Corresponding Author: Anjaneyulu Kowluru
B-4237 Research Service, John D. Dingell VA Medical Center, 4646 John R, Detroit, MI-48201 (USA)
Tel. +1-313-576-4478, +1-313-576-1112, E-Mail akowluru@med.wayne.edu
CARD9 Mediates Pancreatic Islet Beta-Cell Dysfunction Under the Duress of Hyperglycemic Stress
Suhadinie Gamagea,b Mirabela Halia,b Fei Chenb,c Anjaneyulu Kowlurua,b
aBiomedical Research Service, John D. Dingell VA Medical Center, Detroit, MI, USA, bDepartment of Pharmaceutical Sciences, Eugene Applebaum College of Pharmacy and Health Sciences, Wayne State University, Detroit, MI, USA, cStony Brook Cancer Center, and Department of Pathology, Stony Brook University, Stony Brook, NY, USA
Introduction
Chronic exposure of pancreatic beta-cells to metabolic stress conditions, such as hyperglycemia, has been shown to increase the generation of intracellular reactive oxygen species (ROS; oxidative stress), endoplasmic reticulum (ER) stress, and mitochondrial dysregulation (mito stress) culminating in defective glucose-stimulated insulin secretion (GSIS) and apoptotic demise of the effete beta cell [1-10]. Published evidence implicates NADPH oxidases, specifically the phagocyte-like NADPH oxidase (Nox2), as contributors of oxidative stress in pancreatic beta cells following exposure to diabetogenic conditions [1, 11-16]. Regulatory roles of NADPH oxidases in islet beta cell dysfunction were confirmed in insulin-secreting beta cell lines, rodent islets and human islets under in vitro conditions, and in islets from animal models as well as humans with T2DM [1, 11, 17, 18]. Several previous studies have reported sustained (or constitutive) activation of Rac1, a small G protein, in pancreatic beta cells following exposure to chronic hyperglycemic and hyperlipidemic conditions as well as exposure to cell-permeable ceramides and pro-inflammatory cytokines [19-25]. Interestingly, hyperactivation of Rac1 under these pathological conditions has been shown to promote activation of Nox2 and stress kinases (e.g., p38MAPK) leading to dysregulation of beta cell function [17, 18, 26]. Several mechanisms have been put-forth to explain sustained activation of Rac1 under conditions of metabolic stress; these include increase in the dissociation of complexes of Rac1 and its GDP-dissociation inhibitors (RhoGDIs, e.g., RhoGDIβ) and accelerated activation of “free” Rac1 by its respective guanine nucleotide exchange factors (GEFS, e.g., Tiam1 and Vav2). The reader is referred to recent reviews on contributory roles of various GEFs, GDIs and other G protein regulatory factors in the activation-deactivation of candidate G proteins (e.g., Rac1) in pancreatic beta cells in normal health and under metabolic stress [21, 27-29].
Extant evidence from multiple laboratories implicates key roles for CARD9 in innate immunity [30-33] . Studies by Jia and coworkers have demonstrated that CARD9 promotes antifungal immunity via a mechanism involving activation of H-Ras, which, in turn, mediates Dectin-1 induced ERK activation [30]. Data accrued in studies by Wu et al. [31] have implicated CARD9 in microbe-mediated generation of ROS via promoting dissociation of Rac1-RhoGDIβ complex leading to activation of Nox2 and the onset of oxidative stress. Together, these studies suggest novel roles for CARD9 in G protein regulated cell function.
In the context of potential roles of CARD9 in islet beta cell function, we recently reported expression of CARD9 in a variety of insulin secreting cells, including human islets, rat islets, mouse islets and INS-1 832/13 cells. Interestingly, siRNA-mediated depletion of CARD9 markedly suppressed GSIS without significantly affecting glucose-induced activation of Rac1 under those conditions. We also noticed that CARD9 deletion markedly inhibited glucose-induced p38MAPK activation under conditions of GSIS inhibition [34]. These findings have led us to postulate that CARD9 might regulate GSIS via a Rac1-independent, but p38-dependent signaling module. Lastly, in an attempt to investigate potential regulatory roles of RhoGDIβ (also referred to as LyGDI, D4-GDI, RhoGDI2) in glucose-induced activation of Rac1 and insulin secretion, we recently demonstrated that siRNA-mediated knockdown of RhoGDIβ in INS-1 832/13 cells significantly attenuated glucose-induced Rac1 activation without affecting its translocation and membrane association. In addition, no significant effects of RhoGDIβ-depletion were observed on GSIS; these findings suggested differential regulatory roles of RhoGDIβ in Rac1 activation and GSIS [35].
Based on the findings highlighted above in other cell types and, albeit limited, in pancreatic beta cells, we undertook the current investigations to test the hypothesis that CARD9 plays key regulatory roles in the onset of beta cell dysfunction under the duress of chronic hyperglycemic conditions by regulating the RhoGDIβ-Rac1-p38MAPK-NF-κB signaling module. The first evidence in support of this hypothesis is presented below.
Materials and Methods
Materials
Antibodies directed against CARD9 (sc-374569), RhoGDIβ [Ly-GDI (D-7); sc-271108], BCL-10 (sc-5273) and agarose beads were from Santa Cruz Biotechnology (Dallas, TX, USA). Rac1 antibody (05-389-25UG) was from EMD Millipore (Burlington, MA, USA). Total and phospho antisera for p38MAPK [p38 MAPK; 9212S and P-p38 MAPK (Thr180/Tyr182); 9211S], total and phospho-JNK ½ antibodies (SAPK/ JNK; 9258S and P-SAPK/JNK (Thr183/Tyr185); 9251S), total ERK ½ antibody [p44/42 MAPK (ERK1/2) (137F5); 4695S] and phospho- ERK ½ antibody [P-p44/42 MAPK (ERK1/2) (Thr202/Tyr204) (D13.14.4E); 4370S], total and phospho antisera for p65 [NF-kappaBp65 (D14E12) XP(R); 8242S and P-NF-kappaB-p65 (Ser36) (93H1); 3033S], cleaved Caspase-3 antibody (Asp175; 9661S), CHOP antibody (2895S) and HRP-conjugated secondary antibodies were from Cell Signaling Technology, Inc (Danvers, MA, USA). β-actin antibody (A1978), sodium palmitate, BRD5529 inhibitor were from Sigma Aldrich (St. Louis, MO, USA). ON-TARGETplus Non-targeting siRNA (Control siRNA: Con-si; D001810-01-20), ON-TARGETplus Rat CARD9 siRNA-SMARTpool (CARD9-si; L-096926-02-20) and DharmaFect1 transfection reagent (T-2001-03) were from Horizon Discovery (Lafayette, CO, USA). Rac1 activation assay kits (pull-down) were from Cytoskeleton (Denver, CO, USA). Ceramide (C-2) was from Cayman Chemicals (Ann Arbor, MI, USA). Co-IP kit was from Thermo Scientific Inc. (Waltham, MA, USA).
Culture of INS-1 832/13 cells and mouse islets
INS-1 832/13 cells (passage #s 50-60) were cultured in RPMI-1640 medium containing 10% FBS supplemented with 100 IU/ ml penicillin and 100 IU/ml streptomycin, 1 mM sodium pyruvate, 50 µM 2-mercapto-ethanol, and 10 mM HEPES (pH 7.4). As indicated in the text, cells were treated overnight with low serum/low glucose media prior to each experiment. Cells were incubated in the presence of low glucose (LG; 2.5mM) or high glucose (HG; 20mM) or HG and palmitic acid (PA; 500 µM; glucolipotoxic [GLT] condition) for 24 hrs unless specified otherwise.
All protocols for mouse islet isolation were reviewed and approved by Institutional Animal Care and Use Committees of Wayne State University and John D. Dingell Veterans Affairs Medical Center. Approximately 10 week-old (male and female) C57BL/6 mice (Charles River, Wilmington, MA, USA) were used for islet isolation using the Collagenase digestion [34]. Islets were hand-picked, and incubated overnight in islet media (RPMI-1640 medium containing 10% FBS supplemented with 100 IU/ ml penicillin and 100 IU/ml streptomycin, 1 mM sodium pyruvate, and 10 mM HEPES). These islets were then exposed to LG or HG treatment for 24 hrs. and cell lysates were prepared using the RIPA buffer and employed in studies described herein.
siRNA-mediated knockdown of expression of CARD9 in INS-1 832/13 cells
Endogenous expression of CARD9 was suppressed using CARD9-si as we recently reported in [34]. Briefly, cells were transfected with siRNA at a final concentration of 100 nM using the DharmaFect1 reagent. Specificity of RNA interference was accessed using cells transfected with non-targeting siRNA (i.e., control siRNA; Con-si). Following specific treatments, as indicated in the text, the cell lysates were prepared in RIPA lysis buffer containing protease and phosphatase inhibitors. Efficiency of the knockdown was determined by western blotting. Additional details on expression of CARD9 in various insulin-secreting cells, and characterization of the antibody directed against CARD9 can be found in one of our recent publications [34].
Western Blotting
Cell lysates (30-50 μg for INS-1 832/13 cells and 50 μg for mouse islets) were resolved by SDS-PAGE and transferred onto nitrocellulose membranes. Membranes were blocked in 3% BSA for one hour and probed overnight with appropriate primary antibodies. The membranes were then washed and then probed with the appropriate secondary antibody for 1 hr. The immune complexes were detected using ECL detection kit (ThermoScientific, Waltham, MA, USA). The band intensities were quantified using Image Studio Lite imaging software (Li- COR Biosciences, Lincoln, NE, USA).
