Corresponding Author: Naglaa K. Idriss
Department of Medical Biochemistry, Faculty of Medicine, Assiut University, Assiut, 71516 (Egypt)
Tel. +201003830234, E-Mail naglaaidriss@hotmail.com; naglaaidriss@aun.edu.eg
Spastic Paraplegia 20 and Serine/Threonine Protein Kinase 31 Expression for the Detection of Colorectal Cancer
Nivin A. Hassana
Naglaa K. Idrissb
Noha Gaberc
Abeer Ibrahimd
Mariana A. Tawfeeka
Eman Mossadc
Aliaa A. Mosab
Eman H. Ahmedc
Sally A. Sayede
Heba A. Ahmedf
Amal A. M. Mohamedb
aPharmacology and Experimental Oncology Unit, Cancer Biology Department, South Egypt Cancer Institute, Assiut University, Assiut, Egypt, bDepartment of Medical Biochemistry, Faculty of Medicine, Assiut University, Assiut, Egypt, cOncological Clinical Pathology Department, South Egypt Cancer Institute, Assiut University, Assiut, Egypt, dMedical Oncology and Hematological Malignancy Department, South Egypt Cancer Institute, Assiut University, Assiut, Egypt, eMedical Physiology Department, Faculty of Medicine, Assiut University, Assiut, Egypt, fClinical Pathology Department, Faculty of Medicine, Sohag University, Sohag, Egypt
Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed cancers worldwide [1], with a high incidence in North America, Australia, and parts of Europe and low incidence in Asian countries [2]. Additionally, men show a higher incidence of CRC than women [3]. In Egypt, colon cancer, with an incidence of 2.98%, is the 8th most common cancer and the 9th most common cause of death. Meanwhile, rectal cancer, with an incidence of 1.35 %, is the 17th most common cancer and cause of death [4]. Risk factors for CRC include aging, family history, smoking, alcohol consumption, high caloric intake, physical inactivity, sedentary lifestyle, obesity, and diabetes [5]. Hereditary CRC syndromes, such as Lynch syndrome, hereditary nonpolyposis colorectal cancer, and familial adenomatous polyposis, influence screening recommendations [6]. While CRC is associated with a variety of serum markers, all of them demonstrate a low ability of detecting primary CRC owing to significant overlap with benign disease and low sensitivity for early-stage CRC [7]. The SPG20 gene is located at 13q13.3 andencodes the spartin protein, which is a multifunctional protein involved in intracellular epidermal growth factor receptor trafficking [8], lipid droplet turnover [9], and bone morphogenetic protein signaling inhibition [10]. It is also identified as an adaptor for E3 ubiquitin ligases [11]. Aberrant methylation of the SPG20 promoter is associated with gene silencing, subsequently causing cytokinesis arrest and aneuploidy, which is possibly correlated with tumorigenesis [12]. SPG20 promoter hypermethylation was reported as a biomarker for CRC, with a sensitivity of 89% and 78% in CRC and adenomas, respectively, and a specificity of 99% [13].
The serine-threonine kinase 31 (STK31) gene was initially identified through cDNA subtraction as a testis-specific protein kinase gene expressed in mouse spermatogonia [14]. Since then, STK31 has been described as a novel cancer-testis (CT) antigen that is highly expressed in gastrointestinal cancer cells (colorectal, gastric, and esophageal cancer) [15], while being restricted to the testis and fetal brain in normal tissues [16], making it a potential diagnostic biomarker for CRC. An STK31-derived peptide can trigger specific cytotoxic T-lymphocytes and induce their lysis, which makes STK31 a good candidate for targeted therapy. In addition, it is shown to be a predictive and prognostic factor for early-stage and metastatic CRC [17].
The aim of the present study was to determine whether the quantity of circulating SPG20 and STK31 mRNA could help distinguish between patients with CRC and healthy individuals. In addition, we aimed to investigate whether there is any correlation between the expression patterns of SPG20 and STK31 and tumor stage and grade in patients with CRC.
Materials and Methods
Study subjects
Fifty patients with CRC and 50 control subjects were included in this study. All subjects were from the medical oncology department of the South Egypt Cancer Institute between 2018 and 2020. The clinical and pathological data for the patients, including the patient’s age, sex, history of chronic illness, tumor stage and grade, tumor marker, treatment, disease-free survival (DFS), and overall survival (OS), were obtained from the registry. The control group included healthy, age-matched volunteers who did not receive regular medications and had no evidence of neoplastic or chronic inflammatory disease, as determined upon obtaining their detailed medical history and clinical examination. After recording their complete medical history, the patients were subjected to physical examination, MRI or multi-slice computed tomography (MSCT), and pathological examination of the excised tumor.
The study protocol was approved by the Ethics Committee of the Assiut University, Egypt (IRB no:17100560), and the study was conducted according to the code of ethics of the World Medical Association (Declaration of Helsinki). Written informed consent was obtained from all participants before their participation.
Quantitative real-time PCR
Venous blood (2 ml) was collected, from both patients and control subjects, in EDTA tubes under aseptic conditions. Blood sample collection and RNA purification from blood cells were carried out on the same day and the samples were stored at -80 °C until further use. Total cellular RNA was extracted using the Gene JET RNA extraction kit (Gene JET RNA Purification Kit Catalog number: K0731_Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s protocol. Briefly, blood samples were centrifuged and the supernatant was discarded. The solution was spun for a further 10 min at 2500 RPM, and the supernatant was aspirated. Pellets were resuspended in lysis buffer, incubated, and centrifuged, and the supernatant was transferred into a new vial. The RNA concentration was measured by Nanodrop ND-1000 Spectrophotometer SPG20 and STK31 mRNA levels were determined using the Verso SYBR Green 1-Step qRT-PCR kit plus ROX Vial, SYBR Green qPCR Master Mix (No ROX). Catalog No. C0006 according to the manufacturer’s protocol. The Primers for each gene are listed as sequences for each gene in Table 1.
