Corresponding Author: Gabriela P. Diniz
Laboratory of Cellular Biology and Functional Anatomy, Department of Anatomy, Institute of Biomedical Sciences, University of Sao Paulo,
Av. Prof. Lineu Prestes 2415. Sao Paulo, SP, 05508-000 (Brazil)
Tel. +55 (11) 2648-8236 , E-Mail gpdiniz@usp.br
Set7 Deletion Prevents Glucose Intolerance and Improves the Recovery of Cardiac Function After Ischemia and Reperfusion in Obese Female Mice
Juliane B. Mirandaa Guilherme Lunardona Vanessa M. Limaa Tábatha de Oliveira Silvaa Caroline A. Linoa Leonardo Jensenb Maria Cláudia Irigoyenb Ivson Bezerra da Silvac Yao Wei Lud Jianming Liud Jose Donato Júniore Maria Luiza M. Barreto-Chavesa Da-Zhi Wangd,f Gabriela P. Diniza
aDepartment of Anatomy, Institute of Biomedical Sciences, University of Sao Paulo, Sao Paulo, Brazil, bHypertension Unit, Heart Institute, University of Sao Paulo, Sao Paulo, Brazil, cDepartment of Morphology, Federal University of Paraiba, Paraiba, Brazil, dDepartment of Cardiology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA, eDepartment of Physiology and Biophysics, Institute of Biomedical Sciences, University of Sao Paulo, Sao Paulo, Brazil, fCenter for Regenerative Medicine, USF Health Heart Institute, University of South Florida, Tampa, FL, USA
Introduction
Obesity increases the risk of several diseases, such as cancer, hepatic steatosis, type 2 diabetes mellitus, and cardiovascular disorders [1]. Diverse studies have demonstrated that men develop cardiovascular diseases at an earlier age than women [2]. However, obese and insulin resistant women have a higher risk of cardiovascular disease than men [3–5].
The major cause of obesity results from an energy imbalance between calories consumed and calories expended. Mice fed an obesogenic diet, which contains high levels of carbohydrate and fat, is an effective method to model diet-induced obesity in rodents since it recapitulates many obesity-related disorders found in overweight and obese individuals [6]. Despite the increase in obesity levels worldwide, most of the studies related to obesity has been performed in men and male rodents. Therefore, understanding the biological mechanisms involved in obesity-related cardiometabolic disorders in both sexes is necessary to develop new strategies for prevention and treatment.
Studies have demonstrated the role of epigenetic mechanisms, such as DNA methylation, microRNAs, and histone modifications in obesity-related cardiovascular and metabolic disorders [7–10]. The histone-lysine N-methyltransferase Set7 (also known as Setd7, Set9, Set7/9, or Kmt7) was originally described as a histone H3-lysine 4 (H3K4)-specific methyltransferase [11]. The methylation of H3K4 by Set7 prevents chromatin condensation and increases transcription [12]. Set7-dependent methylation of H3K4 has been associated with transcription of genes related to muscle differentiation [13], oxidative stress, inflammation [14], insulin secretion [15], and extracellular matrix proteins [16]. Over the past few years, researchers have determined that Set7 can also methylate non-histone substrates involved in diverse physiological and pathological processes, such as the DNA damage response, cell cycle regulation, chromatin modulation, gene transcription, metabolism, and cell differentiation [17]. Through methylation of proteins, Set7 can influence their function, intracellular localization, and degradation [17]. Several proteins can be methylated by Set7, including estrogen receptor alpha [18], P53 [19], Foxo [20], Akap6 [21], β-catenin [22], Pdx1 [23], Pgc-1α [24], Sirt1 [25], and Nfκb [26]. Functional studies have reported that Set7 affects glucose homeostasis [23, 27] and the response to renal ischemic injury [28]. In addition, streptozotocin-induced type 1 diabetic rats have enhanced Set7 protein levels in the heart, suggesting that Set7 may play a role in diabetic cardiomyopathy [29]. However, the impact of Set7 in obesity-related metabolic and cardiovascular disorders remains unclear.
