Corresponding Author: Patrycja Dajnowicz-Brzezik
Department of Physiology, Medical University of Bialystok, Mickiewicza 2C Str., 15-959 Bialystok (Poland)
Tel. +48600445459, E-Mail patrycja.dajnowicz@umb.edu.pl
The Effect of α-Lipoic Acid on Oxidative Stress in Adipose Tissue of Rats with Obesity-Induced Insulin Resistance
Patrycja Dajnowicz-Brzezika Ewa Żebrowskaa Mateusz Maciejczykb Anna Zalewskac Adrian Chabowskia
aDepartment of Physiology, Medical University of Bialystok, Bialystok, Poland, bDepartment of Hygiene, Epidemiology and Ergonomics, Medical University of Bialystok, Poland, cIndependent Laboratory of Experimental Dentistry, Medical University of Bialystok, Poland
Introduction
Overnutrition and sedentary life style reinforce the rising trend of worldwide obesity and its metabolic consequences such as insulin resistance and type 2 diabetes [1, 2]. However, the exact mechanisms underlying these negative outcomes as well as their treatment are still under debate. Currently the role of adipose tissue has gained a lot of attention, mainly due to its recently recognized endocrine function. Many studies have shown that white adipose tissue, besides being just an energy reservoir, synthesizes biologically active adipokines (e.g. adiponectin, leptin, resistin, visfatin) involved in glucose and lipid metabolism, as well as regulating blood pressure, angiogenesis and the immune response of the body [2, 3].
Nearly 65–70% of body fat is stored in the subcutaneous adipose tissue (SAT), while the remaining 30–35% in the visceral adipose tissue (VAT). VAT and SAT differ greatly in their structure, metabolic activity as well as their role in eliciting metabolic consequences of abdominal obesity [4]. Indeed, obesity induced VAT accumulation has been considered to predispose to the metabolic dysregulation and overall mortality [5, 6]. What is more, recent research emphasizes the role of oxidative stress and inflammation as a link between obesity and its metabolic comorbidities [7]. Furthermore, the use of antioxidants as potent drugs ameliorating systemic insulin resistance is being extensively studied.
α-lipoic acid (ALA) is an eight-carbon saturated fatty acid essential for mitochondrial metabolic pathways [8]. Pleiotropic pharmacological, antioxidant, anti-inflammatory as well as antiapoptotic properties of ALA are used in the treatment of diabetes and its complications [9-11]. Antioxidant ALA activity relays mainly on free radical scavenging, chelation of prooxidant metal ions, or regeneration of various antioxidants (reduced glutathione (GSH), coenzyme Q10, or vitamins C and E) [12-14]. Moreover, recently proven ALA effects include glycemic control improvement and alleviation of neuropathy, retinopathy as well as nephropathy symptoms accompanying diabetes [15]. Besides, ALA used in obesity reduces total cholesterol and low-density lipoprotein in obese individuals and animals [16, 17]. However, previous studies were focused mainly on the systemic effects of ALA treatment. In the present study, we were the first to investigate ALA role in the alleviation of oxidative stress directly in the adipose tissue of high fat diet induced insulin resistant rats. What is more, we have compared the response of VAT and SAT to ALA supplementation.
Materials and Methods
Animals
Three-week-old male Wistar cmdb/outbred rats (weighing initially approx. 50-70 g) were maintained with unlimited access to water and food, under 12 h light / 12 h dark cycle, 21°C ± 2. After a week of acclimatization, the animals were divided into four independent groups as follows:
1. CTRL group (n = 6) – the rats fed the control diet (CTRL; Agropol, Motycz, Poland; 10.3 kcal% fat, 24.2 kcal% protein, and 65.5 kcal% carbohydrate) for 10 weeks.
2. HFD group (n = 6) – the rats fed the high fat diet (D12492; Research Diets, Inc. New Brunswick, USA; 60 kcal% fat, 20 kcal% proteins and 20 kcal% carbohydrate) for 10 weeks.
After 6 weeks of the experiment, both groups received intragastrically saline solution additionally for the next 4 weeks.
3. HFD+ALA group (n = 6) – the rats fed the high fat diet for 10 weeks. Additionally, after 6 weeks of the experiment, the animals received intragastrically ALA solution at a dose of 30 mg/kg body weight (in 0.9% NaCl) for the next 4 weeks.
Animal body weight and food consumption was monitored during the experiment. After 10 weeks overnight fasted animals were anesthetized with intraperitoneal, phenobarbital injection at a dose of 80 mg/kg body weight. Subsequently, the subcutaneous and visceral adipose tissue was excised and immediately freeze-clamped in liquid nitrogen and stored at -80°C until analysis. Meanwhile, blood from the abdominal aorta was collected into the heparinized tubes (to obtain plasma) and centrifuged (3000 × g, 4°C, 10 min). The above-mentioned procedures were done by the same experienced technician.
Plasma glucose and insulin concentrations
The fasting plasma glucose concentration was measured with a glucometer (Accu-Chek, Bayer, Germany). Insulin concentration was measured in the plasma with commercially available ELISA kit according to the manufacturer’s instructions (Abbot, USA). The insulin sensitivity was evaluated using the homeostasis model assessment of insulin resistance (HOMA-IR) = fasting insulin (U/mL) × fasting glucose (mM)/22.5.
Oxidative stress parameters
The assay included determination of enzymatic and nonenzymatic antioxidants; proteins and lipids oxidation products; proteins oxidative damage products; prooxidant enzymes and proinflammatory and proapoptotic proteins. All measurements were performed in homogenates of subcutaneous and visceral adipose tissue. Absorbance and fluorescence were estimated using microplate reader Infinite M200 PRO Multimode Tecan (Tecan Group Ltd., Männedorf, Switzerland), while all the biochemical reagents were from Sigma-Aldrich Germany/Sigma-Aldrich USA.
Colorimetric bicinchoninic acid (BCA) assay with bovine serum albumin as a standard (Pierce BCA Protein Assay Kit, Rockford, USA) was used to assess the total protein content. The standardisation of the final results was made to one milligram of the total protein. All assays were performed in duplicates.
Enzymatic and nonenzymatic antioxidants
Catalase (CAT) spectrophotometric analysis was conducted at 340 nm and was based on the decomposition rate of hydrogen peroxide (H2O2), where 1 micromole of H2O2 is degraded by one unit of CAT in one minute. Glutathione peroxidase (GPx) was assayed based of NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) conversion to NADP+, where one millimole of NADPH was catalyzed for one minute by one unit of GPx. During GPx spectrophotometric analysis, the absorbance was assayed at 340 nm. Total glutathione level was investigated with reaction of DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)), and glutathione reductase. The level of oxidized glutathione (GSSG) was determined in the same way, however, prior to analysis, the samples were thawed and neutralized to pH 6-7 with 1M hydrochloric triethanolamine and then incubated with 2-vinylpyridine. The level of reduced glutathione (GSH) was calculated from the difference between the total glutathione and GSSG, and the redox status was evaluated using the formula [GSH2/GSSG]. Superoxide dismutase (SOD) activity was measured spectrophotometrically at the absorbance of 340 nm. Cytosolic activity of superoxide dismutase was measured by inhibiting oxidation of epinephrine to adrenochrome and the amount of enzyme inhibiting oxidation of epinephrine by 50% was defining one unit of SOD activity.
Lipid oxidation
Lipid hydroperoxides (LOOH) concentration was analyzed colorimetrically at 560 nm using a complex formation reaction involving a mixture of xylenol orange, butylated hydroxytoluene (BHT), sulfuric acid, and FOX2 reagent (ferrous ammonium sulfate). Concentrations were calculated based on the calibration curve for the hydrogen peroxide solution [18].
Malondialdehyde (MDA) was assayed spectrophotometrically (at 535 nm) based on thiobarbituric acid reactive substances (TBARS) method with 1,3,3,3-tetraethoxypropane as a standard [19].
Protein oxidation
The advanced oxidation protein products (AOPP) concentration was assessed colorimetrically, measuring the total iodide ion oxidizing capacity of the samples. Absorbance was measured at 340 nm. The protein carbonyl groups (PC) concentration was estimated spectrophotometrically at 355 nm using 2,4-dinitrophenylhydrazine (2,4-DNPH) forming stable complexes with carbonyl groups in oxidatively damaged proteins [20]. PCs were calculated using the absorption coefficient for 2,4-DNPH (22,000 M-1cm-1).