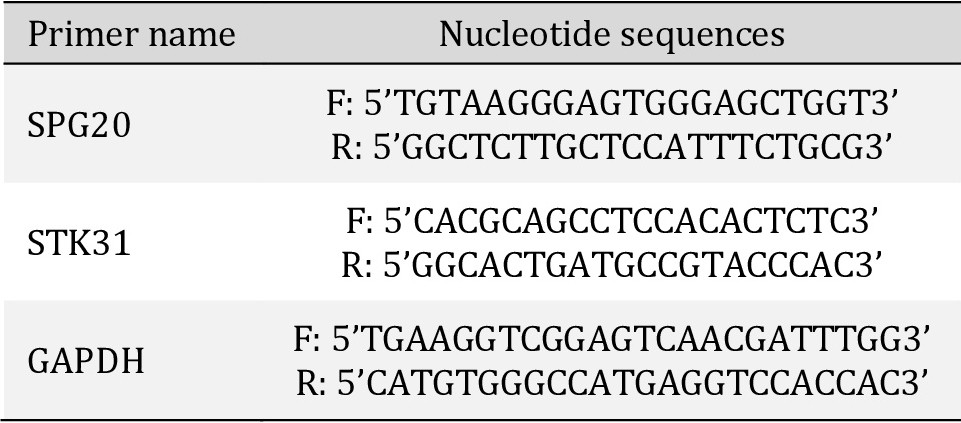
Primer sequences
Relative quantification of gene expression
Relative quantification (RQ) was performed using the comparative CT (ΔΔCt) method. An RQ > 1 implies that the target’s expression is higher than that of the controls (upregulated), and RQ < 1 implies that the target’s expression is lower than that of the control (downregulated) (Thermo Fisher, Life Technologies, Applied Biosystems. Product Name: Step-One-Plus Real-Time PCR. Catalog Number: 4376600).
Statistical analysis
Sample size calculations were conducted using the G power program (University of Kiel, Kiel, Germany). All statistical calculations were performed using the Statistical Package for the Social Sciences version 22 (SPSS Inc., Chicago, IL, USA). Normally distributed data were statistically described in terms of mean ± standard deviation (±SD); frequencies (number of cases) and percentages were used for qualitative data. The Student’s t-test was used to compare continuous variables. To compare categorical data, the chi-square (χ2) test was performed. The exact test was used when the expected frequency was less than 5. Correlations between both tumor biomarkers were assessed using the Pearson (R) correlation test. The Kaplan-Meier test was used to compare survival between the two study groups. The Mann-Whitney U test was used to test the median differences of the data that did not follow a normal distribution. The Kruskal-Wallis test was used to test the median differences of the data that did not follow a normal distribution, and a post-hoc test was calculated using Bonferroni corrections. Statistical significance was set at p ≤ 0.05.
Results
SPG20 and STK31 expression levels in patients and controls
Our study included 50 CRC patients and 50 controls. The age of patients (range: 26–72 y, mean: 47.64 ± 10.43 y) and control subjects (range: 23–72 y, mean: 45.16 ± 11.70 y) showed no significant differences (p=0.266). Demographic data (Table 2) did not show any significant differences between patients and controls as well.
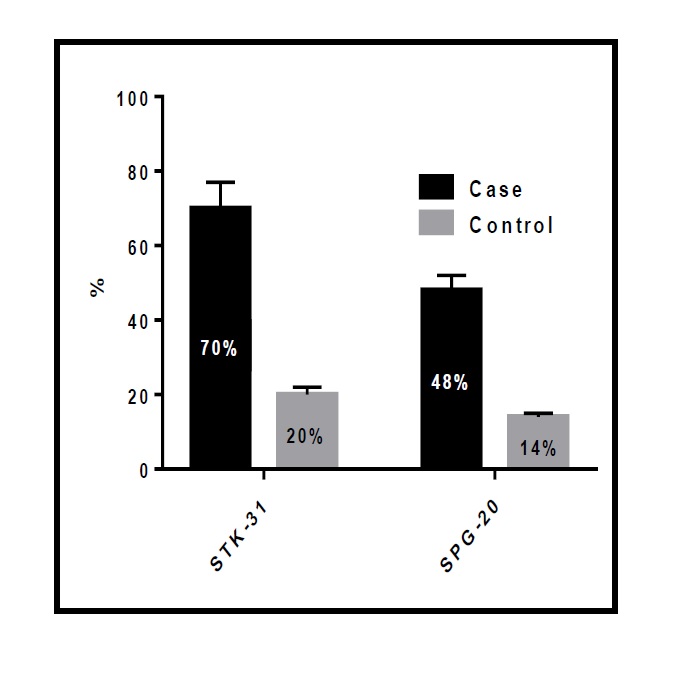
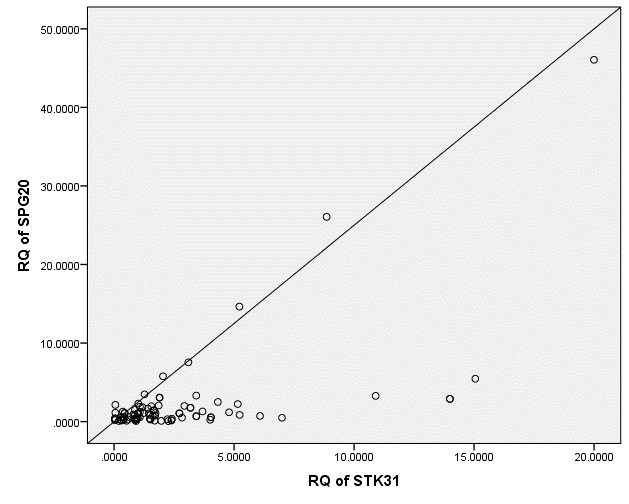
Our study showed that the percentage of STK31 and SPG20 upregulation was significantly higher (p=0.000 for each) in patients than in controls (Fig. 1). There was a significant positive correlation between the expression of the two tumor biomarkers, STK31 and SPG20 (R=0.636, p=0.000; Fig. 2).
