Corresponding Author: Itsuro Kazama
Miyagi University, School of Nursing, 1-1 Gakuen, Taiwa-cho, Kurokawa-gun, Miyagi 981-3298 (Japan)
Tel. +81-22-377-8246; Fax +81-22-377-8290 , E-Mail kazamai@myu.ac.jp
Pyridoxine Synergistically Potentiates Mast Cell-Stabilizing Property of Ascorbic Acid
Itsuro Kazamaa Yukine Satoa,b Tsutomu Tamadac
aMiyagi University, School of Nursing, Gakuen, Taiwa-cho, Kurokawa-gun, Miyagi, Japan, bTohoku University Hospital, Seiryo-cho, Aoba-ku, Sendai, Miyagi, Japan, cDepartment of Respiratory Medicine, Tohoku University Graduate School of Medicine, Seiryo-cho, Aoba-ku, Sendai, Miyagi, Japan
Introduction
Vitamins are essential nutrients that the body needs to function properly [1]. Among them, vitamin C (ascorbic acid) and B6 (pyridoxine) are water-soluble vitamins that play roles in immune function, protein metabolism, growth and organ development [1]. Besides these physiological properties, previous studies revealed their roles in ameliorating the symptoms of allergic disorders, such as anaphylaxis and bronchial asthma, indicating their anti-allergic properties [2-4]. To elucidate the mechanisms underlying the anti-allergic properties of these vitamins, previous studies tried to evaluate the amount of histamine released from mast cells [2, 5-7]. However, since mast cells also release other chemical mediators or various types of growth factors [8], the exocytotic process itself must be monitored directly to determine the ability of vitamins in stabilizing mast cells. In our previous studies, by continuously monitoring the changes in whole-cell membrane capacitance (Cm) in mast cells, we provided electrophysiological evidence for the first time that anti-allergic drugs, anti-microbial drugs, corticosteroids and catecholamines exert mast cell-stabilizing properties [9-13]. Recently, we have additionally revealed that food constituents, such as caffeine and catechin, inhibit the process of exocytosis in mast cells in a dose-dependent manner [14]. In the present study, employing the standard patch-clamp whole-cell recording technique in rat peritoneal mast cells, we examined the effects of vitamins, such as ascorbic acid and pyridoxine, on the changes in the Cm to quantify their ability to stabilize mast cells. Here, this study provides electrophysiological evidence that ascorbic acid and pyridoxine inhibit the process of exocytosis in a dose-dependent manner for the first time. This study also shows that low concentrations of pyridoxine synergistically potentiate the mast cell-stabilizing property of ascorbic acid, which may be attributable to the synergistic interaction of their antioxidant properties.
Materials and Methods
Cell Sources and Preparation
Male Wistar rats no less than 25 weeks old were purchased from CLEA Japan Inc. (Tokyo, Japan). We profoundly anaesthetized the rats with isoflurane and sacrificed them by cervical dislocation. The protocols for the use of animals were approved by the Animal Care and Use Committee of Miyagi University. As we previously described [9-12, 15], we washed rat peritoneum using standard external (bathing) solution which consists of (in mM): NaCl, 145; KCl, 4.0; CaCl2, 1.0; MgCl2, 2.0; HEPES, 5.0; bovine serum albumin, 0.01 % (pH 7.2 adjusted with NaOH) and isolated mast cells from the peritoneal cavity. We maintained the isolated mast cells at room temperature (22-24 °C) for about 8 hours until use. The suspension of mast cells was spread on a chamber placed on the headstage of an inverted microscope (Nikon, Tokyo, Japan). Mast cells were easily distinguished from other cell types since they included characteristic secretory granules within the cells [9-15].
Quantification of Mast Cell Degranulation
Ascorbic acid, purchased from Wako Pure Chem Ind. (Osaka, Japan), and pyridoxine hydrochloride, from Kanto Chemical Co., Inc. (Tokyo, Japan), were separately dissolved in the external solution at final concentrations of 1, 2, 5 and 10 mM. After we incubated mast cells in these solutions or a solution without the substances, exocytosis was externally induced by compound 48/80 (Sigma-Aldrich Co., St. Louis, MO, USA; final concentration 10 µg/ml) [9-15]. We obtained bright-field images from randomly chosen 0.1-mm2 fields of view (10 views from each condition), as described previously [9-15]. We counted the number of degranulated mast cells (definition; cells surrounded by more than 8 granules outside the cell membrane) and calculated their ratio to all mast cells.
Electrical Setup and Membrane Capacitance Measurements
As we described in our previous studies [9-13, 15], we employed an EPC-9 patch-clamp amplifier system (HEKA Electronics, Lambrecht, Germany) and conducted standard whole-cell patch-clamp recordings. Briefly, we maintained the patch pipette resistance between 3-5 MΩ when plugged with internal (patch pipette) solution which consists of (in mM): K-glutamate, 145; MgCl2, 2.0; Hepes, 5.0 (pH 7.2 adjusted with KOH). We added 100 µM guanosine 5’-o-(3-thiotriphosphate) (GTP-γ-S) (Sigma Aldrich Co.) into the internal solution to endogenously induce exocytosis in mast cells [9-13, 15]. We induced a giga-seal formation on a single mast cell spread in the external solutions with or without different concentrations of ascorbic acid or pyridoxine hydrochloride (1, 2, 5 and 10 mM). Then we briefly sucked the pipette to rupture the patch membrane and perfused GTP-γ-S into the cells. To monitor the membrane capacitance of mast cells, we conducted a sine plus DC protocol employing the Lock-in amplifier of an EPC-9 Pulse program. We superimposed an 800-Hz sinusoidal command voltage on the holding potential of -80 mV. We continuously monitored the membrane capacitance (Cm), membrane conductance (Gm) and series conductance (Gs) during the whole-cell recording configuration. We performed all experiments at room temperature.