Rac1 activation assay
Con-siRNA or CARD9-siRNA transfected INS-1 832/13 cells were exposed to either LG or HG for 24 hrs. and the magnitude of Rac1 activation was determined using a pull-down assay kit as we reported previously [18, 19, 34].
Co-immunoprecipitation assay
Immunoprecipitation was performed according to the manufacture’s protocol. Briefly, 500 μg of lysates from LG- or HG-treated INS-1 832/13 cells were incubated overnight with respective primary antibody as indicated in the text. Pre-cleared lysates were incubated (continuous agitation) with agarose beads for 3 hrs. at 4°C. After multiple washes with lysis buffer, the resulting immunoprecipitates were subjected to SDS-PAGE and western blotting to detect and quantify the abundance of proteins of interest in the immunoblots [36].
Statistical analysis
Data are presented as mean ± SEM of three-six independent experiments. Statistical analysis was done using the student t-test. A p-value of < 0.05 was considered statistically significant.
Results
We previously reported that exposure of INS-1 832/13 cells to HG (20mM; 24 hrs.) results in dysregulation of mitochondrial function (Caspase-3 activation) and nuclear abnormalities (nuclear lamin-B degradation) leading to impaired GSIS and beta cell demise [26, 37, 38]. We employed this experimental model in the following investigations to assess the regulatory roles of CARD9 in the induction of metabolic dysfunction under conditions of chronic hyperglycemic conditions.
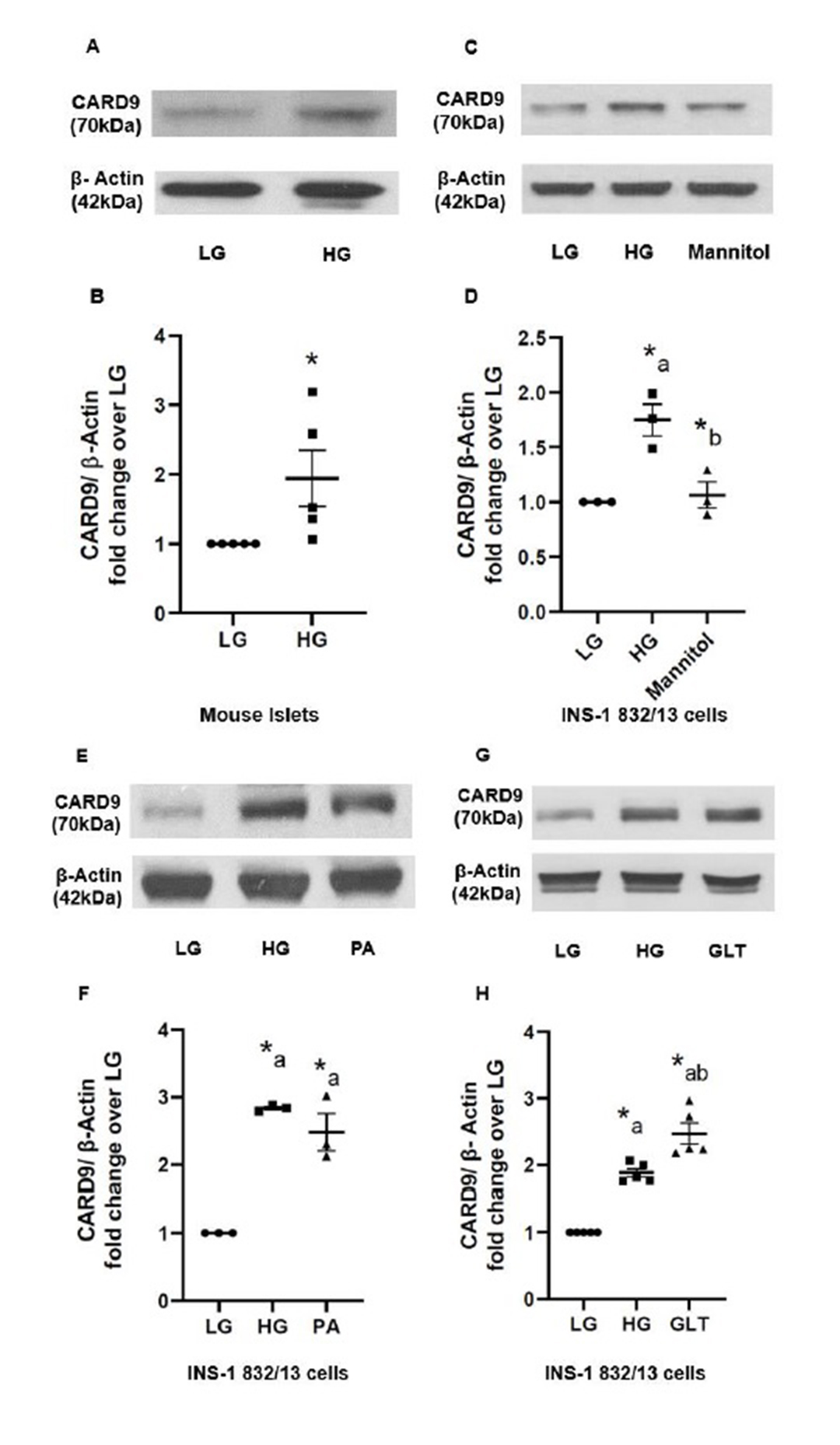
Diabetogenic stimuli increase the expression of CARD9 in insulin-secreting INS-1 832/13 cells and mouse islets
Overall objective of the studies described herein is to determine contributory roles of CARD9 in metabolic dysregulation of the islet beta cell exposed to metabolic stress conditions. To address this, at the outset, we determined the effects of a variety of diabetogenic stimuli on the expression of CARD9 in mouse islets and INS-1 832/13 cells. Data depicted in Fig. 1 (Panels A and B) indicate a significant increase (~2-fold) in the expression of CARD9 following exposure of mouse islets to chronic hyperglycemic conditions (20 mM; 24 hrs.). A significant increase in the expression of CARD9 (~1.7-fold) was also seen in INS-1 832/13 cells (Fig. 1; Panels C and D) following exposure to hyperglycemic conditions. Mannitol (20 mM; 24 hrs.), used as an osmotic control, elicited no effects on CARD9 expression suggesting that the effects of HG conditions are specific to glucose, not due to osmolality (Fig. 1; Panels C and D). In addition, exposure of INS-1 832/13 cells to palmitate (PA; 500 μM; 24 hrs.; lipotoxic conditions; LT), also increased (~2.5-fold) the expression of CARD9 (Fig. 1; Panels E and F). Lastly, exposure of these cells to a combination of glucose plus palmitate (to mimic gluco-lipotoxic conditions; GLT) exerted similar effects (~2.5-fold) on the expression of CARD9 in INS-1 832/13 cells (Fig. 1; Panels G and H). Interestingly however, we failed to see any significant effects on the expression of CARD9 in INS-1 832/13 cells following exposure to ceramide (50 µM; 24 hrs.) or IL-1β (25ng/ml; 24 hrs.; additional data not shown). Taken together, data represented in Fig. 1 suggest significant effects of HG, LT or GLT conditions on the expression of CARD9 in insulin-secreting cells.