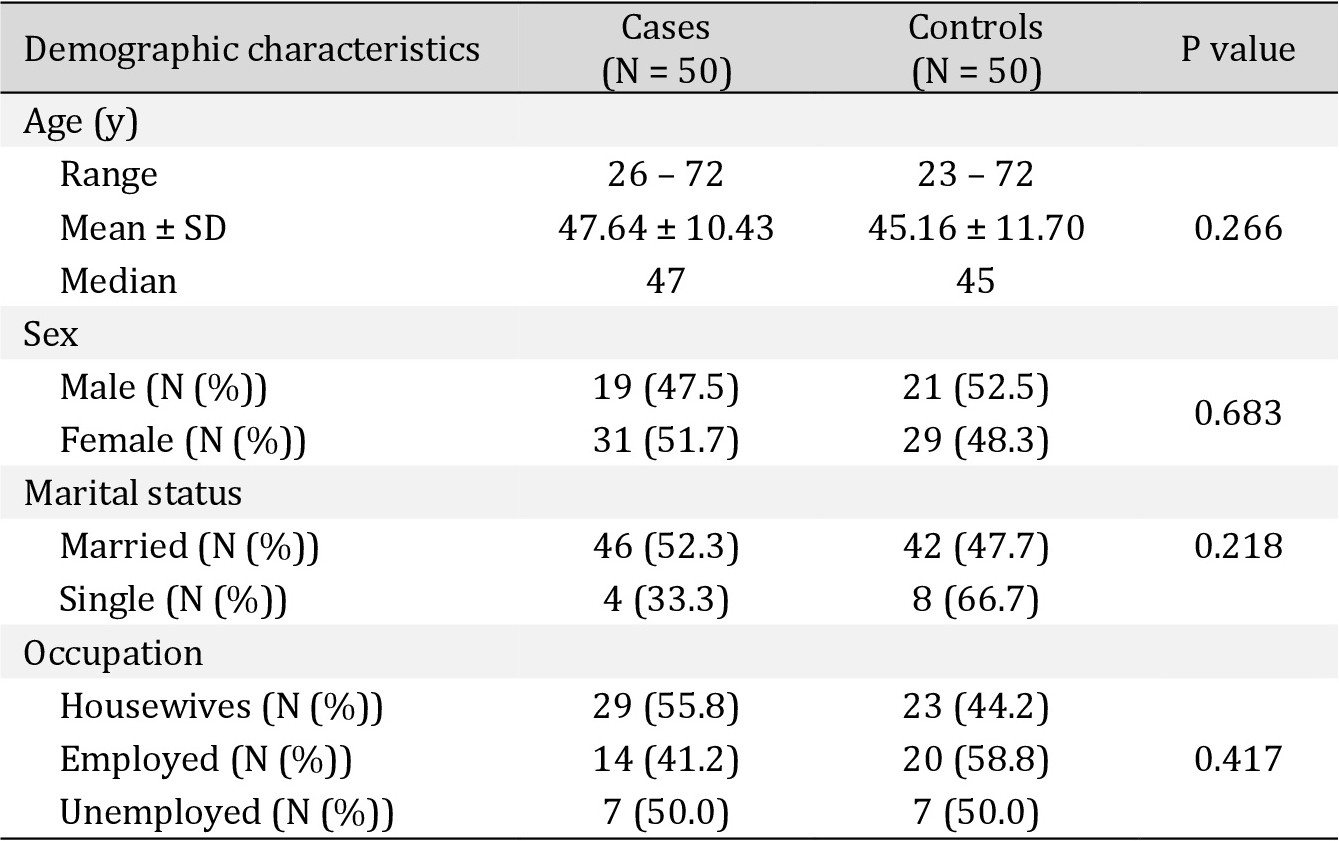
Demographic characteristics of the studied participants (CRC patients N = 50, control N = 50). *Data are mean ± SD and median (range) or n (%). *Student t test was used for the continuous variables (Age). Chi-square analysis was used for the categorical variables (Sex, marital status and occupation). Significance defined by p < 0.05
Disease-free survival and overall survival
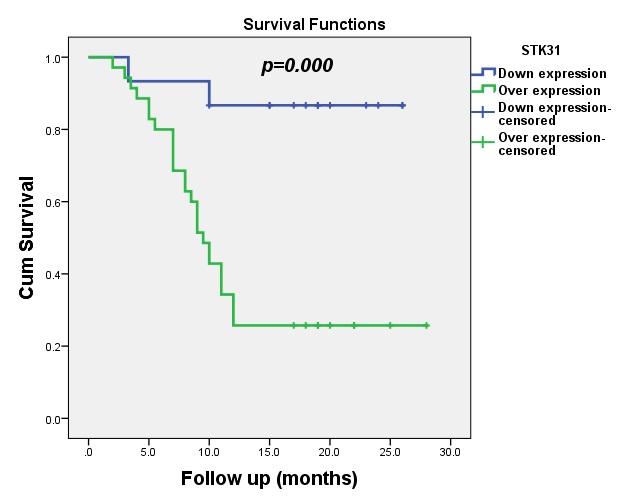
The median DFS for patients with upregulated expression of STK31 was 9.5 months (95% CI: 8.05-10.95), which was statistically significant (p=0.000), as shown in Fig. 3.
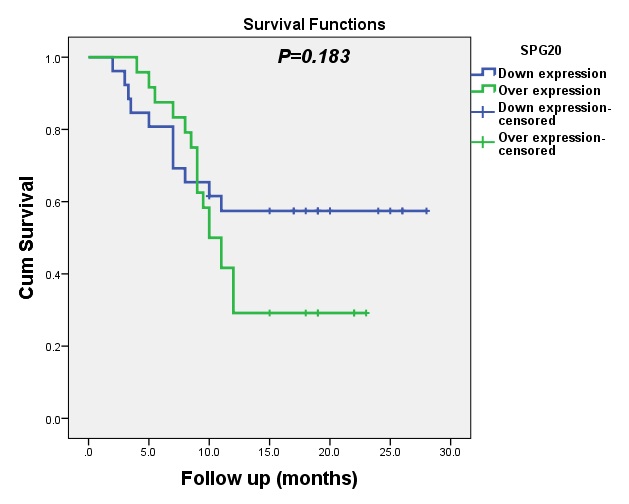
The median DFS for patients with upregulated expression of SPG20 was 10 months (95% CI: 8.20-11.80). With minor variation between the levels of SPG20 tumor biomarker and disease status was observed in this study (p=0.183, p<0.05), as shown in Fig. 4.
