Corresponding Author: André Soares Leopoldo
Center for Physical Education and Sports, Federal University of Espírito Santo, Av. Fernando Ferrari 514, Vitória, Espírito Santo, 29075-910 (Brazil)
Tel. +55 (27) 4009-7882, Fax +55 (27) 4009-2620 , E-Mail andre.leopoldo@ufes.br
High-Fat and Combined High-Fat and Sucrose Diets Promote Cardiac Oxidative Stress Independent of Nox2 Redox Regulation and Obesity in Rats
Patrícia Vasconcelos Fontana Gasparinia
Amanda Martins Matiasa
Suellem Torezani-Salesa
Jéssika Butcovsky Botto Sarter Kobia
Juliana Silva Siqueirab
Camila Renata Corrêab
Ana Paula Lima Leopoldoa,c
André Soares Leopoldoa,c
aPostgraduate Program in Nutrition and Health, Health Sciences Center, Federal University of Espírito Santo, Vitória, Espírito Santo, Brazil, bSão Paulo State University (UNESP), Medical School, Botucatu, São Paulo, Brazil, cCenter for Physical Education and Sports, Federal University of Espírito Santo, Vitória, Espírito Santo, Brazil
Introduction
Food consumption has been modified over the past few decades, so that different dietary patterns modulate cardiovascular risk factors [1]. Excessive intake of saturated fat, simple carbohydrate or the combination of both, show alterations in body composition with concomitant adipokine dysregulation, sympathetic nervous activity, pathological cardiac remodeling, systolic dysfunction, and increased myocardial stiffness [2-5]. Among all the mechanisms that may be involved, the production of reactive oxygen species (ROS) is one of the most investigated, although it has been widely believed that electron leakage from the mitochondrial electron transport chain is the primary source of oxidative stress in heart injury, increasing lines of evidence suggest that enzymes which produce ROS may also contribute to it [6].
NADPH oxidases are transmembrane enzymes dedicated to producing superoxide (O2•-)
by transferring an electron from NAD(P)H to molecular oxygen [7]. At the cardiovascular level, the isoform NADPH oxidase 2 (Nox2) stands out as one of the most important [8], and its activation occurs mainly by the hormonal action of angiotensin II. This hormone triggers a variety of intracellular signaling by protein kinase C (PKC) activation, tyrosine kinases, and transcription factors, with a consequent increase in the production of O2•- radicals [9].
Under conditions of positive energy balance, excess calories are stored in the form of triglycerides in adipose tissue, which leads to organ expansion through cellular adaptations such as adipocyte hypertrophy and hyperplasia [10]. These processes are physiologically treated; the increase in triglycerides in the cytoplasm of these cells compromises their function, causing adipose tissue to respond by increasing the secretion of substances such as inflammatory mediators, ROS, and pro-angiotensin II, which may favor an oxidizing scenario for the organism defiling the heart, and toxic metabolites can accumulate in cardiomyocytes leading to cardiac redox imbalance, impairing homeostasis and cardiac function [11, 12].
The consumption of high-calorie diets is indirectly related to the exacerbated production of angiotensin II, as it is associated with excessive expansion of adipose tissue, which secretes bioactive molecules generated in the renin-angiotensin system from its precursor angiotensinogen, such as the inflammatory hormone angiotensin II, thus inducing the production of ROS mainly through its regulation of Nox2 activation [5, 12].
However, knowing the particularities of each nutrient, the literature is scarce regarding studies that point to the effects of fat and sucrose consumption specifically on cardiac tissue, not providing sufficient bases to define whether high levels of ROS are related to the kind of nutrients offered per se or are due to obesity. In addition, there is a lack of consistent information related to possible signaling pathways involved in this process that can be stimulated by hypercaloric diets. It is suggested that the excess of nutrients is related to the greater formation of free radicals and the accumulation of adipose tissue, contributing to the increase in the hormonal production of angiotensin II, which, when bound to its type 1 angiotensin receptor (AT1), activates NADPH oxidase and causes the formation of ROS [9, 12]; however, antioxidant defenses, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) would not be sufficient, resulting in lipid and protein damage and cellular dysfunction. In this context, this study aims to investigate the effects of different hypercaloric diets on cardiac oxidative stress, testing the hypothesis that these diets promote cardiac oxidative stress, resulting in increased lipid peroxidation products and protein carbonylation. This process will be mediated by greater expression of AT1 and Nox2, as well as by the reduction of the enzymes SOD, CAT, and GPX, and will be more evident in the high-fat hypercaloric diet with sucrose.
Materials and Methods
Animal care and experimental design
Twenty male Wistar rats, 30 days of age, were obtained from the Central Biotério of Federal University of Espirito Santo (UFES), Vitória, Espírito Santo, Brazil. The experimental procedures were carried out in accordance with the Manual of Care and Use of Laboratory Animals and approved by the Animal Use Ethics Committee (CEUA) of UFES under number 52/2019.
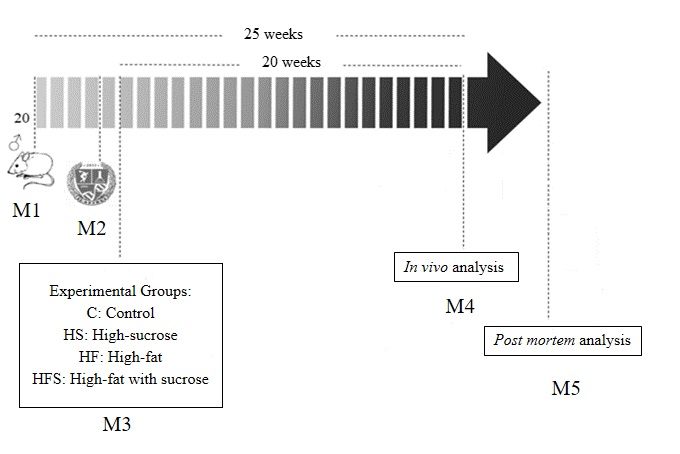
Rats were housed on a 12/12 dark/light cycle with controlled temperature (24 ± 2°C) and humidity (55 ± 5%), allowed to acclimatize for 7 days, and then randomly distributed into four dietary groups: a Control (C) that received a standard chow diet (n = 5; 3.55 kcal/g); high-sucrose (HS): fed with a high-sucrose diet and water with sucrose (300 g/L) in alternate weeks (n = 5; 4.25 kcal/g); high-fat (HF): fed a high-fat diet with lard (n = 5; 4.59 kcal/g); and high-fat with sucrose (HFS): fed a high-fat diet with lard plus sucrose (n = 5; 4.49 kcal/g). The duration of the experimental protocol was 20 weeks (Fig. 1).
All animals had free access to water and chow (40 g/day), and daily food consumption was measured. The feed efficiency (FE) was calculated by dividing the total weight gain of the animals (g) by the total ingested energy (kcal) [13, 14]. Caloric intake was calculated by daily food consumption multiplied by the caloric value of each diet (g x kcal) [15]. To calculate the caloric intake of the HS group, the energy from the intake of water with sucrose was also quantified (1.2 kcal/mL).
The diets used in the current study were formulated by Nutriave Alimentos®, Vitória, Espírito Santo, Brazil [16]. The composition (g/kg) and macronutrients for each experimental diet are described in Table 1.
At the end of the 20th week, the animals were fasted for 12–15 hours, heparinized (500U/kg/intraperitoneal; Hepamax-S®, Blau Farmacêutica SA, Cotia, São Paulo, Brazil), and anesthetized with ketamine hydrochloride (50 mg/kg/intraperitoneal; Dopalen®, Sespo Indústria e Comércio Ltda., Vetbrands Division, Jacareí, São Paulo, Brazil) and xylazine hydrochloride (10 mg/kg/intraperitoneal; Anasedan®, Sespo Indústria e Comércio Ltda., Vetbrands Division, Jacareí, São Paulo, Brazil). When the animal did not have an adequate anesthetic plan for the surgical intervention, a single higher dose (20%–30% of the initial dose of anesthetics) was administered [17]. After euthanasia, the animals were submitted to median thoracotomy for blood and tissue sample collection.
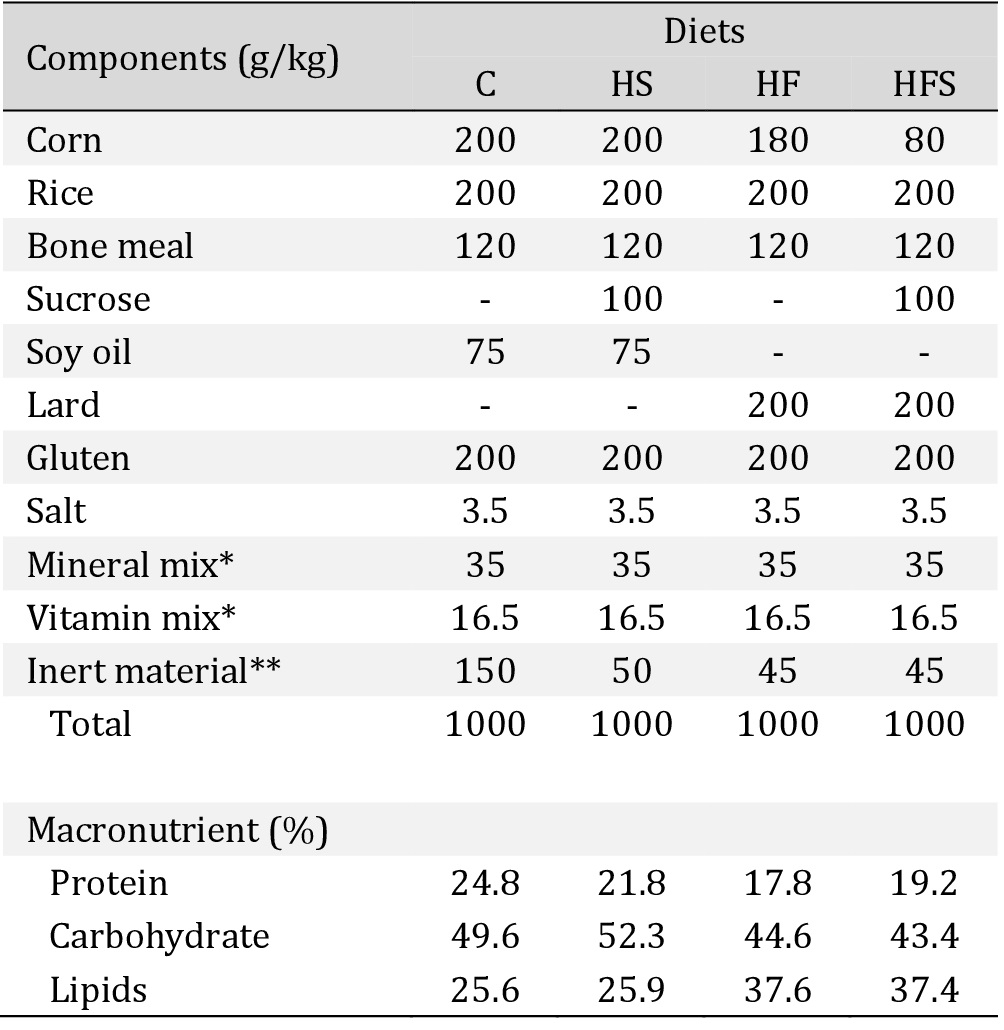
Composition and macronutrient values of diets. C: standard chow diet; HS: high-sucrose diet; HF: high-fat diet; HFS: high-fat diet with sucrose. *Mineral and Vitamin mix: selenium, iron, copper, manganese, iodine, zinc, cobalt, calcium, phosphorus, vit. A, vit. D3, vit. E, vit. K3, vit. C, B complex, choline. **Inert material, without nutritional value and calories
Nutritional assessment
Body weight was measured weekly, and body fat (BF) amount was determined from the dissection of the epididymal, mesenteric, and retroperitoneal fat pads. The adiposity index (AI) was calculated using the following formula: AI = (total BF/final body weight) × 100 [18, 19].