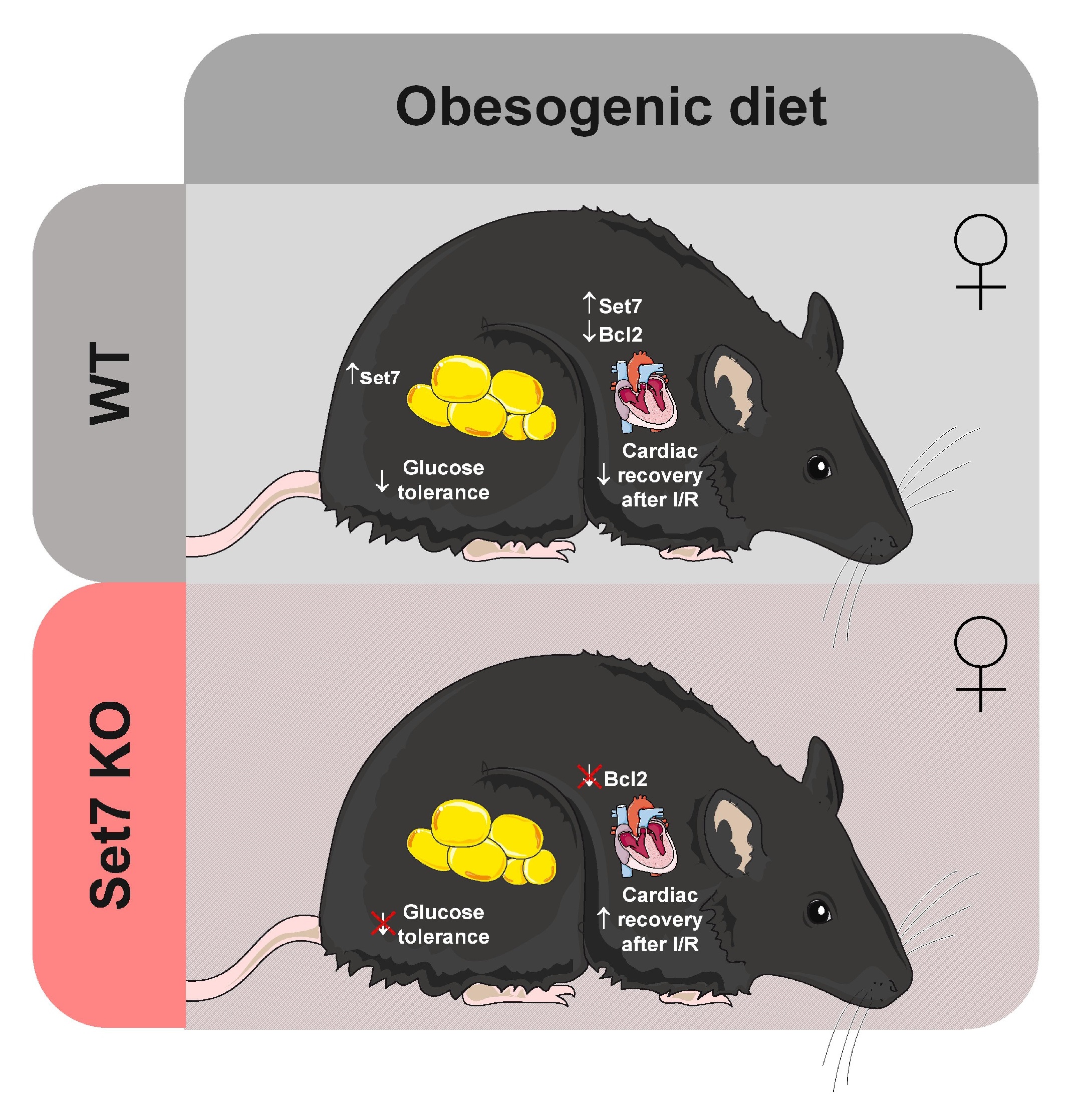
In this study, we explored the role of Set7 in obesogenic diet-induced metabolic and cardiovascular complications. We found that obese female mice fed an obesogenic diet exhibited higher Set7 protein levels in the heart and perigonadal adipose tissue (PAT). Deletion of Set7 did not affect body weight gain, adiposity, and cardiac hypertrophy in response to an obesogenic diet in female mice. However, loss of Set7 prevented obesogenic diet-induced glucose intolerance and the impaired recovery of cardiac function following I/R injury.