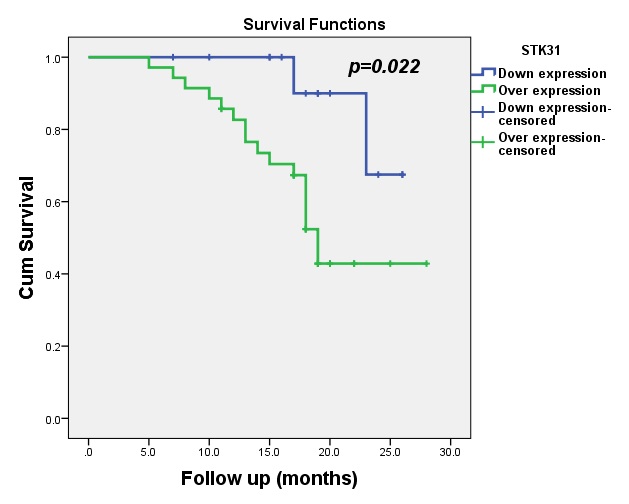
The median OS for patients with upregulated expression of STK31 was 19 months (95% CI: 17.46-20.54). Statistically significant differences between STK31 levels and OS were observed in this study (p=0.022), as shown in Fig. 5.
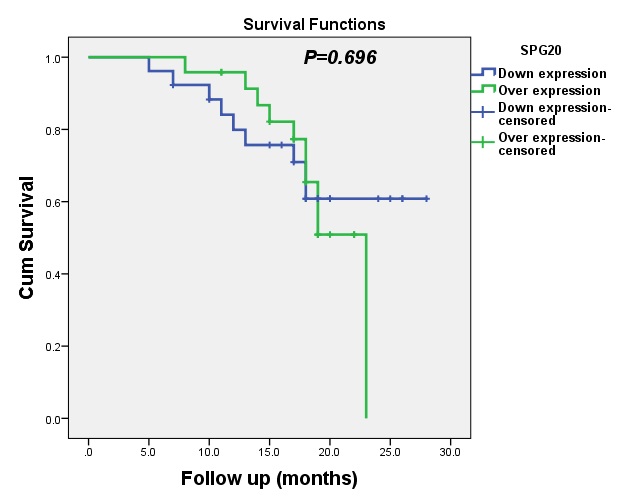
At 6 months, the OS for patients with upregulated expression of SPG20 expression was 100%, whereas the OS for patients with downregulated expression of SPG20 expression was 96%. Similarly, the values of OS for patients at 12, 18 and 22 months with upregulated and downregulated expression of SPG20 was 96%and 80%, 65% and 61%, and 60% and 61%, respectively. Therefore, this gene does not affect the OS of patients, as shown in Fig. 6.
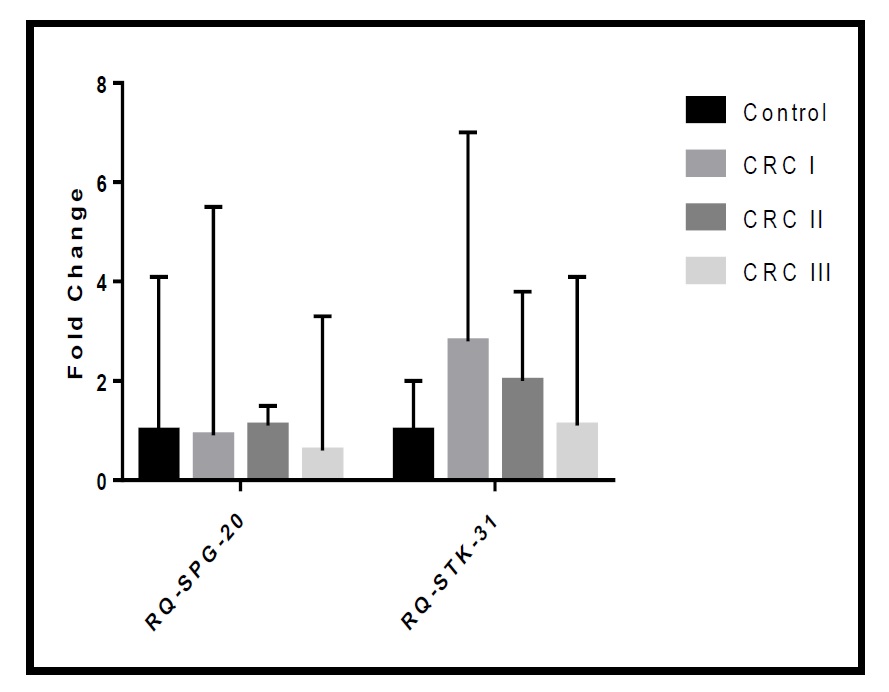
Relationship between CRC stage and grade and the biomarkers
There were no significant differences in the fold change of expression of both markers according to tumor stage in the studied cohort (p=0.713 for RQ-SPG20 and p=0.270 for RQ-STK31; as demonstrated in Table 3, Fig. 7). Additionally, there were no significant differences in the fold change of SPG20 expression according to tumor grade in the studied cohort (p=0.248). However, there was a significant difference in the fold change of STK31 expression according to tumor grade in the studied cohort (p=0.010). The median RQ-STK31 of grade I CRC was triple that of the control, that of grade II CRC was double that of the control, and that of grade III CRC was equal to that of the control.
More specifically, when we compared each grade with the others, we found significant differences in STK31 expression levels between controls and patients with grade I CRC (p=0.037), between controls and patients with grade II CRC (p=0.046), and between patients with grade I and grade III CRC (p=0.044), as shown in Table 4 and Fig. 8.
