Corresponding Author: Ahmed Chraibi
Department of Pharmacology & Physiology, Université de Sherbrooke, 3001, 12th Av North, Sherbrooke, QC, J1H 5N4 (Canada)
Tel. +1 819 821-8000-75304, Fax +1 819 564-5399 , E-Mail Ahmed.chraibi@usherbrooke.ca
Apelin-13 Decreases Epithelial Sodium Channel (ENaC) Expression and Activity in Kidney Collecting Duct Cells
Houda Ayaria Ahmed Chraibia,b
aDepartment of Pharmacology and Physiology, Faculté de Médecine et des Sciences de la Santé, Université de Sherbrooke, Sherbrooke, QC, Canada, bResearch Center of the Centre Hospitalier Universitaire de Sherbrooke (CR-CHUS), Université de Sherbrooke, Sherbrooke, QC, Canada
Introduction
Apelin is a vasoactive peptide isolated from bovine stomach recognised as the endogenous ligand for a putative receptor related to the angiotensin receptor AT1 (APJ), which belongs to the G protein coupled receptor family [1, 2]. It originates from a 77-aminoacid precursor, preproapelin, which encompasses several active fragments including apelin-13, -17, and -36 [3, 4]. Apelin and its receptor are widely expressed in a number of tissues such as central nervous system, heart and blood vessels [5, 6]. In the kidney, the apelin/APJ system is expressed in glomeruli, vascular endothelial cells and all nephron segments including collecting tubules [7-9]. Recent studies show that a second peptide ligand of APJ, elabela, discovered in 2014, is localised in collecting duct principal cells [10-12]. A large body of evidence indicates that the apelin/APJ system plays a crucial role in numerous pathophysiological and physiological processes [13-15]. In the kidney, it is a critical mediator of renal fibrosis, renal ischemia, diabetic nephropathy and hemodialysis [16-18]. In addition, the apelin/APJ system exerts a central role in the regulation of blood pressure, glomerular haemodynamics and acts on the tubule to promote diuresis [8, 19, 20]. Recently, we have determined that the effect on diuresis is due to inhibition of aquaporin-2 (AQP-2) water channel insertion into the apical plasma membrane of the collecting duct cells [21]. The central role of these cells in water and sodium transport is governed by AQP-2 and epithelial sodium channel (ENaC).
ENaC is a protein complex consisting of three homologous subunits (α, β, γ) encoded by three different genes [22]. It is expressed at the apical membrane of a variety of tissues, such as the distal nephron of the kidney, the lungs and distal colon [23-25]. This channel is a major regulator of salt and water reabsorption and blood pressure in the aldosterone-sensitive distal nephron (ASDN) [26, 27]. Thus, ENaC is regulated in a very stringent manner, including the involvement of several hormonal factors and mechanisms. For instance, arginine vasopressin and its analogues bind to V2 receptors and increase the activity and translocation of ENaC to the apical membrane from intracellular stores through activation of protein kinase A [28]. Aldosterone stimulates ENaC activity and expression through different genomic responses [29, 30]. Aldosterone regulation of ENaC occurs through receptor-mediated modulation of intracellular signalling pathways involving various kinase cascades, such as inhibition of extracellular signal-regulated kinase (ERK1/2), stimulation of serum and glucocorticoid-regulated kinase (SGK1) or inhibition of the neural precursor cell-expressed developmentally down-regulated protein 4-2 (Nedd4-2), an E3 ubiquitin ligase inducing internalisation and degradation of ENaC [31-34]. The coordination of ENaC and AQP-2 channels is essential for the control of extracellular fluid volume, sodium homeostasis and blood pressure. The action of the apelin/APJ system on the renal tubule in promoting diuresis is mediated by several signalling pathways, including those involved in the regulation of sodium homeostasis. In light of the above, we aimed to investigate the role of apelin in the regulation of sodium balance in the distal nephron, more specifically its involvement in modulating the expression and activity of ENaC in collecting duct principal cells, as well as elucidate the mechanisms implicated in this regulation.
Materials and Methods
Cell culture
Immortalised mouse kidney cortical collecting duct cells (mpkCCDc14) were maintained in DMEM-F12 (Dulbecco’s modified Eagle’s medium / Ham’s F-12 (v/v), Wisent) supplemented with 5 μg/ml transferrin, 20 mM D-glucose, 2% foetal bovine serum (FBS), 20 mM HEPES, 1% penicillin/streptomycin, 5μg/ml insulin, 50 nM dexamethasone, 60 nM sodium selenate, 1 nM triiodothyronine, and 10 ng/ml epidermal growth factor (EGF). Cells were plated in 100 mm dishes at 37°C and 5% CO2, 95% air atmosphere until 80% confluency, after which cells were transferred into 4.5 cm2 semi-permeable filter-containing pores measuring 0.4 μm (Greiner-Bio-One, Germany) at a density of 80 000 cells/cm2. Upon reaching a transepithelial resistance of 1200 Ω.cm2 (4-5 days after seeding), cells were pretreated for 8h or 24h with 1μM aldosterone (Sigma-Aldrich, USA) to induce endogenous ENaC expression and subsequently treated, in the continued presence of aldosterone, with or without 200 nM apelin-13 for varying time intervals. All treatments were added to the basolateral compartment and culture medium was renewed daily. Apelin-13 was generously provided by P. Sarret, IPS, FMSS, Université de Sherbrooke.
Transepithelial current measurement
Transepithelial voltage (Vte) and resistance (Rte) of each filter were measured under sterile conditions with an EndOhm-6 coupled to an EVOM2 (WPI, Sarasota, FL, USA) before and after treatment. Briefly, Vte was measured by means of a set of 2 Ag-AgCl electrodes and determined with the apical electrode as reference. Rte was measured by passage of current through the cell monolayer and measurement of the resulting voltage gradient across the cells. The transepithelial current (Isc) was calculated using Ohm’s law. 10 µM amiloride was added in the apical compartment to determine the magnitude of ENaC-mediated sodium transport across the cell monolayer.
RNA isolation and QPCR
Total RNA was isolated using Trizol Reagent (Life Technologies, Burlington, ON, Canada) according to the manufacturer’s instructions. To remove potentially contaminating DNA, total RNA was treated with DNase (Promega, Madison, WI) for 1 hour at 37°C. RNA was reverse-transcribed according to the manufacturer’s procedure (Quantitect Qiagen, CA) and cDNA samples were amplified using the specific primers for αENaC forward, 5’CGGAGTTGCTAAACTCAACATC3’, and reverse, 5’TGGAGACCAGTACCGGCT3’, and for GAPDH forward, 5’TGGTGCCAAAAGGGTCATC3’, and reverse 5’CTTCGACGATGCCAAAGTTG3’. The resulting cDNAs were used as templates in duplicate qPCR reactions each in triplicate, using a Brilliant II SYBR Green qPCR Master Mix kit (Stratagene, Mississauga, Ontario) in a MX3000P Real-Time PCR system to assess changes in expression of several transcripts. Fold-change values were calculated in “relative change” compared to the control condition, normalised with the expression of mGAPDH, and calculated according to the Pfaffl mathematical model [35].