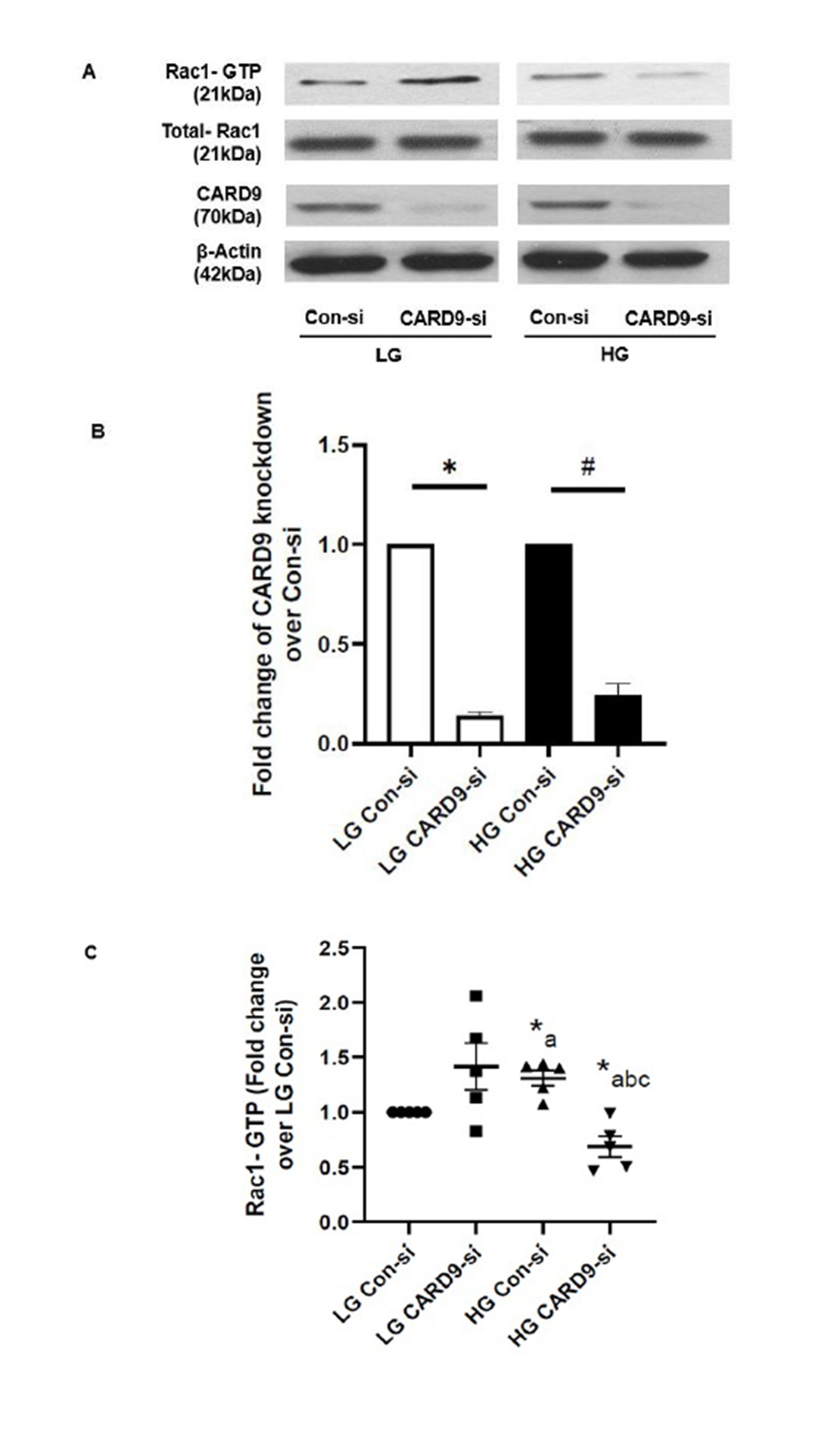
CARD9 is involved in the sustained activation of Rac1 in INS-1 832/13 cells exposed to chronic hyperglycemic conditions
Several recent investigations in pancreatic beta cells and other cell types have shown that chronic metabolic stress promotes activation of Rac1, a small G protein belonging to Rho subfamily of G Proteins, leading to activation of phagocyte-like NADPH oxidases (Nox2) and stress kinases (e.g., p38 MAPK). Therefore, in the current set of studies, we determined putative roles of CARD9 in HG-induced activation of Rac1 in INS-1 832/13 cells. As shown in Fig. 2 (Panels A and C), siRNA-mediated knockdown of CARD9 abolished HG-induced Rac1 activation. Interestingly, knockdown of CARD9 promoted activation of Rac1 under LG conditions (i.e., inappropriate activation under basal conditions). Data in Fig. 2 (Panel B) indicate ~87% knockdown of CARD9 in cells exposed to LG conditions using our transfection protocol. Under these conditions, we noticed nearly 76% knockdown of CARD9 expression in INS-1 832/13 cells exposed to HG conditions. Together, data in Fig. 2 suggest key regulatory roles for CARD9 in the sustained activation of Rac1 in pancreatic beta cells under the duress of chronic metabolic stress conditions.
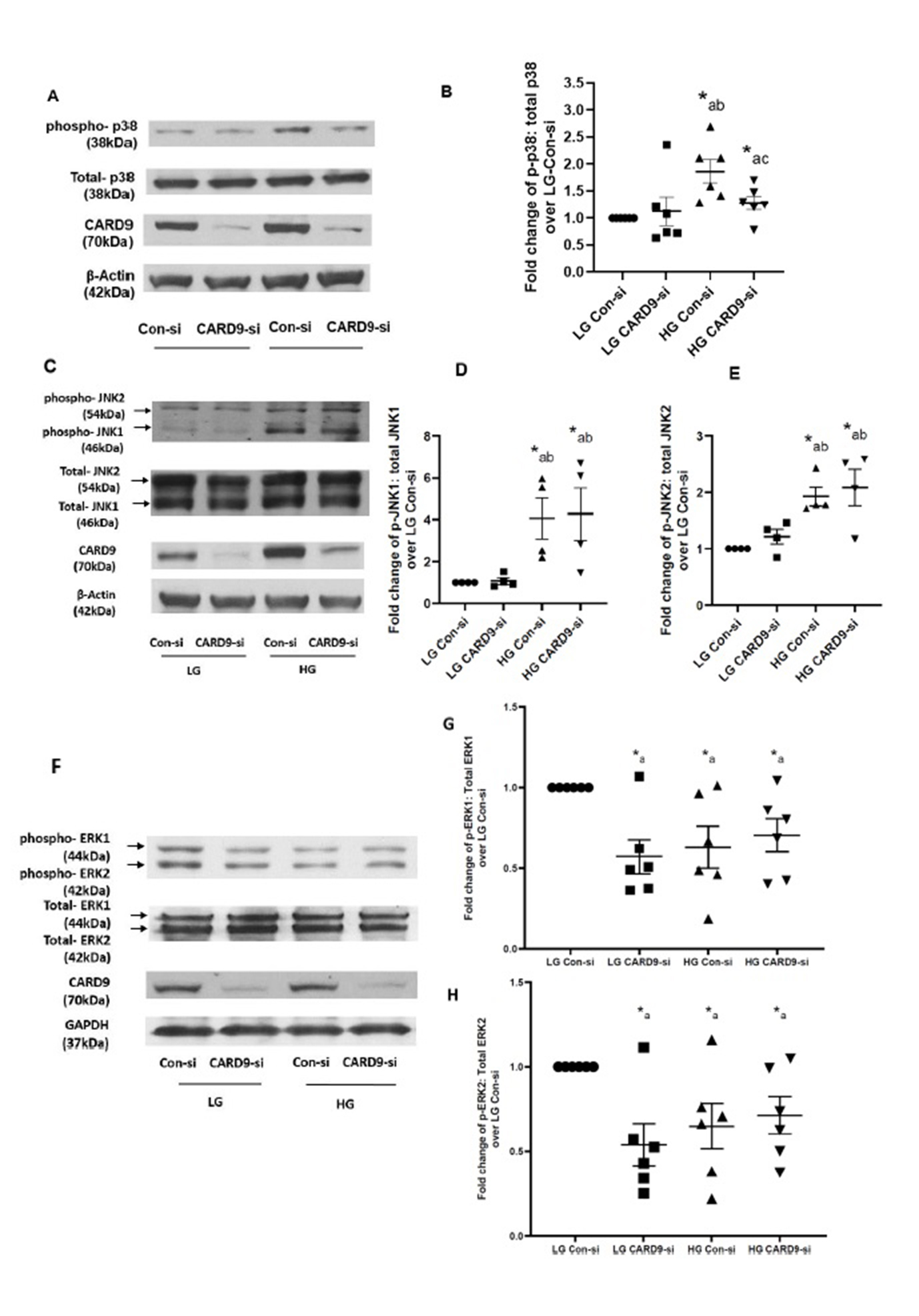
CARD9 promotes p38MAPK activation under chronic hyperglycemic conditions without significantly affecting glucose-induced regulatory effects on JNK1/2 and ERK1/2 in pancreatic beta cells
As indicated above, earlier studies have suggested that Rac1 activation represents an upstream signaling event for the activation of stress kinases, such as p38 MAPK, in pancreatic beta cells exposed to metabolic stress conditions [17, 18, 26]. Therefore, in the next set of studies, we investigated contributory roles of CARD9-Rac1 signaling axis in p38MAPK activation in INS-1 832/13 cells incubated under HG conditions. Data shown in Fig. 3 (Panel A) demonstrate significant inhibitory effects of HG-induced activation of p38MAPK in CARD9-depleted INS-1 832/13 cells. Pooled data from multiple studies are provided in Fig. 3 (Panel B). It is noteworthy that HG-induced activation of JNK1/2 appear to be resistant to CARD9 knockdown under our current experimental conditions (Fig. 3; Panels C, D and E). Lastly, no significant effects of CARD9 deletion on ERK1/2 phosphorylation were demonstrable under LG and HG exposure conditions (Fig. 3; Panels F, G and H). Taken together, data accrued from studies highlighted in Fig. 3 suggest key roles for CARD9-Rac1 signaling module in HG-induced activation of p38MAPK in pancreatic beta cells.
