today%2C%20the%20U.S.%20Food%20and,year%20of%20age%20and%20older. | ||||
| 25 Establishment of a New Drug Code for Marihuana Extract, 2016. URL: https://www.federalregister.gov/documents/2016/12/14/2016-29941/establishment-of-a-new-drug-code-for-marihuana-extract. | ||||
| 26 Barnes MP: Sativex®: clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Expert Opin Pharmacother 2006;7:607-615. https://doi.org/10.1517/14656566.7.5.607 |
||||
| 27 Atalay S, Jarocka-Karpowicz I, Skrzydlewska E: Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants (Basel) 2019;9:21. https://doi.org/10.3390/antiox9010021 |
||||
| 28 Hampson AJ, Grimaldi M, Lolic M, Wink D, Rosenthal R, Axelrod J: Neuroprotective antioxidants from marijuana. Ann N Y Acad Sci 2000;899:274-282. https://doi.org/10.1111/j.1749-6632.2000.tb06193.x |
||||
| 29 Paolino MC, Ferretti A, Papetti L, Villa MP, Parisi P: Cannabidiol as potential treatment in refractory pediatric epilepsy. Expert Rev Neurother 2016;16:17-21. https://doi.org/10.1586/14737175.2016.1121098 |
||||
| 30 Leo A, Russo E, Elia M: Cannabidiol and epilepsy: Rationale and therapeutic potential. Pharmacol Res 2016;107:85-92. https://doi.org/10.1016/j.phrs.2016.03.005 |
||||
| 31 Campos AC, Fogaça MV, Sonego AB, Guimarães FS: Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res 2016;112:119-127. https://doi.org/10.1016/j.phrs.2016.01.033 |
||||
| 32 Kis B, Ifrim FC, Buda V, Avram S, Pavel IZ, Antal D, Paunescu V, Dehelean CA, Ardelean F, Diaconeasa Z, Soica C, Danciu C: Cannabidiol-from Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer. Int J Mol Sci 2019;20 https://doi.org/10.3390/ijms20235905 |
||||
| 33 Stanley CP, Hind WH, O'Sullivan SE: Is the cardiovascular system a therapeutic target for cannabidiol? Br J Clin Pharmacol 2013;75:313-322. https://doi.org/10.1111/j.1365-2125.2012.04351.x |
||||
| 34 Passani A, Posarelli C, Sframeli AT, Perciballi L, Pellegrini M, Guidi G, Figus M: Cannabinoids in Glaucoma Patients: The Never-Ending Story. J Clin Med 2020;9:3978. https://doi.org/10.3390/jcm9123978 |
||||
| 35 Pena J, Jimenez C, Schmidt J: Do cannabinoids play a role in the control of glaucoma? Medwave 2018;18:e7144. https://doi.org/10.5867/medwave.2018.01.7144 |
||||
| 36 Schwitzer T, Schwan R, Angioi-Duprez K, Giersch A, Laprevote V: The Endocannabinoid System in the Retina: From Physiology to Practical and Therapeutic Applications. Neural Plast 2016;2016:2916732. https://doi.org/10.1155/2016/2916732 |
||||
| 37 Kokona D, Georgiou PC, Kounenidakis M, Kiagiadaki F, Thermos K: Endogenous and Synthetic Cannabinoids as Therapeutics in Retinal Disease. Neural Plast 2016;2016:8373020. https://doi.org/10.1155/2016/8373020 |
||||
| 38 Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J: What Causes Eye Pain? Curr Ophthalmol Rep 2015;3:111-121. https://doi.org/10.1007/s40135-015-0073-9 |
||||
| 39 Thapa D, Cairns EA, Szczesniak AM, Toguri JT, Caldwell MD, Kelly MEM: The Cannabinoids Delta(8)THC, CBD, and HU-308 Act via Distinct Receptors to Reduce Corneal Pain and Inflammation. Cannabis Cannabinoid Res 2018;3:11-20. https://doi.org/10.1089/can.2017.0041 |
||||
| 40 Rashid K, Akhtar-Schaefer I, Langmann T: Microglia in Retinal Degeneration. Front Immunol 2019;10:1975. https://doi.org/10.3389/fimmu.2019.01975 |
||||
| 41 Wang AL, Albert C, Lau LT, Lee C, Tso MO: Minocycline inhibits LPS-induced retinal microglia activation. Neurochem Int 2005;47:152-158. https://doi.org/10.1016/j.neuint.2005.04.018 |