Statistical Analyses
Data were analyzed using PulseFit software (HEKA Electronics, Lambrecht, Germany) and Microsoft Excel (Microsoft Corporation, Redmond, Wash., USA) and reported as means ± SEM. Statistical significance was assessed by two-way ANOVA. A value of p<0.05 was considered significant.
Results
Effects of ascorbic acid and pyridoxine on degranulation of rat peritoneal mast cells
Mast cells incubated in the external solution alone or 1 mM ascorbic acid showed many wrinkles on their cell surface and released secretory granules as a consequence of exocytosis (Fig. 1Ab, c vs. a). However, in the case of 2 mM or higher concentration of ascorbic acid, such findings suggestive of exocytosis were partially or completely suppressed (Fig. 1Ad, e, f). Quantitatively, 1 mM ascorbic acid did not affect the numbers of degranulating mast cells (Fig. 1B). However, 2 mM ascorbic acid significantly decreased the number of degranulating mast cells (external solution, 97.6 ± 0.71 % vs. 2 mM, 51.5 ± 5.03 %; n=10, p<0.05; Fig. 1B). Five and 10 mM ascorbic acid further reduced the numbers of degranulating cells (5 mM, 7.64 ± 1.41 %; 10 mM, 4.82 ± 1.22 %; n=10, p<0.05; Fig. 1B).
Like these effects of ascorbic acid (Fig. 1), 1 mM pyridoxine did not affect the degranulation of mast cells (Fig. 2Ab, c vs. a) and the numbers of them were almost comparable to those incubated in the external solution alone (Fig. 2B). However, 2 mM or higher concentration of pyridoxine remarkably suppressed the process of exocytosis (Fig. 2Ad, e, f) and significantly suppressed the numbers of degranulating mast cells (external solution, 97.5 ± 0.80 % vs. 2 mM, 73.9 ± 8.50 %; n=10, p<0.05; Fig. 2B). Of note, 5 and 10 mM pyridoxine showed a marked reduction in the numbers of degranulating mast cells (5 mM, 11.3 ± 0.75 %; 10 mM, 5.82 ± 1.27 %; n=10, p<0.05; Fig. 2B).
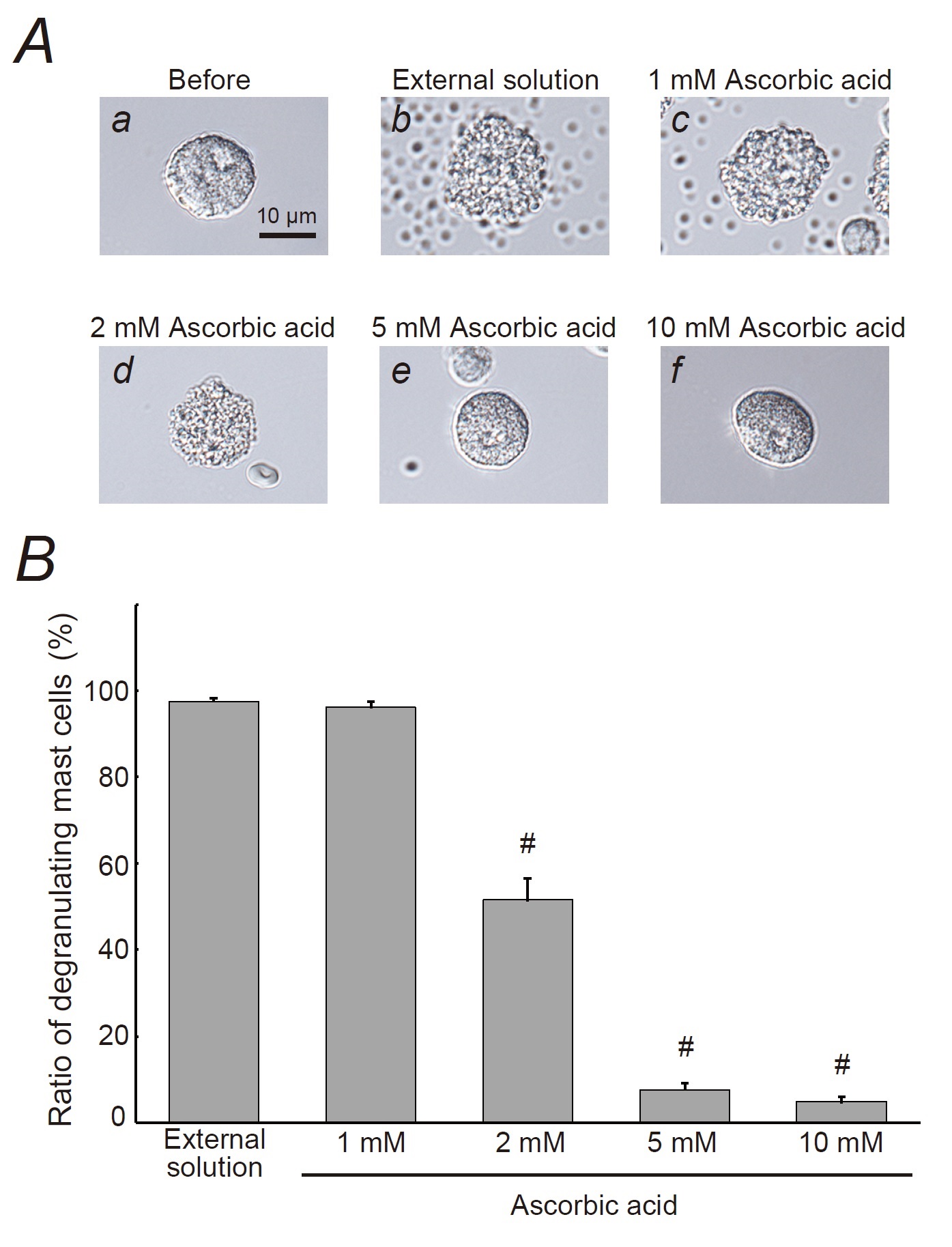
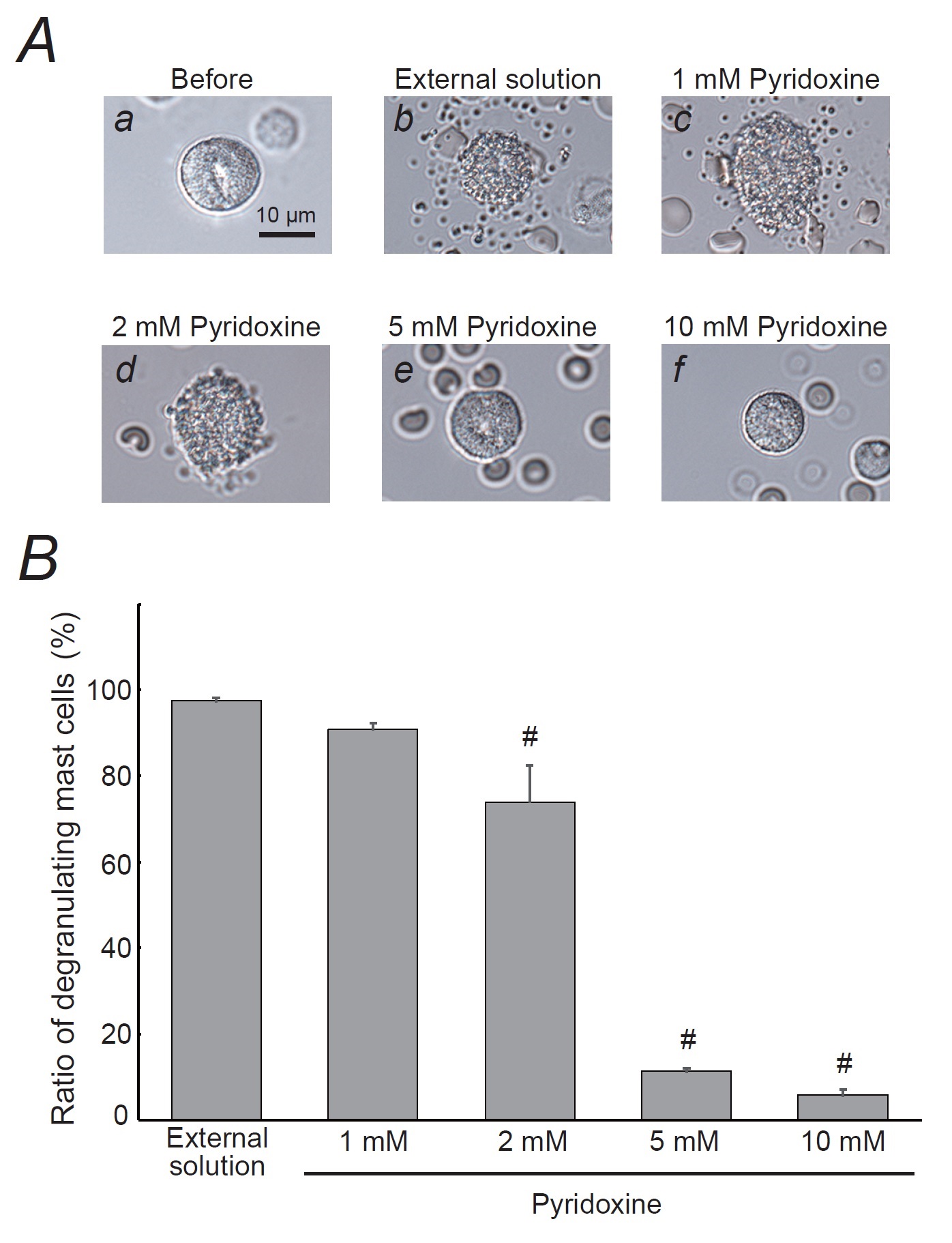