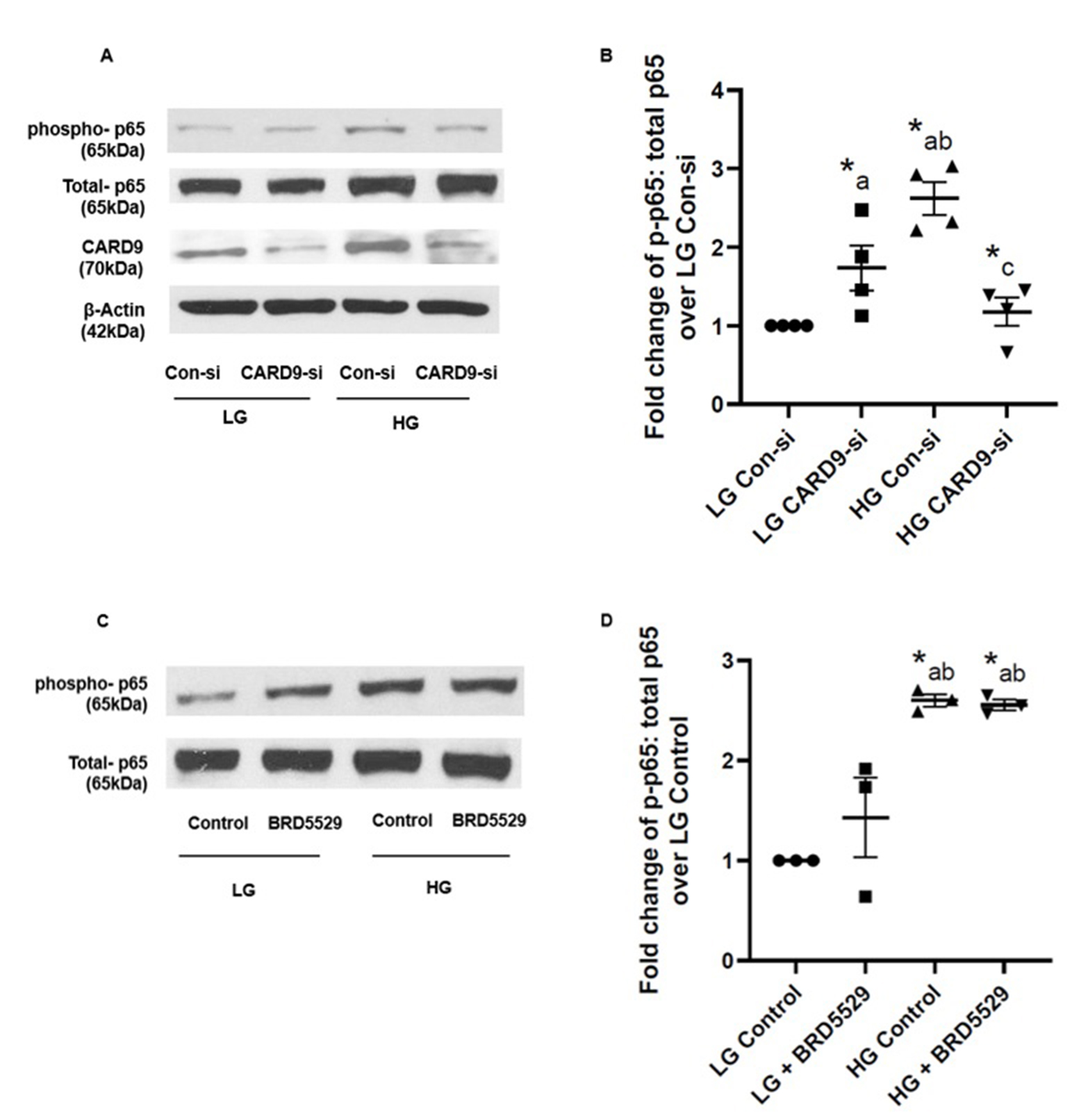
CARD9-TRIM62 module may not be involved in p38MAPK activation in INS-1 832/13 cells under chronic hyperglycemic conditions
Earlier studies by Leshchiner et al. have indicated BRD5529 as a selective inhibitor of CARD9 activation [39]. Mechanistically, BRD5529 binds to CARD9, thereby preventing cross-talk/interaction between CARD9 and TRIM62, a known E3 ubiquitin ligase for CARD9, resulting in functional inactivation of CARD9. Therefore, we examined the effects of BRD5529 (50 μM; 24 hrs.) on p38MAPK activation in INS-1 832/13 cells following exposure to high glucose conditions. Data accrued in these studies have indicated no clear effects of BRD5529 on high glucose-induced activation of p38MAPK. Ratios of phospho-p38MAPK to total-p38MAPK in high glucose exposed cells were 1.534 ± 0.16 and 1.53 ± 0.27 in the absence and presence of BRD5529, respectively (n=8 experiments; p= not significant). Based on these data, we conclude that CARD9-TRIM62 signaling pathway may not be involved in high glucose-induced p38MAPK activation under conditions of the current study.
CARD9 is necessary for increased phosphorylation of the p65 NF-κB (RelA) subunit in INS-1 832/13 cells exposed to hyperglycemic conditions
Several lines of evidence have affirmed regulatory roles of CARD proteins in the induction of cellular dysfunction and apoptosis via modulation of NF-κB activity [40]. Furthermore, CARD9 has been implicated in promoting inflammation via activation of MAPK and NF-κB signaling modules in innate immune cells, which, in turn, promotes multiple metabolic diseases, including obesity and insulin resistance [41]. Therefore, we next asked if CARD9 contributes to increased phosphorylation of p65 subunit under conditions of exposure to high glucose conditions. Data shown in Fig. 4 (Panel A) demonstrate increased phosphorylation of p65 under high glucose conditions, which was markedly attenuated in INS-1 832/13 cells following siRNA-mediated knockdown of CARD9. Pooled data from multiple experiments are provided in Fig. 4 (Panel B). Together, data presented in Fig. 3 and 4 suggest that CARD9 plays important regulatory roles in high glucose-induced p38MAPK and NF-κB signaling modules. Furthermore, as in the case of p38MAPK (above), BRD5529, a known inhibitor of CARD9-TRIM62 signaling step, exerted no significant effects on high glucose-induced p65 phosphorylation (Fig. 4; Panels C and D). Taken together, our observations suggest minimal regulatory roles of CARD9-TRIM62 module in the activation of p38MAPK-NF-κB signaling pathways in INS-1 832/13 cells exposed to hyperglycemic conditions.
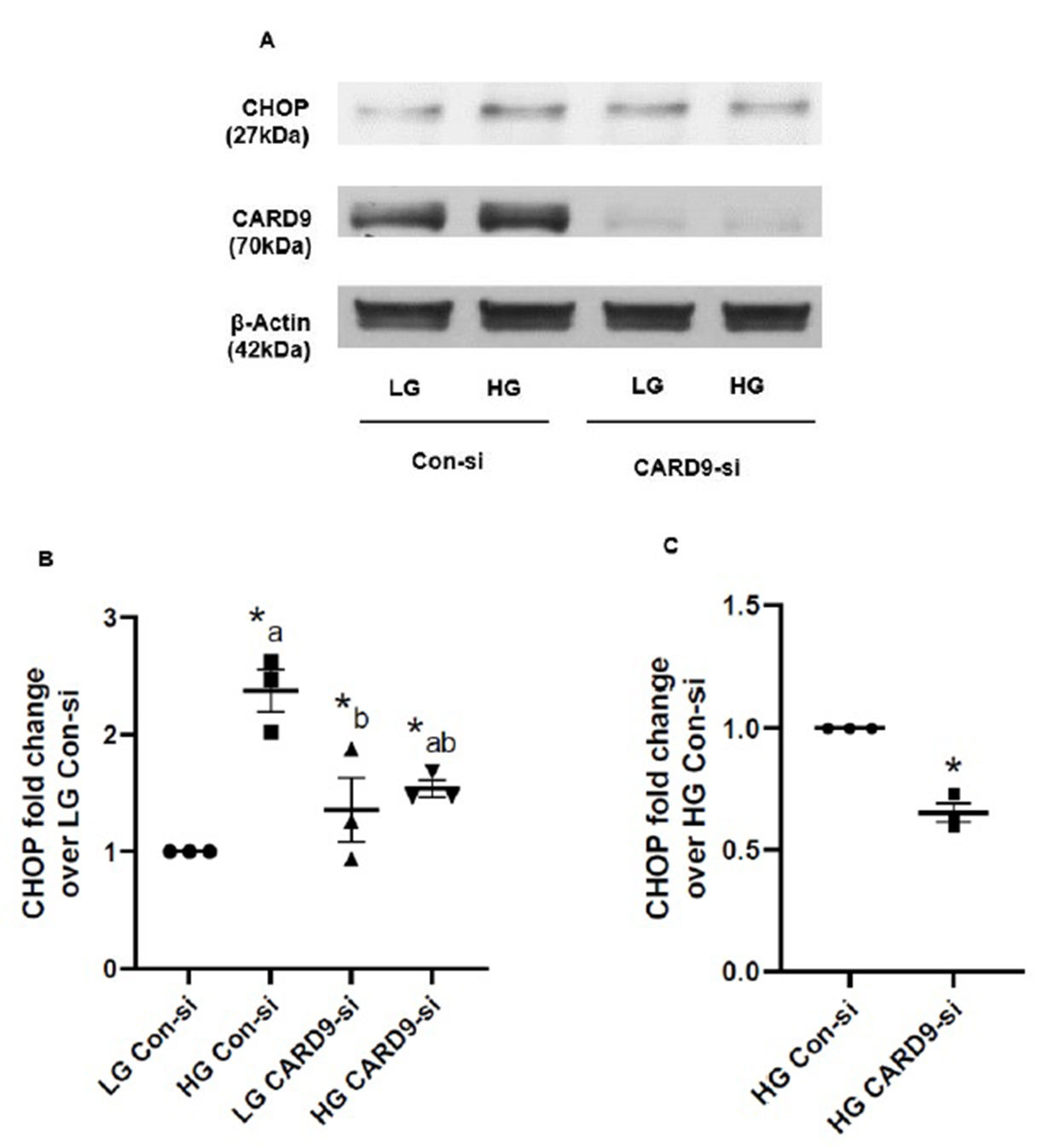
CARD9 promotes ER stress (i.e., CHOP expression) under chronic hyperglycemic conditions in INS-1 832/13 cells
Based on the existing evidence that HG exposure conditions induce endoplasmic reticulum stress (ER stress) resulting in mitochondrial and nuclear dysregulation in the pancreatic beta cell [37, 38, 42, 43], we next investigated if CARD9 contributes to ER stress response in INS-1 832/13 cells exposed to HG conditions. Western blot data shown in Fig. 5 (Panel A) indicate a significant increase in CHOP (a marker for ER stress) expression in these cells under the duress of HG conditions. siRNA-mediated knockdown of CARD9 significantly attenuated HG-induced CHOP expression. Pooled data from multiple experiments are included in Fig. 5 (Panel B). Data in Fig. 5 (Panel C) demonstrate ~40% inhibition of HG-induced CHOP expression following CARD9 knockdown in these cells under our current experimental conditions. Altogether, data depicted in Fig. 3 and 4 implicate key roles for CARD9 in HG-induced stress kinase (p38MAPK) and ER stress signaling pathways.