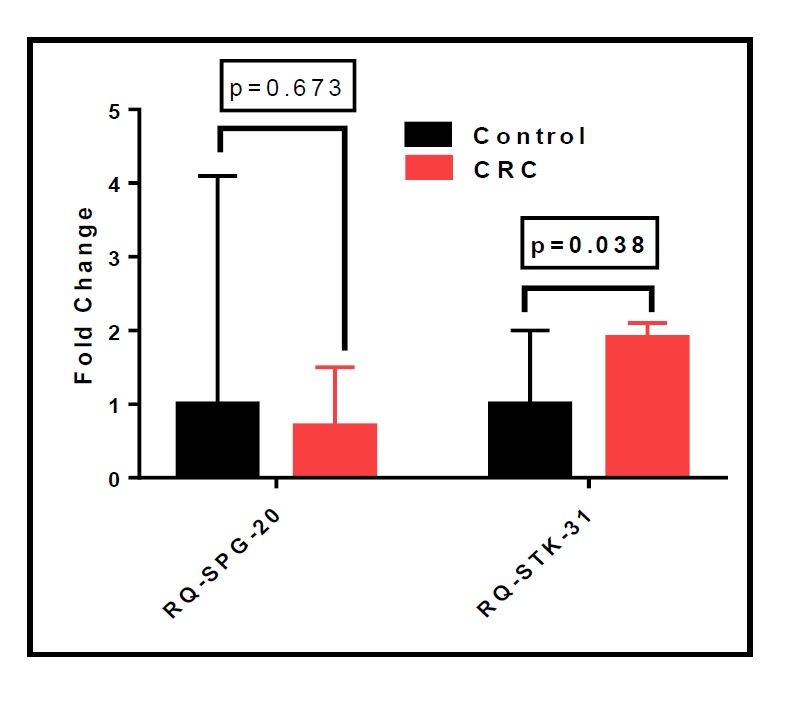
Nevertheless, in the studied cohort, we found no significant differences in SPG20 expression between patients and controls. However, there were significant differences in the fold change of STK31 expression in patients double the control (Median Interquartile range (IQR): patients=1.9, controls=1), as shown in Supplementary Table S1 and Fig. 9 (for all supplementary material see www.cellphysiolbiochem.com).
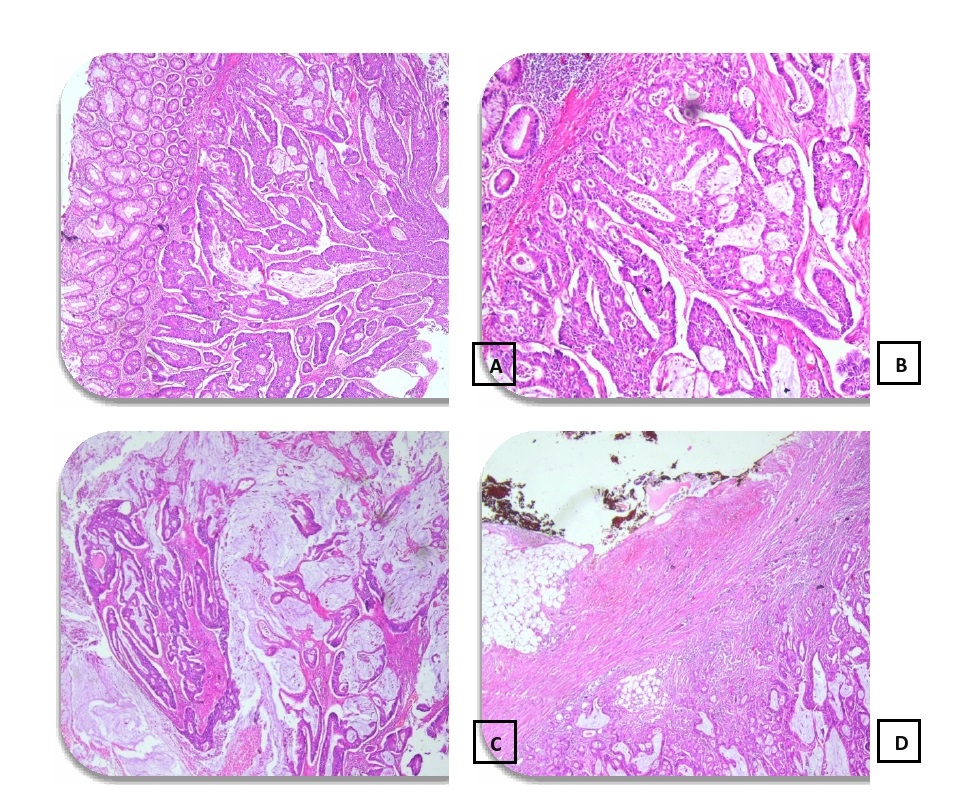
Conventionally, pathologists perform CRC diagnosis by visually investigating resected tissue samples that are fixed and stained with hematoxylin and eosin (H&E). The presence and level of malignancy were measured by observing the structural alterations in the tissues, as shown in Fig. 10.
Discussion
Colorectal cancer (CRC) is a major worldwide public health issue and one of the most frequent types of solid cancers diagnosed in developed countries. Early detection of CRC can aid in decreasing the associated mortality, and detection of its precursor lesion can even reduce the incidence; however, the current CRC screening strategies present many limitations [18]. Both basic and clinical scientists are constantly searching for more reliable individual biomarkers to prevent disease relapse, occurrence of severe side effects caused by chemotherapy, and development of treatment resistance, which would ultimately lead to improved patient survival and quality of life [19].
Therefore, it is important to find new noninvasive, well-accepted biomarkers that could detect tumors in asymptomatic early stages, when CRC is still curable.
Our results showed a significantly higher level of STK31 expression in newly diagnosed patients than in controls. These findings are consistent with those reported previously [15], where overexpression of STK31 was observed in CRC tissues compared to that in adjacent non-cancerous tissues. Since STK31 is known to regulate the cell cycle, it might play a role in tumorigenicity [15]. This was also confirmed by Xiong et al, who found that STK31 knockdown induced apoptosis [20].
However, a recent study by Watany et al. [21] contradicts our findings, as STK31 was found to be highly expressed in patients with benign colorectal polyps compared to that in controls. This could be explained by the fact that CRC may develop during the follow-up of these benign polyps as a result of a sequence of genetic alterations, known as the adenoma-carcinoma sequence [22].