Materials and Methods
Mice and diets
All animal experiments were carried out in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. The experimental procedures were approved by the Ethic Committee for Animal Research at the Institute of Biomedical Sciences of the University of Sao Paulo (ICB/USP) (CEUA/3979120418) and were performed in the Department of Anatomy at the ICB/USP. The Set7 global knockout (Set7KO) and wild type (WT) mice used in this study were characterized previously [30]. Five-week-old Set7KO and WT female mice were obtained from heterozygous breeding and fed a normal diet (Nd; 10% kcal from fat, 20% kcal from protein and 70% kcal from carbohydrate; PragSolucoes) or an obesogenic diet containing a high-fat diet (HFD; 60% kcal from fat, 20% kcal from protein and 20% kcal from carbohydrate; Research Diets) and sweetened condensed milk (Nestlé) supplemented with mineral and vitamin mix (AIN 93G) for 12 weeks [31]. Five-week-old WT male mice were fed a normal diet or a HFD for 12 weeks. The mice were housed in a temperature- and light- controlled room (22 °C; 12h light-dark cycle) and had water and food ad libitum. Body weight gain was monitored weekly. After euthanasia using a CO2 chamber, heart, PAT, subcutaneous adipose tissue (SAT), retroperitoneal adipose tissue (RAT), and liver were collected, weighted, and stored at -80 °C for further analysis. The weight of the tissues was normalized by tibia length.
Nuclear magnetic resonance
Nuclear magnetic resonance was used to evaluate the body composition (percentages of fat mass and lean mass) of the mice at the last week of dietary feeding (Bruker’s minispec LF50 Body Composition Analyzer).
Glucose homeostasis
Intraperitoneal glucose tolerance test (iGTT) and insulin tolerance test (ITT) were performed to assess glucose homeostasis during the last week of the dietary regimen. Female mice were fasted for 6 h. For iGTT, blood glucose levels were measured at baseline (before glucose injection) and after an intraperitoneal injection of glucose (2 g/kg body weight) using a glucometer (Accu‐Chek Active, Roche Diagnostics). After 72 hours, ITT was performed in mice fasted for 6 h. Blood glucose levels were assessed before and after insulin injection (0.5 U insulin/kg body weight). The blood glucose disappearance rate (KITT) was calculated to determine insulin sensitivity [32].
Hemodynamic parameters
Systolic blood pressure (SBP) and heart rate (HR) were measured during the last week of the dietary regimen using tail cuff plethysmography (BP-2000 Blood Pressure Analysis System™ of Visitech Systems©). Female mice were conditioned to tail cuff for 7 days before data acquisition. Ten measurements of SBP and HR were obtained per mouse during the morning.
Echocardiography assessment
Echocardiography was performed to evaluate cardiac function and morphology during the last week of the dietary feeding. Female mice were anesthetized with 1.5% isoflurane and echocardiographic analyses were performed using VEVO 2100 system (Visual Sonics) and a 13-24-MHz MicroScan transducer (model MS-550D). The measurements obtained were end-diastolic interventricular septum (IVS;d), end-systolic interventricular septum (IVS;s), LV end-diastolic posterior wall thickness (LVPW;d), LV end-systolic posterior wall thickness (LVPW;s), ejection fraction (EF), fractional shortening (FS), isovolumetric relaxation time (IVRT), isovolumetric contraction time (IVCT), E/A ratio, and E/E’ ratio.