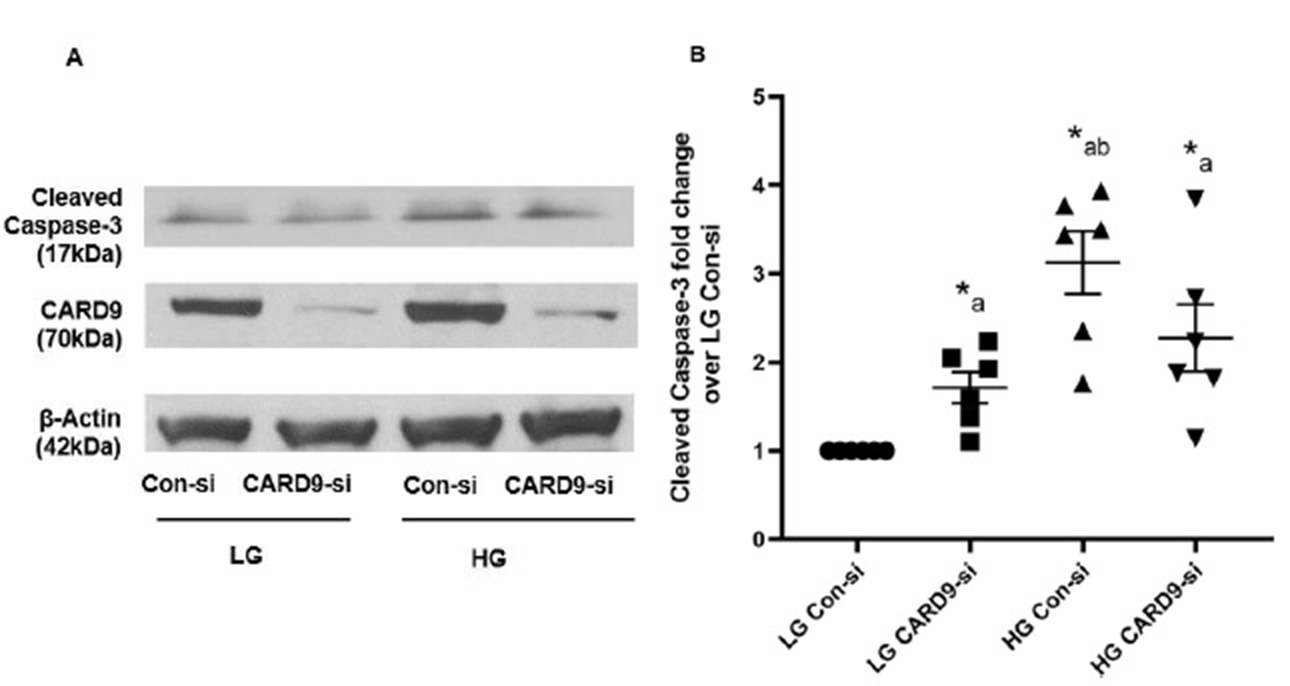
Lack of clear regulatory roles of CARD9 in high glucose-induced activation of Caspase-3 in INS-1 832/13 cells
Next set of experiments were aimed at determining if CARD9-mediated stress kinase activation and/or induction of ER stress contributes to Caspase-3 activation in pancreatic beta cells exposed to HG. Data highlighted in Fig. 6 (Panels A and B), as expected, suggest a significant increase in Caspase-3 activation in Control-siRNA transfected cells incubated under HG conditions. Interestingly however, knockdown of CARD9 appears to have modest effects on HG-induced activation of Caspase-3. Even though there appears to be directional evidence for modest suppression of Caspase-3 activation in CARD9-siRNA treated cells under HG conditions, they did not achieve statistical significance compared to Control-siRNA transfected cells under those conditions. Based on these data we conclude that CARD9 contributes to HG-induced Rac1 activation and associated oxidative and ER stress in pancreatic beta cells. However, its role in HG-mediated Caspase-3 activation needs further examination. The following experiments were conducted to gain additional mechanistic insights in CARD9-mediated, HG-induced metabolic dysregulation of the islet beta cell.
Co-IP studies suggest alterations in the interaction between CARD9, RhoGDIβ and Rac1 in INS-1 832/13 cells exposed to chronic hyperglycemic conditions
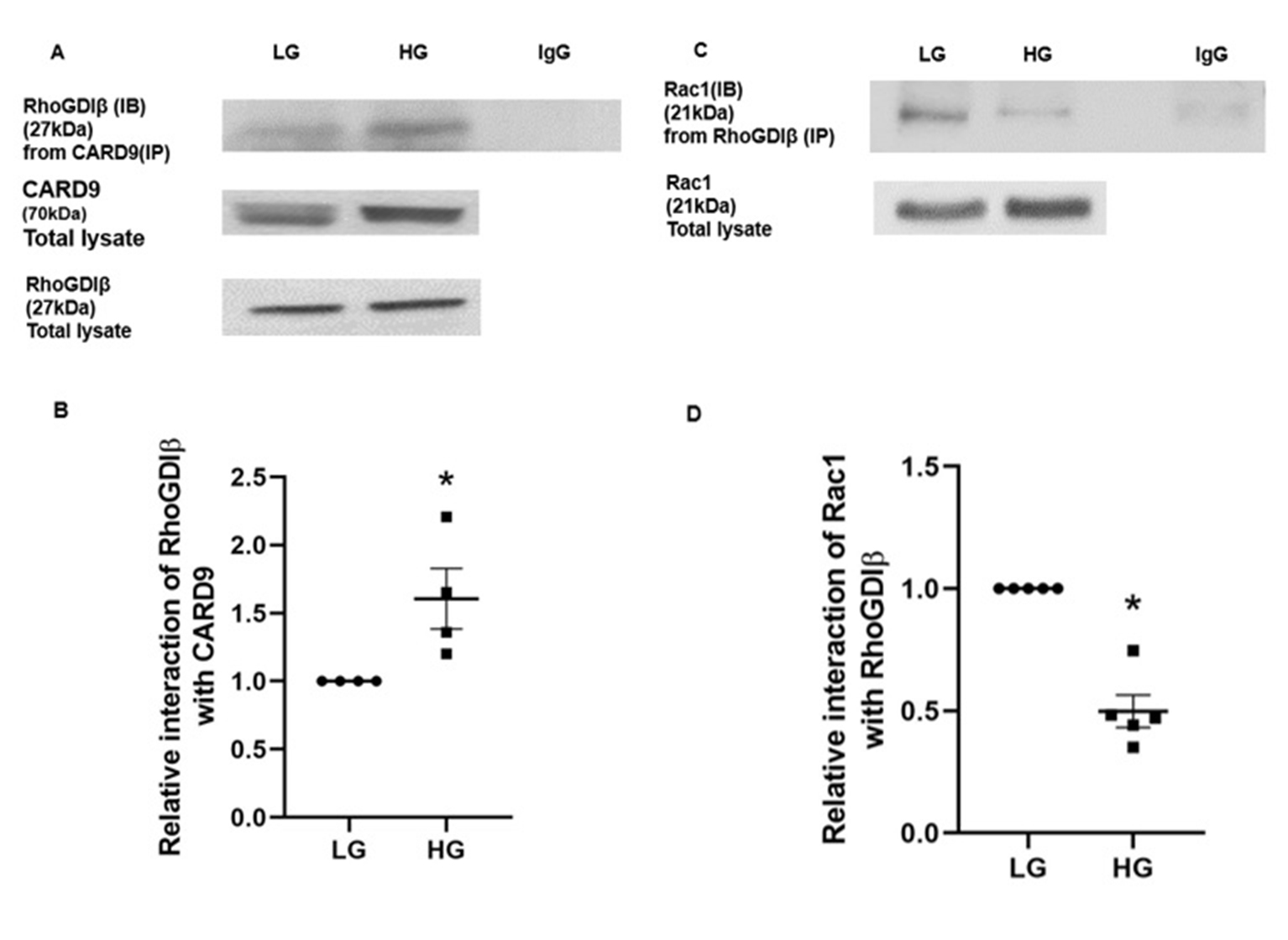
It is well established that activation-deactivation cycles of small G proteins (e.g., Rac1) is mediated by concerted regulatory roles of GTP/GDP exchange factors (GEFs), GDP dissociation inhibitors (GDIs), and GTPase activating proteins (GAPs). Several of these regulatory factors have been identified and characterized in relation to their roles in physiological insulin secretion and metabolic dysfunction of the islet beta cell [21, 28]. In the context of CARD9-mediated regulation of Rac1 functions, a recent report by Wu and coworkers have identified RhoGDIβ-Rac1 complex as one of the target sites for CARD9-mediated effects in microbial killing in macrophages [31]. They provided compelling evidence to suggest that CARD9 promotes sustained activation of Rac1 via its binding (i.e., complexation) with RhoGDIβ thereby dissociating the RhoGDβI-Rac1 complex. Such a signaling step was shown to promote activation of Rac1 leading to activation of downstream signaling steps including generation of reactive oxygen species [31]. We recently reported expression of RhoGDIβ in human islets, rodent islets and INS-1 832/13 cells and demonstrated key regulatory roles for glucose-induced activation of Rac1, but not insulin secretion [35, 44]. Based on our evidence for RhoGDIβ’s role in Rac1 functional regulation [44], and the data from studies by Wu et al. [31], we aimed at understanding potential interactions (alterations in these signaling steps, if any) between CARD9, RhoGDIβ and Rac1 in INS-1 832/13 cells incubated with LG and HG. We employed Co-IP approach to decipher those interactions.