Western blot analysis
Total cell lysates were collected in cold lysis buffer (20 mM Tris HCl, pH 7.4, 5 mM EDTA, 40 mM beta-glycerophosphate, 30 mM NaF, and 1% Triton X-100) supplemented with 200 µM sodium orthovanadate and completeTM EDTA-free protease inhibitors (Roche Diagnostics, Laval, QC, Canada). Protein quantification assays were determined using the BCA (bicinchoninic acid) procedure. Lysates were separated on 12% SDS-PAGE gel and transferred onto a PVDF membrane (Polyvinylidene difluoride; Perkin Elmer, Woodbridge, ON). The membrane was blocked in 5% BSA (Bovine Serum Albumin) in TBS-T (20 mM Tris, 150 mM NaCl, 0.1% Tween-20) for 2 h and then incubated overnight at 4°C with the following primary antibodies: anti-αENaC (rabbit polyclonal, 1/2000, SPC403D, Biosciences Inc, Burlington, ON, Canada); anti-actin (mouse monoclonal, 1/10000, MAB1501, Millipore, CA); anti-p-SGK1 (rabbit monoclonal, ab-55281, 1/1000, Abcam, CA); anti-pNedd4-2 (rabbit polyclonal, 1/1000, ab-73386, Abcam, CA); anti p-ERK (rabbit monoclonal, 1/1000, Cell Signaling 9100, CA); anti-ERK total (rabbit monoclonal, 1/1000, Cell Signaling 9100, CA). Membranes were then incubated with 1/1000 of suitable HRP-conjugated secondary antibody: anti-mouse (NA931V, Santa Cruz, CA); anti-rabbit (NA934V, Amersham, CA) for 2 hours at room temperature. Following the primary and secondary antibody incubations, the blot was washed in TBST three times for at least 10 min. Detection was performed using the Western Lightning Chemiluminescence Reagent Plus kit (Perkin Elmer, Woodbridge, ON, Canada) and revelation carried out using the LICOR system. Densitometric protein analysis was performed using the STUDIO Image software.
Statistical analyses
Results are expressed as the mean ± SEM from independent experiments. Each experiment was carried out on cells having the same number of passages and all experiments were performed in quadruple. The number of experiments is indicated in the figure legends. All statistical analyses were performed using Prism 9 software (Graph Pad Software, San Diego, CA). A two-way ANOVA, with the Bonferroni multiple comparison test, were used to analyse the data. Data were considered to be statistically significant at the 95% confidence level (p <0.05).
Results
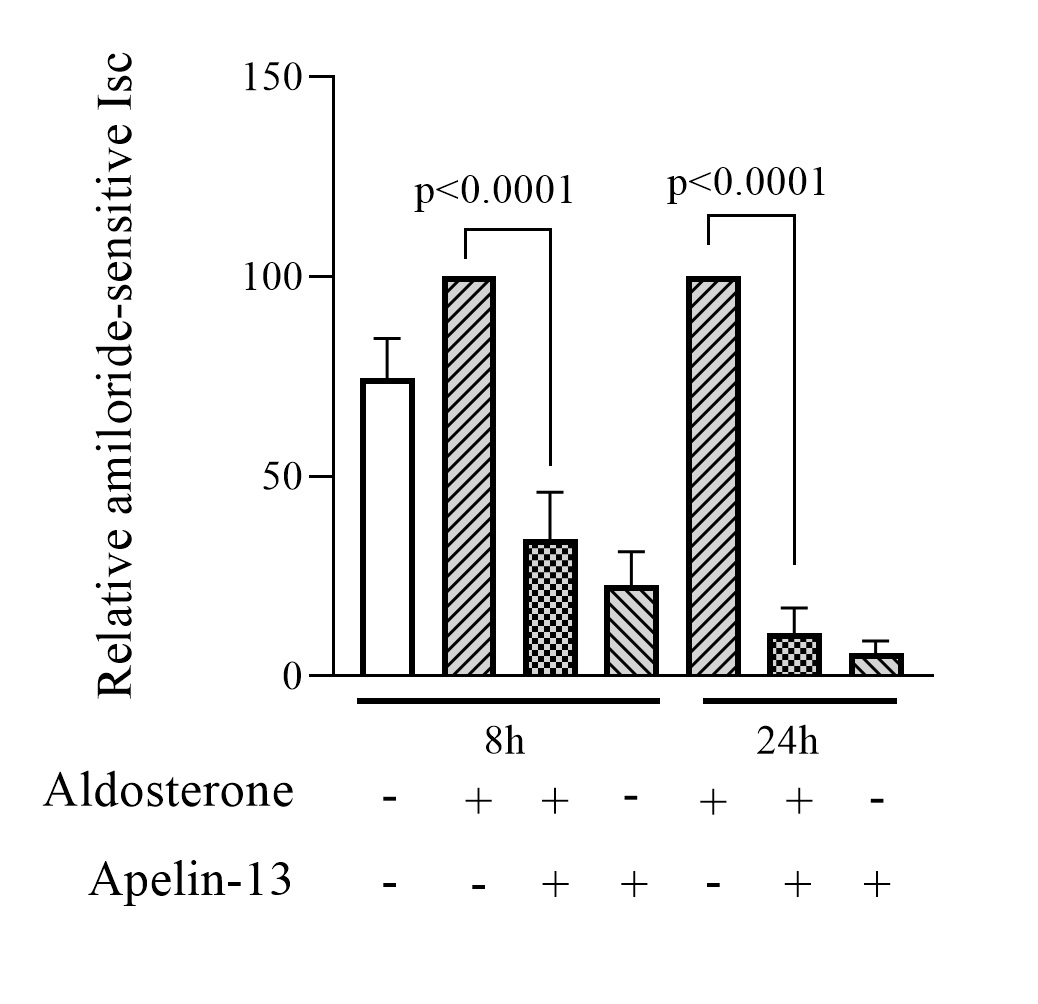
Apelin-13 reduces amiloride-sensitive sodium current transport in mpkCCD cells
We first investigated whether apelin affects the amiloride-sensitive sodium current. To achieve the latter, electrophysiological measurements of transepithelial sodium current (Isc) were performed in monolayers of mpkCCD cells with high transepithelial resistance (≥ 1200 Ω.cm2). The epithelial cell line was then pretreated for 8h or 24h with 1μM aldosterone to induce endogenous ENaC expression and subsequently treated, in the continued presence of aldosterone, with or without 200 nM apelin-13 for 8h or 24h. Aldosterone treatment produces a significant change in basal sodium Isc of 19% and 33% after 8h and 24h respectively (p = 0.022 and 0.010). Basal sodium Isc values were 79.31 ± 6.29 μA (n = 72) in absence of aldosterone, and 94.32 ± 3.39 μA (n = 60) and 105.61 ± 4.55 μA (n = 60) in the presence of aldosterone 8h and 24h, respectively. Amiloride (10 μM) was added to the apical membrane at the end of the experiments to confirm that the transepithelial current was mediated by ENaC. In all experimental conditions, amiloride strongly decreased sodium current showing that the majority of basal sodium transport in mpkCCD cells is amiloride-sensitive. As illustrated in Fig. 1, treatment with 200 nM apelin-13, in the continued presence of aldosterone, produced a significant decrease in Isc amiloride-sensitive sodium current after 8h and 24h (76% ± 12% and 89% ± 6.3% respectively, n=6) compared to aldosterone alone (positive control). A similar decrease was observed when mpkCCD cells were treated with apelin-13 without aldosterone, suggesting that apelin is able to reduce, significantly, the transepithelial sodium current mediated by ENaC channels in the presence or absence of aldosterone (Fig. 1).