||||
| 42 Hasko G, Pacher P, Vizi ES, Illes P: Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci 2005;26:511-516. https://doi.org/10.1016/j.tips.2005.08.004 |
||||
| 43 Noji T, Takayama M, Mizutani M, Okamura Y, Takai H, Karasawa A, Kusaka H: KF24345, an adenosine uptake inhibitor, suppresses lipopolysaccharide-induced tumor necrosis factor-alpha production and leukopenia via endogenous adenosine in mice. J Pharmacol Exp Ther 2002;300:200-205. https://doi.org/10.1124/jpet.300.1.200 |
||||
| 44 Liou GI, Auchampach JA, Hillard CJ, Zhu G, Yousufzai B, Mian S, Khan S, Khalifa Y: Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest Ophthalmol Vis Sci 2008;49:5526-5531. https://doi.org/10.1167/iovs.08-2196 |
||||
| 45 Dreyer EB: A proposed role for excitotoxicity in glaucoma. J Glaucoma 1998;7:62-67. https://doi.org/10.1097/00061198-199802000-00012 |
||||
| 46 Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA: Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol 1996;114:299-305. https://doi.org/10.1001/archopht.1996.01100130295012 |
||||
| 47 Choi DW: Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988;1:623-634. https://doi.org/10.1016/0896-6273(88)90162-6 |
||||
| 48 Waxman EA, Lynch DR: N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist 2005;11:37-49. https://doi.org/10.1177/1073858404269012 |
||||
| 49 Coyle JT, Puttfarcken P: Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993;262:689-695. https://doi.org/10.1126/science.7901908 |
||||
| 50 Misko TP, Highkin MK, Veenhuizen AW, Manning PT, Stern MK, Currie MG, Salvemini D: Characterization of the cytoprotective action of peroxynitrite decomposition catalysts. J Biol Chem 1998;273:15646-15653. https://doi.org/10.1074/jbc.273.25.15646 |
||||
| 51 El-Remessy AB, Khalil IE, Matragoon S, Abou-Mohamed G, Tsai NJ, Roon P, Caldwell RB, Caldwell RW, Green K, Liou GI: Neuroprotective effect of (-)Delta9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: involvement of peroxynitrite. Am J Pathol 2003;163:1997-2008. https://doi.org/10.1016/S0002-9440(10)63558-4 |
||||
| 52 Li C, Miao X, Li F, Wang S, Liu Q, Wang Y, Sun J: Oxidative Stress-Related Mechanisms and Antioxidant Therapy in Diabetic Retinopathy. Oxid Med Cell Longev 2017;2017:9702820. https://doi.org/10.1155/2017/9702820 |
||||
| 53 Aiello LP, Wong JS: Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int Suppl 2000;77:S113-119. https://doi.org/10.1046/j.1523-1755.2000.07718.x |
||||
| 54 Kamiuchi K, Hasegawa G, Obayashi H, Kitamura A, Ishii M, Yano M, Kanatsuna T, Yoshikawa T, Nakamura N: Intercellular adhesion molecule-1 (ICAM-1) polymorphism is associated with diabetic retinopathy in Type 2 diabetes mellitus. Diabet Med 2002;19:371-376. https://doi.org/10.1046/j.1464-5491.2002.00694.x |
||||
| 55 Semeraro F, Cancarini A, dell'Omo R, Rezzola S, Romano MR, Costagliola C: Diabetic Retinopathy: Vascular and Inflammatory Disease. J Diabetes Res 2015;2015:582060. https://doi.org/10.1155/2015/582060 |
||||
| 56 Igarashi M, Wakasaki H, Takahara N, Ishii H, Jiang ZY, Yamauchi T, Kuboki K, Meier M, Rhodes CJ, King GL: Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clin Invest 1999;103:185-195. https://doi.org/10.1172/JCI3326 |
||||
| 57 Purves T, Middlemas A, Agthong S, Jude EB, Boulton AJ, Fernyhough P, Tomlinson DR: A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J 2001;15:2508-2514. https://doi.org/10.1096/fj.01-0253hyp |