Protein glycoxidation
The content of glycoxidatively modified proteins (dityrosine, kynurenine, N-formylokynurenine and tryptophan) were determined spectrofluorimetrically at wavelengths: 330/415, 365/480, 325/434 and 295/340 nm respectively. Advanced glycation end products (AGE) were determined spectrofluorometrically at 440/350 nm.
Nitrosative stress
NADPH oxidase (NOX) analysis was carried out immediately after sampling with the luminescence test using lucigenin as the luminophore. The amount of enzyme required to release 1 nmol of peroxide anion defines one unit of NOX activity per minute. Total nitric oxide (NO) concentration was measured spectrofluorimetrically with the use of sulfanilamide and N-(1-naphthyl)-ethylenediamine dihydrochloride at 490 nm. The peroxynitrite content was determined colorimetrically at 320 nm. The assessment was based on the peroxynitrite-mediated nitration which resulted in the formation of nitrophenol. The content of S-nitrosothiols was determined with Griess reagent and Cu2+ ions based reaction, whose product was analyzed spectrophotometrically at 490 nm [21, 22].
Proinflammatory and proapoptotic proteins
The concentrations of tumor necrosis factor α (TNF-α), Bcl-2-associated X protein (Bax) and B-cell lymphoma 2 (Bcl-2) in the adipose tissue were determined with commercial ELISA kits (from EIAab Science Inc. Wuhan (Wuhan, China)), according to the manufacturer’s instructions.
Statistical analysis
The data were processed using GraphPad Prism 7 (GraphPad Software, La Jolla, USA). Data was verified with The Shapiro–Wilk, while the Levene test was used to the homogeneity of the variance. With the one-way ANOVA and the post hoc Tukey test detailed analysis of honestly significant differences (HSDs) was conducted. The multiplicity-adjusted p value was also calculated. The threshold for statistical significance was p < 0.05.
Results
The effect of ALA supplementation on body weight, plasma metabolic parameters and food intake
HFD caused significant increase in body weight, plasma glucose, plasma insulin and HOMA-IR (+36%, +69%, +123%, +777% respectively) when compared to CTRL. However, decreased food intake in HFD was observed (-37%) when compared to CTRL. ALA administration to HFD animals increased plasma glucose (+9%) and decreased food intake (-30%) when compared to CTRL, while it led to a decrease in body weight, plasma glucose, insulin and HOMA-IR when compared to HFD (-19%, -36%, -38% and -88%, respectively) (Table 1).
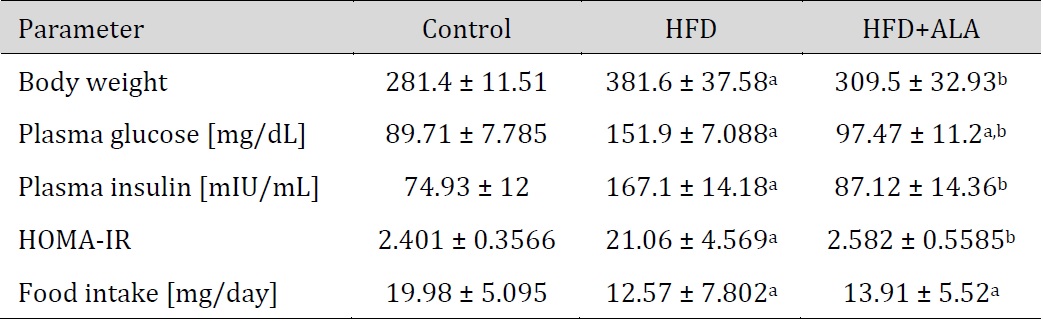
The effect of ALA supplementation on body weight, plasma metabolic parameters and food intake; Control – control rats; HFD – rats fed high-fat diet; HFD+ALA – rats fed high-fat diet + α-lipoic acid; HOMA-IR – homeostasis model assessment of insulin resistance. a p < 0.05 vs. Control, b p < 0.05 vs. HFD.
The effects of ALA supplementation on enzymatic antioxidants during HFD regime
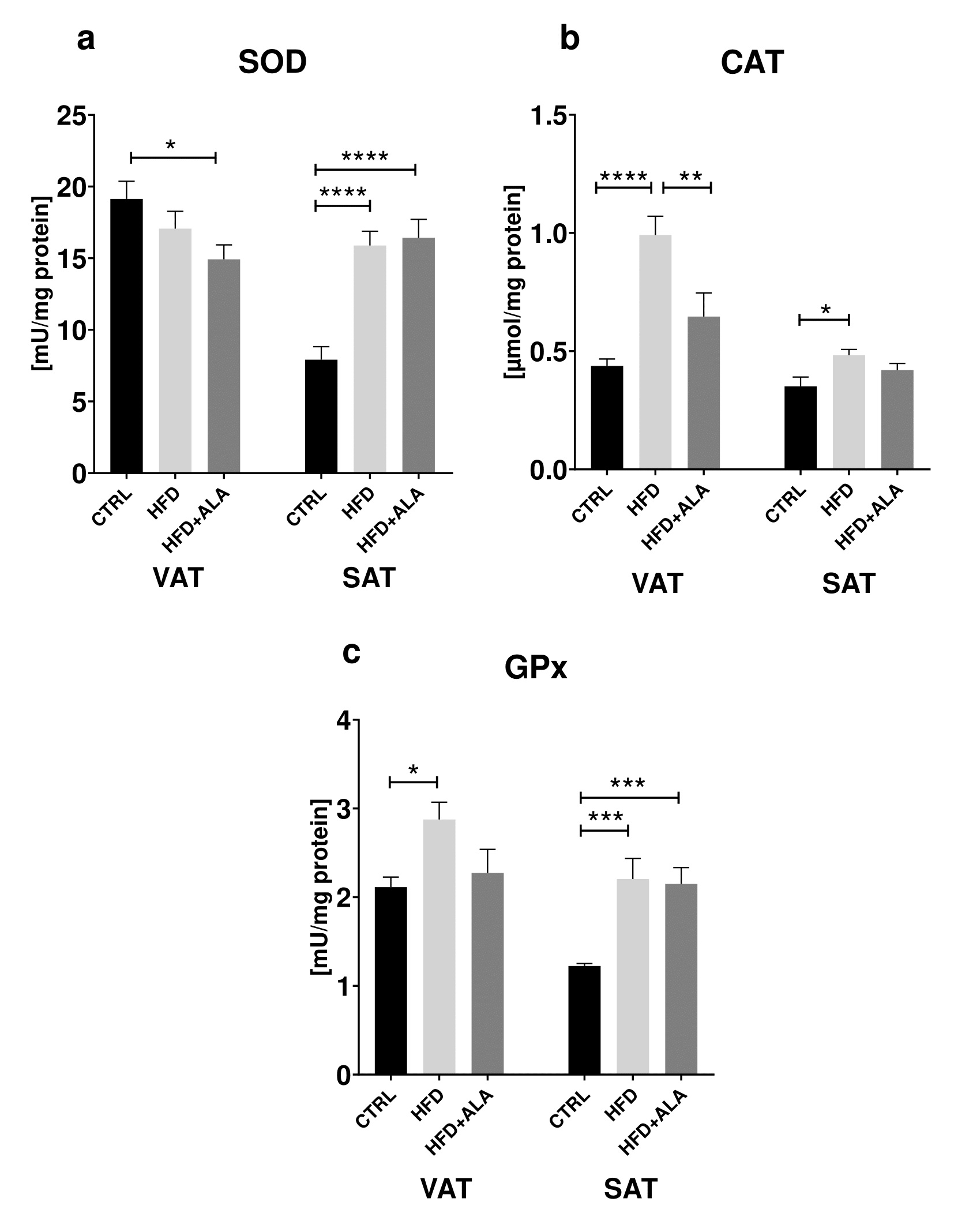
HFD caused an increase in SOD activity only in the subcutaneous adipose tissue when compared to the control (+101%), while in HFD+ALA animals we showed a significant decrease (-22%) in VAT and increase (+107%) in SOD activity in SAT when compared to CTRL. Moreover, HFD caused an increase in CAT in both locations (+127% in VAT and +28% in SAT), while decrease in CAT activity (-26%) in HFD+ALA was observed when compared to HFD only in VAT. Activity of GPx increased in VAT and SAT (+36%, +80% respectively) of HFD animals when compared to CTRL group. We also showed that GPx level increased (75%) in HFD+ALA rats when compared to CTRL (Fig. 1).