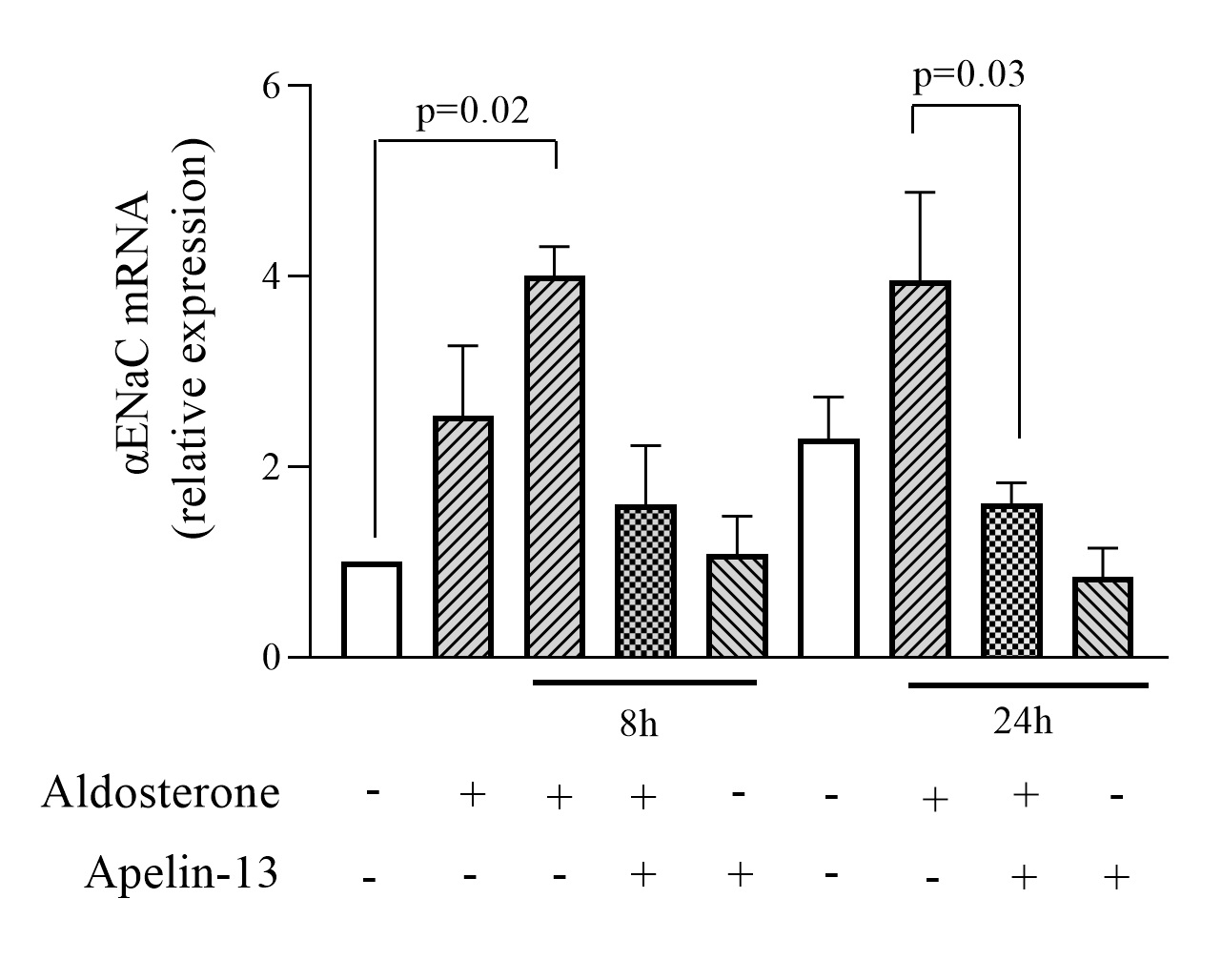
Apelin-13 decreases αENaC mRNA expression
To assess whether apelin is able to regulate the expression of αENaC, we first tested the effect of apelin-13 on αENaC mRNA expression. mpkCCD cells were stimulated with aldosterone to induce endogenous ENaC expression and then treated or not with apelin-13 for 8h or 24h in the continued presence of aldosterone as described previously. As determined by quantitative RT-PCR (Fig. 2), treatment with aldosterone for 8h and 24h induced a 4.01 ± 0.31 and 3.96 ± 0.92 fold increase in αENaC mRNA compared to untreated cells. These results are consistent with previous findings showing that aldosterone stimulates ENaC transcription [30, 36, 37]. Treatment of mpkCCD cells with apelin-13 for 8h and 24h in the presence of aldosterone decreased αENaC mRNA by 61% and 60% (n=4), respectively, suggesting that apelin-13 induces a inhibitory effect on αENaC transcription in mpkCCD cells.