Our results also showed significantly higher expression of SPG20 in the serum of patients with CRC compared to that in the control subjects. These results are in agreement with a previous study, where a high percentage of methylated reference (PMR) values for the SPG20 promoter was reported in the plasma and tissue samples obtained from patients with CRC [23]. The use of SPG20 as a screening method for CRC was first suggested by Zhang et al., who explored the feasibility of detecting hypermethylated SPG20 in the stool. However, measuring plasma levels is considered a cheaper and simpler method for screening [24]. Thus, both markers (STK31 and SPG20) could be used to screen for CRC using simple noninvasive maneuvers.
In the present study, we did not find any significant correlation between the expression levels of both markers and the clinicopathological data of patients, such as cancer stage, pathological subtype, and grade of differentiation.
For SPG20, our results were similar to those reported previously by Rezvani et al. [23] and Zhang et al., who found no significant differences in age, gender, and tumor location either [24].
For STK31, our results are consistent with previous reports that showed no correlation between the STK31 mRNA levels and features such as age, gender, tumor size, histologic grade, primary tumor invasion depth, or Duke’s stage of the carcinoma [15].
STK31 was previously found to be robustly expressed in colon cancer tissues, playing a critical role in differentiation [25]; however, this was not confirmed by others [15]. This discrepancy might become clearer in the future with a better understanding of the role of STK31 in the mechanism of tumorigenesis. A recent study by Kwak et al. suggests that STK31 does not inhibit apoptosis directly but is instead responsible for the stability of programmed cell death 5 (PDCD5), a positive regulator of p53 during DNA damage. Similarly, other unknown mechanisms might be responsible for STK31 maintaining the undifferentiated state of the tumor [26].
Nevertheless, there were no significant differences between STK31 expression and CEA (the commonly used tumor marker for CRC). A previous study showed significantly higher levels of CEA in CRC patients compared to those in patients with benign polyps and controls; however, no significant difference was found between patients with benign polyps and controls. Additionally, a significant positive correlation was found between STK31 and CEA expression [21]. Since our study did not explore the levels of CEA, we do not have adequate data to compare with the previous reports.
Our study showed significant differences between SPG20 and CEA expression, in line with the study by Rezvani et al., which found that SPG20 methylation exhibited a significantly higher sensitivity than the CEA tumor marker [23].
We found a significant negative impact of STK31 on both DFS (p=0.000) and OS (p=0.022). This was consistent with the results of Zhong et al. who showed significantly high expression of STK31in metastatic patients [15]. Kuo et al. previously reported that overexpression of STK31 enhances cell migration and invasion, leading to metastasis [27]. This was confirmed in other tumors, such as pancreatic cancer and lung cancer, wherepatients with high expression of STK31 showed poorer survival [20, 28].
Our results did not show any significant association between the expression levels of SPG20 and DFS (p=0.183) or OS (p=0.696). This is in contrast to previous reports of a significant correlation between SPG20 and the poor prognosis of patients with gastric cancer [29] and hepatocellular carcinoma (HCC) [30].
We found no significant differences in the fold change of SPG20 expression between patients and controls. In contrast, Rezvani et al. found that the median PMR values for plasma samples from patients were 12 times higher than thosein plasma samples from healthy individuals [23].
However, there were significant differences in the fold change of STK31 expression, with patients exhibiting double the values of control subjects. Our results are in agreement with previous studies, where significantly higher STK31 levels were found in patients with CRC compared to those in controls or patients with benign polyps [21], and overexpression of STK31 was observed in colorectal cancerous tissues compared to the adjacent non-cancerous tissues [15].