The effects of ALA supplementation on non-enzymatic antioxidants during HFD regime
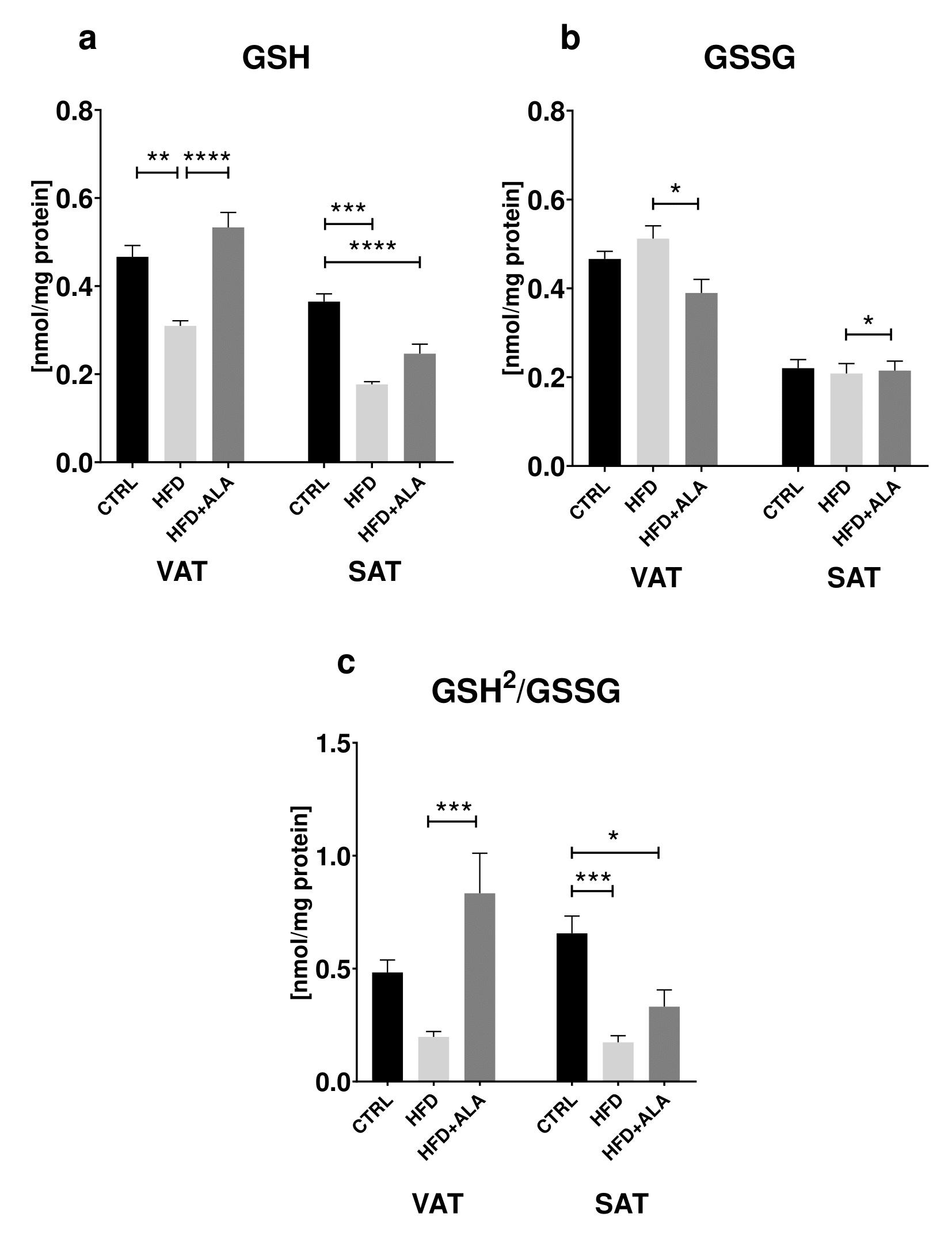
Both in the VAT and SAT of HFD animals we showed a decrease in GSH content (-33%, -25% respectively) when compared to CTRL. There was also an increase of GSH in HFD+ALA when compared to HFD (+48%) in visceral AT. The concentration of GSH in SAT was lower (-32%) when compared to CTRL. A decrease in GSSG in VAT (-26%) of HFD+ALA animals was observed when compared to HFD. In subcutaneous adipose tissue of HFD animals, [GSH2]/[GSSG] content decreased (-52%) when compared to CTRL. Its concentration in HFD+ALA was also lower (-49%) in SAT when compared to CTRL. In HFD+ALA rats, we observed its increased concentration (+114%) when compared to HFD (Fig. 2).
The effects of ALA supplementation on protein oxidation during HFD regime
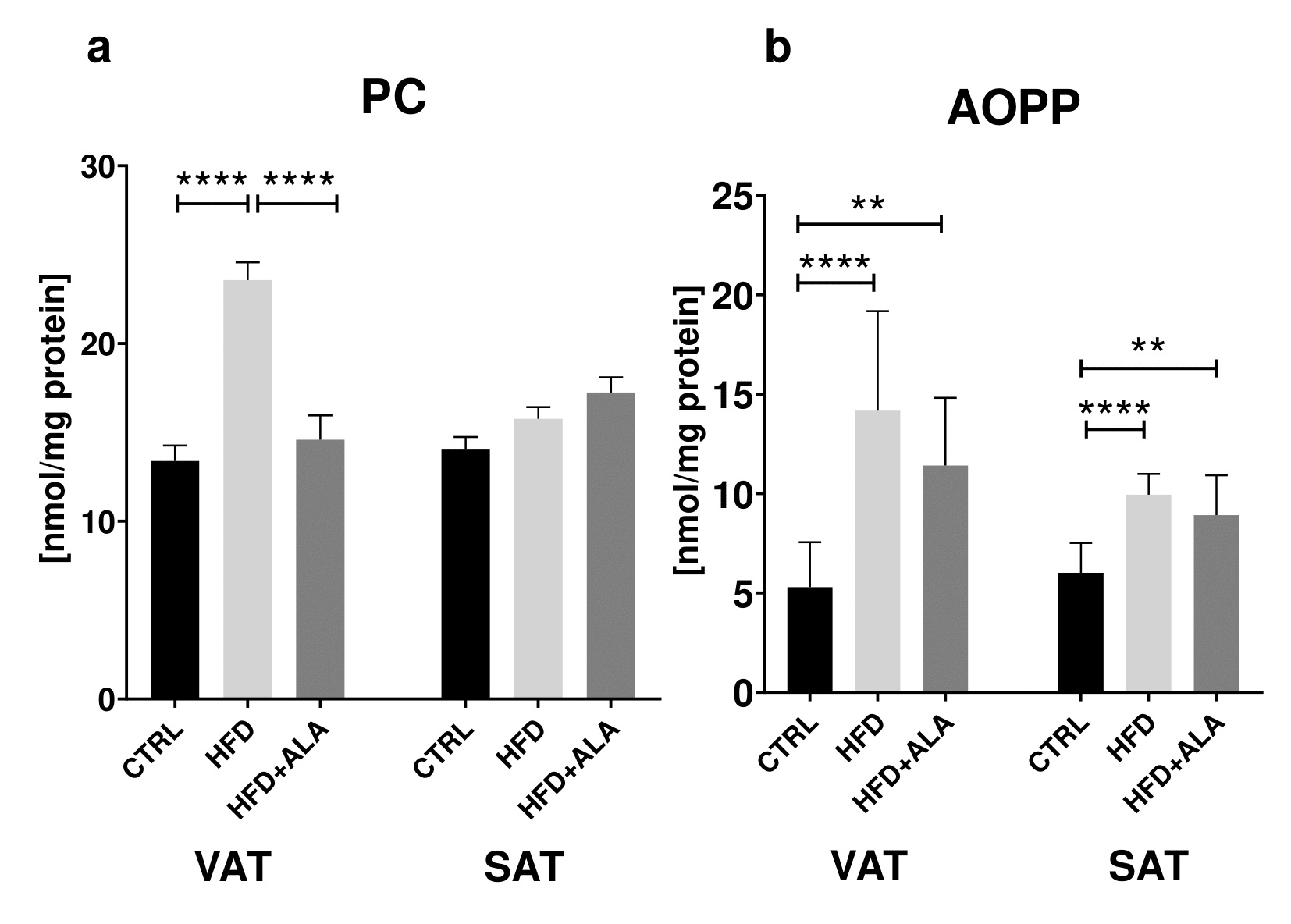
In the visceral adipose tissue of HFD animals, concentration of PC decreased as compared to both CTRL and HFD+ALA (-76% and -67% respectively). In VAT and SAT, the concentration of AOPP was increased in HFD (+168%, +65 respectively) and HFD+ALA (+116% and +48% respectively) when compared to CTRL. LOOH in VAT and SAT of HFD animals increased significantly (+58%, +176% respectively) when compared to CTRL. Similarly, higher concentration of AOPP was observed in SAT of HFD+ALA rats (+102%) when compared to CTRL group. In VAT, AOPP concentration in HFD+ALA animals decreased when compared to HFD (-45%). In both visceral and subcutaneous AT, concentration of MDA in HFD (+126%, +158% respectively) and HFD+ALA (+65% and +136% respectively) rats increased when compared to CTRL. In VAT, there was a decrease in MDA concentration in HFD+ALA when compared to HFD rats (-66%) (Fig. 3, 4).