Effects of ascorbic acid and pyridoxine on whole-cell membrane capacitance in rat peritoneal mast cells
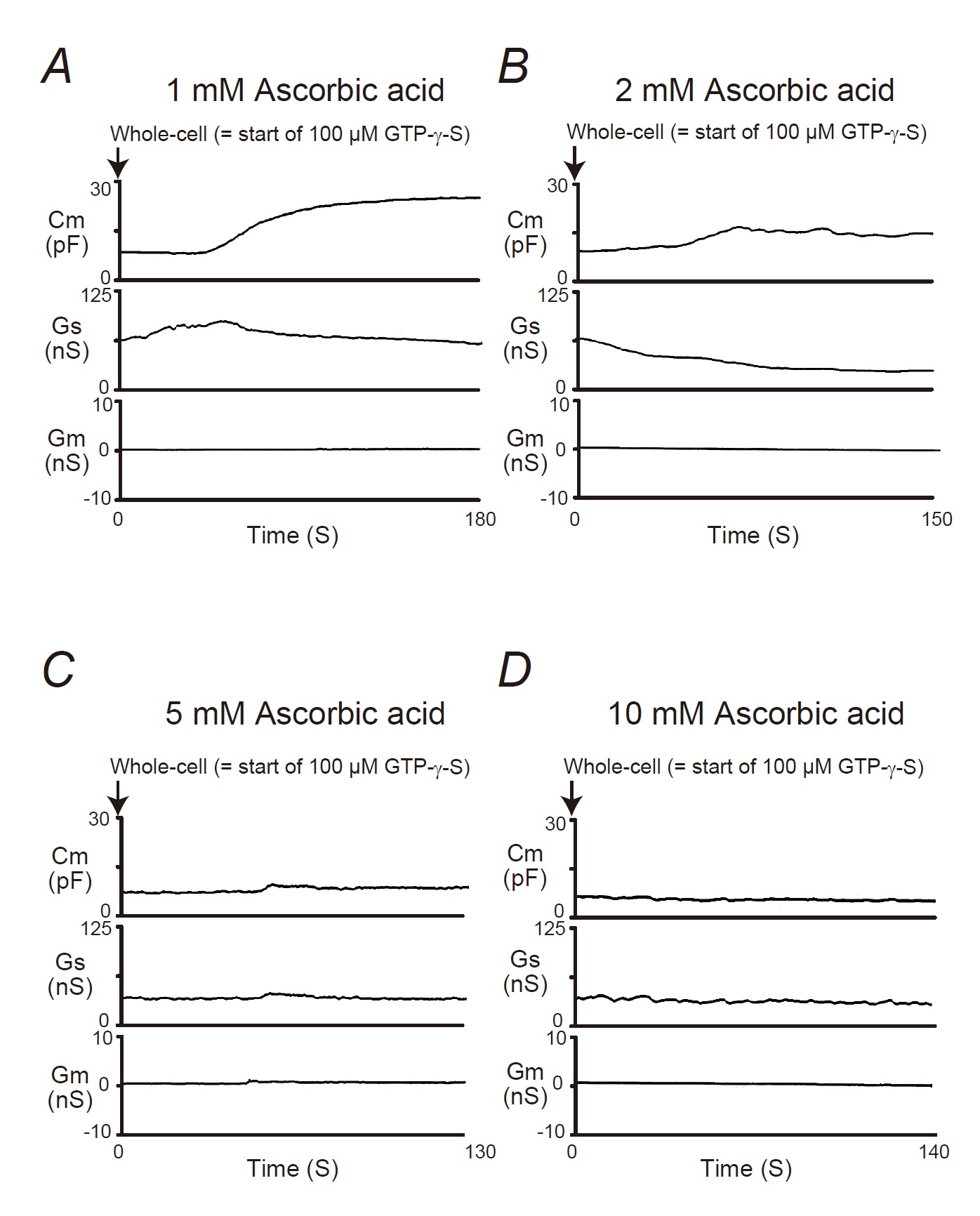
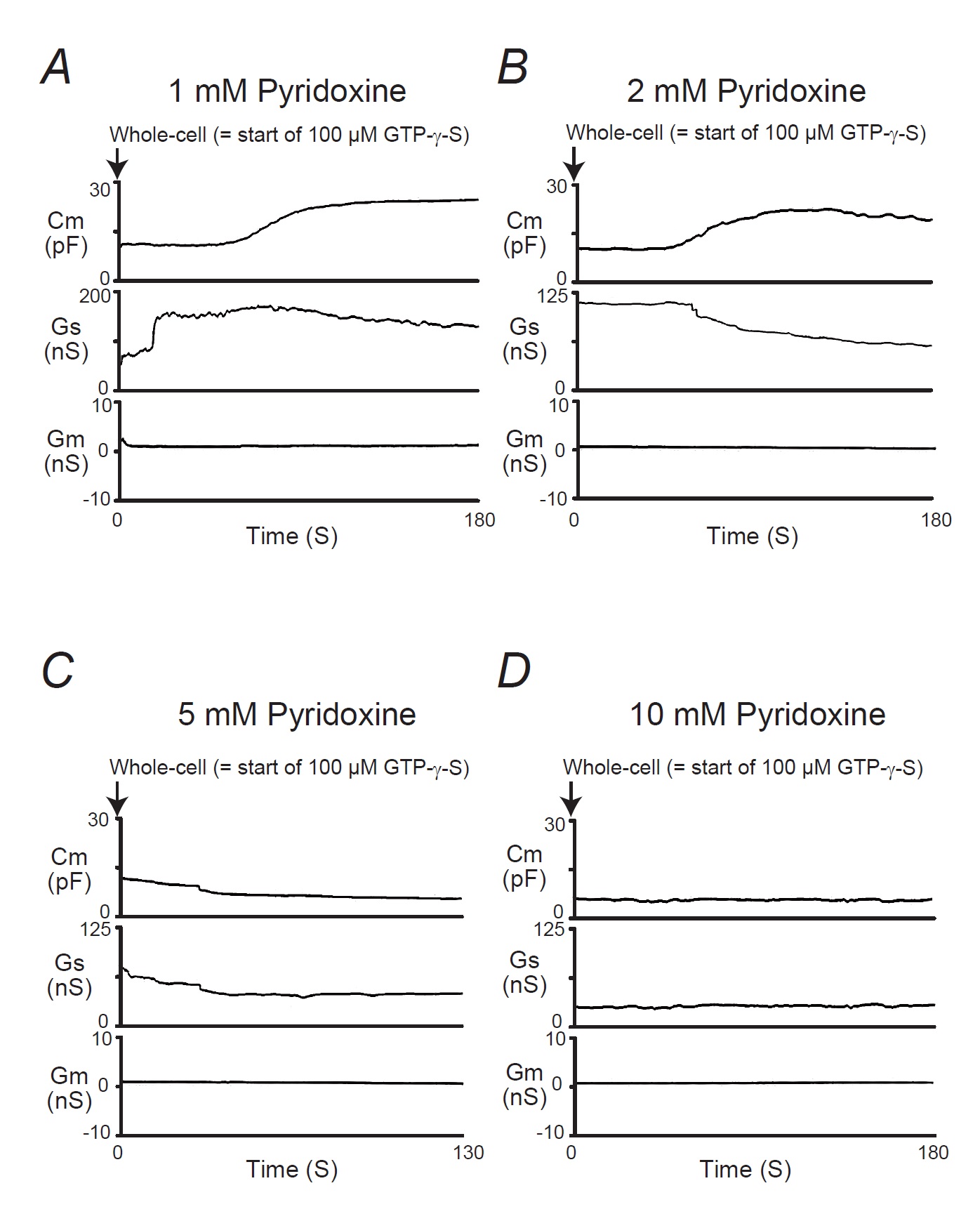
In our previous studies, microscopic changes in megakaryocyte or lymphocyte membranes were exactly monitored by measuring the whole-cell membrane capacitance (Cm) [16-24]. Of note, in mast cells, the process of degranulation during exocytosis was continuously monitored using the degree of the increase in the Cm [9-13, 15, 25, 26]. Hence, in our study, to investigate the effects of ascorbic acid or pyridoxine on the process of exocytosis, we pre-incubated mast cells in ascorbic acid- or pyridoxine- containing external solutions and monitored the changes in Cm (Fig. 3 and 4). In these figures, we showed the effects of 1, 2, 5 and 10 mM ascorbic acid (Fig. 3) and pyridoxine (Fig. 4) on the Cm, series conductance (Gs) and membrane conductance (Gm). Table 1 summarizes the changes in the Cm.