Cardiac ischemia-reperfusion model
An ex vivo Langendorff perfusion system was used to model cardiac ischemia/reperfusion (I/R), as described previously [33, 34]. After euthanasia, hearts from female mice were removed and cannulated on a non-recirculating Langendorff system (ADInstruments, Castle Hill, NSW, Australia). The hearts were perfused via the aorta with a modified Krebs–Henseleit buffer [NaCl (118 mM), KCl (4.7 mM), CaCl2 (1.75 mM), MgSO4 (1.66 mM), NaHCO3 (24.88 mM), KH2PO4 (1.18 mM), dextrose 2 g/l and bidistilled water with a pH of 7.4] under a constant flow rate (3 ml/min) at 37 ± 1 °C and constant oxygenation (5% CO2 and 95% O2). The fresh buffer was prepared and filtered (47 mm Swinnex®, membrane pore 0.22 mM) immediately before heart infusion. The hearts were perfused for 30 min (stabilization period) to establish a baseline recording and then submitted to a global ischemia (zero flow) for 20 min. The flow was restarted and the hearts were reperfused for 45 min (reperfusion period). Ventricular function was determined by a pressure transducer inserted into the left ventricle. Left ventricular developed pressure (LVDP), positive first derivative of left ventricular pressure (+dP/dT), and negative first derivative of left ventricular pressure (−dP/dT) were constantly monitored (PowerLab Chart 7-Lab, AD Instruments, Australia). Functional recovery values are presented for the stabilization period and after 45 min of reperfusion.
Western blotting
Total protein from cell cultures, heart, and PAT was isolated using Ripa Buffer (Tris-HCl 50 mM, NaCl 150 mM, Sodium deoxycholate 0.5%, and Triton 1%) with protease inhibitors. Protein concentration of the samples was quantified using the Bradford method. Fifty mg of each sample was resolved by electrophoresis on polyacrylamide-SDS gels and transferred onto nitrocellulose membrane (Bio-Rad). The membrane was stained with ponceau to evaluate protein transfer efficiency, followed by incubation with the primary antibody overnight at 4ºC and with the secondary antibody at room temperature for 1 h. The membrane was washed three times with TBST (NaCl 150 mM, Tris-base 50 mM, Tween 20 0.1%) between antibodies incubation. The antibodies used were Set7 (#2813, Cell Signaling), α-actinin (sc-15335, Santa Cruz Biotechnology), Gapdh (sc-32233, Santa Cruz Biotechnology), me2-Rpl29 (#19495S, Cell Signaling), β-tubulin (sc-23949), Pparg (sc-7273, Santa Cruz Biotechnology), Fabp4 (sc-271529, Santa Cruz Biotechnology), Cebpα (sc-365318, Santa Cruz Biotechnology), Serca2 (#9580, Cell Signaling), α-tubulin (sc-5286, Santa Cruz Biotechnology), Casp1 (sc-398715, Santa Cruz Biotechnology), Bcl2 (sc-7382, Santa Cruz Biotechnology), and phospho-Stat3 (#9145, Cell Signalling). The protein bands were visualized using the UVITEC Cambridge (Aliance 9.7) system using a chemiluminescent reagent (Luminata™ Forte) and quantified by densitometry. The results are expressed as relative levels.
Histology
Transverse heart sections were fixed in paraformaldehyde 4% for 24 h and stored in 70% ethanol. The heart samples were dehydrated, embedded in paraffin and sectioned into 5 mm-thick slices. Transverse heart sections were stained with wheat germ agglutinin for quantification of cardiomyocyte area at the papillary muscle level (n=30-50 cardiomyocytes were evaluated per animal). Cardiac fibrosis was evaluated using picrosirius red staining and measured as a relative area in relation to the total cardiac area. Images were analyzed using a light microscope (Nikon®) and quantified using ImageJ software.
White adipocyte culture
White adipocyte cultures were prepared as previously described [35]. The 3T3-L1 cells were differentiated into white adipocytes in medium containing 1 mM dexamethasone, 0.125 mM indomethacin, 1 mM insulin, and 0.5 mM isobutylmethylxanthine for 7 days. To evaluate the role of Set7 in white adipogenesis, 3T3-L1 cells were induced to differentiate into white adipocytes in medium supplemented with (R)-PFI-2 (5 mM), which is a specific Set7 inhibitor [36]. Oil Red O staining was used to assess the intracellular lipid content during adipocyte differentiation.