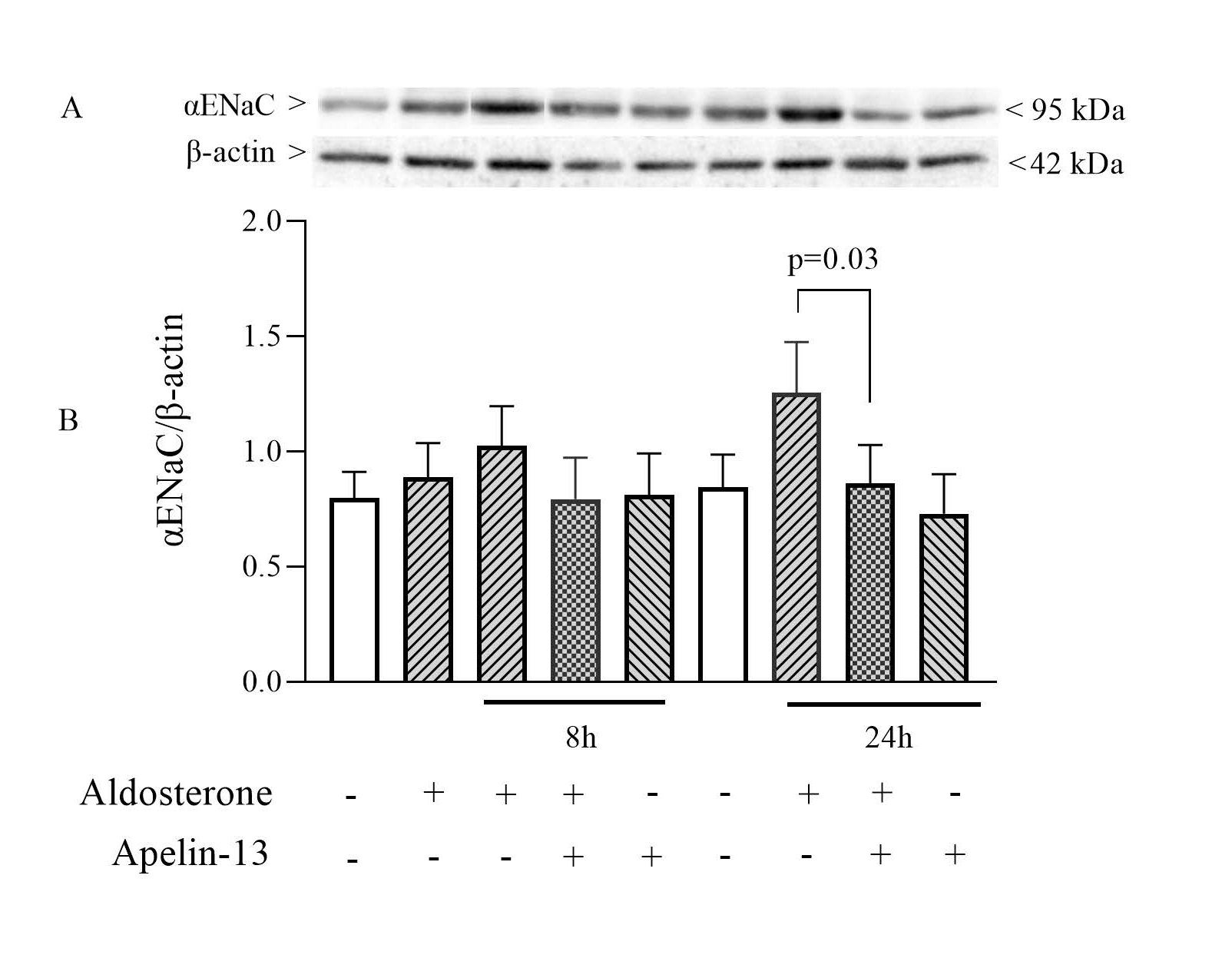
Effect of apelin-13 on aldosterone-induced αENaC protein expression
We next assessed whether apelin-13 treatment induces a decrease in αENaC protein expression. To this end, mpkCCD cells were stimulated with aldosterone and then treated or not with apelin-13 for 8 h or 24 h in the continued presence of aldosterone. As shown in
Fig. 3, there was no significant change in the expression of ENaC detected at a molecular weight of 95 kDa (as corrected for β-actin) induced after 8h aldosterone treatment compared with unstimulated cells. However, a significant 31% decrease in the expression of the αENaC protein was observed when mpkCCD cells were treated with apelin-13 for 24h (n=8). This reduction is consistent with the inhibitory effect of apelin on ENaC mRNA expression.
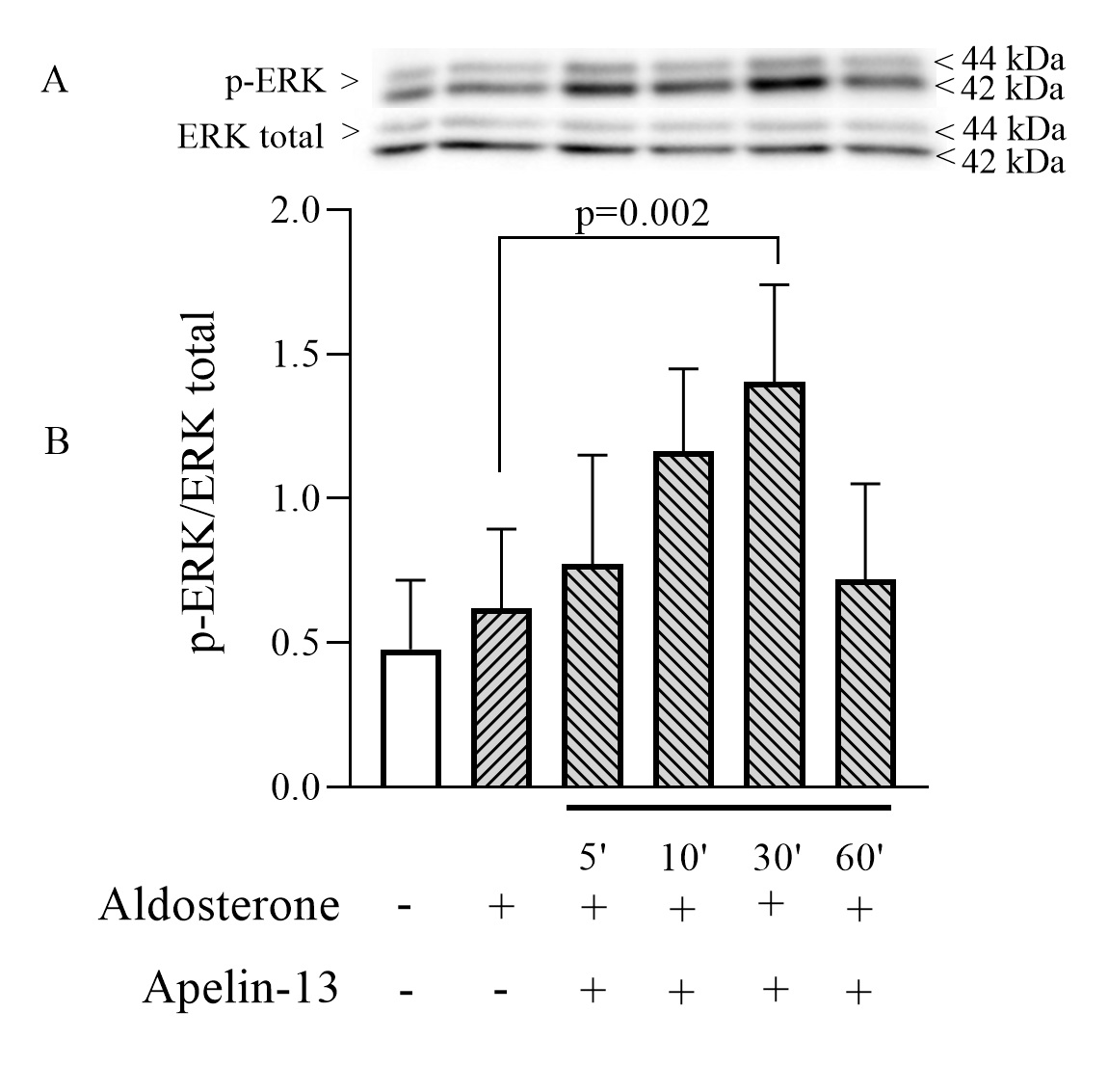
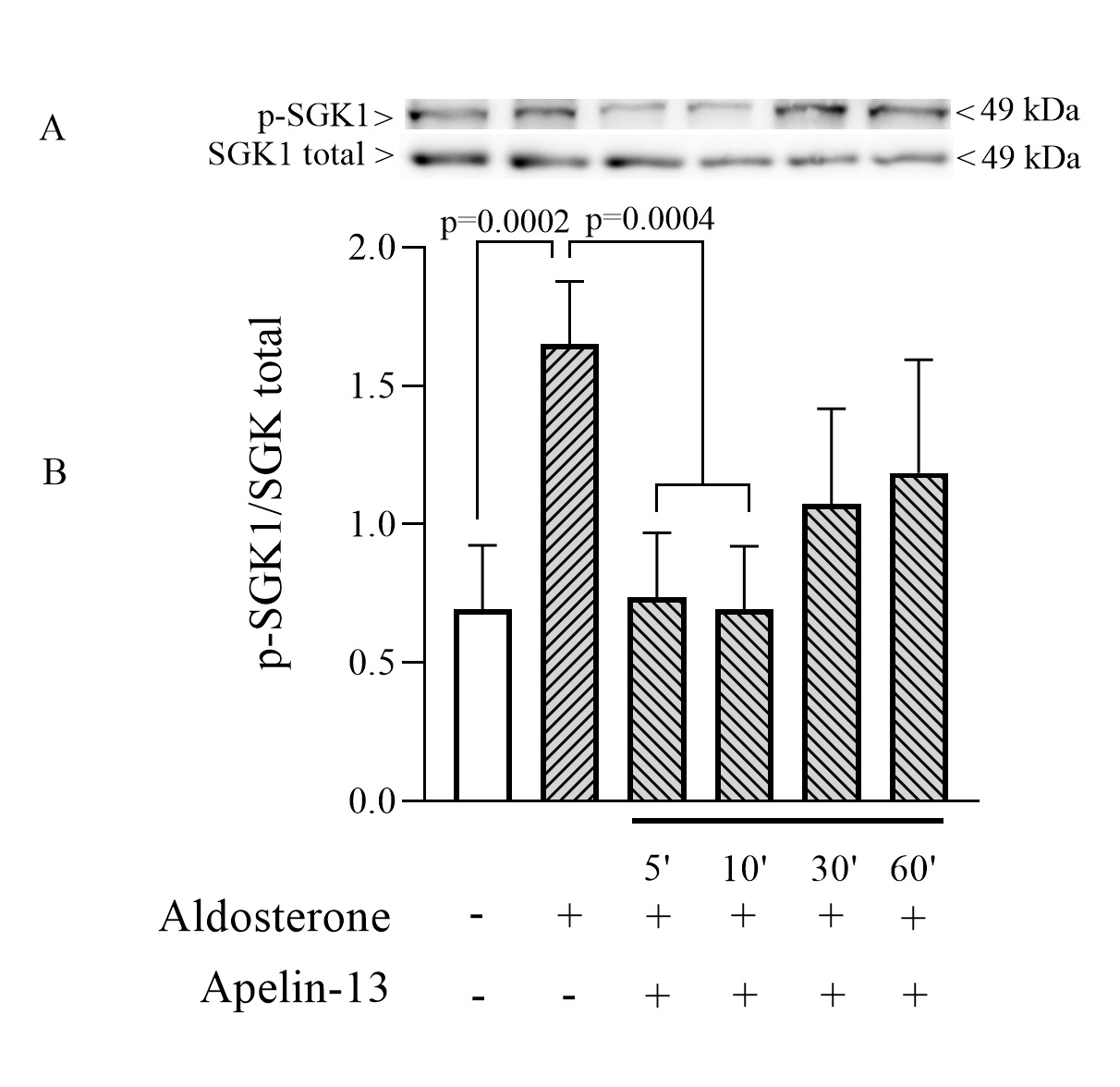
Effect of apelin-13 on SGK-1 phosphorylation in mpkCCD cells
We subsequently investigated whether the SGK pathway is involved in the inhibition of ENaC by apelin. mpkCCD cells were accordingly treated with apelin-13 for 5, 10, 30 and 60 min in the presence of aldosterone; the non-phosphorylated and phosphorylated forms of SGK-1 were assessed by Western blot. Cells treated with aldosterone alone showed a strong increase in p-SGK/SGKtotal ratio (Fig. 5) in accordance with previously described data showing the central role of SGK-1 in the regulation of ENaC by aldosterone [42, 43]. This large activation of the SGK pathway was significantly reduced when cells were treated with apelin-13 for 5 and 10 min suggesting that the decrease in p-SGK/SGKtotal ratio could be responsible for the reduction in ENaC activity by apelin.
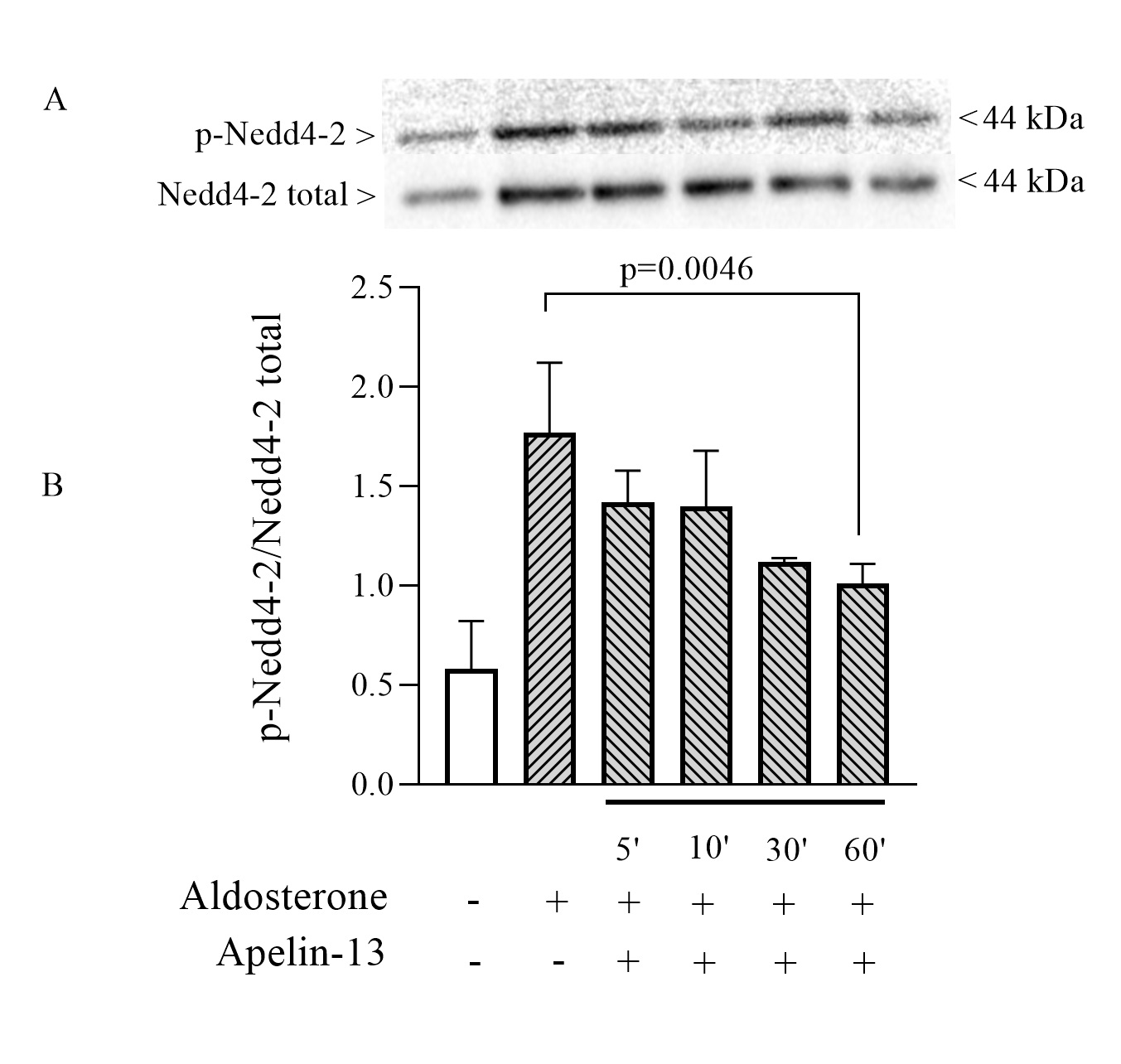
Effect of apelin-13 on Nedd4-2 phosphorylation in mpkCCD cells
An acknowledged central mechanism by which Sgk1 stimulates ENaC is the phosphorylation of the ubiquitin ligase Nedd4-2 which is thus prevented from ubiquitinating this channel [32, 44]. In order to verify whether the inhibitory effect of apelin on ENaC is in part due to a decrease in Nedd4-2 phosphorylation, experiments were performed to detect the presence of phosphorylated and non-phosphorylated forms of Nedd4-2 following treatment of mpkCCD cells for 5, 10, 30 and 60 minutes. As illustrated in Fig. 6, apelin-13 treatment in the presence of aldosterone showed a progressive decrease in the phosphorylated form of Nedd4-2 compared to that obtained after treatment with aldosterone alone. The p-Nedd4-2/Nedd4-2 ratio was respectively 1.7 and 0.9 when cells were treated with aldosterone in the absence or presence of apelin-13 for 60 minutes.
These data suggest that the inhibition of ENaC by apelin may be explained, in part, by the stimulation of ENaC ubiquitination.
Author Contributions
HA and AC conceived the study and experiments, performed and analysed the data. HA contributed to the writing of the manuscript. AC wrote the paper and supervised the project.
Funding
This work was supported by the CR-CHUS, the FMSS and by the Canada Foundation for Innovation (CFI), Kidney Foundation of Canada grants (KFOC) and Natural Sciences and Engineering Research Council of Canada (NSERC) grants to Ahmed Chraibi.
The authors declare that they have no conflicts of interest.
| 1 O'Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T: A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 1993;136:355-360. https://doi.org/10.1016/0378-1119(93)90495-O |
||||
| 2 Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M: Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 1998;251:471-476. https://doi.org/10.1006/bbrc.1998.9489 |
||||