Reflecting the endogenous induction of exocytosis [9-13, 15, 27, 28], the internal addition of GTP-γ-S into mast cells markedly increased the value of Cm (from 7.85 ± 1.44 to 30.5 ± 5.11 pF, n=5, p<0.05; Table 1). When mast cells were pre-incubated with 1 or 2 mM ascorbic acid, the addition of GTP-γ-S tended to increase the Cm similarly to that of mast cells pre-incubated with the external solution alone (Fig. 3A, B). However, compared to the external solution alone, 2 mM ascorbic acid significantly suppressed the increase in the Cm (∆Cm) (external solution, 22.7 ± 4.39 vs. 2 mM, 8.87 ± 3.22 pF; n=16, p<0.05; Table 1). In the case of relatively higher concentrations (5, 10 mM) of ascorbic acid, the GTP-γ-S-induced increase in the Cm was markedly suppressed (Fig. 3C and D; 5 mM, -0.93 ± 1.35 pF, n=6, p<0.05; 10 mM, -0.34 ± 1.22 pF, =7, p<0.05; Table 1).
Similarily to the effects of ascorbic acid (Fig. 3), in mast cells pre-incubated with 1 or 2 mM pyridoxine, the addition of GTP-γ-S tended to increase the Cm (Fig. 4A, B). However, compared to the external solution alone, 2 mM pyridoxine significantly suppressed the increase in the Cm (external solution, 22.7 ± 4.39 vs. 2mM, 12.8 ± 1.65 pF; n=13, p<0.05; Table 1). In the case of higher concentrations (5, 10 mM) of pyridoxine, the GTP-γ-S-induced increase in the Cm was markedly suppressed (Fig. 4C and D; 5 mM, 0.65 ± 1.34 pF, n=6, p <0.05; 10 mM, 0.56 ± 1.43 pF, n=6, p<0.05; Table 1). These findings suggest that vitamins, such as ascorbic acid and pyridoxine, inhibit the exocytotic process of mast cells in a dose-dependent manner.
In Fig. 3A and 4A, the Gs initially increased immediately after the rupture the patch membrane, which preceded the increase in the Cm (Fig. 3A and 4A). This reflected the decrease in the series resistance due to the generation of the electrical connection between the pipette electrodes and the cells interior [27]. Then, as previously demonstrated in degranulating mast cells [26, 27], the Gs tended to decrease with the internalization of GTP-γ-S, reflecting the gradual increase in the series resistance. However, the Gs was not largely affected for the rest of the observation period, suggesting that the cells were accurately voltage-clamped during the measurement of the Cm.
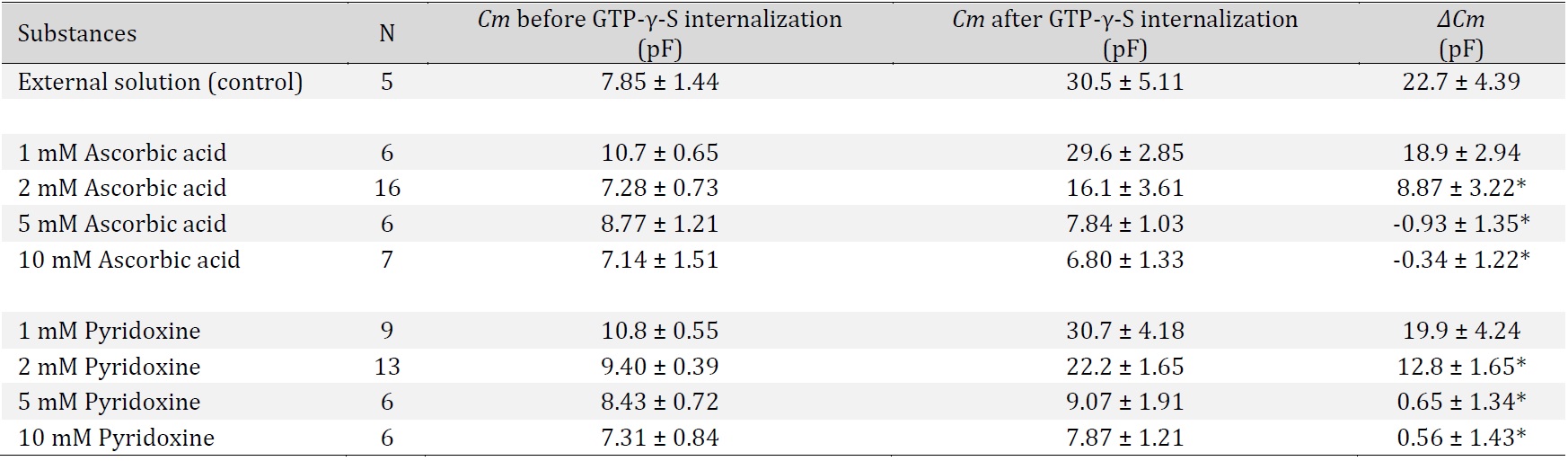
Combined effects of ascorbic acid with low concentrations of pyridoxine on degranulation of rat peritoneal mast cells
As shown in Fig. 2 and 4, the suppressive effects of relatively lower concentrations of pyridoxine (1, 2 mM) on the degranulation of mast cells and the increase in the Cm were much smaller than those of relatively higher concentrations of pyridoxine (5, 10 mM) (Fig. 2, 4). However, we recently revealed that lower concentrations of food substances, such as catechin, could synergistically potentiate the mast cell-stabilizing property of caffeine [14]. To investigate the similar additive therapeutic efficacy of low dose substances, we examined the effects of 1 or 2 mM pyridoxine on the ascorbic acid-induced inhibition of exocytosis (Fig. 5).