Histological analysis
Histological analyses were performed on mesenteric adipose tissue fixed for 24 hours in 4% paraformaldehyde with 0.1 M phosphate buffer (pH = 7.4). After dehydration in ethanol and clearing in xylol, the tissue was embedded in paraffin to form blocks. Sections 5 µm thick were obtained using a LEICA RM2125 microtome (LEICA Biosystems Inc., Richmond, Illinois, USA) and stained with hematoxylin-eosin (H&E). Images were captured with a video camera (Evolution, Media Cybernetics, Inc., Bethesda, MD) coupled to an optical microscope (Eclipse 400, Nikon) under 40× magnification. Measurements were performed using the specific software (ImageJ Pro-Plus®, Media Cybernetics, Silver Spring, Maryland, USA). The cell area (µm2) was obtained, and the examiner was blinded to the experimental groups.
Comorbidities
Arterial blood pressure was assessed indirectly using the tail plethysmography method, coupled to a data acquisition system (IITC INC, Life Science, Woodland Hills, CA, USA), and the mean of three recordings was obtained for each animal.
Glucose intolerance was determined by the glucose tolerance test after 6 hours of fasting. Glycemic levels were analyzed under baseline conditions (time 0) and after intraperitoneal overload of 25% glucose (Sigma-Aldrich®, St Louis, MO, USA), equivalent to 2 g/kg [20] after 30, 60, 90, and 120 minutes and were evaluated by the area under the curve (AUC) for glucose.
The presence of dyslipidemia was assessed by triglycerides (TG), total cholesterol (T- Chol), and high-density lipoprotein (HDL) concentrations using specific kits (Bioclin Bioquímica®, Belo Horizonte, Minas Gerais, Brazil, and Synermed do Brasil Ltda., São Paulo, Brazil).
Cardiac characteristics
The cardiac remodeling process was assessed by means of macroscopic structural analysis by determining the weights of the heart, left ventricle (LV), right ventricle (RV), and total atrium (AT), and relationships with tibia length [21]. In addition, markers of cardiac injury were added by means of creatine kinase (CK), creatine kinase fraction MB (CK-MB), and lactate dehydrogenase (LDH) using specific kits (Bioclin Bioquímica®, Belo Horizonte, Minas Gerais, Brazil) and analyzed by the BS-200 automated biochemical equipment (Mindray do Brasil - Trade and Distribution of Medical Equipment Ltda., São Paulo, Brazil).
Cardiac oxidative stress markers
Fragments of LV (100 mg) were homogenized in 1:10 sodium phosphate buffer and transferred to Eppendorf microcentrifuge tubes. Then, the samples were centrifuged at 3000 rpm for 10 minutes, and the supernatant was used for measuring thiobarbituric acid reactive substance (TBARS) and carbonylated protein.
To evaluate the peroxidation of membrane lipids, the TBARS method was used. For this purpose, 250 μL of cardiac tissue homogenate was placed in a test tube and 750 μL of 10% trichloroacetic acid was added. After vortexing, the samples were centrifuged at 3000 rpm for 5 minutes, in test tubes, 500 μL of the supernatant and 500 μL of 0.67% thiobarbituric acid (TBA) were placed. Then, the samples were heated in a water bath at 100 °C for 15 minutes. The malondialdehyde (MDA) reacted with the TBA in a 1:2 MDA-TBA ratio. After cooling in an ice bath, the reading at 535 nm was performed on the Spectra Max 190 microplate reader (Molecular Devices®, Sunnyvale, CA, USA). The MDA concentration was obtained by the molar extinction coefficient (1.56 x 105 M-1 cm-1) and the absorbance of the samples, the results being expressed in nmol/g of protein [22-23].
Carbonylated proteins were measured using the method adapted from Mesquita et al. [24], 100 μL of cardiac tissue homogenate were used for 100 μL of the derivatizer 2, 4-dinitrophenyl-hydrazine. The samples were incubated for 10 minutes at room temperature, and 50 µL of NaOH (6 M) were added and incubated again for 10 minutes. The reading was performed at 450 nm in a Spectra Max 190 microplate reader (Molecular Devices®, Sunnyvale, CA, USA), and the result was obtained (nmol/mg of protein) through the absorbance of the samples and the molar extinction coefficient (22 000 M-1 cm-1).
Oxidizing and Antioxidizing Signaling Pathways
The oxidant and antioxidant signaling pathways were analyzed using the western blot technique, with determination of the oxidant proteins AT-1 and Nox2 and antioxidants SOD-1, SOD-2, CAT, and GPX, using the β-actin protein for normalization.
Homogenization of frozen cardiac tissues was performed in plastic tubes containing lysis buffer (Tris-HCL 10 mM pH = 7.4, 1 mM NaVO3, 1% SDS, 0.05 mM DTT, 5 mM EDTA, 1 mM PMSF, 10 mM NaF) with protease inhibitor (1: 100) added in the proportion 1 mL/100 mg. The samples were centrifuged for 20 minutes at 14 000 rpm at 4 °C. The pellet formed was discarded and the protein concentration of the supernatant quantified by the method of Bradford [25]. The absorbance was measured at 595 nm using a spectrophotometer.
Then, aliquots were prepared containing the necessary volume for a 50 μg protein load, in addition to the sample buffer (Laemmli 4×). All aliquots were standardized to a final volume of 15 μL, completing the volume of each one with deionized water. The samples were heated at 95 °C for 10 minutes and then loaded onto 10% SDS-polyacrylamide gels, and then electrophoresis was performed. Subsequently, the protein was transferred electrophoretically to a polyvinylidene fluoride membrane (Bio-Rad, CA, USA).
At the end of the transfer, the membranes were incubated for 1 hour, at room temperature, with 5% bovine serum albumin blocking solution. Then, the membranes were incubated overnight at 4 °C, under agitation, with the primary antibodies AT1 receptor through the AT-1R anti-mouse monoclonal antibody (1:500, Santa Cruz Biotechnology) and Nox 2 by the antibody monoclonal anti-mouse for Nox 2 (Gp91phox) (1:500, Santa Cruz Biotechnology), SOD, by the monoclonal anti-mouse antibody for the SOD-1 isoform (1:1000, Santa Cruz Biotechnology) and the polyclonal anti-rabbit for SOD-2 (1:500 Sigma-Aldrich), CAT through monoclonal anti-mouse antibody for CAT (1: 2000, Sigma-Aldrich) and GPX with the antibody polyclonal anti-rabbit for GPX-1 (1:3000 Sigma-Aldrich). The binding of the primary antibody was detected with secondary antibodies conjugated with peroxidase anti-mouse IgG (1:10000, Santa Cruz Biotechnology) or an anti-rabbit IgG (1: 10000, Santa Cruz Biotechnology), and the detection of the bands of the proteins of interest was carried out using the ECL chemiluminescence detection reagent (GE Healthcare, UK).
To quantify the density of the bands, the software ImageJ (National Institute of Health, NIH, USA) was used and the results calculated using the relationship between the density of the proteins of interest corrected by the intensity of the protein used as a control (β-actin: 1: 1000, Cell Signaling).
Statistical Analysis
Data were expressed using the mean ± standard error of the mean (SEM) and subjected to analysis of variance (ANOVA) one way (diet factor) for independent samples. When significant differences were found (p < 0.05), a Tukey post hoc test was carried out. The level of significance was 5%.
Results
The nutritional assessment demonstrated no statistical difference between the groups regarding initial body weight, final body weight, and body weight gain after the end of the experimental protocol (Table 2). Only the HF group showed a significant increase in retroperitoneal fat pad (97.8%; p = 0.03) and total body fat (69.2%; p = 0.04), as well as in the adiposity index (33.8%; p = 0.02), relative to the C group. Considering the other parameters, all dietary interventions showed similar results to the C.
The total body fat (109%; p < 0.001) as well as the mesenteric (92%; p = 0.02) and retroperitoneal (156%; p = 0.08) fat pads were significantly elevated in the HF when compared to the HS group, consequently reflecting a higher adiposity index (62%). This index was also significantly higher in the HFS group when compared to the HS (45.4%;
p = 0.01); however, it was not followed by significant changes in fat pads and total body fat. The HF and HFS groups showed similar behavior for all evaluated adiposity parameters
(Table 2).