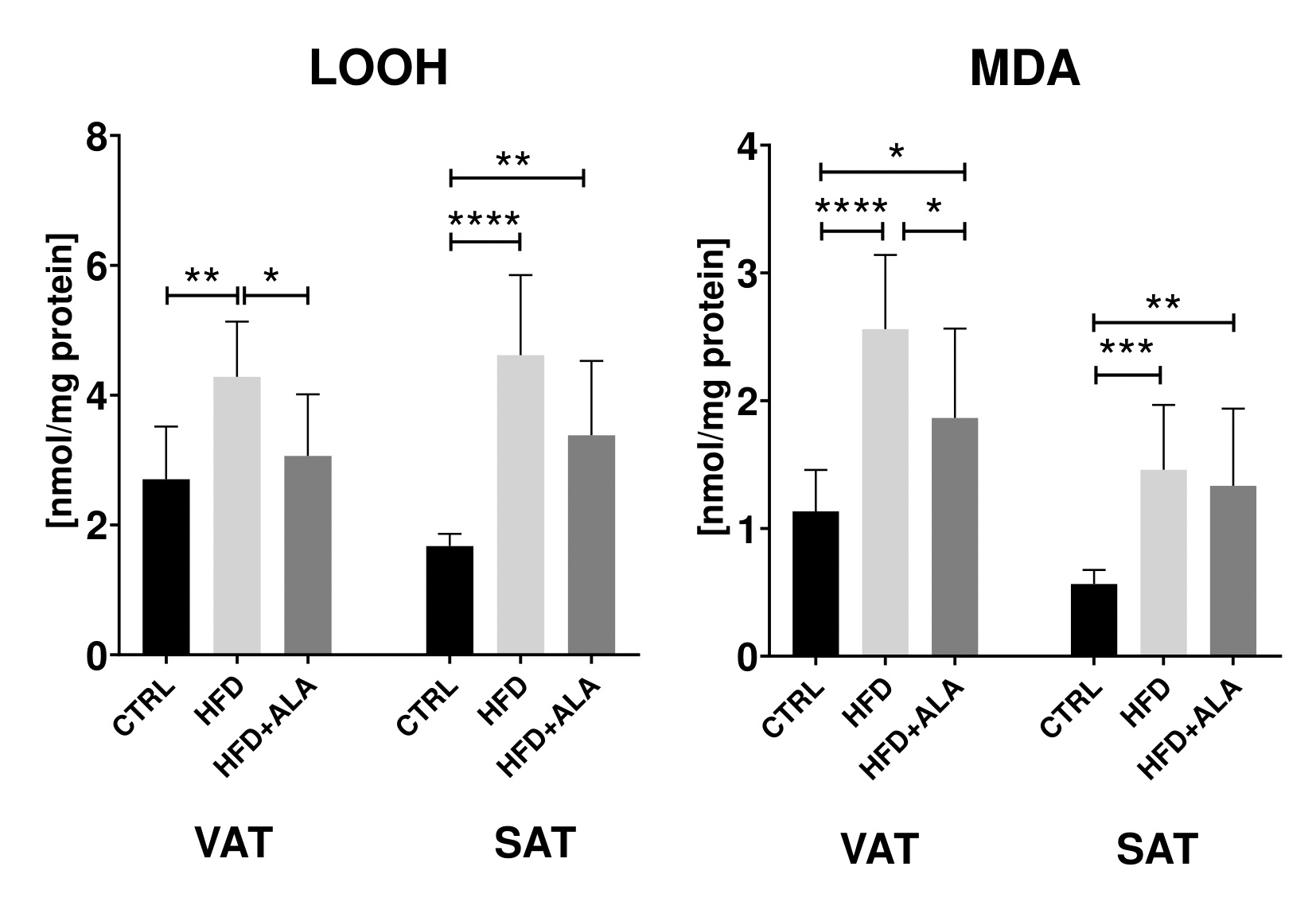
The effects of ALA supplementation on protein glycoxidation during HFD regime
We showed significant changes in the protein glycoxidation products concentrations. In visceral adipose tissue, the concentration of tryptophan was lower (-46%) in HFD when compared to CTRL. Tryptophan content in HFD+ALA group decreased in VAT (-34%), while increased in SAT (+46%) when compared do CTRL. In VAT, kynurenine concentration increased in HFD when compared to CTRL (+59%). Its concentration in HFD+ALA rats decreased in VAT when compared to HFD (-69%) and increased in SAT when compared to CTRL (+38%). In both VAT and SAT, concentration of N-formylkynurenine increased in HFD when compared to CTRL (+43% and +92% respectively). In VAT its concentration decreased in HFD+ALA when compared to HFD (-46%). In SAT there was an increase in N-formylkynurenine in HFD+ALA when compared to CTRL (+84%). In visceral adipose tissue, concentration of dityrosine increased both in HFD and HFD+ALA when compared to CTRL (+140% and +87% respectively). In the same location, dityrosine concentration decreased in HFD+ALA group when compared to HFD (-53%). In subcutaneous AT, concentration of dityrosine increased in both HFD and HFD+ALA when compared to CTRL (+136% and +144% respectively). In VAT and SAT of HFD animals, there was a significant increase in AGE concentrations (+46% and +55% respectively) when compared to CTRL (Fig. 5).