In conclusion, our study is the first attempt to analyze the expression of both STK31 and SPG20 in Egyptian patients with CRC in relation to diverse clinicopathological features. We found a higher relative gene expression level in patients with lymph node metastasis and established the investigative and predictive potential of both transcripts in patients with CRC. While STK31 is a potential diagnostic biomarker for CRC, a good candidate for targeted therapy and monitoring, as well as an assumptive predictive and prognostic factor for early-stage and metastatic CRC, SPG20 is a beneficial noninvasive biomarker for early diagnosis, rather than the prognosis of CRC. Overall, the use of either genes represents a potential tool for the diagnosis of CRC and could assist oncologists in recommending suitable management strategies for individual patients.

Differences in Fold Change of the Marker according to Tumour Stage among the studied Cohort. * Kruskal Wallis test was used to compare the mean difference between groups. **Post-hoc test with Bonferroni Corrections was used to compare the mean difference between groups
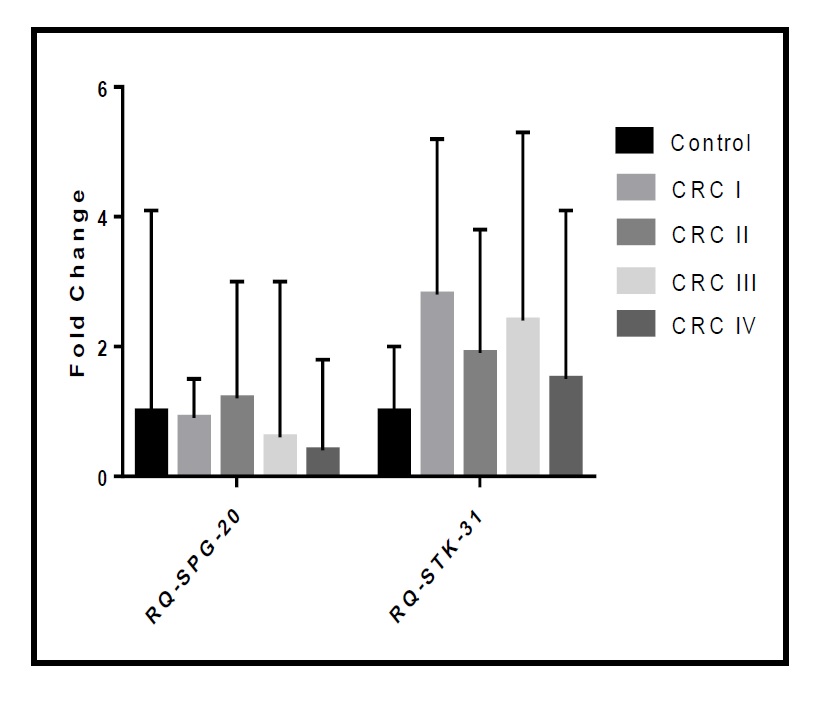
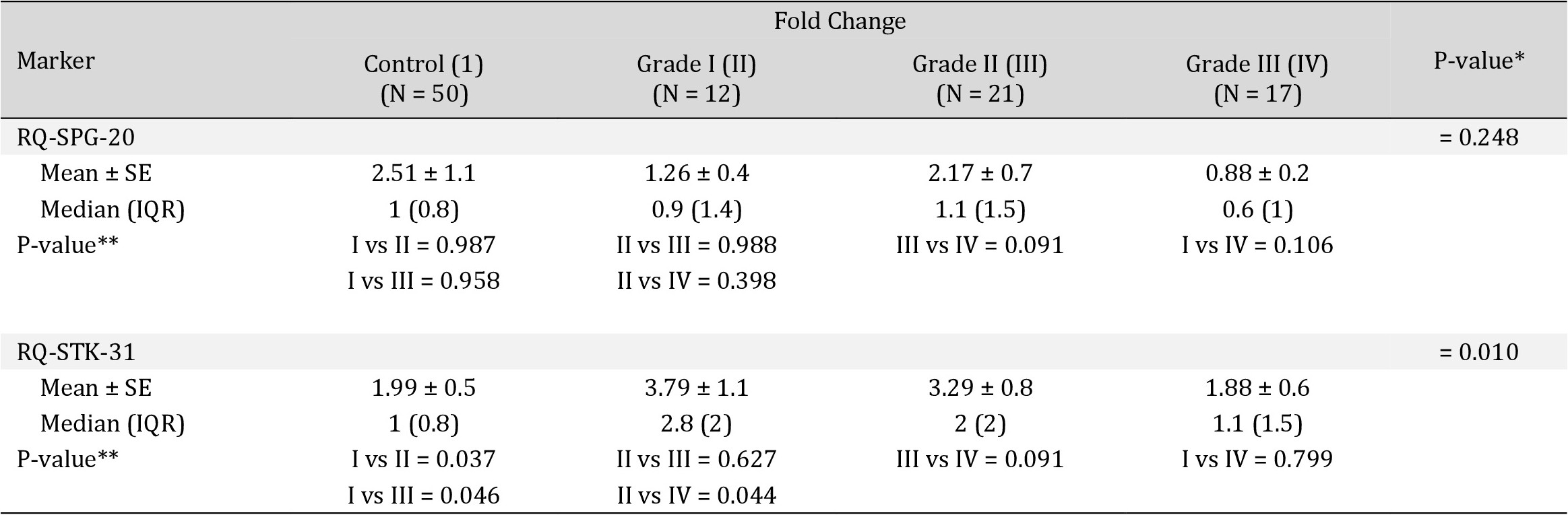
Differences in Fold Change of the Marker according to Tumour Grade among the studied Cohort. * Kruskal Wallis test was used to compare the mean difference between groups. **Post-hoc test with Bonferroni Corrections was used to compare the mean difference between groups
Author Contributions
Dr. Nivin Hassan: Conceptualization, methodology, Investigation, Writing-review & editing; Prof. Naglaa K. Idriss: Designing concept, laboratory and genetic testing of the biomarkers, editing of the original draft, critical review, and submission; Dr. Noha Gaber & Dr. Aliaa Aly Mosa: acquisition of data, analysis and interpretation; Mariana A. Tawfeek: writing – drafting & revision; E. Mossad: Editing of manuscript; Abeer Elsayed A. Ibrahim: Methodology, Writing and Formal analysis; Amal Mohamed: Writing – critical revision, genetic testing of the biomarkers and analysis; Eman Ahmed, Sally Sayed & Heba A. Ahmed: Interpretation of data and drafting the work.
Statement of Ethics
Approval for this study was obtained from the Institutional Review Board of the Faculty of Medicine, Assiut University prior to the study. Written informed consent was obtained from all the study participants. Informed consent was clear and designated the persistence of the study and their freedom to participate or exit the study at any time without any responsibility. Furthermore, participants’ confidentiality and anonymity were assured by assigning each participant with a code number. No inducements or rewards were provided for any of the participants.
The authors declare that no conflicts of interest exist.
| 1 Miller KD, Sauer AG, Ortiz AP, Fedewa SA, Pinheiro PS, Tortolero-Luna G, Martinez-Tyson D, Jemal A, Siegel RL: Cancer Statistics for Hispanics/Latinos. CA Cancer J Clin 2018;68:425-445. https://doi.org/10.3322/caac.21494 |
||||
| 2 Safari A, Shariff ZM, Kandiah M, Rashidkhani B, Fereidooni F: Dietary patterns and risk of colorectal cancer in Tehran Province: a case-control study. BMC Public Health 2013;13:222. https://doi.org/10.1186/1471-2458-13-222 |
||||
| 3 Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary DL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA: SEER Cancer Statistics Review, 1975-2010. National Cancer Institute, Bethesda, MD. URL: https://seer.cancer.gov/archive/csr/1975_2010/. | ||||
| 4 Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A: Global Cancer Statistics 2018: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2018;68:394-424. https://doi.org/10.3322/caac.21492 |
||||
| 5 Huxley R, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M: The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer 2009;125:171-180. https://doi.org/10.1002/ijc.24343 |
||||
| 6 Katona BW, Yurgelun MB, Garber JE, Offit K, Domchek SM, Robson ME, Stadler ZK: A counseling framework for moderate-penetrance colorectal cancer susceptibility genes. Genet Med 2018;20:1324-1327. https://doi.org/10.1038/gim.2018.12 |
||||
| 7 De Jong MC, Beckers RC, Woerden VV, Sijmons JM, Bemelmans MH, Dam RM, Dejong CH: The liver-first approach for synchronous colorectal liver metastases: more than a decade of experience in a single centre. HPB (Oxford) 2018;20:631-640. https://doi.org/10.1016/j.hpb.2018.01.005 |
||||
| 8 Bakowska JC, Jupille H, Fatheddin P, Puertollano R, Blackstone C: Troyer syndrome protein spartin is mono-ubiquitinated and functions in EGF receptor trafficking. Mol Biol Cell 2007;18:1683-1692. https://doi.org/10.1091/mbc.e06-09-0833 |