Quantitative Real Time RT-PCR (qPCR)
Total RNA from heart was isolated with TRIzol (Life Technologies) following the manufacture’s recommendations. cDNA was made using SuperScript II RNase H Transcriptase (Invitrogen). The cDNA was amplified by qPCR using SYBR Green Master Mix (Applied Biosystems) and specific primers (Exxtend). The primers used were: 18S: 5’-GCC ACT TGT CCC TCT AAG-3’ and 5’-GTG CAT CGT TCT TAG TTG-3’; for Myh6: 5’-TGA CGT CAC CCT CCA ACA TGG-3’ and 5’-CAA CTC CCC GTT CTC TGT C-3’; for Acta1: 5’-GCT CGG TGA GGA TTT TCA TCA G-3’ and 5’-CCT GCC ACA CGC CAT CAT-3’. 18S was used as an internal control since its expression levels were similar between the experimental groups. The relative gene expression levels were calculated using the formula (2-∆∆Ct).
Statistical analyses
The results are presented as mean ± SD. Statistical significance was calculated using two-way ANOVA followed by Bonferroni post hoc test and Student’s t-test using GraphPad® Prism software. Statistical significance was set at p≤0.05.
Results
Set7 levels are increased in the heart and PAT of obese female mice
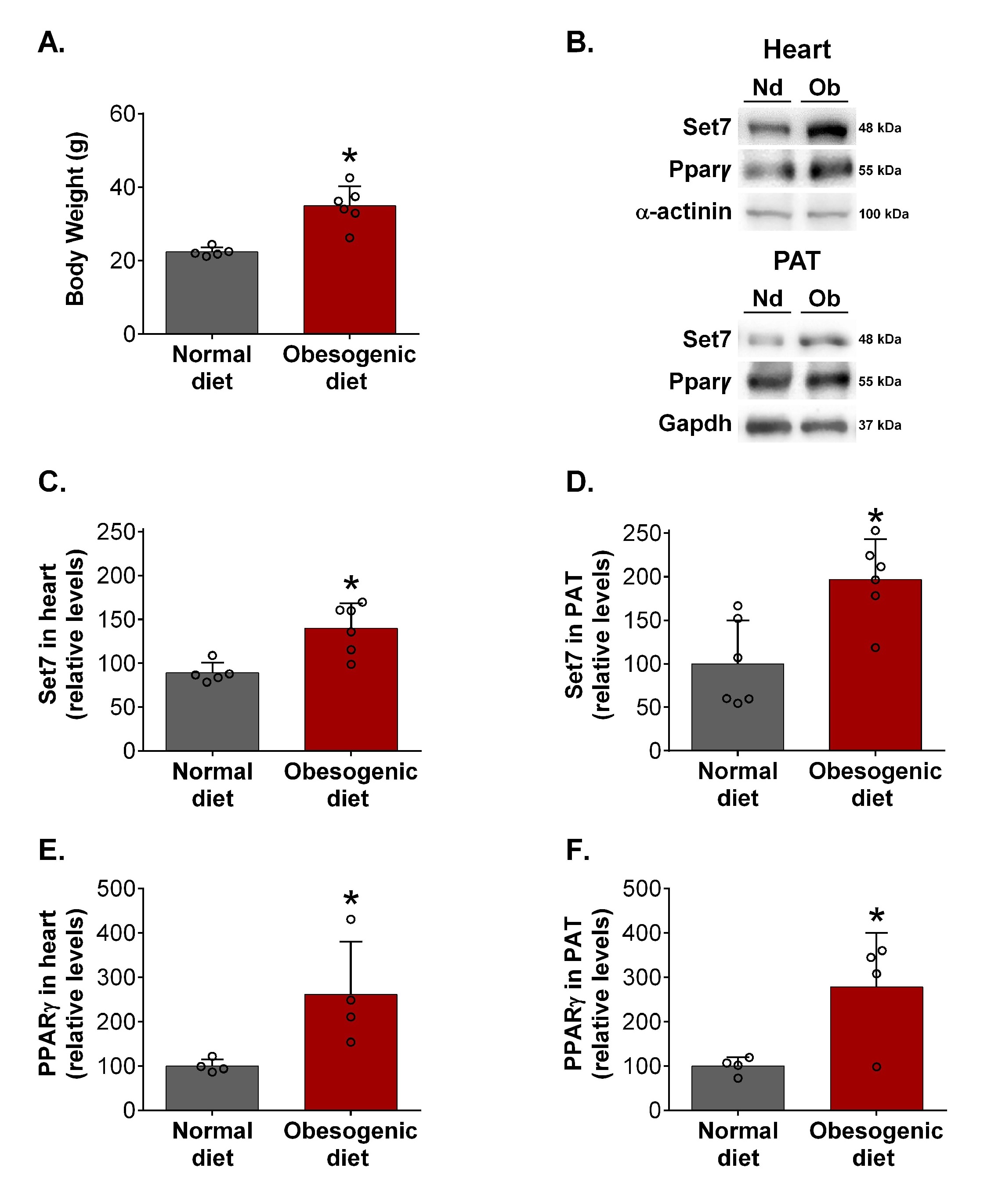