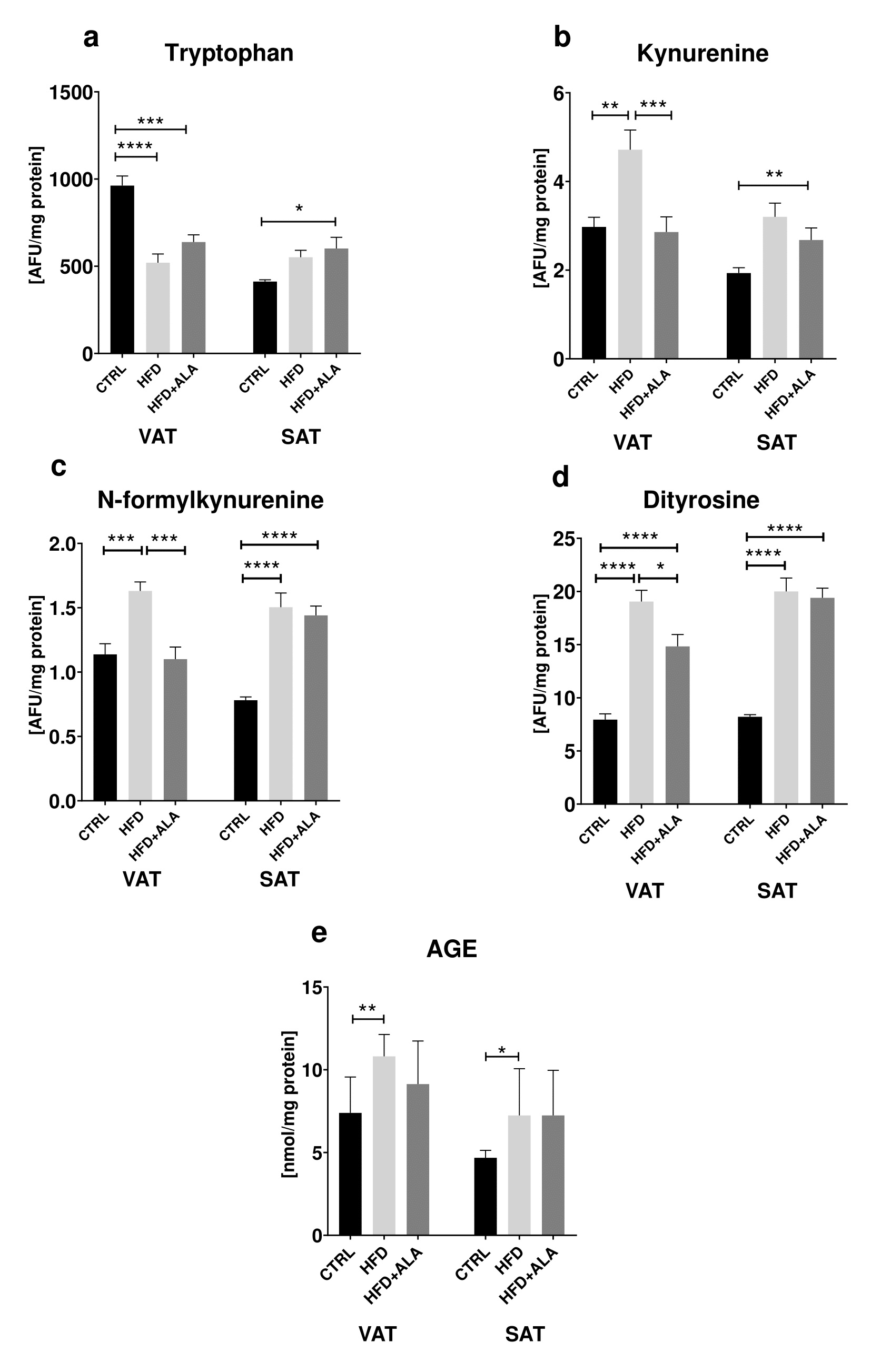
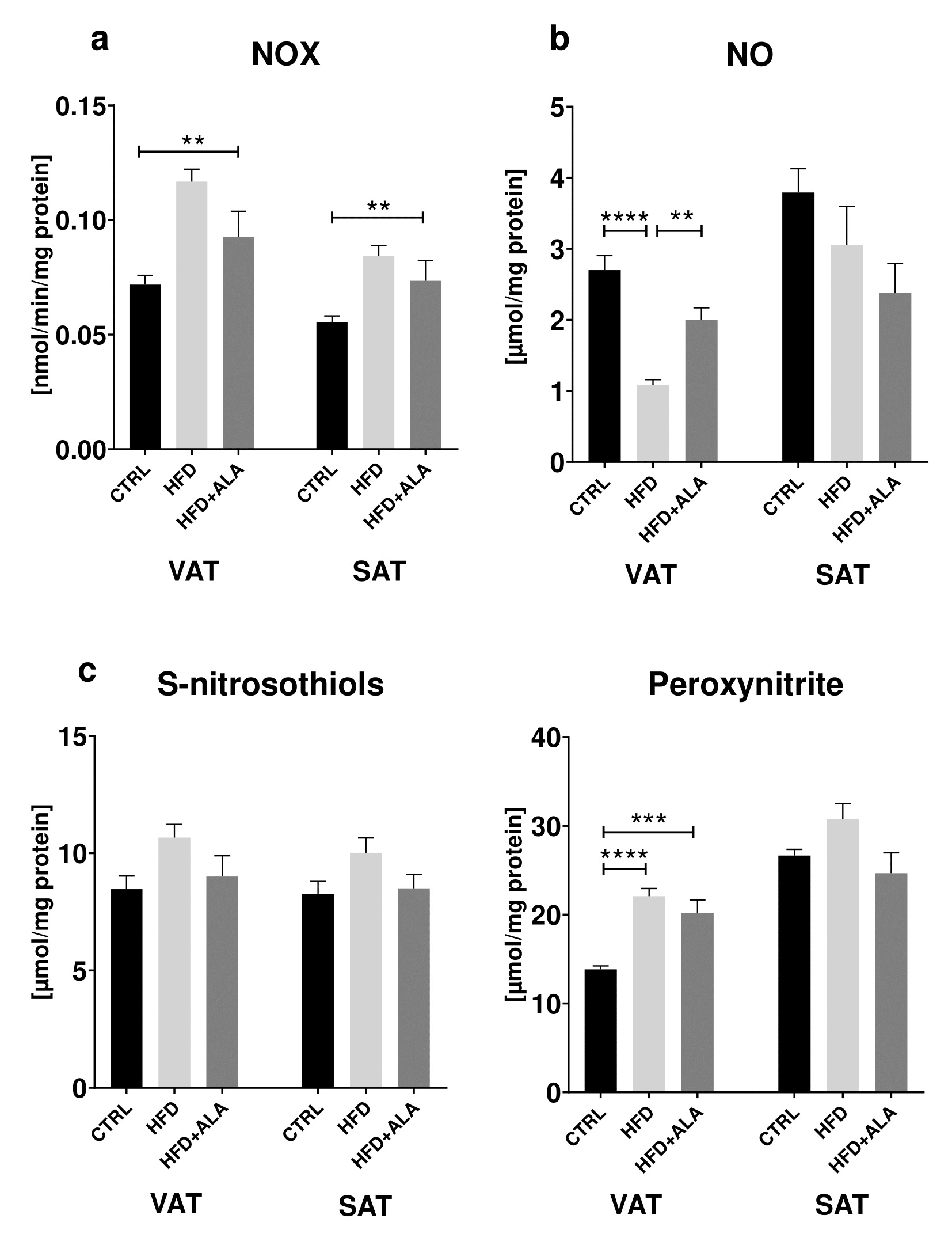
The effects of ALA supplementation on nitrosative stress during HFD regime
In both visceral and subcutaneous adipose tissue, we showed that activity of NOX in HFD+ALA animals was significantly higher (+52% and +46% respectively), when compared to CTRL. Concentration of NO in VAT increased in HFD+ALA (+34%) when compared to HFD and decreased in HFD when compared to CTRL (-60%). In VAT, the concentration of peroxynitrite was increased in HFD+ALA (+60%) and in HFD (+63%), when compared to CTRL (Fig. 6).