Data shown in Fig. 7 (Panel A) demonstrate increased interaction between CARD9 and RhoGDIβ in INS-1 832/13 cells under HG conditions. Data from 4 independent experiments are shown in Fig. 7 (Panel B). Furthermore, under the same experimental conditions, we noticed a marked reduction in the interaction between RhoGDIβ and Rac1 in cells exposed to HG conditions. These data provide the first experimental evidence to indicate that HG conditions promote a significant increase in the interaction between CARD9 and RhoGDIβ, resulting in dissociation of RhoGDIβ-Rac1 complex to enable subsequent activation of Rac1 by candidate GEF proteins.
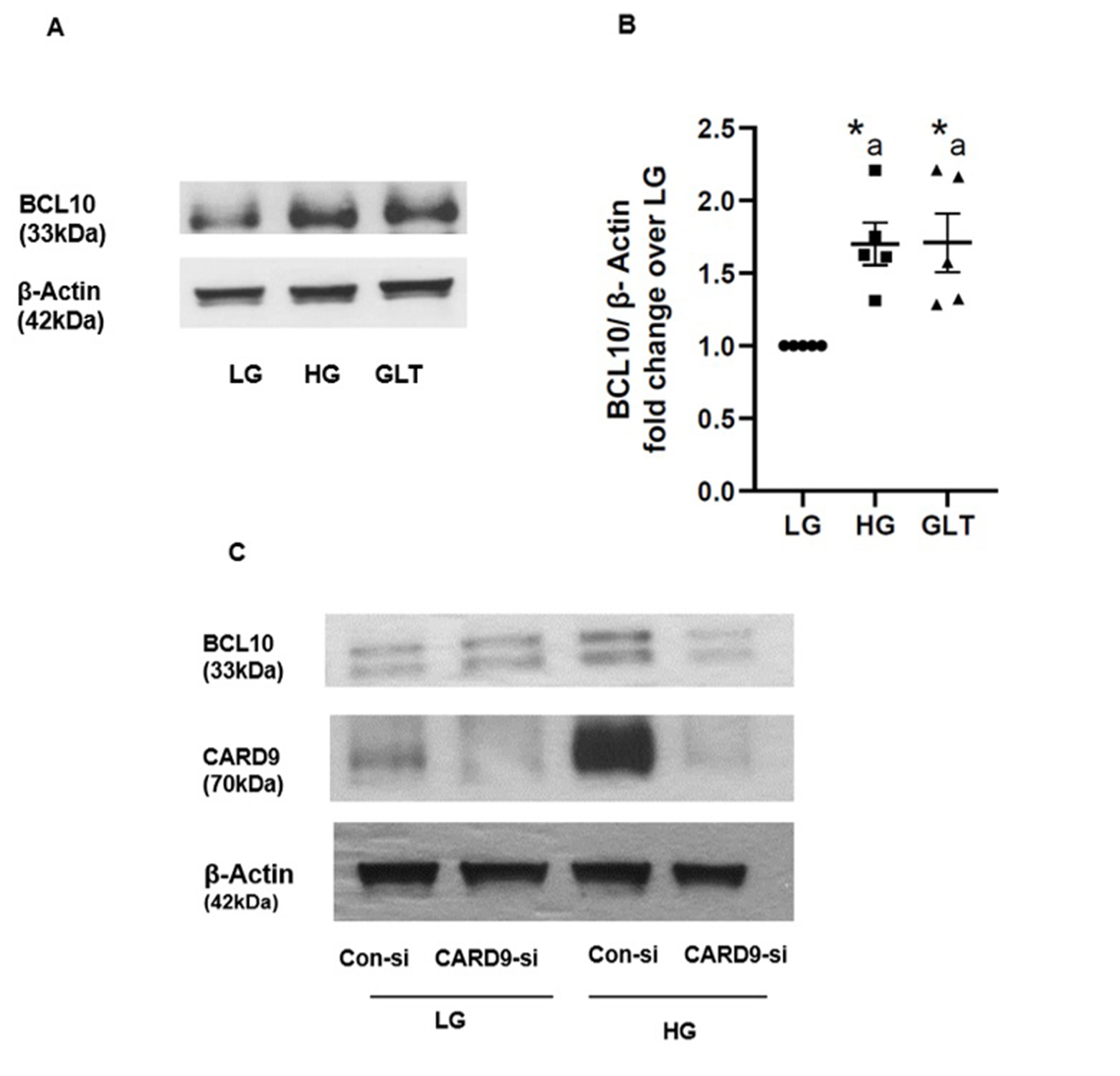
Evidence to implicate CARD9 in the increased expression of BCL 10 in pancreatic beta cells exposed to chronic hyperglycemic conditions
Several lines of evidence provide strong support for involvement of specific CARD9 associated proteins (CARD9 signalome) for innate antifungal immunity [32, 33, 45]. For example, studies have shown that signaling complexes, comprising of CARD9-BCL10-Malt 1, play critical roles in the activation of NF-κB signaling pathway in C-type lectin receptor (CLR) induced inflammatory responses [46]. Published evidence also suggests strong connection between over expression of CARD9 in the activation of p38 MAPK during inflammation [47, 48]. Lastly, studies by Wang and coworkers have demonstrated that obesity promotes activation of p38MAPK through the upregulation of CARD9-BCL10 complex [49]. As a logical extension to our current experimental findings supporting CARD9-mediated regulation of p38MAPK in pancreatic beta cells under HG conditions, we quantified BCL10 expression levels in INS-1 832/13 cells following exposure to HG or GLT conditions. Data depicted in Fig. 8, ~ 1.75-fold increase in the expression of BCL10 was seen under HG or GLT conditions (Panels A and B). Lastly, in two independent experiments, we observed inhibition (43-77%) of HG-induced expression of BCL10 following siRNA-mediated knockdown of CARD9. A representative blot of the two studies is provided in Panel C. Taken together, these findings support a close relationship between CARD9 and BCL10 in HG-mediated effects on islet beta cell dysregulation (see below).
The authors thank Dr. Vijayalakshmi Thamilselvan for assistance in the initial stages of this project. This research work is conducted by SG for the partial fulfillment of requirements for a PhD degree in Pharmaceutical Sciences at Wayne State University.
Portions of the findings in this article were presented in the Annual Meeting of Experimental Biology (April 2021) and Annual Meetings of Cell Biology and EMBO December 2021).
Author Contributions
Suhadinie Gamage: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization. Mirabela Hali: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization. Fei Chen: Scientific input, Writing-review and editing. Anjaneyulu Kowluru: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Vali-dation, Visualization, Writing-original draft, Writing-review and editing.
Funding
These studies are supported (to AK) by Merit Review (I01 BX004663) and Senior Research Career Scientist (IK6 BX005383) awards from the US Department of VA, and an R01 grant from the NIH/NEI (EY022230). AK also thanks Wayne State University for the Distinguished Professorship award. FC is supported by grants from the NIH R01 ES031822.
Statement of Ethics
All protocols involving animal care and use were reviewed and approved by Wayne State University and John D. Dingell VA Medical Center Institutional Animal Care and Use Committees. Studies involving human islets were approved by the Biosafety Committee at the John D. Dingell VA Medical Center.
The authors declare that no conflicts of interest exist.