||||
| 9 Eastman SW, Yassaee M, Bieniasz PD: A role for ubiquitin ligases and Spartin/SPG20 in lipid droplet turnover. J Cell Biol 2009;184:881-894. https://doi.org/10.1083/jcb.200808041 |
||||
| 10 Tsang HT, Edwards TL, Wang X, Connell JW, Davies RJ, Durrington HJ, O'Kane CJ, Luzio JP, Reid E: The hereditary spastic paraplegia proteins NIPA1, spastin and spartin are inhibitors of mammalian BMP signalling. Hum Mol Genet 2009;18:3805-3821. https://doi.org/10.1093/hmg/ddp324 |
||||
| 11 Hooper C, Puttamadappa SS, Loring Z, Shekhtman A, Bakowska JC: Spartin activates atrophin-1-interacting protein 4 (AIP4) E3 ubiquitin ligase and promotes ubiquitination of adipophilin on lipid droplets. BMC Biol 2010;8:72. https://doi.org/10.1186/1741-7007-8-72 |
||||
| 12 Sagona AP, Stenmark H: Cytokinesis and cancer. FEBS Lett 2010;584:2652-2661. https://doi.org/10.1016/j.febslet.2010.03.044 |
||||
| 13 Lind GE, Raiborg C, Danielsen SA, Rognum TO, Thiis-Evensen E, Hoff G, Nesbakken A, Stenmark H, Lothe RA: SPG20, a novel biomarker for early detection of colorectal cancer, encodes a regulator of cytokinesis. Oncogene 2011;30:3967-3978. https://doi.org/10.1038/onc.2011.109 |
||||
| 14 Wang PJ, Page DC, McCarrey JR: Differential expression of sex-linked and autosomal germ-cell-specific genes during spermatogenesis in the mouse. Hum Mol Genet 2005;14:2911-2918. https://doi.org/10.1093/hmg/ddi322 |
||||
| 15 Zhong L, Liu J, Hu Y, Wang W, Xu F, Xu W, Han J, Biskup E: STK31 as novel biomarker of metastatic potential and tumorigenicity of colorectal cancer. Oncotarget 2017;8:24354-24361. https://doi.org/10.18632/oncotarget.15396 |
||||
| 16 Petit FG, Kervarrec C, Jamin SP, Smagulova F, Hao C, Becker E, Jégou B, Chalmel F, Primig M: Combining RNA and protein profiling data with network interactions identifies genes associated with spermatogenesis in mouse and human. Biol Reprod 2015;92:71. https://doi.org/10.1095/biolreprod.114.126250 |
||||
| 17 Yokoe T, Tanaka F, Mimori K, Inoue H, Ohmachi T, Kusunoki M, Mori M: Efficient identification of a novel cancer/testis antigen for immunotherapy using three-step microarray analysis. Cancer Res 2008;68:1074-1082. https://doi.org/10.1158/0008-5472.CAN-07-0964 |
||||
| 18 Thorsteinsson M, Jess P: The clinical significance of circulating tumor cells in non-metastatic colorectal cancer -- a review. Eur J Surg Oncol 2011;37:459-465. https://doi.org/10.1016/j.ejso.2011.01.025 |
||||
| 19 Marcuello M, Vymetalkova V, Neves RP, Duran-Sanchon S, Vedeld HM, Tham E, Dalum GV, Flügen G, Garcia-Barberan V, Fijneman RJ, Castells A, Vodicka P, Lind GE, Stoecklein NH, Heitzer E, Gironella M: Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol Aspects Med 2019;69:107-122. https://doi.org/10.1016/j.mam.2019.06.002 |
||||
| 20 Xiong J, Xing S, Dong Z, Niu L, Xu Q, Liu P, Yang P: STK31 regulates the proliferation and cell cycle of lung cancer cells via the Wnt/β catenin pathway and feedback regulation by c myc. Oncol Rep 2020;43:395-404. https://doi.org/10.3892/or.2019.7441 |
||||
| 21 Watany MM, Elmashad NM, Badawi R, Hawash N: Serum FBLN1 and STK31 as biomarkers of colorectal cancer and their ability to noninvasively differentiate colorectal cancer from benign polyps. Clin Chim Acta 2018;483:151-155. https://doi.org/10.1016/j.cca.2018.04.038 |
||||
| 22 Shussman N, Wexner SD: Colorectal polyps and polyposis syndromes. Gastroenterol Rep (Oxf) 2014;2:1-15. https://doi.org/10.1093/gastro/got041 |
||||
| 23 Rezvani N, Alibakhshi R, Vaisi-Raygani A, Bashiri H, Saidijam M: Detection of SPG20 gene promoter-methylated DNA, as a novel epigenetic biomarker, in plasma for colorectal cancer diagnosis using the MethyLight method. Oncol Lett 2017;13:3277-3284. https://doi.org/10.3892/ol.2017.5815 |
||||
| 24 Zhang H, Song YC, Dang CX: Detection of hypermethylated spastic paraplegia-20 in stool samples of patients with colorectal cancer. Int J Med Sci 2013;10:230-234. https://doi.org/10.7150/ijms.5278 |
||||
| 25 Fok KL, Chung CM, Yi SQ, Jiang X, Sun X, Chen H, Chen YC, Kung H, Tao Q, Diao R, Chan H, Zhang XH, Chung YW, Cai Z, Chan HC: STK31 maintains the undifferentiated state of colon cancer cells. Carcinogenesis 2012;33:2044-2053. https://doi.org/10.1093/carcin/bgs246 |
||||
| 26 Kwak S, Lee S, Han E, Park S, Jeong M, Seo J, Park S, Sung G, Yoo J, Yoon H, Choi K: Serine/threonine kinase 31 promotes PDCD5‐mediated apoptosis in p53‐dependent human colon cancer cells. J Cell Physiol 2019;234:2649-2658. https://doi.org/10.1002/jcp.27079 |
||||