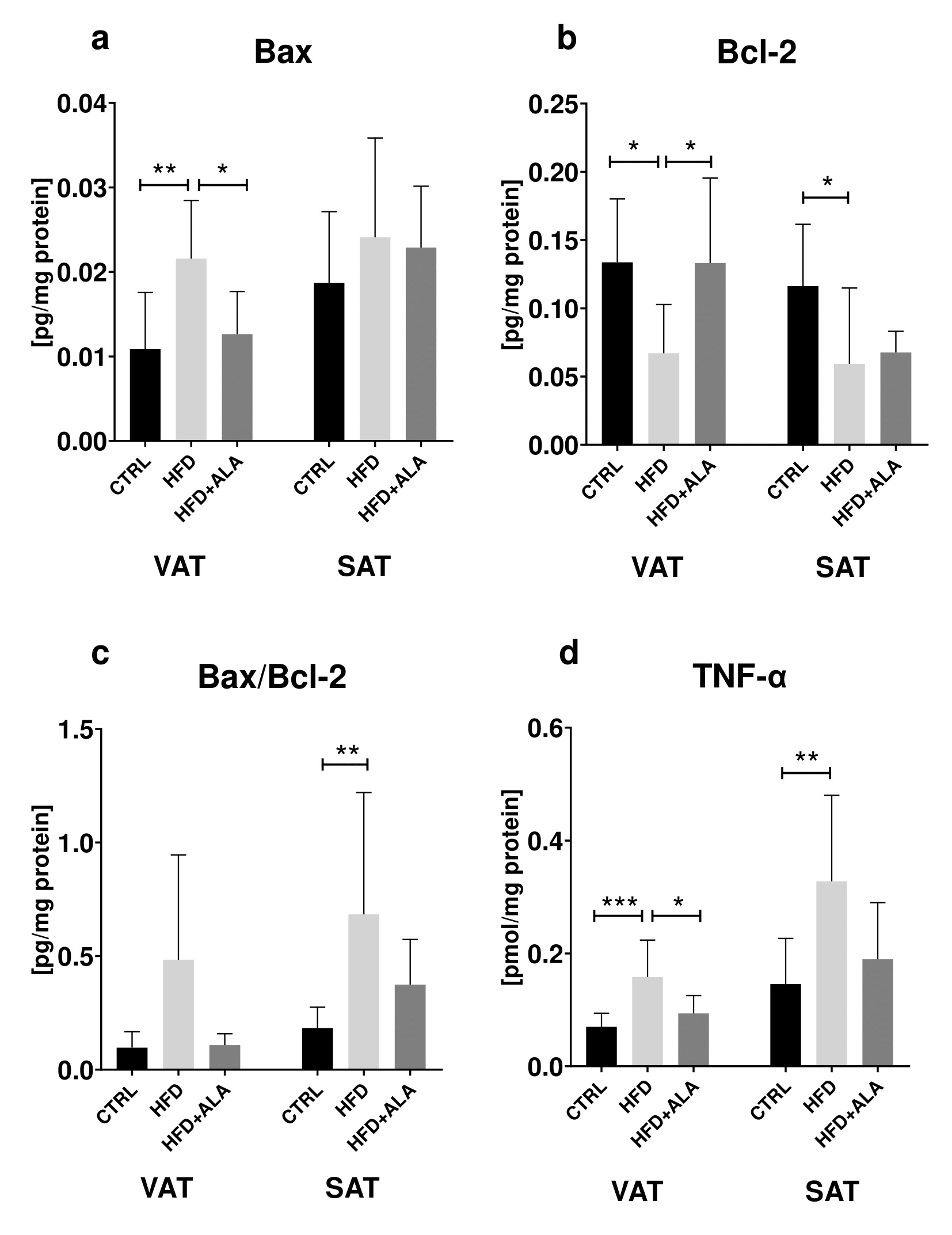
The effects of ALA supplementation on proinflammatory and proapoptotic proteins during HFD regime
In the visceral and subcutaneous AT, concentration of Bcl-2 decreased in HFD (-50% and -49% respectively) when compared to CTRL. In VAT, its concentration in HFD+ALA rats increased (+50%) when compared to HFD. In VAT, concentration of Bax was lower in CTRL (-98%) and HFD+ALA (-82%) when compared to HFD (Fig. 7).
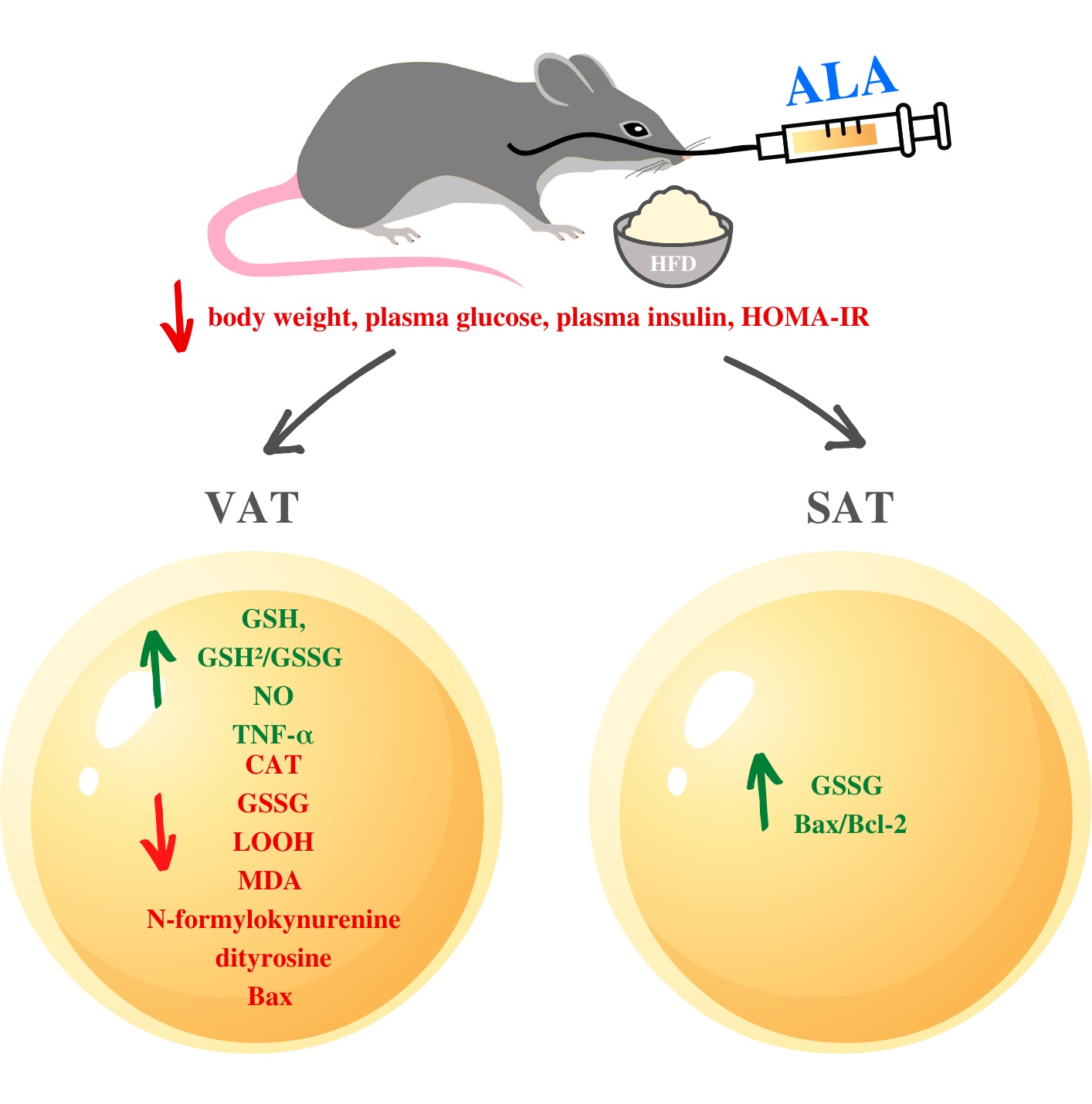
• ALA normalizes body weight, blood glucose level as well as systemic HOMA-IR in the insulin resistant rats.
• ALA inhibits the activity of pro-oxidant and pro-nitrating enzymes mainly in VAT in the insulin resistant rats.
• ALA improves GSH biosynthesis in both adipose tissue deposits (VAT and SAT) in the insulin resistant rats.
• ALA prevents oxidation, carbonylation and glycation of proteins and lipids mainly in VAT in the insulin resistant rats.
Author Contributions
Conceptualization, P.D-B., E.Ż., M.M., A.Z. and A.C.; data curation, P.D-B., E.Ż., and M.M.; funding acquisition, P.D-B., E.Ż. and A.C.; investigation, P.D-B., E.Ż., and M.M.; methodology, M.M.; visualization, P.D-B.; writing—original draft, P.D-B., E.Ż., M.M.; writing—review and editing, P.D-B., E.Ż., M.M. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding Sources
This work was supported by the Medical University of Bialystok, grants number
SUB/1/NN/21/001/1118 and SUB/1/DN/21/011/1118.
Statement of Ethics
Animal experiments conform to internationally accepted standards and have been approved by the appropriate institutional review body. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Local Ethics Committee of University of Warmia and Mazury in Olsztyn, Poland (protocol code 21/2017 approved on 28 March 2017).