||||
| 58 El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai NT, Caldwell RB, Liou GI: Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol 2006;168:235-244. https://doi.org/10.2353/ajpath.2006.050500 |
||||
| 59 Phu J, Agar A, Wang H, Masselos K, Kalloniatis M: Management of open-angle glaucoma by primary eye-care practitioners: toward a personalised medicine approach. Clin Exp Optom 2021;104:367-384. https://doi.org/10.1111/cxo.13114 |
||||
| 60 Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S: Glaucoma. Lancet 2017;390:2183-2193. https://doi.org/10.1016/S0140-6736(17)31469-1 |
||||
| 61 Shalaby WS, Shankar V, Razeghinejad R, Katz LJ: Current and new pharmacotherapeutic approaches for glaucoma. Expert Opin Pharmacother 2020;21:2027-2040. https://doi.org/10.1080/14656566.2020.1795130 |
||||
| 62 Lu LJ, Tsai JC, Liu J: Focus: Drug Development: Novel Pharmacologic Candidates for Treatment of Primary Open-Angle Glaucoma. Yale J Biol med 2017;90:111-118. | ||||
| 63 Miller S, Daily L, Leishman E, Bradshaw H, Straiker A: Delta9-Tetrahydrocannabinol and Cannabidiol Differentially Regulate Intraocular Pressure. Invest Ophthalmol Vis Sci 2018;59:5904-5911. https://doi.org/10.1167/iovs.18-24838 |
||||
| 64 Tomida I, Azuara-Blanco A, House H, Flint M, Pertwee RG, Robson PJ: Effect of sublingual application of cannabinoids on intraocular pressure: a pilot study. J Glaucoma 2006;15:349-353. https://doi.org/10.1097/01.ijg.0000212260.04488.60 |
||||
| 65 Crawford WJ, Merritt JC: Effects of tetrahydrocannabinol on arterial and intraocular hypertension. Int J Clin Pharmacol Biopharm 1979;17:191-196. | ||||
| 66 Cooler P, Gregg JM: Effect of delta-9-tetrahydrocannabinol on intraocular pressure in humans. South Med J 1977;70:951-954. https://doi.org/10.1097/00007611-197708000-00016 |
||||
| 67 Colasanti BK, Powell SR, Craig CR: Intraocular pressure, ocular toxicity and neurotoxicity after administration of delta 9-tetrahydrocannabinol or cannabichromene. Exp Eye Res 1984;38:63-71. https://doi.org/10.1016/0014-4835(84)90139-8 |
||||
| 68 Fischer KM, Ward DA, Hendrix DV: Effects of a topically applied 2% delta-9-tetrahydrocannabinol ophthalmic solution on intraocular pressure and aqueous humor flow rate in clinically normal dogs. Am J Vet Res 2013;74:275-280. https://doi.org/10.2460/ajvr.74.2.275 |
||||
| 69 Purnell WD, Gregg JM: Delta(9)-tetrahydrocannabinol,, euphoria and intraocular pressure in man. Ann Ophthalmol 1975;7:921-923. | ||||
| 70 Waller CW, Benigni DA, Harland E, Bedford JA, Murphy JC, ElSohly MA: Cannabinoids in Glaucoma III: The Effects of Different Cannabinoids on Intraocular Pressure in the Monkey, in Agurell S, et al (eds): The Cannabinoids: Chemical, Pharmacologic, and Therapeutic Aspects 1984, pp 871-880. https://doi.org/10.1016/B978-0-12-044620-9.50062-2 |
||||
| 71 Liu JH, Dacus AC: Central nervous system and peripheral mechanisms in ocular hypotensive effect of cannabinoids. Arch Ophthalmol 1987;105:245-248. https://doi.org/10.1001/archopht.1987.01060020099037 |
||||
| 72 Green K, Symonds CM, Oliver NW, Elijah RD: Intraocular pressure following systemic administration of cannabinoids. Curr Eye Res 1982;2:247-253. https://doi.org/10.3109/02713688209011626 |
||||
| 73 ElSohly MA, Harland EC, Benigni DA, Waller CW: Cannabinoids in glaucoma II: the effect of different cannabinoids on intraocular pressure of the rabbit. Curr Eye Res 1984;3:841-850. https://doi.org/10.3109/02713688409000797 |