| 3 Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, Kitada C, Honda S, Kurokawa T, Onda H, Nishimura O, Fujino M: Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem 2000;275:21061-21067. https://doi.org/10.1074/jbc.M908417199 |
||||
| 4 Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, BF OD: Characterization of apelin, the ligand for the APJ receptor. J Neurochem 2000;74:34-41. https://doi.org/10.1046/j.1471-4159.2000.0740034.x |
||||
| 5 Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, Nishizawa N, Kitada C, Onda H, Nishimura O, Fujino M: Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta 2001;1538:162-171. https://doi.org/10.1016/S0167-4889(00)00143-9 |
||||
| 6 O'Carroll AM, Selby TL, Palkovits M, SJ. L: Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim Biophys Acta 2000;1492:72-80. https://doi.org/10.1016/S0167-4781(00)00072-5 |
||||
| 7 De Falco M, De Luca L, Onori N, Cavallotti I, Artigiano F, Esposito V, De Luca B, Laforgia V, Groeger AM, De Luca A: Apelin expression in normal human tissues. In vivo 2002;16:333-336. | ||||
| 8 Hus-Citharel A, Bouby N, Frugiere A, Bodineau L, Gasc JM, Llorens-Cortes C: Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int 2008;74:486-494. https://doi.org/10.1038/ki.2008.199 |
||||
| 9 O'Carroll AM, Salih S, Griffiths PR, Bijabhai A, Knepper MA, Lolait SJ: Expression and functional implications of the renal apelinergic system in rodents. PLoS One 2017;12:e0183094. https://doi.org/10.1371/journal.pone.0183094 |
||||
| 10 Chen H, Wan D, Wang L, Peng A, Xiao H, Petersen RB, Liu C, Zheng L, Huang K: Apelin protects against acute renal injury by inhibiting TGF-beta1. Biochim Biophys Acta 2015;1852:1278-1287. https://doi.org/10.1016/j.bbadis.2015.02.013 |
||||
| 11 Chen H, Wang L, Wang W, Cheng C, Zhang Y, Wang C, Miao X, Wang J, Li J, Zheng L, Huang K: ELABELA and an ELABELA fragment protect against AKI. J Am Soc Nephrol 2017;28:2694-2707. https://doi.org/10.1681/ASN.2016111210 |
||||
| 12 Xu C, Wang F, Chen Y, Xie S, Sng D, Reversade B, Yang T: ELABELA antagonizes intrarenal renin-angiotensin system to lower blood pressure and protects against renal injury. Am J Physiol Renal Physiol 2020;318:F1122-F1135. https://doi.org/10.1152/ajprenal.00606.2019 |
||||
| 13 Reaux A, De Mota N, Skultetyova I, Lenkei Z, El Messari S, Gallatz K, Corvol P, Palkovits M, Llorens-Cortès C: Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem 2001;77.:1085-1096. https://doi.org/10.1046/j.1471-4159.2001.00320.x |
||||
| 14 Szokodi I, Tavi P, Foldes G, Voutilainen-Myllyla S, Ilves M, Tokola H, Pikkarainen S, Piuhola J, Rysa J, Toth M, Ruskoaho H: Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res 2002;91:434-440. https://doi.org/10.1161/01.RES.0000033522.37861.69 |
||||
| 15 Tatemoto K, Takayama K, Zou M, Kumaki I, Zhang W, Kumano K, Fujimiya M: The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept 2001;99:87-92. https://doi.org/10.1016/S0167-0115(01)00236-1 |
||||
| 16 Guo C, Liu Y, Zhao W, Wei S, Zhang X, Wang W, Zeng X: Apelin promotes diabetic nephropathy by inducing podocyte dysfunction via inhibiting proteasome activities. J Cell Mol Med 2015;19:2273-2285. https://doi.org/10.1111/jcmm.12619 |
||||
| 17 Mafra D, Lobo JC, Farage NE, Stockler-Pinto MB, Leal VO, Calixto A, Geloneze B: The relationship between apelin and parathyroid hormone in hemodialysis patients. Ren Fail 2012;34:970-973. https://doi.org/10.3109/0886022X.2012.700675 |
||||
| 18 Nishida M, Okumura Y, Oka T, Toiyama K, Ozawa S, Itoi T, Hamaoka K: The role of apelin on the alleviative effect of Angiotensin receptor blocker in unilateral ureteral obstruction-induced renal fibrosis. Nephron Extra 2012;2:39-47. https://doi.org/10.1159/000337091 |
||||