The authors declare that no conflicts of interest exist.
| 1 Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047-1053. https://doi.org/10.2337/diacare.27.5.1047 |
||||
| 2 Yazıcı D, Sezer H: Insulin Resistance, Obesity and Lipotoxicity. Adv Exp Med Biol 2017;960:277-304. https://doi.org/10.1007/978-3-319-48382-5_12 |
||||
| 3 Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res 2007;48:1253-1262. https://doi.org/10.1194/jlr.R700005-JLR200 |
||||
| 4 Bays HE, González-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR: Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther 2008;6:343-368. https://doi.org/10.1586/14779072.6.3.343 |
||||
| 5 Boden G: Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes 2011;18:139-143. https://doi.org/10.1097/MED.0b013e3283444b09 |
||||
| 6 Itani SI, Ruderman NB, Schmieder F, Boden G: Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002;51:2005-2011. https://doi.org/10.2337/diabetes.51.7.2005 |
||||
| 7 Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I: Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752-1761. https://doi.org/10.1172/JCI21625 |
||||
| 8 Thirunavukkarasu V, Anuradha CV: Influence of alpha-lipoic acid on lipid peroxidation and antioxidant defence system in blood of insulin-resistant rats. Diabetes Obes Metab 2004;6:200-207. https://doi.org/10.1111/j.1462-8902.2004.00332.x |
||||
| 9 Estrada DE, Ewart HS, Tsakiridis T, Volchuk A, Ramlal T, Tritschler H, Klip A: Stimulation of glucose uptake by the natural coenzyme alpha-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway. Diabetes 1996;45:1798-1804. https://doi.org/10.2337/diabetes.45.12.1798 |
||||
| 10 Maddux BA, See W, Lawrence JC Jr, Goldfine AL, Goldfine ID, Evans JL: Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by mircomolar concentrations of alpha-lipoic acid. Diabetes 2001;50:404-410. https://doi.org/10.2337/diabetes.50.2.404 |
||||
| 11 Jeffrey S, Isaac Samraj P, Sundara Raj B: Therapeutic Benefits of Alpha-Lipoic Acid Supplementation in Diabetes Mellitus: A Narrative Review. J Diet Suppl 2021; DOI: 10.1080/19390211.2021.2020387. https://doi.org/10.1080/19390211.2021.2020387 |
||||
| 12 Thaakur S, Himabindhu G: Effect of alpha lipoic acid on the tardive dyskinesia and oxidative stress induced by haloperidol in rats. J Neural Transm 2009;116:807-814. https://doi.org/10.1007/s00702-009-0232-y |
||||
| 13 Myzak MC, Carr AC: Myeloperoxidase-dependent caspase-3 activation and apoptosis in HL-60 cells: protection by the antioxidants ascorbate and (dihydro)lipoic acid. Redox Rep 2002;7:47-53. https://doi.org/10.1179/135100002125000181 |
||||
| 14 Marsh SA, Pat BK, Gobe GC, Coombes JS: Evidence for a non-antioxidant, dose-dependent role of α -lipoic acid in caspase-3 and ERK2 activation in endothelial cells. Apoptosis 2005;10:657-665. https://doi.org/10.1007/s10495-005-1901-4 |
||||
| 15 Gomes MB, Negrato CA: Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol Metab Syndr 2014;6:80. https://doi.org/10.1186/1758-5996-6-80 |
||||
| 16 Erickson N, Zafron M, Harding S V., Marinangeli CPF, Rideout TC: Evaluating the Lipid-Lowering Effects of α-lipoic Acid Supplementation: A Systematic Review. J Diet Suppl 2020;17:753-767. https://doi.org/10.1080/19390211.2019.1651436 |
||||
| 17 Fiedler SE, Spain RI, Kim E, Salinthone S: Lipoic acid modulates inflammatory responses of monocytes and monocyte‐derived macrophages from healthy and relapsing‐remitting multiple sclerosis patients. Immunol Cell Biol 2021;99:107-115. https://doi.org/10.1111/imcb.12392 |
||||
| 18 Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff SP: Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem 1994;220:403-409. https://doi.org/10.1006/abio.1994.1357 |
||||
| 19 Borys J, Maciejczyk M, Krȩtowski AJ, Antonowicz B, Ratajczak-Wrona W, Jabłońska E, Załęski P, Waszkiel D, Ładny JR, Żukowski P, Zalewska A: The Redox Balance in Erythrocytes, Plasma, and Periosteum of Patients with Titanium Fixation of the Jaw. Front Physiol 2017;8:386. https://doi.org/10.3389/fphys.2017.00386 |
||||
| 20 Reznick AZ, Packer L: Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 1994;233:357-363. https://doi.org/10.1016/S0076-6879(94)33041-7 |
||||
| 21 Borys J, Maciejczyk M, Antonowicz B, Krętowski A, Sidun J, Domel E, Dąbrowski JR, Ładny JR, Morawska K, Zalewska A: Glutathione Metabolism, Mitochondria Activity, and Nitrosative Stress in Patients Treated for Mandible Fractures. J Clin Med 2019;8:127. https://doi.org/10.3390/jcm8010127 |
||||
| 22 Wink DA, Kim S, Coffin D, Cook JC, Vodovotz Y, Chistodoulou D, Jourd'heuil D, Grisham MB: Detection of S-nitrosothiols by fluorometric and colorimetric methods. Methods Enzymol 1999;301:201-211. https://doi.org/10.1016/S0076-6879(99)01083-6 |
||||
| 23 Akbari M, Ostadmohammadi V, Tabrizi R, Mobini M, Lankarani KB, Moosazadeh M, Heydari ST, Chamani M, Kolahdooz F, Asemi Z: The effects of alpha-lipoic acid supplementation on inflammatory markers among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab (Lond) 2018;15:39. https://doi.org/10.1186/s12986-018-0274-y |
||||
| 24 Muellenbach EA, Diehl CJ, Teachey MK, Lindborg KA, Archuleta TL, Harrell NB, Andersen G, Somoza V, Hasselwander O, Matuschek M, Henriksen EJ: Interactions of the advanced glycation end product inhibitor pyridoxamine and the antioxidant alpha-lipoic acid on insulin resistance in the obese Zucker rat. Metabolism 2008;57:1465-1472. https://doi.org/10.1016/j.metabol.2008.05.018 |
||||
| 25 Borcea V, Nourooz-Zadeh J, Wolff SP, Klevesath M, Hofmann M, Urich H, Wahl P, Ziegler R, Tritschler H, Halliwell B, Nawroth PP: alpha-Lipoic acid decreases oxidative stress even in diabetic patients with poor glycemic control and albuminuria. Free Radic Biol Med 1999;26:1495-1500. https://doi.org/10.1016/S0891-5849(99)00011-8 |
||||
| 26 Mohammadi V, Khorvash F, Feizi A, Askari G: Does Alpha-lipoic Acid Supplementation Modulate Cardiovascular Risk Factors in Patients with Stroke? A Randomized, Double-blind Clinical Trial. Int J Prev Med 2018;5:9-34. https://doi.org/10.4103/ijpvm.IJPVM_32_17 |
||||
| 27 Scherer PE: Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 2006;55:1537-1545. https://doi.org/10.2337/db06-0263 |
||||
| 28 Gómez-Hernández A, Beneit N, Díaz-Castroverde S, Escribano Ó: Differential Role of Adipose Tissues in Obesity and Related Metabolic and Vascular Complications. Int J Endocrinol 2016;2016:1216783. https://doi.org/10.1155/2016/1216783 |
||||
| 29 Lee BC, Lee J: Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta 2014;1842:446-462. https://doi.org/10.1016/j.bbadis.2013.05.017 |
||||
| 30 Mittendorfer B. Origins of metabolic complications in obesity: adipose tissue and free fatty acid trafficking. Curr Opin Clin Nutr Metab Care 2011;14:535-541. https://doi.org/10.1097/MCO.0b013e32834ad8b6 |
||||
| 31 Kucukgoncu S, Zhou E, Lucas KB, Tek C: Alpha-lipoic acid (ALA) as a supplementation for weight loss: results from a meta-analysis of randomized controlled trials. Obes Rev 2017;18:594-601. https://doi.org/10.1111/obr.12528 |