Consistent with our results shown in Fig. 1, 2 mM ascorbic acid partially halted the process of exocytosis in mast cells (Fig. 5A b vs. a) and significantly but not markedly reduced the number of degranulating mast cells (control, 97.4 ± 0.70 % vs. 2 mM ascorbic acid, 45.7 ± 7.38 %; n=10, p<0.05; Fig. 5B). However, surprisingly enough, in the presence of 1 or 2 mM pyridoxine, the exocytotic process of mast cells was almost completely halted (Fig. 5Ac, d vs. b). Regarding the numbers of degranulating mast cells, they were more markedly decreased than those with 2 mM ascorbic acid alone (2 mM ascorbic acid + 1 mM pyridoxine, 2.19 ± 0.81 %, n=10, p<0.05; 2 mM ascorbic acid + 2 mM pyridoxine, 2.53 ± 1.08 %, n=10, p<0.05; Fig. 5B). These findings suggest that the inhibitory effect of ascorbic acid on exocytosis was augmented, and that lower concentrations of pyridoxine synergistically potentiated the mast cell-stabilizing property of ascorbic acid.
The data used to support the findings of this study are available from the corresponding author upon request.
Author Contributions
IK and YS performed the experiments and analyzed the data. IK designed the experiments, interpreted the results and wrote the paper. TT provided logistical support and advised on the project. All authors read and approved the final manuscript.
Funding
This study was supported by the Salt Science Research Foundation, No. 2123 to IK.
Statement of Ethics
This study was performed in accordance with the guide for the care and use of laboratory animals of Miyagi University, which included ethical considerations. The protocols for the use of the animals were approved by the Animal Care and Use Committee of Miyagi University (Protocol numbers: 2020-04-1, 2021-05-2, 2022-02-01).
The authors declare no conflicts of interest.
| 1 Ofoedu CE, Iwouno JO, Ofoedu EO, Ogueke CC, Igwe VS, Agunwah IM, Ofoedum AF, Chacha JS, Muobike OP, Agunbiade AO, Njoku NE, Nwakaudu AA, Odimegwu NE, Ndukauba OE, Ogbonna CU, Naibaho J, Korus M, Okpala COR: Revisiting food-sourced vitamins for consumer diet and health needs: a perspective review, from vitamin classification, metabolic functions, absorption, utilization, to balancing nutritional requirements. PeerJ 2021; DOI: :10.7717/peerj.11940. https://doi.org/10.7717/peerj.11940 |
||||
| 2 Alvarez RG, Mesa MG: Ascorbic acid and pyridoxine in experimental anaphylaxis. Agents Actions 1981;11:89-93. https://doi.org/10.1007/BF01991466 |
||||
| 3 Hagel AF, Layritz CM, Hagel WH, Hagel HJ, Hagel E, Dauth W, Kressel J, Regnet T, Rosenberg A, Neurath MF, Molderings GJ, Raithel M: Intravenous infusion of ascorbic acid decreases serum histamine concentrations in patients with allergic and non-allergic diseases. Naunyn Schmiedebergs Arch Pharmacol 2013;386:789-793. https://doi.org/10.1007/s00210-013-0880-1 |
||||
| 4 Ubbink JB, Vermaak WJ, Delport R, Serfontein WJ, Bartel P: The relationship between vitamin B6 metabolism, asthma, and theophylline therapy. Ann N Y Acad Sci 1990;585:285-294. https://doi.org/10.1111/j.1749-6632.1990.tb28061.x |
||||
| 5 Clemetson CA: Histamine and ascorbic acid in human blood. J Nutr 1980;110:662-668. https://doi.org/10.1093/jn/110.4.662 |
||||
| 6 Anogeianaki A, Castellani ML, Tripodi D, Toniato E, De Lutiis MA, Conti F, Felaco P, Fulcheri M, Theoharides TC, Galzio R, Caraffa A, Antinolfi P, Cuccurullo C, Ciampoli C, Felaco M, Cerulli G, Pandolfi F, Sabatino G, Neri G, Shaik-Dasthagirisaheb YB: Vitamins and mast cells. Int J Immunopathol Pharmacol 2010;23:991-996. https://doi.org/10.1177/039463201002300403 |
||||
| 7 Garcia M, Gonzalez R: [Effect of pyridoxine on histamine liberation and degranulation of rat mast cells]. Allergol Immunopathol (Madr) 1979;7:427-432. | ||||
| 8 Gruber BL: Mast cells in the pathogenesis of fibrosis. Curr Rheumatol Rep 2003;5:147-153. https://doi.org/10.1007/s11926-003-0043-3 |
||||
| 9 Baba A, Tachi M, Maruyama Y, Kazama I: Olopatadine inhibits exocytosis in rat peritoneal mast cells by counteracting membrane surface deformation. Cell Physiol Biochem 2015;35:386-396. https://doi.org/10.1159/000369704 |
||||
| 10 Baba A, Tachi M, Ejima Y, Endo Y, Toyama H, Matsubara M, Saito K, Yamauchi M, Miura C, Kazama I: Anti-Allergic Drugs Tranilast and Ketotifen Dose-dependently Exert Mast Cell-Stabilizing Properties. Cell Physiol Biochem 2016;38:15-27. https://doi.org/10.1159/000438605 |
||||
| 11 Mori T, Abe N, Saito K, Toyama H, Endo Y, Ejima Y, Yamauchi M, Goto M, Mushiake H, Kazama I: Hydrocortisone and dexamethasone dose-dependently stabilize mast cells derived from rat peritoneum. Pharmacol Rep 2016;68:1358-1365. https://doi.org/10.1016/j.pharep.2016.09.005 |