| 19 De Mota N, Reaux-Le Goazigo A, El Messari S, Chartrel N, Roesch D, Dujardin C, Kordon C, Vaudry H, Moos F, Llorens-Cortes C: Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci U S A 2004;101:10464-10469. https://doi.org/10.1073/pnas.0403518101 |
||||
| 20 Hus-Citharel A, Bodineau L, Frugiere A, Joubert F, Bouby N, Llorens-Cortes C: Apelin Counteracts Vasopressin-Induced Water Reabsorption via Cross Talk Between Apelin and Vasopressin Receptor Signaling Pathways in the Rat Collecting Duct. Endocrinology 2014;155:4483-4493. https://doi.org/10.1210/en.2014-1257 |
||||
| 21 Boulkeroua C, Ayari H, Khalfaoui T, Lafrance M, Besserer-Offroy E, Ekindi N, Sabbagh R, Dumaine R, Lesur O, Sarret P, Chraibi A: Apelin-13 Regulates Vasopressin-Induced Aquaporin-2 Expression and Trafficking in Kidney Collecting Duct Cells. Cell Physiol Biochem 2019;53:687-700. https://doi.org/10.33594/000000165 |
||||
| 22 Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC: Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 1994;367:463-467. https://doi.org/10.1038/367463a0 |
||||
| 23 Bhalla V, Hallows KR: Mechanisms of ENaC Regulation and Clinical Implications. J Am Soc Nephrol 2008;19 1845-1854. https://doi.org/10.1681/ASN.2008020225 |
||||
| 24 Duc C, Farman N, Canessa CM, Bonvalet JP, Rossier BC: Cell-specific expression of epithelial sodium channel alpha, beta, and gamma subunits in aldosterone-responsive epithelia from the rat: localization by in situ hybridization and immunocytochemistry. J Cell Biol 1994;127:1907-1921. https://doi.org/10.1083/jcb.127.6.1907 |
||||
| 25 Kunzelmann K, Mall M: Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev 2002;82:245-289. https://doi.org/10.1152/physrev.00026.2001 |
||||
| 26 Kleyman TR, Kashlan OB, Hughey RP: Epithelial Na(+) Channel Regulation by Extracellular and Intracellular Factors. Annu Rev Physiol 2018;80:263-281. https://doi.org/10.1146/annurev-physiol-021317-121143 |
||||
| 27 Frindt G, Yang L, Bamberg K, Palmer LG: Na restriction activates epithelial Na channels in rat kidney through two mechanisms and decreases distal Na(+) delivery. J Physiol 2018;596:3585-3602. https://doi.org/10.1113/JP275988 |
||||
| 28 Ecelbarger CA, Kim GH, Wade JB, Knepper MA: Regulation of the abundance of renal sodium transporters and channels by vasopressin. Exp Neurol 2001;171:227-234. https://doi.org/10.1006/exnr.2001.7775 |
||||
| 29 Frindt G, Palmer LG: Surface expression of sodium channels and transporters in rat kidney: effects of dietary sodium. Am J Physiol Renal Physiol 2009;297:F1249-1255. https://doi.org/10.1152/ajprenal.00401.2009 |
||||
| 30 Verrey F: Early aldosterone effects. Exp Nephrol 1998;6:294-301. https://doi.org/10.1159/000020536 |
||||
| 31 Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D: Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci U S A 1999;96:2514-2519. https://doi.org/10.1073/pnas.96.5.2514 |
||||
| 32 Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O: Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. Embo J 2001;20:7052-7059. https://doi.org/10.1093/emboj/20.24.7052 |
||||
| 33 Ishigami T, Kino T, Minegishi S, Araki N, Umemura M, Ushio H, Saigoh S, Sugiyama M: Regulators of Epithelial Sodium Channels in Aldosterone-Sensitive Distal Nephrons (ASDN): Critical Roles of Nedd4L/Nedd4-2 and Salt-Sensitive Hypertension. Int J Mol Sci 2020;21:3871. https://doi.org/10.3390/ijms21113871 |
||||
| 34 Shi H, Asher C, Chigaev A, Yung Y, Reuveny E, Seger R, Garty H: Interactions of beta and gamma ENaC with Nedd4 can be facilitated by an ERK-mediated phosphorylation. J Biol Chem 2002;277:13539-13547. https://doi.org/10.1074/jbc.M111717200 |
||||