| 1 Elumalai S, Karunakaran U, Moon JS, Won KC: NADPH Oxidase (NOX) Targeting in Diabetes: A Special Emphasis on Pancreatic β-Cell Dysfunction. Cells 2021;10:1573. https://doi.org/10.3390/cells10071573 |
||||
| 2 Poitout V, Robertson RP: Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008;29:351-366. https://doi.org/10.1210/er.2007-0023 |
||||
| 3 Prentki M, Peyot ML, Masiello P, Madiraju SRM: Nutrient-Induced Metabolic Stress, Adaptation, Detoxification, and Toxicity in the Pancreatic β-Cell. Diabetes 2020;69:279-290. https://doi.org/10.2337/dbi19-0014 |
||||
| 4 Weir GC: Glucolipotoxicity, β-Cells, and Diabetes: The Emperor Has No Clothes. Diabetes 2020;69:273-278. https://doi.org/10.2337/db19-0138 |
||||
| 5 Lytrivi M, Castell AL, Poitout V, Cnop M: Recent Insights Into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes. J Mol Biol 2020;432:1514-1534. https://doi.org/10.1016/j.jmb.2019.09.016 |
||||
| 6 Mukherjee N, Lin L, Contreras CJ, Templin AT: β-Cell Death in Diabetes: Past Discoveries, Present Understanding, and Potential Future Advances. Metabolites 2021;11:796. https://doi.org/10.3390/metabo11110796 |
||||
| 7 Christensen AA, Gannon M: The Beta Cell in Type 2 Diabetes. Curr Diab Rep 2019;19:81. https://doi.org/10.1007/s11892-019-1196-4 |
||||
| 8 Hasnain SZ, Prins JB, McGuckin MA: Oxidative and endoplasmic reticulum stress in β-cell dysfunction in diabetes. J Mol Endocrinol 2016;56:R33-54. https://doi.org/10.1530/JME-15-0232 |
||||
| 9 Fonseca SG, Urano F, Burcin M, Gromada J: Stress hypERactivation in the β-cell. Islets 2010;2:1-9. https://doi.org/10.4161/isl.2.1.10456 |
||||
| 10 Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, Powers AC, Rhodes CJ, Sussel L, Weir GC: β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 2014;37:1751-1758. https://doi.org/10.2337/dc14-0396 |
||||
| 11 Kowluru A, Kowluru RA: Phagocyte-like NADPH oxidase [Nox2] in cellular dysfunction in models of glucolipotoxicity and diabetes. Biochem Pharmacol 2014;88:275-283. https://doi.org/10.1016/j.bcp.2014.01.017 |
||||
| 12 Kowluru A: Oxidative Stress in Cytokine-Induced Dysfunction of the Pancreatic Beta Cell: Known Knowns and Known Unknowns. Metabolites 2020;10:480. https://doi.org/10.3390/metabo10120480 |
||||
| 13 Newsholme P, Morgan D, Rebelato E, Oliveira-Emilio HC, Procopio J, Curi R, Carpinelli A: Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia 2009;52:2489-2498. https://doi.org/10.1007/s00125-009-1536-z |
||||
| 14 Morgan D, Oliveira-Emilio HR, Keane D, Hirata AE, Santos da Rocha M, Bordin S, Curi R, Newsholme P, Carpinelli AR: Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia 2007;50:359-369. https://doi.org/10.1007/s00125-006-0462-6 |
||||
| 15 Vilas-Boas EA, Nalbach L, Ampofo E, Lucena CF, Naudet L, Ortis F, Carpinelli AR, Morgan B, Roma LP: Transient NADPH oxidase 2-dependent H(2)O(2) production drives early palmitate-induced lipotoxicity in pancreatic islets. Free Radic Biol Med 2021;162:1-13. https://doi.org/10.1016/j.freeradbiomed.2020.11.023 |
||||
| 16 Vilas-Boas EA, Carlein C, Nalbach L, Almeida DC, Ampofo E, Carpinelli AR, Roma LP, Ortis F: Early Cytokine-Induced Transient NOX2 Activity Is ER Stress-Dependent and Impacts β-Cell Function and Survival. Antioxidants 2021;10:1305. https://doi.org/10.3390/antiox10081305 |
||||
| 17 Sidarala V, Veluthakal R, Syeda K, Vlaar C, Newsholme P, Kowluru A: Phagocyte-like NADPH oxidase (Nox2) promotes activation of p38MAPK in pancreatic β-cells under glucotoxic conditions: Evidence for a requisite role of Ras-related C3 botulinum toxin substrate 1 (Rac1). Biochem Pharmacol 2015;95:301-310. https://doi.org/10.1016/j.bcp.2015.04.001 |
||||
| 18 Syed I, Kyathanahalli CN, Jayaram B, Govind S, Rhodes CJ, Kowluru RA, Kowluru A: Increased phagocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: role of Rac1-JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet. Diabetes 2011;60:2843-2852. https://doi.org/10.2337/db11-0809 |
||||
| 19 Kowluru A: Friendly, and not so friendly, roles of Rac1 in islet β-cell function: lessons learnt from pharmacological and molecular biological approaches. Biochem Pharmacol 2011;81:965-975. https://doi.org/10.1016/j.bcp.2011.01.013 |
||||
| 20 Kowluru A: Roles of GTP and Rho GTPases in pancreatic islet beta cell function and dysfunction. Small GTPases 2020:1-13. https://doi.org/10.1080/21541248.2020.1815508 |
||||
| 21 Kowluru A: GPCRs, G Proteins, and Their Impact on β-cell Function. Compr Physiol 2020;10:453-490. https://doi.org/10.1002/cphy.c190028 |
||||
| 22 Kowluru A, Kowluru RA: RACking up ceramide-induced islet beta-cell dysfunction. Biochem Pharmacol 2018;154:161-169. https://doi.org/10.1016/j.bcp.2018.04.026 |
||||
| 23 Syed I, Jayaram B, Subasinghe W, Kowluru A: Tiam1/Rac1 signaling pathway mediates palmitate-induced, ceramide-sensitive generation of superoxides and lipid peroxides and the loss of mitochondrial membrane potential in pancreatic beta-cells. Biochem Pharmacol 2010;80:874-883. https://doi.org/10.1016/j.bcp.2010.05.006 |
||||
| 24 Subasinghe W, Syed I, Kowluru A: Phagocyte-like NADPH oxidase promotes cytokine-induced mitochondrial dysfunction in pancreatic β-cells: evidence for regulation by Rac1. Am J Physiol Regul Integr Comp Physiol 2011;300:R12-20. https://doi.org/10.1152/ajpregu.00421.2010 |
||||
| 25 Gendaszewska-Darmach E, Garstka MA, Błażewska KM: Targeting Small GTPases and Their Prenylation in Diabetes Mellitus. J Med Chem 2021;64:9677-9710. https://doi.org/10.1021/acs.jmedchem.1c00410 |
||||
| 26 Sidarala V, Kowluru A: Exposure to chronic hyperglycemic conditions results in Ras-related C3 botulinum toxin substrate 1 (Rac1)-mediated activation of p53 and ATM kinase in pancreatic beta-cells. Apoptosis 2017;22:597-607. https://doi.org/10.1007/s10495-017-1354-6 |
||||
| 27 Kowluru A: Multiple Guanine Nucleotide Exchange Factors Mediate Glucose-Induced Rac1 Activation and Insulin Secretion: Is It Precise Regulatory Control or a Case of Two Peas from the Same Pod? ACS Pharmacol Transl Sci 2021;4:1702-1704. https://doi.org/10.1021/acsptsci.1c00190 |
||||
| 28 Veluthakal R, Thurmond DC: Emerging Roles of Small GTPases in Islet β-Cell Function. Cells 2021;10:1503. https://doi.org/10.3390/cells10061503 |
||||
| 29 Wang Z, Thurmond DC: Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci 2009;122:893-903. https://doi.org/10.1242/jcs.034355 |
||||
| 30 Jia XM, Tang B, Zhu LL, Liu YH, Zhao XQ, Gorjestani S, Hsu YM, Yang L, Guan JH, Xu GT, Lin X: CARD9 mediates Dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity. J Exp Med 2014;211:2307-2321. https://doi.org/10.1084/jem.20132349 |
||||
| 31 Wu W, Hsu YM, Bi L, Songyang Z, Lin X: CARD9 facilitates microbe-elicited production of reactive oxygen species by regulating the LyGDI-Rac1 complex. Nat Immunol 2009;10:1208-1214. https://doi.org/10.1038/ni.1788 |
||||
| 32 Ruland J: CARD9 signaling in the innate immune response. Ann N Y Acad Sci 2008;1143:35-44. https://doi.org/10.1196/annals.1443.024 |
||||
| 33 Vornholz L, Ruland J: Physiological and Pathological Functions of CARD9 Signaling in the Innate Immune System. Curr Top Microbiol Immunol 2020;429:177-203. https://doi.org/10.1007/82_2020_211 |
||||
| 34 Gamage S, Hali M, Kowluru A: CARD9 mediates glucose-stimulated insulin secretion in pancreatic beta cells. Biochem Pharmacol 2021;192:114670. https://doi.org/10.1016/j.bcp.2021.114670 |
||||
| 35 Thamilselvan V, Kowluru A: Paradoxical regulation of glucose-induced Rac1 activation and insulin secretion by RhoGDIβ in pancreatic β-cells. Small GTPases 2021;12:114-121. https://doi.org/10.1080/21541248.2019.1635403 |
||||
| 36 Damacharla D, Thamilselvan V, Zhang X, Mestareehi A, Yi Z, Kowluru A: Quantitative proteomics reveals novel interaction partners of Rac1 in pancreatic beta cells: Evidence for increased interaction with Rac1 under hyperglycemic conditions. Mol Cell Endocrinol 2019;494:110489. https://doi.org/10.1016/j.mce.2019.110489 |
||||