Regarding the food profile, food consumption in grams (g/day) in the HS (29.5%;
p = 0.009) and HFS (26%; p = 0.02) groups was lower than in the C group; however, there were no changes in caloric intake. In addition, food consumption (p = 0.37) and caloric intake were similar between groups C and HF (p = 0.61). However, the results show that the HF and HFS groups had a significant increase in feed efficiency compared with the C group, reflecting in an elevation of 14.8% (p = 0.007) and 17.4% (p = 0.002), respectively. In relation to HS, all groups showed significantly higher feed efficiency. There was no significant difference between the HF and HFS groups for all food profile parameters evaluated (Table 2).
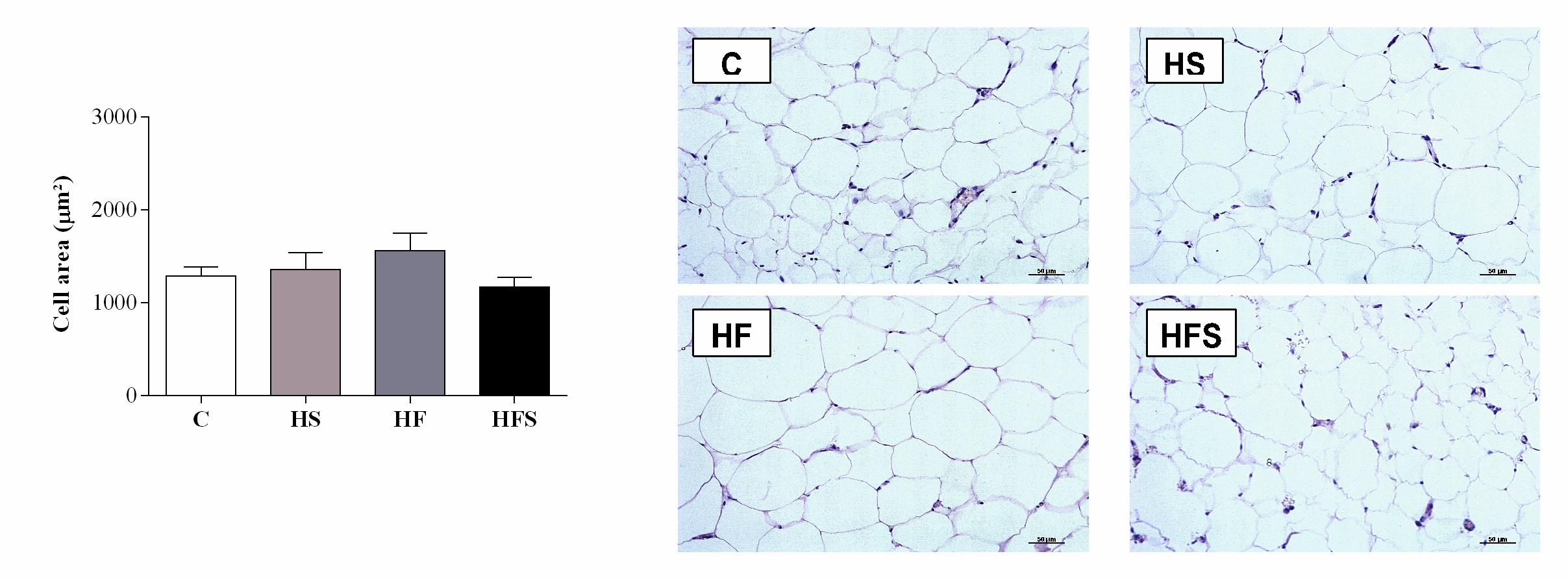
The histological results regarding the mesenteric adipose tissue show that the adipocyte areas were similar between the experimental groups (p = 0.31) (Fig. 2).
There was no statistical difference in systolic blood pressure between the groups (p > 0.05); however, the AUC (mg/dL/min) of the HFS group was higher than that in the C group, representing an increase of 21% in blood glucose (Table 3). It is noteworthy that there was no significant difference in glycemic behavior between the other groups (p > 0.05) in relation to the C group, nor were there any differences in comparisons between other groups. In addition, the results showed no significant differences in relation to lipid profiles (Table 3).
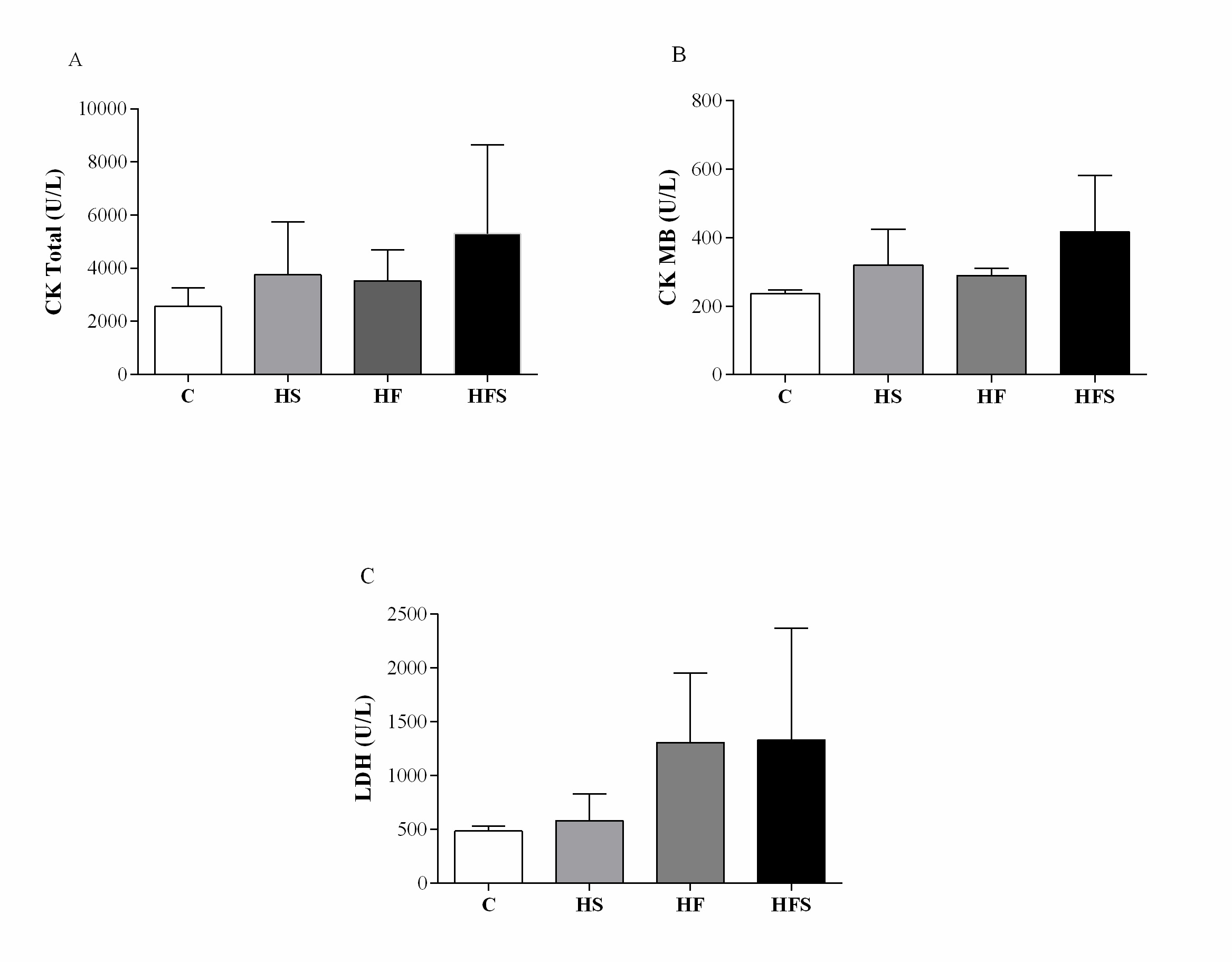
There was no difference between the groups regarding the heart, left ventricle, right ventricle, and atrium weight, and normalization of these variables by tibia length did not change the result. The results demonstrate an absence of cardiac remodeling (Table 4). Similarly, cardiac injury markers also showed no difference between the groups; however, there was an increase in CK-MB of 35.6% in the HS group, 22.8% in the HF, and 76.7% in the HFS in relation to the C group (C = 236 ± 6.65 vs. HS = 320 ± 46.9; HF = 290 ± 10.4; HFS = 417 ± 82.4, p = 0.17), while LDH increased by 19.8%, 170.1%, and 175% respectively (C = 484 ± 26.1 vs. HS = 580 ± 112; HF = 1308 ± 323; HFS = 1332 ± 518, p = 0.16) (Fig. 3).