| 35 Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29:e45. https://doi.org/10.1093/nar/29.9.e45 |
||||
| 36 Chen L, Zhang X, Zhang W: Regulation of alphaENaC transcription. Vitam Horm 2015;98:101-135. https://doi.org/10.1016/bs.vh.2014.12.004 |
||||
| 37 Pearce D, Bhargava A, Cole TJ: Aldosterone: its receptor, target genes, and actions. Vitam Horm 2003;66:29-76. https://doi.org/10.1016/S0083-6729(03)01002-1 |
||||
| 38 Lang F, Pearce D: Regulation of the epithelial Na+ channel by the mTORC2/SGK1 pathway. Nephrol Dial Transplant 2016;31:200-205. https://doi.org/10.1093/ndt/gfv270 |
||||
| 39 Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE: Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 2015;10:135-146. https://doi.org/10.2215/CJN.05760513 |
||||
| 40 Chapman FA, Nyimanu D, Maguire JJ, Davenport AP, Newby DE, Dhaun N: The therapeutic potential of apelin in kidney disease. Nat Rev Nephrol 2021;17:840-853. https://doi.org/10.1038/s41581-021-00461-z |
||||
| 41 Janssens P, Decuypere JP, Bammens B, Llorens-Cortes C, Vennekens R, Mekahli D: The emerging role of the apelinergic system in kidney physiology and disease. Nephrol Dial Transplant 2021; DOI: 10.1093/ndt/gfab070. https://doi.org/10.1093/ndt/gfab070 |
||||
| 42 Vallon V LF: New insights into the role of serum- and glucocorticoid-inducible kinase SGK1 in the regulation of renal function and blood pressure. Curr Opin Nephrol Hypertens 2005;14:59-66. https://doi.org/10.1097/00041552-200501000-00010 |
||||
| 43 Náray-Fejes-Tóth A, Fejes-Tóth G: The sgk, an aldosterone-induced gene in mineralocorticoid target cells, regulates the epithelial sodium channel. Kidney Int 2000;57:1290-1294. https://doi.org/10.1046/j.1523-1755.2000.00964.x |
||||
| 44 Flores SY, Loffing-Cueni D, Kamynina E, Daidie D, Gerbex C, Chabanel S, Dudler J, Loffing J, Staub O: Aldosterone-induced serum and glucocorticoid-induced kinase 1 expression is accompanied by Nedd4-2 phosphorylation and increased Na+ transport in cortical collecting duct cells. J Am Soc Nephrol 2005;16:2279-2287. https://doi.org/10.1681/ASN.2004100828 |
||||
| 45 Blanchard A, Steichen O, De Mota N, Curis E, Gauci C, Frank M, Wuerzner G, Kamenicky P, Passeron A, Azizi M, Llorens-Cortes C: An abnormal apelin/vasopressin balance may contribute to water retention in patients with the syndrome of inappropriate antidiuretic hormone (SIADH) and heart failure. J Clin Endocrinol Metab 2013;98:2084-2089. https://doi.org/10.1210/jc.2012-3794 |
||||
| 46 Galanth C, Hus-Citharel A, Li B, Llorens-Cortes C: Apelin in the control of body fluid homeostasis and cardiovascular functions. Curr Pharm Des 2012;18:789-798. https://doi.org/10.2174/138161212799277770 |
||||
| 47 Sainsily X, Coquerel D, Giguere H, Dumont L, Tran K, Noll C, Ionescu AL, Cote J, Longpre JM, Carpentier A, Marsault E, Lesur O, Sarret P, Auger-Messier M: Elabela Protects Spontaneously Hypertensive Rats From Hypertension and Cardiorenal Dysfunctions Exacerbated by Dietary High-Salt Intake. Front Pharmacol 2021;12:709467. https://doi.org/10.3389/fphar.2021.709467 |
||||
| 48 Urwyler SA, Timper K, Fenske W, de Mota N, Blanchard A, Kuhn F, Frech N, Arici B, Rutishauser J, Kopp P, Stettler C, Muller B, Katan M, Llorens-Cortes C, Christ-Crain M: Plasma Apelin Concentrations in Patients With Polyuria-Polydipsia Syndrome. J Clin Endocrinol Metab 2016;101:1917-1923. https://doi.org/10.1210/jc.2016-1158 |
||||
| 49 Wang J, Li N, Gao F, Song R, Zhu S, Geng Z: Balance between angiotensin converting enzyme and angiotensin converting enzyme 2 in patients with chronic heart failure. J Renin Angiotensin Aldosterone Syst 2015;16:553-558. https://doi.org/10.1177/1470320315576257 |
||||