||||
| 32 Koh EH, Lee WJ, Lee SA, Kim EH, Cho EH, Jeong E, Kim DW, Kim MS, Park JY, Park KG, Lee HJ, Lee IK, Lim S, Jang HC, Lee KH, Lee KU: Effects of alpha-lipoic Acid on body weight in obese subjects. Am J Med 2011;124:85.e1-8. https://doi.org/10.1016/j.amjmed.2010.08.005 |
||||
| 33 Biewenga GP, Haenen GR, Bast A: The pharmacology of the antioxidant lipoic acid. Gen Pharmacol 1997;29:315-331. https://doi.org/10.1016/S0306-3623(96)00474-0 |
||||
| 34 Carbonelli MG, Di Renzo L, Bigioni M, Di Daniele N, De Lorenzo A, Fusco MA: Alpha-lipoic acid supplementation: a tool for obesity therapy?. Curr Pharm Des 2010;16:840-846. https://doi.org/10.2174/138161210790883589 |
||||
| 35 Ansar H, Mazloom Z, Kazemi F, Hejazi N: Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J 2011;32:584-588. | ||||
| 36 Gao Z, Zhang X, Zuberi A, et al.: Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol 2004;18:2024-2034. https://doi.org/10.1210/me.2003-0383 |
||||
| 37 Harford KA, Reynolds CM, McGillicuddy FC, Roche HM: Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc Nutr Soc 2011;70:408-417. https://doi.org/10.1017/S0029665111000565 |
||||
| 38 Mittendorfer B.: Origins of metabolic complications in obesity: adipose tissue and free fatty acid trafficking. Curr Opin Clin Nutr Metab Care 2011;14,:535-541. https://doi.org/10.1097/MCO.0b013e32834ad8b6 |
||||
| 39 Kahn CR, Wang G, Lee KY: Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest 2019;129:3990-4000. https://doi.org/10.1172/JCI129187 |
||||
| 40 Villarroya F, Cereijo R, Gavaldà-Navarro A, Villarroya J, Giralt M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J Intern Med 2018;284:492-504. https://doi.org/10.1111/joim.12803 |
||||
| 41 Nakao C, Ookawara T, Sato Y, Kizaki T, Imazeki N, Matsubara O, Haga S, Suzuki K, Taniguchi N, Ohno H: Extracellular superoxide dismutase in tissues from obese (ob/ob) mice. Free Radic Res 2000;33:229-241. https://doi.org/10.1080/10715760000301401 |
||||
| 42 Farhangi MA, Mesgari-Abbasi M, Hajiluian G, Nameni G, Shahabi P: Adipose Tissue Inflammation and Oxidative Stress: the Ameliorative Effects of Vitamin D. Inflammation 2017;40:1688-1697. https://doi.org/10.1007/s10753-017-0610-9 |
||||
| 43 Amirkhizi F, Siassi F, Minaie S, Djalali M, Rahimi A, Chamari M. Is obesity associated with increased plasma lipid peroxidation and oxidative stress in women. ARYA Atheroscler 2007;2:189-192. | ||||
| 44 Sun H, Yao W, Tang Y, Zhuang W, Wu D, Huang S, Sheng H: Urinary exosomes as a novel biomarker for evaluation of α-lipoic acid's protective effect in early diabetic nephropathy. J Clin Lab Anal 2017;31:e22129. https://doi.org/10.1002/jcla.22129 |
||||
| 45 Kravchenko LV, Aksenov IV, Nikitin NS, Guseva GV, Avrenyeva LI, Trusov NV, Balakina AS, Tutelyan VA: Lipoic Acid Exacerbates Oxidative Stress and Lipid Accumulation in the Liver of Wistar Rats Fed a Hypercaloric Choline-Deficient Diet. Nutrients 2021;10;13:1999. https://doi.org/10.3390/nu13061999 |
||||
| 46 Yang RL, Li W, Shi YH, Le GW: Lipoic acid prevents high-fat diet-induced dyslipidemia and oxidative stress: a microarray analysis. Nutrition 2008;24:582-588. https://doi.org/10.1016/j.nut.2008.02.002 |
||||
| 47 Golbidi S, Badran M, Laher IL Diabetes and alpha lipoic Acid. Front Pharmacol 2011;2:69. https://doi.org/10.3389/fphar.2011.00069 |
||||
| 48 Jamdar SC, Soo E, Cao WF: Effect of glutathione deficiency on the adipocyte sn-glycerol-3-phosphate acyltransferase. Biochim Biophys Acta 1998;1393:41-48. https://doi.org/10.1016/S0005-2760(98)00055-1 |
||||
| 49 Jankovic A, Korac A, Srdic-Galic B, Buzadzic B, Otasevic V, Stancic A, Vucetic M, Markelic M, Velickovic K, Golic I, Korac B: Differences in the redox status of human visceral and subcutaneous adipose tissues--relationships to obesity and metabolic risk. Metabolism 2014;63:661-671. https://doi.org/10.1016/j.metabol.2014.01.009 |
||||
| 50 Hill JH, Solt C, Foster MT: Obesity associated disease risk: the role of inherent differences and location of adipose depots. Horm Mol Biol Clin Investig 2018; DOI: 10.1515/hmbci-2018-0012. https://doi.org/10.1515/hmbci-2018-0012 |
||||
| 51 Ayala A, Muñoz MF, Argüelles S: Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014;2014:360438. https://doi.org/10.1155/2014/360438 |
||||
| 52 Chen J, Zeng L, Xia T, Li S, Yan T, Wu S, Qiu G, Liu Z. Toward a biomarker of oxidative stress: a fluorescent probe for exogenous and endogenous malondialdehyde in living cells. Anal Chem 2015;87:8052-8056. https://doi.org/10.1021/acs.analchem.5b02032 |
||||
| 53 Nomura K, Imai H, Koumura T, Kobayashi T, Nakagawa Y: Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem J 2000;351:183-193. https://doi.org/10.1042/bj3510183 |
||||
| 54 Hauck AK, Bernlohr DA: Oxidative stress and lipotoxicity. J Lipid Res 2016;57:1976-1986. https://doi.org/10.1194/jlr.R066597 |
||||
| 55 Szymczak-Pajor I, Miazek K, Selmi A, Balcerczyk A, Śliwińska A. The Action of Vitamin D in Adipose Tissue: Is There the Link between Vitamin D Deficiency and Adipose Tissue-Related Metabolic Disorders? Int J Mol Sci 2022;23:956. https://doi.org/10.3390/ijms23020956 |
||||
| 56 Choromańska B, Myśliwiec P, Łuba M, Wojskowicz P, Myśliwiec H, Choromańska K, Dadan J, Żendzian-Piotrowska M, Zalewska A, Maciejczyk M. Bariatric Surgery Normalizes Protein Glycoxidation and Nitrosative Stress in Morbidly Obese Patients. Antioxidants (Basel) 2020;9:1087. https://doi.org/10.3390/antiox9111087 |
||||
| 57 Gutiérrez L, García JR, Rincón Mde J, Ceballos GM, Olivares IM: Effect of a hypocaloric diet in the oxidative stress in obese subjects without prescription of exercise and antioxidants. Med Clin (Barc) 2015;145:1-6. https://doi.org/10.1016/j.medcle.2015.12.043 |
||||
| 58 Monden M, Koyama H, Otsuka Y, Morioka T, Mori K, Shoji T, Mima Y, Motoyama K, Fukumoto S, Shioi A, Emoto M, Yamamoto Y, Yamamoto H, Nishizawa Y, Kurajoh M, Yamamoto T, Inaba M: Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of Toll-like receptor 2. Diabetes 2013;62:478-489. https://doi.org/10.2337/db11-1116 |
||||
| 59 Dozio E, Vianello E, Briganti S, Lamont J, Tacchini L, Schmitz G, Corsi Romanelli MM: Expression of the Receptor for Advanced Glycation End Products in Epicardial Fat: Link with Tissue Thickness and Local Insulin Resistance in Coronary Artery Disease. J Diabetes Res 2016;2016:2327341. https://doi.org/10.1155/2016/2327341 |
||||
| 60 Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Chayama K, Oshima T. Effect of obesity on endothelium-dependent, nitric oxide-mediated vasodilation in normotensive individuals and patients with essential hypertension. Am J Hypertens 2001;14:1038-1045. https://doi.org/10.1016/S0895-7061(01)02191-4 |
||||
| 61 Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, Beguinot F, Miele C: Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int J Mol Sci 2019;20:2358. https://doi.org/10.3390/ijms20092358 |
||||