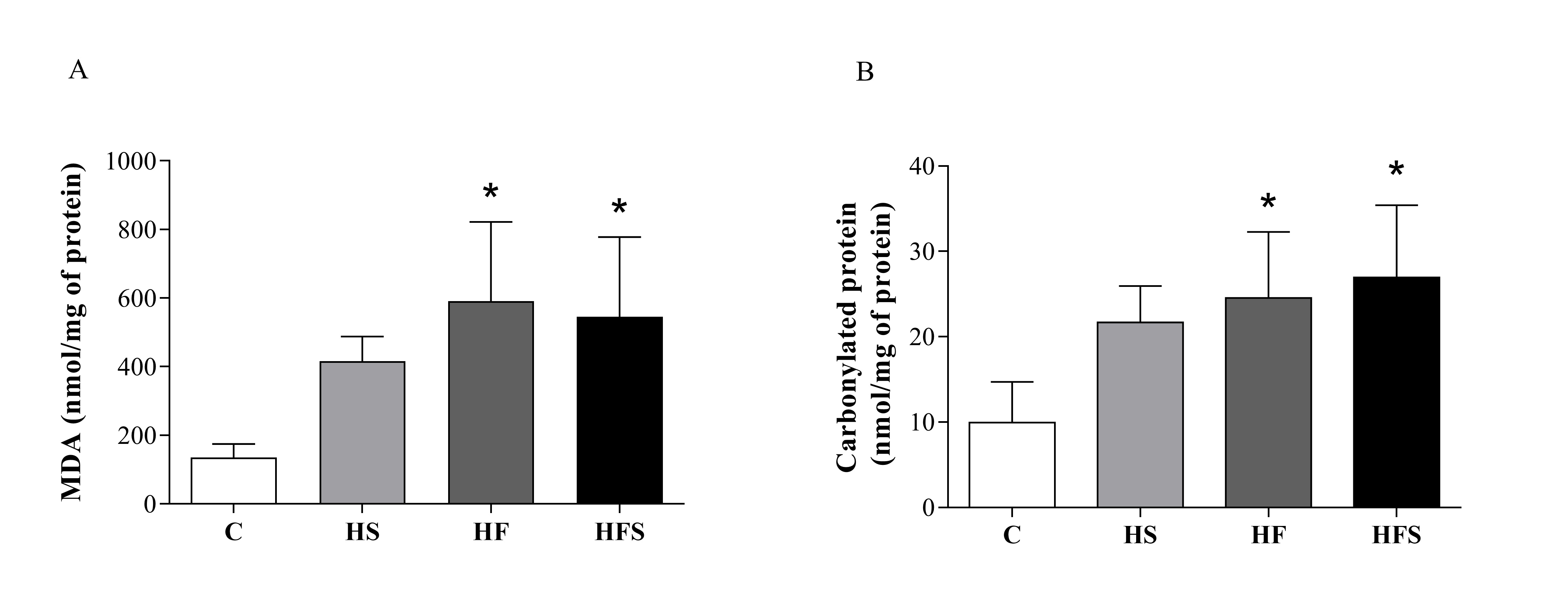
The levels of MDA (nmol/mg protein), a marker of lipid peroxidation, were significantly higher in the HF and HFS groups, representing an increase of approximately four times when compared with the C (C = 136 ± 17 vs. HF = 591 ± 103, p = 0.003; HFS = 545 ± 104, p = 0.007). The same behavior was observed for carbonylated protein, which increased significantly in the HF and HFS groups in relation to the C (C = 9.95 ± 2.13 vs. HF = 24.6 ± 3.5, p = 0.01; HFS = 27.0 ± 3.8, p = 0.004) (Fig. 4), indicating the occurrence of oxidative stress from the supply of a high-fat diet and a high-fat diet with sucrose. However, markers of cardiac oxidative stress were similar between the C and HS groups.
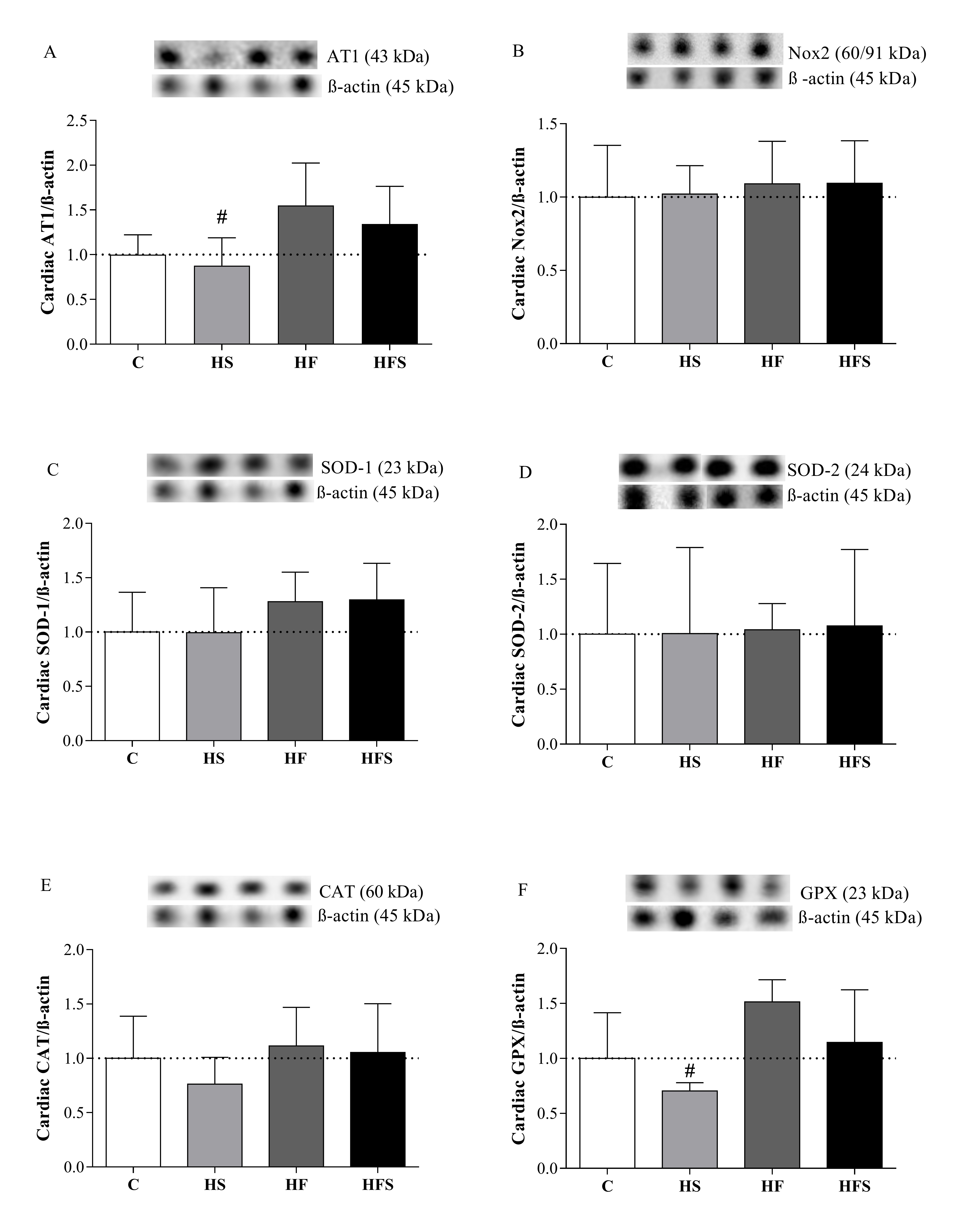
The expression of oxidant proteins AT1 and Nox2 and antioxidants SOD-1, SOD-2, GPX, and CAT did not present statistical differences between the groups when compared with the C group. However, the HS group obtained lower levels of the AT1 receptor (HS = 0.88 ± 0.14 vs. HF = 1.55 ± 0.21, p = 0.04) and GPX (HS = 0.705 ± 0.03 vs. HF = 1.51 ± 0.09, p = 0.007) when compared with the HF. Considering the HS and HFS groups, as well as the HF and HFS groups, the results were similar for all evaluated pathways (Fig. 5).
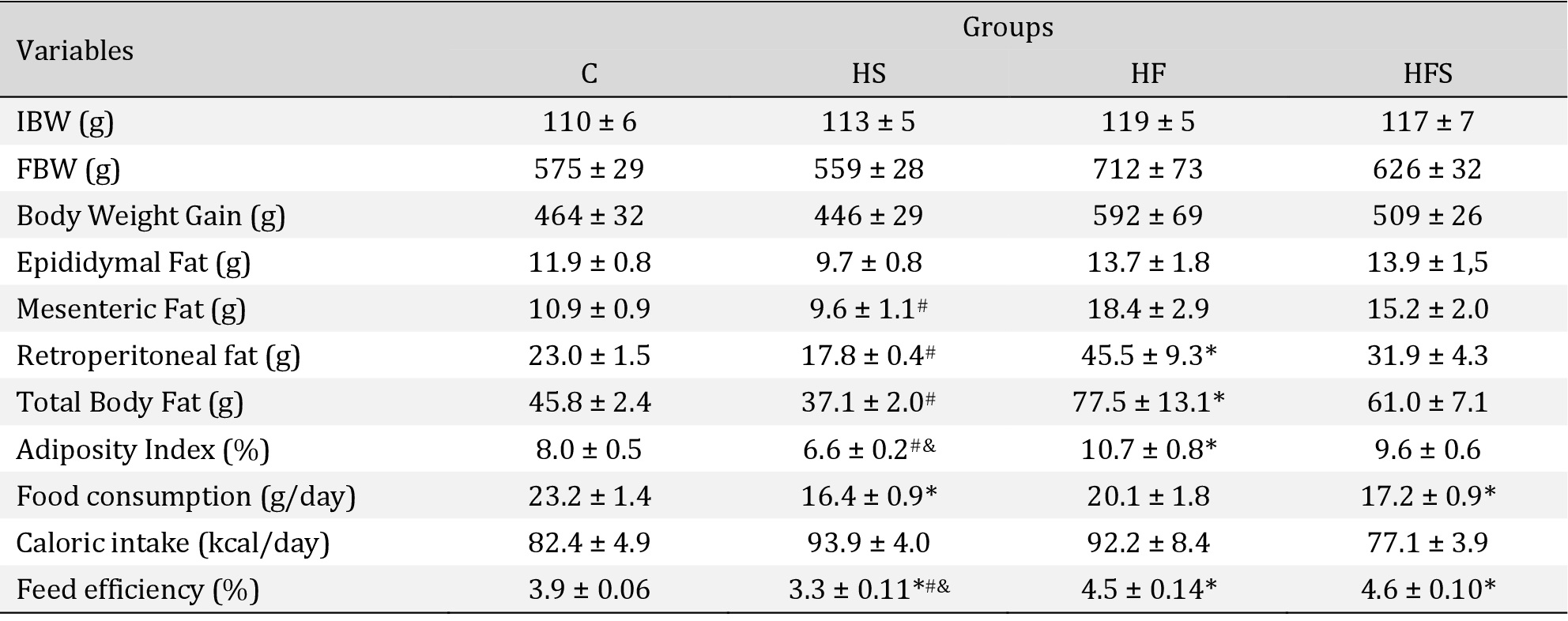
Nutritional assessment and food profile. Values expressed as mean ± SEM of five animals per group; C: control; HS: high-sucrose; HF: high-fat; HFS: high-fat with sucrose. IBW: Initial body weight; FBW: Final body weight. One way ANOVA followed by Tukey's post hoc test. p < 0.05; * vs. C; # HS vs. HF; & HS vs. HFS
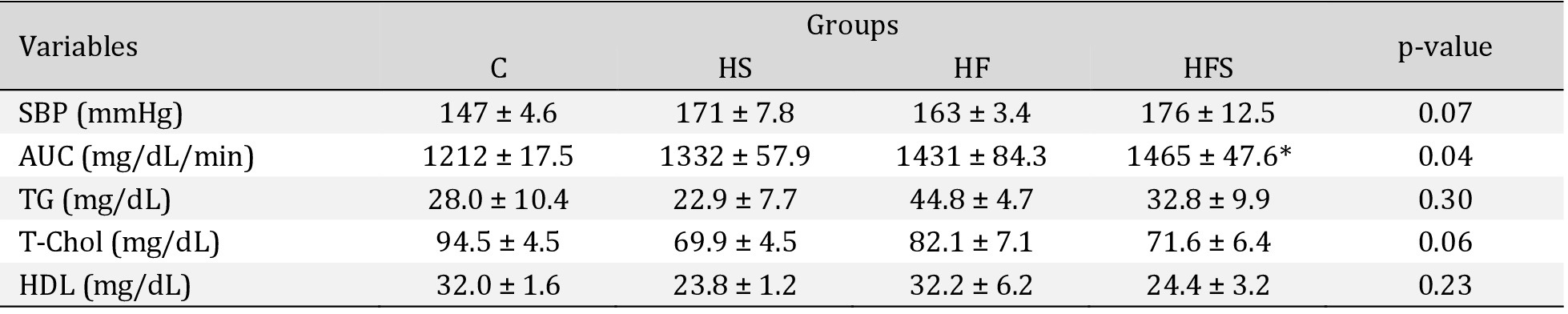
Comorbidities in the experimental groups. Values expressed as mean ± SEM. SBP: systolic blood pressure C: control (n = 5) HS: high-sucrose (n = 5); HF: high-fat (n = 4); HFS: high-fat with sucrose (n = 4). Area under the glycemic curve (AUC). C: control (n = 4); HS: high-sucrose (n = 5); HF: high-fat (n = 5); HFS: high-fat with sucrose (n = 5). TG: triglycerides; T-Chol: total cholesterol; HDL: high-density lipoprotein; C: Control (n = 3); HS: High-sucrose (n = 5); HF: High-fat (n = 4); HFS: High-fat with sucrose (n = 4); One way ANOVA followed by Tukey's post hoc test. p < 0.05; *vs. C
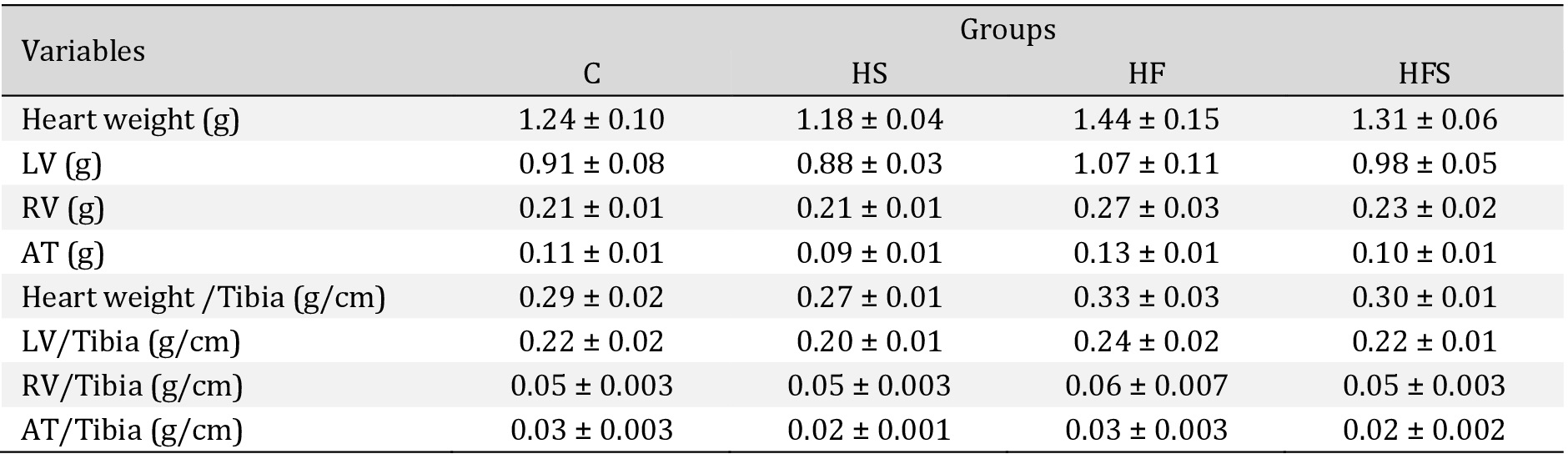
Cardiac morphological characteristics. Values expressed as mean ± SEM of five animals per group; C: control; HS: high-sucrose; HF: high-fat; HFS: high-fat with sucrose. LV: Left ventricle; RV: Right ventricle AT: Atrium. One way ANOVA followed by Tukey's post hoc test
However, despite the high lipid concentrations in the diet, which are associated with promotion of adipocyte hypertrophy, triggering the lipogenesis process and contributing to an increase in fat accumulation capacity [34], it should be noted that the experimental HF model triggered obesity without adipocyte hypertrophy, demonstrating that the increase in adiposity was mild.
The association between diet and increased adiposity has been related to the appearance of several comorbidities, which imply an increased risk for the development of cardiovascular diseases [4, 28]. However, in our study there were no changes in systemic blood pressure or lipid profiles, corroborating other authors [5, 29, 35-38]. It is noteworthy that the animals evaluated in the present study did not show any gain in body mass or accumulation of mesenteric adipose tissue that would justify the development of dyslipidemia or arterial hypertension. Furthermore, lard contains 41.2% monounsaturated fatty acids, which may have a protective effect [39, 40]. Regarding the glycemic profile, the HFS group showed a possible glucose intolerance, corroborating the findings of Apaijai et al. [41]. The authors attribute this to the decrease in the function of protein kinase B (AKT), caused by the addition of simple carbohydrates to a high-fat diet, which would lead to a marked increase in insulin resistance in these animals, a fact not evaluated in the present study.