||||
| 74 Perez-reyes M, Wagner D, Wall ME, Davis KH: Intravenous administration of cannabinoids and intraocular pressure, in: Braude, MC and Szara, S (eds): The Pharmacology of Marihmana, Raven, New York, 1976, pp. 829-832. | ||||
| 75 Green K, Wynn H, Bowman KA: A comparison of topical cannabinoids on intraocular pressure. Exp Eye Res 1978;27:239-246. https://doi.org/10.1016/0014-4835(78)90092-1 |
||||
| 76 Colasanti BK, Brown RE, Craig CR: Ocular hypotension, ocular toxicity, and neurotoxicity in response to marihuana extract and cannabidiol. Gen Pharmacol 1984;15:479-484. https://doi.org/10.1016/0306-3623(84)90202-7 |
||||
| 77 Shelton B: CBD Oil May Worsen Glaucoma. American Academy of Ophthalmology, 2019. URL: https://www.aao.org/eye-health/news/cbd-oil-may-worsen-glaucoma. | ||||
| 78 Russo EB, Burnett A, Hall B, Parker KK: Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res 2005;30:1037-1043. https://doi.org/10.1007/s11064-005-6978-1 |
||||
| 79 De Gregorio D, McLaughlin RJ, Posa L, Ochoa-Sanchez R, Enns J, Lopez-Canul M, Aboud M, Maione S, Comai S, Gobbi G: Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 2019;160:136-150. https://doi.org/10.1097/j.pain.0000000000001386 |
||||
| 80 Pandolfo P, Silveirinha V, dos Santos-Rodrigues A, Venance L, Ledent C, Takahashi RN, Cunha RA, Kofalvi A: Cannabinoids inhibit the synaptic uptake of adenosine and dopamine in the rat and mouse striatum. Eur J Pharmacol 2011;655:38-45. https://doi.org/10.1016/j.ejphar.2011.01.013 |
||||
| 81 Carrier EJ, Auchampach JA, Hillard CJ: Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A 2006;103:7895-7900. https://doi.org/10.1073/pnas.0511232103 |
||||
| 82 Laprairie R, Bagher A, Kelly M, Denovan‐Wright E: Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol 2015;172:4790-4805. https://doi.org/10.1111/bph.13250 |
||||
| 83 Straiker AJ, Maguire G, Mackie K, Lindsey J: Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest Ophthalmol Vis Sci 1999;40:2442-2448. | ||||
| 84 Stamer WD, Golightly SF, Hosohata Y, Ryan EP, Porter AC, Varga E, Noecker RJ, Felder CC, Yamamura HI: Cannabinoid CB(1) receptor expression, activation and detection of endogenous ligand in trabecular meshwork and ciliary process tissues. Eur J Pharmacol 2001;431:277-286. https://doi.org/10.1016/S0014-2999(01)01438-8 |
||||
| 85 Kohno M, Hasegawa H, Inoue A, Muraoka M, Miyazaki T, Oka K, Yasukawa M: Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun 2006;347:827-832. https://doi.org/10.1016/j.bbrc.2006.06.175 |
||||
| 86 McHugh D, Page J, Dunn E, Bradshaw HB: Delta(9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br J Pharmacol 2012;165:2414-2424. https://doi.org/10.1111/j.1476-5381.2011.01497.x |
||||
| 87 Console-Bram L, Brailoiu E, Brailoiu GC, Sharir H, Abood ME: Activation of GPR18 by cannabinoid compounds: a tale of biased agonism. Br J Pharmacol 2014;171:3908-3917. https://doi.org/10.1111/bph.12746 |
||||
| 88 Caldwell MD, Hu SS, Viswanathan S, Bradshaw H, Kelly ME, Straiker A: A GPR18-based signalling system regulates IOP in murine eye. Br J Pharmacol 2013;169:834-843. https://doi.org/10.1111/bph.12136 |
||||
| 89 MacIntyre J, Dong A, Straiker A, Zhu J, Howlett SE, Bagher A, Denovan-Wright E, Yu DY, Kelly ME: Cannabinoid and lipid-mediated vasorelaxation in retinal microvasculature. Eur J Pharmacol 2014;735:105-114. https://doi.org/10.1016/j.ejphar.2014.03.055 |