| 50 Alvarez de la Rosa D, Li H, Canessa CM: Effects of aldosterone on biosynthesis, traffic, and functional expression of epithelial sodium channels in A6 cells. J Gen Physiol 2002;119:427-442. https://doi.org/10.1085/jgp.20028559 |
||||
| 51 Niisato N, Taruno A, Marunaka Y: Aldosterone-induced modification of osmoregulated ENaC trafficking. Biochem Biophys Res Commun 2007;361:162-168. https://doi.org/10.1016/j.bbrc.2007.07.002 |
||||
| 52 Rossier BC, Pradervand S, Schild L, Hummler E: Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol 2002;64:877-897. https://doi.org/10.1146/annurev.physiol.64.082101.143243 |
||||
| 53 Bankir L, Fernandes S, Bardoux P, Bouby N, Bichet DG: Vasopressin-V2 receptor stimulation reduces sodium excretion in healthy humans. J Am Soc Nephrol 2005;16:1920-1928. https://doi.org/10.1681/ASN.2004121079 |
||||
| 54 Shen JP, Cotton CU: Epidermal growth factor inhibits amiloride-sensitive sodium absorption in renal collecting duct cells. Am J Physiol Renal Physiol 2003;284:F57-64. https://doi.org/10.1152/ajprenal.00028.2002 |
||||
| 55 Grossmann C, Freudinger R, Mildenberger S, Krug AW, Gekle M: Evidence for epidermal growth factor receptor as negative-feedback control in aldosterone-induced Na+ reabsorption. Am J Physiol Renal Physiol 2004;286:F1226-1231. https://doi.org/10.1152/ajprenal.00378.2003 |
||||
| 56 Soundararajan R, Zhang T, Wang J, Vandewalle A, Pearce D: A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem 2005;280:39970-39981. https://doi.org/10.1074/jbc.M508658200 |
||||
| 57 Niisato N, Ohta M, Eaton DC, Marunaka Y: Hypotonic stress upregulates beta- and gamma-ENaC expression through suppression of ERK by inducing MKP-1. Am J Physiol Renal Physiol 2012;303:F240-252. https://doi.org/10.1152/ajprenal.00198.2011 |
||||
| 58 Bai B, Cai X, Jiang Y, Karteris E, Chen J: Heterodimerization of apelin receptor and neurotensin receptor 1 induces phosphorylation of ERK(1/2) and cell proliferation via Galphaq-mediated mechanism. J Cell Mol Med 2014;18:2071-2081. https://doi.org/10.1111/jcmm.12404 |
||||
| 59 Li Y, Bai YJ, Jiang YR: Apelin induces the proliferation, migration and expression of cytoskeleton and tight junction proteins in human RPE cells via PI-3K/Akt and MAPK/Erk signaling pathways. Int J Clin Exp Pathol 2017;10:10711-10729. | ||||
| 60 Li Y, Bai YJ, Jiang YR, Yu WZ, Shi X, Chen L, Feng J, Sun GB: Apelin-13 Is an Early Promoter of Cytoskeleton and Tight Junction in Diabetic Macular Edema via PI-3K/Akt and MAPK/Erk Signaling Pathways. Biomed Res Int 2018;2018:3242574. https://doi.org/10.1155/2018/3242574 |
||||
| 61 Wang Y, Song J, Bian H, Bo J, Lv S, Pan W, Lv X: Apelin promotes hepatic fibrosis through ERK signaling in LX-2 cells. Mol Cell Biochem 2019;460:205-215. https://doi.org/10.1007/s11010-019-03581-0 |
||||
| 62 Sun X, Iida S, Yoshikawa A, Senbonmatsu R, Imanaka K, Maruyama K, Nishimura S, Inagami T, Senbonmatsu T: Non-activated APJ suppresses the angiotensin II type 1 receptor, whereas apelin-activated APJ acts conversely. Hypertens Res 2011;34:701-706. https://doi.org/10.1038/hr.2011.19 |
||||
| 63 Soundararajan R, Pearce D, Hughey RP, Kleyman TR: Role of epithelial sodium channels and their regulators in hypertension. J Biol Chem 2010;285:30363-30369. https://doi.org/10.1074/jbc.R110.155341 |
||||
| 64 Lu M, Wang J, Jones KT, Ives HE, Feldman ME, Yao LJ, Shokat KM, Ashrafi K, Pearce D: mTOR complex-2 activates ENaC by phosphorylating SGK1. J Am Soc Nephrol 2010;21:811-818. https://doi.org/10.1681/ASN.2009111168 |
||||
| 65 Kabra R, Knight KK, Zhou R, Snyder PM: Nedd4-2 induces endocytosis and degradation of proteolytically cleaved epithelial Na+ channels. J Biol Chem 2008;283:6033-6039. https://doi.org/10.1074/jbc.M708555200 |
||||