Cardiac remodeling is associated with excess adiposity due to the increased metabolic demand imposed by this condition, which results in increased cardiac output and volume overload, with dilation of the ventricular cavity, leading to eccentric hypertrophy. On the other hand, when pressure overload occurs, there is an increase in the thickness of the ventricular wall called concentric hypertrophy [42]. Thus, when structural myocardial disorders like left ventricular hypertrophy, cardiomyocyte hypertrophy, increased perivascular and interstitial fibrosis, capillary rarefaction, and functional abnormalities like systolic and diastolic dysfunction occur in a sufficient way to cause a heart abnormality, the condition is characterized as cardiomyopathy [43].
Our findings demonstrate that dietary interventions have not been able to promote hypertrophy and cardiac damage. One possible explanation may be related to the absence of volumetric and pressure overload in the evaluated groups. According to Sharma et al. [44], food intake of carbohydrates and lipids largely determines the exposure of the heart to insulin, leading to activation of AKT and mammalian target of rapamycin (mTOR) protein or peroxisome proliferator activated receptors (PPARs) related to the development of hypertrophy. However, in our study, dietary interventions were not able to produce sufficient metabolic changes to activate these pathways, which would induce cardiac remodeling.
Considering the dosages of CK, CK-MB, and LDH, the literature evidences the occurrence of cardiac damage with the consumption of hypercaloric diets. Fouad (2020) [45], when evaluating Wistar rats fed a high-fat diet rich in cholesterol, showed an increase in CK-MB, related to structural and functional changes in the cardiac muscle and disruption of the integrity of the cell membrane; the author also reports that this event may be secondary to lipid peroxidation in cardiac membranes. Similar data were found by Wang et al. [46], who attributed the increase in CK-MB and LDH to the inflammatory effect of saturated fatty acids. However, the data from the present study, in turn, contradict these findings, since there was no significant increase in CK-MB, the most expressed isoform in the heart, and in LDH. However, despite the absence of changes in these cardiac damage markers, it is noteworthy that an important percentage increase was observed in the studied dietary interventions, which may be indicative of the initial triggering of cardiac injury.
Regarding the occurrence of cardiac oxidative stress, studies by several authors have demonstrated the relationship between the consumption of hypercaloric diets and the increase in the production of free radicals, ROS and RNS [41, 47-51]. In our study, cardiac oxidative stress occurred markedly in the HF and HFS dietary interventions, promoting a substantial increase in protein carbonylation and lipid peroxidation. Our findings agree with Apaijai et al. [41] using a high-fat (59.28% of lard and cholesterol) and high-fat diet plus sugar (43.3% of lard and cholesterol, 32.4% of fructose syrup and sweetened condensed milk). Similarly, Emelyanova et al. [52], Ma and Xie (2017) [53] and Yu et al. [54] verified, after intervention with a high-fat diet, an increase in cardiac MDA as well as Noeman et al. [55] who also showed an increase in the levels of carbonylated proteins. It is worth mentioning, however, that despite the lack of studies, some authors have found divergent results [56, 57] relating the absence of oxidative stress to the lack of ectopic accumulation of lipids or inflammatory signaling in cardiac tissue [56].
A possible explanation for the oxidative stress observed in the HF and HFS dietary interventions may be related to the increase of the lipid substrate in the myocardium, which becomes a major target for oxidation by free radicals and mitochondrial utilization, resulting in a greater leakage of electrons in the chain transport and increased production of O2•- [55]. These mitochondrial changes and cardiac oxidative damage have been reported by Sverdlov et al. [48], Yu et al. [54], and Jiménez-González et al. [58]. Thus, the high intake of fats in the present study resulted in an increase in adipose tissue in the HF group and a possible intolerance to glucose in the HFS group, both situations that could contribute to greater uptake of fatty acids by the heart.