||||
| 90 Alhouayek M, Masquelier J, Muccioli GG: Lysophosphatidylinositols, from Cell Membrane Constituents to GPR55 Ligands. Trends Pharmacol Sci 2018;39:586-604. https://doi.org/10.1016/j.tips.2018.02.011 |
||||
| 91 Morales P, Jagerovic N: Advances Towards The Discovery of GPR55 Ligands. Curr Med Chem 2016;23:2087-2100. https://doi.org/10.2174/0929867323666160425113836 |
||||
| 92 Cherif H, Argaw A, Cecyre B, Bouchard A, Gagnon J, Javadi P, Desgent S, Mackie K, Bouchard JF: Role of GPR55 during Axon Growth and Target Innervation. eNeuro 2015; DOI: 10.1523/ENEURO.0011-15.2015. https://doi.org/10.1523/ENEURO.0011-15.2015 |
||||
| 93 Montell C: The TRP superfamily of cation channels. Sci STKE 2005;2005:re3. https://doi.org/10.1126/stke.2722005re3 |
||||
| 94 Muller C, Morales P, Reggio PH: Cannabinoid Ligands Targeting TRP Channels. Front Mol Neurosci 2018;11:487. https://doi.org/10.3389/fnmol.2018.00487 |
||||
| 95 De Petrocellis L, Orlando P, Moriello AS, Aviello G, Stott C, Izzo AA, Di Marzo V: Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol (Oxf) 2012;204:255-266. https://doi.org/10.1111/j.1748-1716.2011.02338.x |
||||
| 96 Hung CY, Tan CH: TRP Channels in Nociception and Pathological Pain. Adv Exp Med Biol 2018;1099:13-27. https://doi.org/10.1007/978-981-13-1756-9_2 |
||||
| 97 Levine JD, Alessandri-Haber N: TRP channels: targets for the relief of pain. Biochim Biophys Acta 2007;1772:989-1003. https://doi.org/10.1016/j.bbadis.2007.01.008 |
||||
| 98 Barro-Soria R, Stindl J, Muller C, Foeckler R, Todorov V, Castrop H, Strauss O: Angiotensin-2-mediated Ca2+ signaling in the retinal pigment epithelium: role of angiotensin-receptor-associated-protein and TRPV2 channel. PLoS One 2012;7:e49624. https://doi.org/10.1371/journal.pone.0049624 |
||||
| 99 Reichhart N, Keckeis S, Fried F, Fels G, Strauss O: Regulation of surface expression of TRPV2 channels in the retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol 2015;253:865-874. https://doi.org/10.1007/s00417-014-2917-7 |
||||
| 100 Gottschling S, Ayonrinde O, Bhaskar A, Blockman M, D'Agnone O, Schecter D, Suarez Rodriguez LD, Yafai S, Cyr C: Safety Considerations in Cannabinoid-Based Medicine. Int J Gen Med 2020;13:1317-1333. https://doi.org/10.2147/IJGM.S275049 |
||||
| 101 Britch SC, Babalonis S, Walsh SL: Cannabidiol: pharmacology and therapeutic targets. Psychopharmacology (Berl) 2020;238:9-28. https://doi.org/10.1007/s00213-020-05712-8 |
||||
| 102 Chesney E, Oliver D, Green A, Sovi S, Wilson J, Englund A, Freeman TP, McGuire P: Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology 2020;45:1799-1806. https://doi.org/10.1038/s41386-020-0667-2 |
||||
| 103 Souza JG, Dias K, Pereira TA, Bernardi DS, Lopez RF: Topical delivery of ocular therapeutics: carrier systems and physical methods. J Pharm Pharmacol 2014;66:507-530. https://doi.org/10.1111/jphp.12132 |
||||
| 104 Yellepeddi VK, Palakurthi S: Recent Advances in Topical Ocular Drug Delivery. J Ocul Pharmacol Ther 2016;32:67-82. https://doi.org/10.1089/jop.2015.0047 |
||||
| 105 Tomida I, Pertwee RG, Azuara-Blanco A: Cannabinoids and glaucoma. Br J Ophthalmol 2004;88:708-713. https://doi.org/10.1136/bjo.2003.032250 |
||||
| 106 Richter A, Anton SF, Koch P, Dennett SL: The impact of reducing dose frequency on health outcomes. Clin Ther 2003;25:2307-2335; discussion 2306. https://doi.org/10.1016/S0149-2918(03)80222-9 |
||||