Initially, we asked whether Set7 protein levels would be changed in the heart and white adipose tissue of obese male and female mice. As expected, male mice fed a high-fat diet (HFD) exhibited increased body weight compared to their respective controls fed a normal diet (Supplementary Fig. 1A – for all supplementary material see www.cellphysiolbiochem.com). Western blotting analysis revealed that Set7 protein levels were unchanged in the heart (Supplementary Fig. 1B, 1C) of male mice fed a HFD compared to those found in male mice fed a normal diet. However, Set7 protein levels were increased in the perigonadal adipose tissue (PAT) of male mice in response to a HFD (Supplementary Fig. 1B, 1D).
The female mice fed an obesogenic diet displayed increased body weight compared to their respective controls (Fig. 1A), indicating the development of obesity. Interestingly, female mice fed an obesogenic diet exhibited higher Set7 protein levels in the heart (Fig. 1B, 1C) and PAT (Fig. 1B, 1D) compared to those fed a normal diet. Together, these results suggest that obese female mice display increased Set7 protein levels in the heart and PAT.
Pparγ may regulate the transcription of diverse proteins containing SET domain [37]. Western blotting analysis revealed that Pparγ protein levels were increased in the heart (Fig. 1B, 1E) and PAT (Fig. 1B, 1F) of female mice fed an obesogenic diet.
Deletion of Set7 does not affect obesogenic diet-induced obesity in female mice, but prevents glucose intolerance
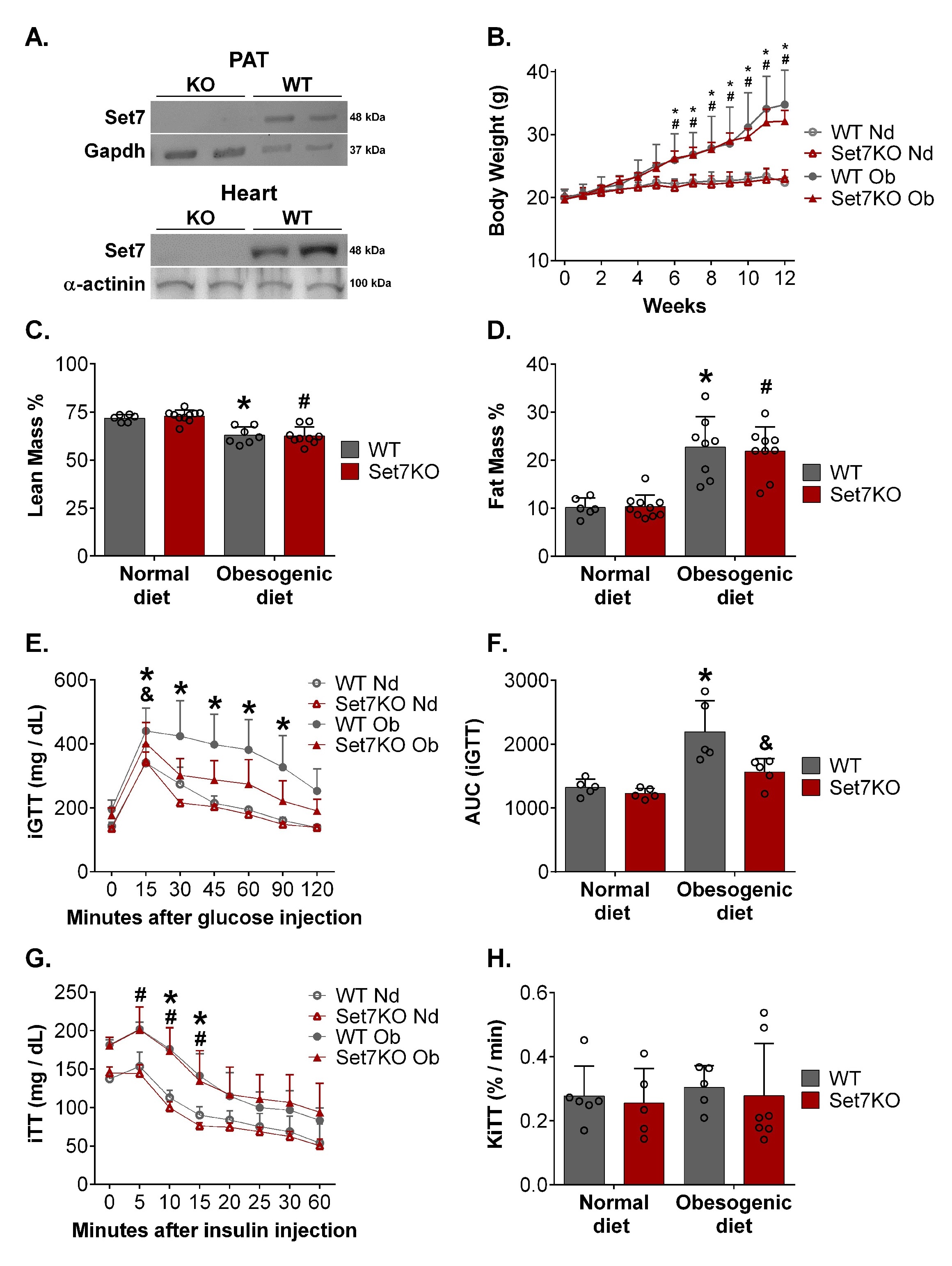
Since Set7 protein levels were increased in the heart and PAT of obese female mice, we examined the role of Set7 in obesogenic diet-induced metabolic and cardiovascular complications by using a global Set7KO mouse model. Western blotting analysis revealed that Set7KO female mice exhibited undetectable Set7 protein levels in the PAT and heart compared to those found in WT mice (Fig. 2A).
Next, we evaluated the impact of Set7 in obesogenic diet-induced metabolic dysfunctions. As expected, the obesogenic diet regimen increased body weight gain in WT female mice (Fig. 2B). Nonetheless, deletion of Set7 did not affect body weight gain in response to an obesogenic diet. No differences were observed between WT and Set7KO female mice fed a normal diet (Fig. 2B).