Lard, the main component of these diets, contains about 39% saturated fatty acids, 23.8% of which is palmitic acid, which has been identified as the main cause of oxidative stress induced by diet [39, 59]. Although saturated fatty acids are less oxidizable, the rest of the unsaturated fatty acids contained in the lard may have promoted lipoperoxidation. However, it is worth mentioning that saturated fatty acids increase ROS, which may enhance lipid peroxidation [60]. In situations of high plasma concentrations of free fatty acids, they are transported to cardiomyocytes, leading to greater formation of ceramides and diacylglycerol, in addition to promoting an increase in unoxidized palmitate in the cytoplasm of cells, suggesting that not only lipid oxidation but also excess lipid accumulation are involved in cardiac lipotoxicity [11, 57, 61].
In disagreement with our initial hypothesis, the HS group did not show an increase in the oxidative markers evaluated, an observation in line with that verified by Szűcs et al. [62]
in animals with a hypercaloric diet rich in fructose (60%). In this sense, sucrose was not able to produce oxidative damage in animals on a HS diet, nor aggravate it in the HFS group, since there was no difference in the levels of cardiac oxidative stress between the HF and HFS groups. These findings differ from the results assessed by Apaijai et al. [41], who showed greater cardiac oxidative damage in animals fed a high-fat diet plus sucrose and fructose. However, it is important to note that isolated cardiac mitochondria produce 23% more hydrogen peroxide (H2O2) when they oxidize fatty acids than mitochondria that use glucose as an energy substrate via the glycolytic pathway [11, 63].
The literature still lacks consistent information about the signaling pathways that may be related to the process of cardiac oxidative stress promoted by hypercaloric diets. Within this context, the excess of lipids and sucrose would act through the metabolic overload caused by the increase of free fatty acids and glucose in the plasma, which, when internalized by cardiomyocytes and transported to the mitochondria, would lead to the formation of ROS with consequent PKC stimulation, which in turn activates Nox2. In addition, the caloric excess contributes to the development of obesity, which can increase sympathetic activity, with greater production of angiotensin II, which binds to the membrane receptor AT1 in the cardiac cell, and this, also via PKC, activates Nox2, which causes an increase in O2•- [9, 64, 65].
In our initial hypothesis, we suggested a possible participation of the pro-oxidant pathway involving the AT1 and Nox2. However, no increase in cardiac expression of these pro-oxidant proteins was observed in dietary interventions. This fact may be associated with the absence of hyperactivity of the renin angiotensin system, given that the animals evaluated did not present arterial hypertension. Among the adipokines released by adipose tissue, angiotensin II, a hypertensive hormone generated from the renin angiotensin system, is associated with the pathogenesis of oxidative stress; angiotensinogen, a precursor of angiotensin II, from adipose tissue, contributes about one third of circulating angiotensinogen in rodents and plays an important role in the function of this tissue through the modulation of adipogenesis and lipid metabolism [12, 66, 67]. The activity of angiotensin II is mediated through its connection with its plasma membrane receptors, among them, the AT1 receptor is the most related to its harmful actions in heart, including the increase in the production of ROS by stimulating NADPH oxidase [68]. In our study, the low levels of adiposity may reflect that there was no hypertrophy, which would justify the lower expression of the AT1 receptor, since angiotensin II is produced in excess by hypertrophied adipose tissue.
In addition, the ROS resulting from this activation can enter the mitochondria, making it dysfunctional, while the organelle is producing more ROS due to the increase in the oxidation processes of nutrients, which in turn activate the PKC which in a cyclic way increases plus the activity of NADPH oxidase, resulting in overproduction of ROS [69]. The involvement of this enzyme in the oxidative stress process was confirmed by Bhatti and Li (2020) [47], who reported Nox2-isoform-dependent higher production of O2•-, in addition to greater phosphorylation of AKT and Extracellular Signal Regulated Protein Kinase (ERK) 1/2, which are involved in hypertrophy processes in C57BL/6J WT mice that received a high-fat diet; these changes are significantly reduced or absent in Nox2 knockout animals. Gamez-Mendez and collaborators (2015) [70] also showed an increase in peroxynitrite (ONOO-) and O2•- in mice fed a high-fat diet; however, when offering the same diet plus apocynin, an antioxidant inhibitor of NADPH oxidase, they observed that the vascular generation of these molecules was significantly lower.
However, according to our findings, there was no evidence of involvement of the
AT1/Nox 2 pathway in the development of cardiac oxidative stress, which points to the participation of other mechanisms as responsible for the increase in oxidative markers observed in the HF and HFS groups; in particular, in addition to the oxidative phosphorylation carried out by the mitochondria, the activation of other enzymatic pathways, such as xanthine oxidase, lipoxygenase, cyclooxygenase, cytochrome p450 enzymes, and uncoupled nitric oxide synthase (eNos), as well as other isoforms of the NADPH oxidase enzyme that can also be found in the heart, especially Nox4, could promote oxidative stress in these
animals [6, 12].
In addition, Drosatos and Schulze (2013) [63] propose that saturated fatty acids, especially palmitate, can inhibit AMP-activated protein kinase (AMPK), which increases levels of malonyl-CoA and inhibits carnitine palmitoyl transferase 1 (CPT-1), causing accumulation of fatty acids and lipotoxicity. Other than that, an increase in diacylglycerol induces over-regulation of PKC, which in turn inhibits the insulin signaling pathway by blocking the insulin substrate 1 receptor (IRS-1), which may exacerbate the accumulation of lipids in the heart, increasing the uptake of fatty acids. This chronic supply of lipids to the heart increases excessive ROS production, contributing to damage to various organelles and macromolecules via oxidative changes [71].
In relation to antioxidant defenses, in disagreement with our initial hypothesis, in which a reduction in the amount of defense enzymes in cardiac tissue was expected, under the effect of HF and HFS dietary interventions, which showed an increase in oxidative stress, no differences were found in protein expression of SOD-1, SOD-2, CAT, and GPX. However, studies have shown that in addition to quantitative determination, the evaluation of enzyme activity plays an important role in the oxidative stress process [50, 54]. In the present study, this assessment was not carried out; however, it is observed that the levels of SOD, CAT, and GPX in the HF and HFS groups were not sufficient to promote redox balance, suggesting a possible decrease in activity. Thus, it is noted that, even though there was no significant difference between the groups, there was an increase in the levels of SOD-1 (27.9% in the HF group and 29.6% in the HFS group), and GPX (51.4% in the HF group and 14.4% in the HFS group), possibly as an attempt to compensate for the reduced capacity of these enzymes to prevent oxidative damage, possibly due to an increase in the transcription of antioxidant enzyme genes, mediated by regulatory systems such as nuclear factor 2 related to
erythroid 2 (Nrf-2), nuclear factor kapa-b (NF-κB), and mitogen activated protein (MAP) kinase [72].
Conclusion
High-fat and combined high-fat with sucrose diets promoted cardiac oxidative stress, in the presence and absence of obesity, respectively. However, this process was not mediated by the AT1 and Nox2 pro-oxidant pathways or by the quantitative reduction of antioxidant enzymes.
We are grateful to Priscilla Spadeto Altoé, Amanda Rangel Madureira, Jóctan Pimentel Cordeiro and Beatriz Crisostomo dos Santos for their assistance.
Author Contributions
P.V.F.G. and A.S.L. conceived the design of the study. P.V.F.G., A.M.M., S.T.-S., J.B.B.S.K., J.S.S., C.R.C., A.P.L.-L, and A.S.L. performed the experiments and statistical analysis. P.V.F.G. and A.S.L. interpreted and discussed the data and wrote the manuscript. P.V.F.G., A.P.L.-L., and A.S.L. refined the final draft and revised the manuscript. All authors read and approved the final version of the manuscript.
Funding Sources
This study was supported by the Brazilian National Council for Scientific and Technological Development – CNPq (grant number 402090/2016-0).
Statement of Ethics
Animal experiments conformed to internationally accepted standards and have been approved by the appropriate institutional review body (Animal Use Ethics Committee (CEUA) of UFES under number 52/2019).
The authors declare that no conflicts of interest exist.