| 37 Syeda K, Mohammed AM, Arora DK, Kowluru A: Glucotoxic conditions induce endoplasmic reticulum stress to cause caspase 3 mediated lamin B degradation in pancreatic β-cells: protection by nifedipine. Biochem Pharmacol 2013;86:1338-1346. https://doi.org/10.1016/j.bcp.2013.08.023 |
||||
| 38 Khadija S, Veluthakal R, Sidarala V, Kowluru A: Glucotoxic and diabetic conditions induce caspase 6-mediated degradation of nuclear lamin A in human islets, rodent islets and INS-1 832/13 cells. Apoptosis 2014;19:1691-1701. https://doi.org/10.1007/s10495-014-1038-4 |
||||
| 39 Leshchiner ES, Rush JS, Durney MA, Cao Z, Dančík V, Chittick B, Wu H, Petrone A, Bittker JA, Phillips A, Perez JR, Shamji AF, Kaushik VK, Daly MJ, Graham DB, Schreiber SL, Xavier RJ: Small-molecule inhibitors directly target CARD9 and mimic its protective variant in inflammatory bowel disease. Proc Natl Acad Sci U S A 2017;114:11392-11397. https://doi.org/10.1073/pnas.1705748114 |
||||
| 40 Jiang C, Lin X: Regulation of NF-κB by the CARD proteins. Immunol Rev 2012;246:141-153. https://doi.org/10.1111/j.1600-065X.2012.01110.x |
||||
| 41 Tian C, Tuo YL, Lu Y, Xu CR, Xiang M: The Role of CARD9 in Metabolic Diseases. Curr Med Sci 2020;40:199-205. https://doi.org/10.1007/s11596-020-2166-4 |
||||
| 42 Veluthakal R, Arora DK, Goalstone ML, Kowluru RA, Kowluru A: Metabolic Stress Induces Caspase-3 Mediated Degradation and Inactivation of Farnesyl and Geranylgeranyl Transferase Activities in Pancreatic β-Cells. Cell Physiol Biochem 2016;39:2110-2120. https://doi.org/10.1159/000447907 |
||||
| 43 Dingreville F, Panthu B, Thivolet C, Ducreux S, Gouriou Y, Pesenti S, Chauvin MA, Chikh K, Errazuriz-Cerda E, Van Coppenolle F, Rieusset J, Madec AM: Differential Effect of Glucose on ER-Mitochondria Ca(2+) Exchange Participates in Insulin Secretion and Glucotoxicity-Mediated Dysfunction of β-Cells. Diabetes 2019;68:1778-1794. https://doi.org/10.2337/db18-1112 |
||||
| 44 Kowluru A, Gleason NF: Underappreciated roles for RhoGDP dissociation inhibitors (RhoGDIs) in cell function: Lessons learned from pancreatic islet beta cell. Biochem Pharmacol 2022;197:114886. https://doi.org/10.1016/j.bcp.2021.114886 |
||||
| 45 Roth S, Bergmann H, Jaeger M, Yeroslaviz A, Neumann K, Koenig PA, Prazeres da Costa C, Vanes L, Kumar V, Johnson M, Menacho-Márquez M, Habermann B, Tybulewicz VL, Netea M, Bustelo XR, Ruland J: Vav Proteins Are Key Regulators of Card9 Signaling for Innate Antifungal Immunity. Cell Rep 2016;17:2572-2583. https://doi.org/10.1016/j.celrep.2016.11.018 |
||||
| 46 Zhao XQ, Zhu LL, Chang Q, Jiang C, You Y, Luo T, Jia XM, Lin X: C-type lectin receptor dectin-3 mediates trehalose 6,6'-dimycolate (TDM)-induced Mincle expression through CARD9/Bcl10/MALT1-dependent nuclear factor (NF)-κB activation. J Biol Chem 2014;289:30052-30062. https://doi.org/10.1074/jbc.M114.588574 |
||||
| 47 Zeng X, Du X, Zhang J, Jiang S, Liu J, Xie Y, Shan W, He G, Sun Q, Zhao J: The essential function of CARD9 in diet-induced inflammation and metabolic disorders in mice. J Cell Mol Med 2018;22:2993-3004. https://doi.org/10.1111/jcmm.13494 |
||||
| 48 Wang J, Tian J, He YH, Yang ZW, Wang L, Lai YX, Xu P: Role of CARD9 in inflammatory signal pathway of peritoneal macrophages in severe acute pancreatitis. J Cell Mol Med 2020;24:9774-9785. https://doi.org/10.1111/jcmm.15559 |
||||
| 49 Wang S, Gu J, Xu Z, Zhang Z, Bai T, Xu J, Cai J, Barnes G, Liu QJ, Freedman JH, Wang Y, Liu Q, Zheng Y, Cai L: Zinc rescues obesity-induced cardiac hypertrophy via stimulating metallothionein to suppress oxidative stress-activated BCL10/CARD9/p38 MAPK pathway. J Cell Mol Med 2017;21:1182-1192. https://doi.org/10.1111/jcmm.13050 |
||||
| 50 Peterson MR, Haller SE, Ren J, Nair S, He G: CARD9 as a potential target in cardiovascular disease. Drug Des Devel Ther 2016;10:3799-3804. https://doi.org/10.2147/DDDT.S122508 |
||||
| 51 Cao L, Qin X, Peterson MR, Haller SE, Wilson KA, Hu N, Lin X, Nair S, Ren J, He G: CARD9 knockout ameliorates myocardial dysfunction associated with high fat diet-induced obesity. J Mol Cell Cardiol 2016;92:185-195. https://doi.org/10.1016/j.yjmcc.2016.02.014 |
||||
| 52 Qin X, Peterson MR, Haller SE, Cao L, Thomas DP, He G: Caspase recruitment domain-containing protein 9 (CARD9) knockout reduces regional ischemia/reperfusion injury through an attenuated inflammatory response. PLoS One 2018;13:e0199711. https://doi.org/10.1371/journal.pone.0199711 |
||||
| 53 Sidarala V, Kowluru A: The Regulatory Roles of Mitogen-Activated Protein Kinase (MAPK) Pathways in Health and Diabetes: Lessons Learned from the Pancreatic beta-Cell. Recent Pat Endocr Metab Immune Drug Discov 2017;10:76-84. https://doi.org/10.2174/1872214810666161020154905 |
||||
| 54 Dib K, Melander F, Axelsson L, Dagher MC, Aspenström P, Andersson T: Down-regulation of Rac activity during beta 2 integrin-mediated adhesion of human neutrophils. J Biol Chem 2003;278:24181-24188. https://doi.org/10.1074/jbc.M302300200 |
||||
| 55 Duan W, Xu Y, Dong Y, Cao L, Tong J, Zhou X: Ectopic expression of miR-34a enhances radiosensitivity of non-small cell lung cancer cells, partly by suppressing the LyGDI signaling pathway. J Radiat Res 2013;54:611-619. https://doi.org/10.1093/jrr/rrs136 |
||||
| 56 Zhang Y, Rivera Rosado LA, Moon SY, Zhang B: Silencing of D4-GDI inhibits growth and invasive behavior in MDA-MB-231 cells by activation of Rac-dependent p38 and JNK signaling. J Biol Chem 2009;284:12956-12965. https://doi.org/10.1074/jbc.M807845200 |
||||
| 57 Kowluru A, Gleason NF: Underappreciated roles for Rho GDP dissociation inhibitors (RhoGDIs) in cell function: Lessons learned from the pancreatic islet β-cell. Biochem Pharmacol 2021;197:114886. https://doi.org/10.1016/j.bcp.2021.114886 |
||||
| 58 Nevins AK, Thurmond DC: Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic beta-cells. J Biol Chem 2006;281:18961-18972. https://doi.org/10.1074/jbc.M603604200 |
||||
| 59 Veluthakal R, Chvyrkova I, Tannous M, McDonald P, Amin R, Hadden T, Thurmond DC, Quon MJ, Kowluru A: Essential role for membrane lipid rafts in interleukin-1beta-induced nitric oxide release from insulin-secreting cells: potential regulation by caveolin-1+. Diabetes 2005;54:2576-2585. https://doi.org/10.2337/diabetes.54.9.2576 |
||||
| 60 Kowluru A: Small G proteins in islet beta-cell function. Endocr Rev 2010;31:52-78. https://doi.org/10.1210/er.2009-0022 |
||||
| 61 Chundru SA: Novel Regulatory Roles of RhoG and IQGAPs in Pancreatic Islet Beta Cell Function, 2020. Wayne State University Theses. 784. URL: https://digitalcommons.wayne.edu/oa_theses/784. | ||||
| 62 Mercader JM, Puiggros M, Segrè AV, Planet E, Sorianello E, Sebastian D, Rodriguez-Cuenca S, Ribas V, Bonàs-Guarch S, Draghici S, Yang C, Mora S, Vidal-Puig A, Dupuis J, Florez JC, Zorzano A, Torrents D: Identification of novel type 2 diabetes candidate genes involved in the crosstalk between the mitochondrial and the insulin signaling systems. PLoS Genet 2012;8:e1003046. https://doi.org/10.1371/journal.pgen.1003046 |
||||
| 63 Akula MK, Ibrahim MX, Ivarsson EG, Khan OM, Kumar IT, Erlandsson M, Karlsson C, Xu X, Brisslert M, Brakebusch C, Wang D, Bokarewa M, Sayin VI, Bergo MO: Protein prenylation restrains innate immunity by inhibiting Rac1 effector interactions. Nat Commun 2019;10:3975. https://doi.org/10.1038/s41467-019-11606-x |
||||
| 64 Garcia-Solis B, Van Den Rym A, Pérez-Caraballo JJ, Al-Ayoubi A, Alazami AM, Lorenzo L, Cubillos-Zapata C, López-Collazo E, Pérez-Martínez A, Allende LM, Markle J, Fernández-Arquero M, Sánchez-Ramón S, Recio MJ, Casanova JL, Mohammed R, Martinez-Barricarte R, Pérez de Diego R: Clinical and Immunological Features of Human BCL10 Deficiency. Front Immunol 2021;12:786572. https://doi.org/10.3389/fimmu.2021.786572 |
||||
| 65 Kaur S, Mirza AH, Overgaard AJ, Pociot F, Størling J: A Dual Systems Genetics Approach Identifies Common Genes, Networks, and Pathways for Type 1 and 2 Diabetes in Human Islets. Front Genet 2021;12:630109. https://doi.org/10.3389/fgene.2021.630109 |
||||