||||
| 12 Kazama I, Saito K, Baba A, Mori T, Abe N, Endo Y, Toyama H, Ejima Y, Matsubara M, Yamauchi M: Clarithromycin Dose-Dependently Stabilizes Rat Peritoneal Mast Cells. Chemotherapy 2016;61:295-303. https://doi.org/10.1159/000445023 |
||||
| 13 Abe N, Toyama H, Ejima Y, Saito K, Tamada T, Yamauchi M, Kazama I: alpha 1-Adrenergic Receptor Blockade by Prazosin Synergistically Stabilizes Rat Peritoneal Mast Cells. Biomed Res Int 2020;2020:3214186. https://doi.org/10.1155/2020/3214186 |
||||
| 14 Yashima M, Sato Y, Kazama I: Catechin synergistically potentiates mast cell-stabilizing property of caffeine. Allergy Asthma Clin Immunol 2021;17:1. https://doi.org/10.1186/s13223-020-00502-5 |
||||
| 15 Kazama I, Maruyama Y, Takahashi S, Kokumai T: Amphipaths differentially modulate membrane surface deformation in rat peritoneal mast cells during exocytosis. Cell Physiol Biochem 2013;31:592-600. https://doi.org/10.1159/000350079 |
||||
| 16 Kazama I, Maruyama Y, Nakamichi S: Aspirin-induced microscopic surface changes stimulate thrombopoiesis in rat megakaryocytes. Clin Appl Thromb Hemost 2014;20:318-325. https://doi.org/10.1177/1076029612461845 |
||||
| 17 Kazama I, Maruyama Y, Murata Y: Suppressive effects of nonsteroidal anti-inflammatory drugs diclofenac sodium, salicylate and indomethacin on delayed rectifier K+-channel currents in murine thymocytes. Immunopharmacol Immunotoxicol 2012;34:874-878. https://doi.org/10.3109/08923973.2012.666249 |
||||
| 18 Kazama I, Maruyama Y, Matsubara M: Benidipine persistently inhibits delayed rectifier K(+)-channel currents in murine thymocytes. Immunopharmacol Immunotoxicol 2013;35:28-33. https://doi.org/10.3109/08923973.2012.723011 |
||||
| 19 Kazama I, Maruyama Y: Differential effects of clarithromycin and azithromycin on delayed rectifier K(+)-channel currents in murine thymocytes. Pharm Biol 2013;51:760-765. https://doi.org/10.3109/13880209.2013.764539 |
||||
| 20 Kazama I, Baba A, Maruyama Y: HMG-CoA reductase inhibitors pravastatin, lovastatin and simvastatin suppress delayed rectifier K(+)-channel currents in murine thymocytes. Pharmacol Rep 2014;66:712-717. https://doi.org/10.1016/j.pharep.2014.03.002 |
||||
| 21 Baba A, Tachi M, Maruyama Y, Kazama I: Suppressive Effects of Diltiazem and Verapamil on Delayed Rectifier K+-Channel Currents in Murine Thymocytes. Pharmacol Rep 2015 67:959-964. https://doi.org/10.1016/j.pharep.2015.01.009 |
||||
| 22 Kazama I, Ejima Y, Endo Y, Toyama H, Matsubara M, Baba A, Tachi M: Chlorpromazine-induced changes in membrane micro-architecture inhibit thrombopoiesis in rat megakaryocytes. Biochim Biophys Acta 2015;1848:2805-2812. https://doi.org/10.1016/j.bbamem.2015.08.013 |
||||
| 23 Kazama I, Baba A, Endo Y, Toyama H, Ejima Y, Matsubara M, Tachi M: Salicylate Inhibits Thrombopoiesis in Rat Megakaryocytes by Changing the Membrane Micro-Architecture. Cell Physiol Biochem 2015;35:2371-2382. https://doi.org/10.1159/000374039 |
||||
| 24 Saito K, Abe N, Toyama H, Ejima Y, Yamauchi M, Mushiake H, Kazama I: Second-Generation Histamine H1 Receptor Antagonists Suppress Delayed Rectifier K(+)-Channel Currents in Murine Thymocytes. Biomed Res Int 2019;2019:6261951. https://doi.org/10.1155/2019/6261951 |
||||
| 25 Fernandez JM, Neher E, Gomperts BD: Capacitance measurements reveal stepwise fusion events in degranulating mast cells. Nature 1984;312:453-455. https://doi.org/10.1038/312453a0 |
||||
| 26 Lorenz D, Wiesner B, Zipper J, Winkler A, Krause E, Beyermann M, Lindau M, Bienert M: Mechanism of peptide-induced mast cell degranulation. Translocation and patch-clamp studies. J Gen Physiol 1998;112:577-591. https://doi.org/10.1085/jgp.112.5.577 |
||||
| 27 Neher E: The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol 1988;395:193-214. https://doi.org/10.1113/jphysiol.1988.sp016914 |
||||
| 28 Penner R, Neher E: Secretory responses of rat peritoneal mast cells to high intracellular calcium. FEBS Lett 1988;226:307-313. https://doi.org/10.1016/0014-5793(88)81445-5 |
||||
| 29 Kakavas S, Karayiannis D, Mastora Z: The Complex Interplay between Immunonutrition, Mast Cells, and Histamine Signaling in COVID-19. Nutrients 2021;13:3458. https://doi.org/10.3390/nu13103458 |
||||
| 30 Chatterjee IB, Gupta SD, Majumder AK, Nandi BK, Subramanian N: Effect of ascorbic acid on histamine metabolism in scorbutic guinea-pigs. J Physiol 1975;251:271-279. https://doi.org/10.1113/jphysiol.1975.sp011091 |
||||