| 1 Santos RD, Gagliardi ACM, Xavier HT, Magnoni CD, Cassani R, Lottenberg AMP, Casella Filho A, Araújo DB, Cesena FY, Alves RJ, Fenelon G, Nishioka SAD, Faludi AA, Geloneze B, Scherr C, Kovacs C, Tomazzela C, Carla C, Barrera-Arellano D, Cintra D, et al.: I Diretriz sobre o consumo de gorduras e saúde cardiovascular. Arq Bras Cardiol 2013;100:1-40. https://doi.org/10.5935/abc.2013S003 |
||||
| 2 Barnes MJ, Lapanowski K, Conley A, Rafols JA, Jen KL, Dunbar JC: High fat feeding is associated with increased blood pressure, sympathetic nerve activity and hypothalamic mu opioid receptors. Brain Res Bull 2003;61:511-519. https://doi.org/10.1016/S0361-9230(03)00188-6 |
||||
| 3 Leopoldo AS, Sugizaki MM, Lima-Leopoldo AP, do Nascimento AF, Luvizotto RA, de Campos DH, Okoshi K, Dal Pai-Silva M, Padovani CR, Cicogna AC: Cardiac remodeling in a rat model of diet-induced obesity. Can J Cardiol 2010;26:423-429. https://doi.org/10.1016/S0828-282X(10)70440-2 |
||||
| 4 Gonçalves N, Silva AF, Rodrigues PG, Correia E, Moura C, Eloy C, Roncon-Albuquerque R Jr, Falcão-Pires I, Leite-Moreira AF: Early cardiac changes induced by a hypercaloric Western-type diet in "subclinical" obesity. Am J Physiol Heart Circ Physiol 2016;310:H655-H666. https://doi.org/10.1152/ajpheart.00684.2015 |
||||
| 5 Moreno-Fernández S, Garcés-Rimón M, Vera G, Astier J, Landrier JF, Miguel M: High fat/high glucose diet induces metabolic syndrome in an experimental rat model. Nutrients 2018;10:1502. https://doi.org/10.3390/nu10101502 |
||||
| 6 Panth N, Paudel KR, Parajuli K: Reactive oxygen species: a key hallmark of cardiovascular disease. Adv Med 2016;2016:9152732. https://doi.org/10.1155/2016/9152732 |
||||
| 7 Griendling KK, Sorescu D, Ushio-Fukai M: NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 2000;86:494-501. https://doi.org/10.1161/01.RES.86.5.494 |
||||
| 8 Sirker A, Murdoch CE, Protti A, Sawyer GJ, Santos CX, Martin D, Zhang X, Brewer AC, Zhang M, Shah AM: Cell-specific effects of Nox2 on the acute and chronic response to myocardial infarction. J Mol Cell Cardiol 2016;98:11-17. https://doi.org/10.1016/j.yjmcc.2016.07.003 |
||||
| 9 Cai H, Griendling KK, Harrison DG: The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 2003;24:471-478. https://doi.org/10.1016/S0165-6147(03)00233-5 |
||||
| 10- Rutkowski JM, Stern JH, Scherer PE: The cell biology of fat expansion. J Cell Biol 2015;208:501-512. https://doi.org/10.1083/jcb.201409063 |
||||
| 11 Lacerda DDS, Bock PM, Funchal, C: Consumo exacerbado de lipídeos provoca dano celular em algumas doenças metabólicas e cardiovasculares. Nutrire Rev Soc Bras Aliment Nutr 2015;40:200-213. https://doi.org/10.4322/2316-7874.41214 |
||||
| 12 Ramalingam L, Menikdiwela K, LeMieux M, Dufour JM, Kaur G, Kalupahana N, Moustaid-Moussa N: The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim Biophys Acta Mol Basis Dis 2017;1863:1106-1114. https://doi.org/10.1016/j.bbadis.2016.07.019 |
||||
| 13 Irving BA, Weltman JY, Patrie JT, Davis CK, Brock DW, Swift D, Barrett EJ, Gaesser GA, Weltman A: Effects of exercise training intensity on nocturnal growth hormone secretion in obese adults with the metabolic syndrome. J Clin Endocrinol Metab 2009;94:1979-1986. https://doi.org/10.1210/jc.2008-2256 |
||||
| 14 Kim CH, Youn JH, Park JY, Hong SK, Park KS, Park SW, Suh KI, Lee KU: Effects of high-fat diet and exercise training on intracellular glucose metabolism in rats. Am J Physiol Endocrinol Metab 2000;278:E977-E984. https://doi.org/10.1152/ajpendo.2000.278.6.E977 |
||||
| 15 Koch CE, Lowe C, Pretz D, Steger J, Williams LM, Tups A: High-fat diet induces leptin resistance in leptin-deficient mice. J Neuroendocrinol 2014;26:58-67. https://doi.org/10.1111/jne.12131 |
||||
| 16 Matias AM, Estevam WM, Coelho PM, Haese D, Kobi JBBS, Lima-Leopoldo AP, Leopoldo AS: Differential effects of high sugar, high lard or a combination of both on nutritional, hormonal and cardiovascular metabolic profiles of rodents. Nutrients 2018;10:1071. https://doi.org/10.3390/nu10081071 |
||||
| 17 Freeman PL, Luff AR: Contractile properties of hindlimb muscles in rat during surgical overload. Am J Physiol 1982;242:C259-C264. https://doi.org/10.1152/ajpcell.1982.242.5.C259 |
||||
| 18 Rolls BJ, Shide DJ: The influence of dietary fat on food intake and body weight. Nutr Rev 1992;50:283-290. https://doi.org/10.1111/j.1753-4887.1992.tb02466.x |
||||
| 19 Taylor BA, Phillips SJ: Detection of obesity QTLs on mouse chromosomes 1 and 7 by selective DNA pooling. Genomics 1996;34:389-398. https://doi.org/10.1006/geno.1996.0302 |
||||
| 20 Akiyama T, Tachibana I, Shirohara H, Watanabe N, Otsuki M: High-fat hypercaloric diet induces obesity, glucose intolerance and hyperlipidemia in normal adult male Wistar rat. Diabetes Res Clin Pract 1996;31:27-35. https://doi.org/10.1016/0168-8227(96)01205-3 |
||||
| 21 Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG: Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol 1982;243:H941-H947. https://doi.org/10.1152/ajpheart.1982.243.6.H941 |
||||
| 22 Uchiyama M, Mihara M: Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 1978;86:271-278. https://doi.org/10.1016/0003-2697(78)90342-1 |
||||
| 23 Samarghandian S, Farkhondeh T, Samini F, Borji A: Protective Effects of Carvacrol against Oxidative Stress Induced by Chronic Stress in Rat's Brain, Liver, and Kidney. Biochem Res Int 2016;2016:2645237. https://doi.org/10.1155/2016/2645237 |
||||
| 24 Mesquita CS, Oliveira R, Bento F, Geraldo D, Rodrigues JV, Marcos JC: Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal Biochem 2014;458:469-471. https://doi.org/10.1016/j.ab.2014.04.034 |
||||
| 25 Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-254. https://doi.org/10.1016/0003-2697(76)90527-3 |
||||
| 26 Tan BL, Norhaizan ME, Liew WP: Nutrients and Oxidative Stress: Friend or Foe? Oxid Med Cell Longev 2018;2018:9719584. https://doi.org/10.1155/2018/9719584 |
||||
| 27 Després JP: Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012;126:1301-1313. https://doi.org/10.1161/CIRCULATIONAHA.111.067264 |
||||
| 28 Pinheiro-Castro N, Silva LBAR, Novaes GM, Ong TP: Hypercaloric Diet-Induced Obesity and Obesity-Related Metabolic Disorders in Experimental Models. Adv Exp Med Biol 2019;1134:149-161. https://doi.org/10.1007/978-3-030-12668-1_8 |
||||
| 29 Ferron AJ, Jacobsen BB, Sant'Ana PG, de Campos DH, de Tomasi LC, Luvizotto Rde A, Cicogna AC, Leopoldo AS, Lima-Leopoldo AP: Cardiac Dysfunction Induced by Obesity Is Not Related to β-Adrenergic System Impairment at the Receptor-Signalling Pathway. PLoS One 2015;10:e0138605. https://doi.org/10.1371/journal.pone.0138605 |
||||
| 30 Crescenzo R, Bianco F, Mazzoli A, Giacco A, Cancelliere R, di Fabio G, Zarrelli A, Liverini G, Iossa S: Fat Quality Influences the Obesogenic Effect of High Fat Diets. Nutrients 2015;7:9475-9491. https://doi.org/10.3390/nu7115480 |
||||
| 31 Her TK, Lagakos WS, Brown MR, LeBrasseur NK, Rakshit K, Matveyenko AV: Dietary carbohydrates modulate metabolic and β-cell adaptation to high-fat diet-induced obesity. Am J Physiol Endocrinol Metab 2020;318:E856-E865. https://doi.org/10.1152/ajpendo.00539.2019 |
||||
| 32 Sheludiakova A, Rooney K, Boakes RA: Metabolic and behavioural effects of sucrose and fructose/glucose drinks in the rat. Eur J Nutr 2012;51:445-454. https://doi.org/10.1007/s00394-011-0228-x |
||||
| 33 Vileigas DF, de Deus AF, da Silva DC, de Tomasi LC, de Campos DH, Adorni CS, Oliveira SM, Sant'Ana PG, Okoshi K, Padovani CR, Cicogna AC: Saturated high-fat diet-induced obesity increases adenylate cyclase of myocardial β-adrenergic system and does not compromise cardiac function. Physiol Rep 2016;4:e12914. https://doi.org/10.14814/phy2.12914 |
||||
| 34 González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, Martinez JA: Obesity. Nat Rev Dis Primers 2017;3:17034. https://doi.org/10.1038/nrdp.2017.34 |
||||
| 35 Pranprawit A, Wolber FM, Heyes JA, Molan AL, Kruger MC: Short-term and long-term effects of excessive consumption of saturated fats and/or sucrose on metabolic variables in Sprague Dawley rats: a pilot study. J Sci Food Agric 2013;93:3191-3197. https://doi.org/10.1002/jsfa.6240 |
||||
| 36 Castellanos JAK, Rodríguez PSM, Cardoso SG, Díaz Díaz E, Tejero BME, del Bosque PL, Carbó Zabala R: Adipose tissue redistribution caused by an early consumption of a high sucrose diet in a rat model. Nutr Hosp 2015;31:2546-2553. | ||||
| 37 Gómez-Crisóstomo NP, De la Cruz-Hernández EN, Méndez MER, Hernández-Landero MF, Camacho LJU, Martínez-Abundis E: Differential effect of high-fat, high-sucrose and combined high-fat/high-sucrose diets consumption on fat accumulation, serum leptin and cardiac hypertrophy in rats. Arch Physiol Biochem 2020;126:258-263. https://doi.org/10.1080/13813455.2018.1517181 |
||||
| 38 Fonseca CSM, Basford JE, Kuhel DG, Konaniah ES, Cash JG, Lima VLM, Hui DY: Distinct Influence of Hypercaloric Diets Predominant with Fat or Fat and Sucrose on Adipose Tissue and Liver Inflammation in Mice. Molecules 2020;25:4369. https://doi.org/10.3390/molecules25194369 |
||||
| 39 Izar MCO, Lottenberg AM, Giraldez VZR, Santos Filho RD, Machado RM, Bertolami A, Assad MHV, Saraiva JFK, Faludi AA, Moreira ASB, Geloneze B, Magnoni CD, Scherr C, Amaral CK, Araújo DB, Cintra DEC, Nakandakare ER, Fonseca FAH, Mota ICP, Santos JE, et al.: Posicionamento sobre o Consumo de Gorduras e Saúde Cardiovascular - 2021. Arq Bras Cardiol 2021;116:160-212. https://doi.org/10.36660/abc.20201340 |
||||
| 40 Barroso WKS, Rodrigues CIS, Bortolotto LA, Mota-Gomes, MA, Brandão AA, Feitosa ADM, Machado CA, Poli-de-Figueiredo CE, Amodeo C, Mion Júnior D, Barbosa ECD, Nobre F, Guimarães ICB, Vilela-Martin JF, Yugar-Toledo JC, Magalhães MEC, Neves MFT, Jardim PCBV, Miranda RD, Póvoa RMS, et al.: Diretrizes Brasileiras de Hipertensão Arterial - 2020. Arq Bras Cardiol 2021;116:516-658. https://doi.org/10.36660/abc.20201238 |
||||
| 41 Apaijai N, Arinno A, Palee S, Pratchayasakul W, Kerdphoo S, Jaiwongkam T, Chunchai T, Chattipakorn SC, Chattipakorn N: High-saturated fat high-sugar diet accelerates left-ventricular dysfunction faster than high-saturated fat diet alone via increasing oxidative stress and apoptosis in obese-insulin resistant rats. Mol Nutr Food Res 2019;63:e1800729. https://doi.org/10.1002/mnfr.201800729 |
||||
| 42 Matsubara LS, Narikawa S, Ferreira ALA, Paiva SAR, Zornoff LM, Matsubara BB: Remodelação miocárdica na sobrecarga crônica de pressão ou de volume no coração de ratos. Arq Bras Cardiol 2006;86:126-130. https://doi.org/10.1590/S0066-782X2006000200008 |
||||
| 43 Aboumsallem JP, Muthuramu I, Mishra M, Kempen H, De Geest B: Effective Treatment of Diabetic Cardiomyopathy and Heart Failure with Reconstituted HDL (Milano) in Mice. Int J Mol Sci 2019;20:1273. https://doi.org/10.3390/ijms20061273 |
||||
| 44 Sharma N, Okere IC, Duda MK, Chess DJ, O'Shea KM, Stanley WC: Potential impact of carbohydrate and fat intake on pathological left ventricular hypertrophy. Cardiovasc Res 2007;3:257-268. https://doi.org/10.1016/j.cardiores.2006.11.007 |
||||
| 45 Fouad IG: Synergistic anti-atherosclerotic role of combined treatment of omega-3 and co-enzyme Q10 in hypercholesterolemia-induced obese rats. Heliyon 2020;6:e03659. https://doi.org/10.1016/j.heliyon.2020.e03659 |
||||
| 46 Wang Y, Qian Y, Fang Q, Zhong P, Li W, Wang L, Fu W, Zhang Y, Xu Z, Li X, Liang G: Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nat Commun 2017;8:13997. https://doi.org/10.1038/ncomms13997 |
||||
| 47 Bhatti SN, Li JM: Nox2 dependent redox-regulation of Akt and ERK1/2 to promote left ventricular hypertrophy in dietary obesity of mice. Biochem Biophys Res Commun 2020;528:506-513. https://doi.org/10.1016/j.bbrc.2020.05.162 |
||||
| 48 Hunter I, Soler A, Joseph G, Hutcheson B, Bradford C, Zhang FF, Potter B, Proctor S, Rocic P: Cardiovascular function in male and female JCR:LA-cp rats: effect of high-fat/high-sucrose diet. Am J Physiol Heart Circ Physiol 2017;312:H742-H751. https://doi.org/10.1152/ajpheart.00535.2016 |
||||
| 49 Sverdlov AL, Elezaby A, Qin F, Behring JB, Luptak I, Calamaras TD, Siwik DA, Miller EJ, Liesa M, Shirihai OS, Pimentel DR, Cohen RA, Bachschmid MM, Colucci WS: Mitochondrial Reactive Oxygen Species Mediate Cardiac Structural, Functional, and Mitochondrial Consequences of Diet-Induced Metabolic Heart Disease. J Am Heart Assoc 2016;5:e002555. https://doi.org/10.1161/JAHA.115.002555 |
||||
| 50 Martínez-Martínez E, Jurado-López R, Valero-Muñoz M, Bartolomé MV, Ballesteros S, Luaces M, Briones AM, López-Andrés N, Miana M, Cachofeiro V: Leptin induces cardiac fibrosis through galectin-3, mTOR and oxidative stress: potential role in obesity. J Hypertens 2014;32:1104-1114. https://doi.org/10.1097/HJH.0000000000000149 |
||||
| 51 Pinotti MF, Silva MD, Sugizaki MM, Novelli YSD, Sant'ana LS, Aragon FF, Padovani CR, Novelli EL, Cicogna AC: Influências de dietas ricas em ácidos graxos saturados e insaturados sobre o miocárdio de ratos. Arq Bras Cardiol 2007;88:346-353. https://doi.org/10.1590/S0066-782X2007000300015 |
||||
| 52 Emelyanova L, Boukatina A, Myers C, Oyarzo J, Lustgarten J, Shi Y, Jahangir A: High calories but not fat content of lard-based diet contribute to impaired mitochondrial oxidative phosphorylation in C57BL/6J mice heart. PLoS One 2019;14:e0217045. https://doi.org/10.1371/journal.pone.0217045 |
||||
| 53 Ma SR, Xie XW: NLRC5 deficiency promotes myocardial damage induced by high fat diet in mice through activating TLR4/NF-Kb. Biomed Pharmacother 2017;91:755-766. https://doi.org/10.1016/j.biopha.2017.03.062 |
||||
| 54 Yu EP, Bennett MR: Mitochondrial DNA damage and atherosclerosis. Trends Endocrinol Metab 2014;25:481-487. https://doi.org/10.1016/j.tem.2014.06.008 |
||||
| 55 Noeman SA, Hamooda HE, Baalash AA: Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndr 2011;3:17. https://doi.org/10.1186/1758-5996-3-17 |
||||
| 56 Enos RT, Velázquez KT, Murphy EA: Insight into the impact of dietary saturated fat on tissue-specific cellular processes underlying obesity-related diseases. J Nutr Biochem 2014;25:600-612. https://doi.org/10.1016/j.jnutbio.2014.01.011 |
||||
| 57 Pakiet A, Jakubiak A, Mierzejewska P, Zwara A, Liakh I, Sledzinski T, Mika A: The effect of a high-fat diet on the fatty acid composition in the hearts of mice. Nutrients 2020;12:824. https://doi.org/10.3390/nu12030824 |
||||
| 58 Jiménez-González S, Marín-Royo G, Jurado-López R, Bartolomé MV, Romero-Miranda A, Luaces M, Islas F, Nieto ML, Martínez-Martínez E, Cachofeiro V: The Crosstalk between Cardiac Lipotoxicity and Mitochondrial Oxidative Stress in the Cardiac Alterations in Diet-Induced Obesity in Rats. Cells 2020;9:451. https://doi.org/10.3390/cells9020451 |
||||
| 59 Drosatos K, Schulze PC: Cardiac lipotoxicity: molecular pathways and therapeutic implications. Curr Heart Fail Rep 2013;10:109-121. https://doi.org/10.1007/s11897-013-0133-0 |
||||
| 60 Wu KM, Hsu YM, Ying MC, Tsai FJ, Tsai CH, Chung JG, Yang JS, Tang CH, Cheng LY, Su PH, Viswanadha VP, Kuo WW, Huang CY: High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in H9c2 cardiomyoblast cells via ROS suppression. Nutr Metab (Lond) 2019;16:36. https://doi.org/10.1186/s12986-019-0356-5 |
||||
| 61 van de Weijer T, Schrauwen-Hinderling VB, Schrauwen P: Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res 2011;92:10-18. https://doi.org/10.1093/cvr/cvr212 |
||||
| 62 Szűcs G, Sója A, Péter M, Sárközy M, Bruszel B, Siska A, Földesi I, Szabó Z, Janáky T, Vígh L, Balogh G, Csont T: Prediabetes induced by fructose-enriched diet influences cardiac lipidome and proteome and leads to deterioration of cardiac function prior to the development of excessive oxidative stress and cell damage. Oxid Med Cell Longev 2019;2019:3218275. https://doi.org/10.1155/2019/3218275 |
||||
| 63 Rindler PM, Plafker SM, Szweda LI, Kinter M: High dietary fat selectively increases catalase expression within cardiac mitochondria. J Biol Chem 2013;288:1979-1990. https://doi.org/10.1074/jbc.M112.412890 |
||||
| 64 Joseph LC, Barca E, Subramanyam P, Komrowski M, Pajvani U, Colecraft HM, Hirano M, Morrow JP: Inhibition of NAPDH Oxidase 2 (NOX2) Prevents Oxidative Stress and Mitochondrial Abnormalities Caused by Saturated Fat in Cardiomyocytes. PLoS One 2016;11:e0145750. https://doi.org/10.1371/journal.pone.0145750 |
||||
| 65 Andreadou I, Daiber A, Baxter GF, Brizzi MF, Di Lisa F, Kaludercic, N, Lazou A, Varga ZV, Zuurbier CJ, Schulz R, Ferdinandy P: Influence of cardiometabolic comorbidities on myocardial function, infarction, and cardioprotection: Role of cardiac redox signaling. Free Radic Biol Med 2021;166:33-52. https://doi.org/10.1016/j.freeradbiomed.2021.02.012 |
||||
| 66 Kalupahana NS, Moustaid-Moussa N: The renin-angiotensin system: a link between obesity, inflammation and insulin resistance. Obes Rev 2012;13:136-149. https://doi.org/10.1111/j.1467-789X.2011.00942.x |
||||
| 67 Feraco A, Armani A, Mammi C, Fabbri A, Rosano GM, Caprio M: Role of mineralocorticoid receptor and renin-angiotensin-aldosterone system in adipocyte dysfunction and obesity. J Steroid Biochem Mol Biol 2013;137:99-106. https://doi.org/10.1016/j.jsbmb.2013.02.012 |
||||
| 68 Nickenig G, Harrison DG: The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesis. Circulation 2002;105:393-396. https://doi.org/10.1161/hc0302.102618 |
||||
| 69 Münzel T, Gori T, Keaney JF Jr, Maack C, Daiber A: Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J 2015;36:2555-2564. https://doi.org/10.1093/eurheartj/ehv305 |
||||
| 70 Gamez-Mendez AM, Vargas-Robles H, Ríos A, Escalante B: Oxidative stress-dependent coronary endothelial dysfunction in obese mice. PLoS One 2015;10:e0138609. https://doi.org/10.1371/journal.pone.0138609 |
||||
| 71 Gong F, Gu J, Wang H: Up regulated Tmbim1 activation promotes high fat diet (HFD)-induced cardiomyopathy by enhancement of inflammation and oxidative stress. Biochem Biophys Res Commun 2018;504:797-804. https://doi.org/10.1016/j.bbrc.2018.08.059 |
||||
| 72 Lushchak VI: Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 2014;224:164-175. https://doi.org/10.1016/j.cbi.2014.10.016 |
||||