| 31 Penner R: Multiple signaling pathways control stimulus-secretion coupling in rat peritoneal mast cells. Proc Natl Acad Sci U S A 1988;85:9856-9860. https://doi.org/10.1073/pnas.85.24.9856 |
||||
| 32 Nishikawa H, Kitani S: Tea catechins have dual effect on mast cell degranulation induced by compound 48/80. Int Immunopharmacol 2008;8:1207-1215. https://doi.org/10.1016/j.intimp.2008.04.010 |
||||
| 33 Kuehn HS, Gilfillan AM: G protein-coupled receptors and the modification of FcepsilonRI-mediated mast cell activation. Immunol Lett 2007;113:59-69. https://doi.org/10.1016/j.imlet.2007.08.007 |
||||
| 34 Chelombitko MA, Fedorov AV, Ilyinskaya OP, Zinovkin RA, Chernyak BV: Role of Reactive Oxygen Species in Mast Cell Degranulation. Biochemistry (Mosc) 2016;81:1564-1577. https://doi.org/10.1134/S000629791612018X |
||||
| 35 Suzuki Y, Yoshimaru T, Inoue T, Niide O, Ra C: Role of oxidants in mast cell activation. Chem Immunol Allergy 2005;87:32-42. https://doi.org/10.1159/000087569 |
||||
| 36 Fukumura H, Sato M, Kezuka K, Sato I, Feng X, Okumura S, Fujita T, Yokoyama U, Eguchi H, Ishikawa Y, Saito T: Effect of ascorbic acid on reactive oxygen species production in chemotherapy and hyperthermia in prostate cancer cells. J Physiol Sci 2012;62:251-257. https://doi.org/10.1007/s12576-012-0204-0 |
||||
| 37 Akram NA, Shafiq F, Ashraf M: Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front Plant Sci 2017;8:613. https://doi.org/10.3389/fpls.2017.00613 |
||||
| 38 Matxain JM, Ristila M, Strid A, Eriksson LA: Theoretical study of the antioxidant properties of pyridoxine. J Phys Chem A 2006;110:13068-13072. https://doi.org/10.1021/jp065115p |
||||
| 39 Natera J, Massad W, Garcia NA: The role of vitamin B6 as an antioxidant in the presence of vitamin B2-photogenerated reactive oxygen species. A kinetic and mechanistic study. Photochem Photobiol Sci 2012;11:938-945. https://doi.org/10.1039/c2pp05318g |
||||
| 40 Tian Y, Zhang X, Du M, Li F, Xiao M, Zhang W: Synergistic Antioxidant Effects of Araloside A and L-Ascorbic Acid on H2O2-Induced HEK293 Cells: Regulation of Cellular Antioxidant Status. Oxid Med Cell Longev 2021;2021:9996040. https://doi.org/10.1155/2021/9996040 |
||||
| 41 Jain DP, Pancholi SS, Patel R: Synergistic antioxidant activity of green tea with some herbs. J Adv Pharm Technol Res 2011;2:177-183. https://doi.org/10.4103/2231-4040.85538 |
||||
| 42 Gruber BL: Mast cells: accessory cells which potentiate fibrosis. Int Rev Immunol 1995;12:259-279. https://doi.org/10.3109/08830189509056717 |
||||
| 43 Holdsworth SR, Summers SA: Role of mast cells in progressive renal diseases. J Am Soc Nephrol 2008;19:2254-2261. https://doi.org/10.1681/ASN.2008010015 |
||||
| 44 Blank U, Essig M, Scandiuzzi L, Benhamou M, Kanamaru Y: Mast cells and inflammatory kidney disease. Immunol Rev 2007;217:79-95. https://doi.org/10.1111/j.1600-065X.2007.00503.x |
||||
| 45 Miyajima A, Asano T, Yoshimura I, Seta K, Hayakawa M: Tranilast ameliorates renal tubular damage in unilateral ureteral obstruction. J Urol 2001;165:1714-1718. https://doi.org/10.1016/S0022-5347(05)66400-2 |
||||
| 46 Kelly DJ, Zhang Y, Gow R, Gilbert RE: Tranilast attenuates structural and functional aspects of renal injury in the remnant kidney model. J Am Soc Nephrol 2004;15:2619-2629. https://doi.org/10.1097/01.ASN.0000139066.77892.04 |
||||
| 47 Doggrell SA, Wanstall JC: Cardiac chymase: pathophysiological role and therapeutic potential of chymase inhibitors. Can J Physiol Pharmacol 2005;83:123-130. https://doi.org/10.1139/y04-136 |
||||
| 48 Shiota N, Kakizoe E, Shimoura K, Tanaka T, Okunishi H: Effect of mast cell chymase inhibitor on the development of scleroderma in tight-skin mice. Br J Pharmacol 2005;145:424-431. https://doi.org/10.1038/sj.bjp.0706209 |
||||
| 49 Kazama I, Baba A, Endo Y, Toyama H, Ejima Y, Matsubara M, Tachi M: Mast cell involvement in the progression of peritoneal fibrosis in rats with chronic renal failure. Nephrology 2015;20:609-616. https://doi.org/10.1111/nep.12489 |
||||
| 50 Kobayashi T, Kessoku T, Ozaki A, Iwaki M, Honda Y, Ogawa Y, Imajo K, Yoneda M, Saito S, Nakajima A: Vitamin B6 efficacy in the treatment of nonalcoholic fatty liver disease: an open-label, single-arm, single-center trial. J Clin Biochem Nutr 2021;68:181-186. https://doi.org/10.3164/jcbn.20-142 |
||||
| 51 Rodrigues da Silva M, Schapochnik A, Peres Leal M, Esteves J, Bichels Hebeda C, Sandri S, Pavani C, Ratto Tempestini Horliana AC, Farsky SHP, Lino-Dos-Santos-Franco A: Beneficial effects of ascorbic acid to treat lung fibrosis induced by paraquat. PLoS One 2018;13:e0205535. https://doi.org/10.1371/journal.pone.0205535 |
||||