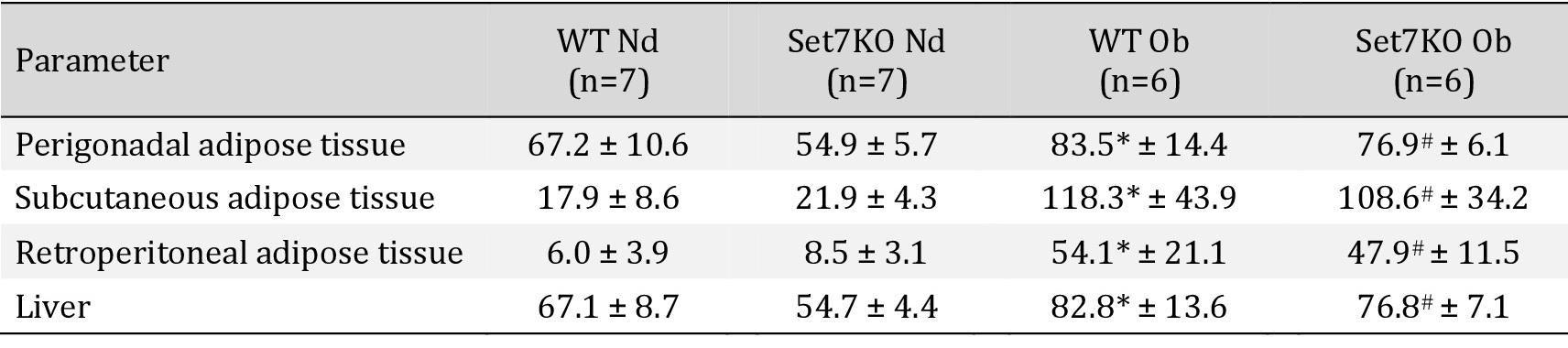
We therefore verified whether Set7 deletion could affect fat mass expansion in response to an obesogenic diet. Nuclear magnetic resonance showed that both WT and Set7KO female mice fed an obesogenic diet exhibited reduced relative lean mass (Fig. 2C) and enhanced fat mass (Fig. 2D) in comparison to their respective controls. In line with this finding, analysis of white adipose tissue depots revealed that the obesogenic diet increased the weight of PAT, SAT, and RAT (Table 1) both in WT and Set7KO female mice compared to those fed a normal diet; however, this increase was unaffected by deletion of Set7. Liver weight was increased both in WT and Set7KO female mice fed an obesogenic diet in relation to their respective controls (Table 1).
Given that obese male and female mice had increased Set7 protein levels in the PAT, but Set7 deletion did not change the white adipose tissue gain in response to an obesogenic diet in female mice, we therefore evaluated whether Set7 could affect white adipogenic differentiation. First, we examined the expression of Set7 upon white adipocyte differentiation in vitro. Western blotting analysis demonstrated that Set7 protein levels were reduced upon induction of adipocyte differentiation (day 7) compared to 3T3-L1 cells (day 0) (Supplementary Fig. 2A). Next, to characterize the impact of Set7 in white adipocyte differentiation, (R)-PFI-2, which is a selective inhibitor of Set7 [36], was added during the course of white adipogenic induction in 3T3-L1 cells. Western blotting analysis revealed that protein levels of me2-Rpl29, which is the major substrate of the Set7 [38], were reduced by (R)-PFI-2 treatment in white adipocytes (Supplementary Fig. 2B, 2F). Inhibition of Set7 did not affect lipid droplet content in white adipocytes, as assessed by Oil Red O staining (Supplementary Fig. 2B). In addition, protein levels of Pparg (Supplementary Fig. 2C, 2F), Fabp4 (Supplementary Fig. 2D, 2F), and Cebpα (Supplementary Fig. 2E, 2F), which are factors involved in differentiation and maturation of adipocytes, were unchanged by (R)-PFI-2, suggesting that Set7 does not affect white adipogenesis in vitro.
Next, we investigated whether Set7 deletion and obesogenic diet could affect glucose homeostasis. WT female mice fed an obesogenic diet exhibited elevated blood glucose levels during iGTT (Fig. 2E), resulting in increased AUC (Fig. 2F) compared to their respective controls. These results indicate that obesogenic diet induced glucose intolerance. However, Set7KO female mice fed an obesogenic diet did not display increased AUC of the iGTT (Fig. 2F) compared to their respective controls, suggesting that deletion of Set7 prevented obesogenic diet-induced glucose intolerance. Insulin tolerance test (ITT) was also performed to evaluate glucose homeostasis (Fig. 2G). WT and Set7KO female mice fed both diets exhibited similar blood glucose disappearance rate (KITT) (Fig. 2H), indicating that neither obesogenic diet nor Set7 deletion affected insulin sensitivity. Together, these results suggest that Set7 does not contribute to obesogenic diet-induced obesity in female mice, but Set7 deletion protects against obesogenic diet-induced glucose intolerance.
Set7 deletion does not affect obesogenic-diet induced cardiac hypertrophy in female mice
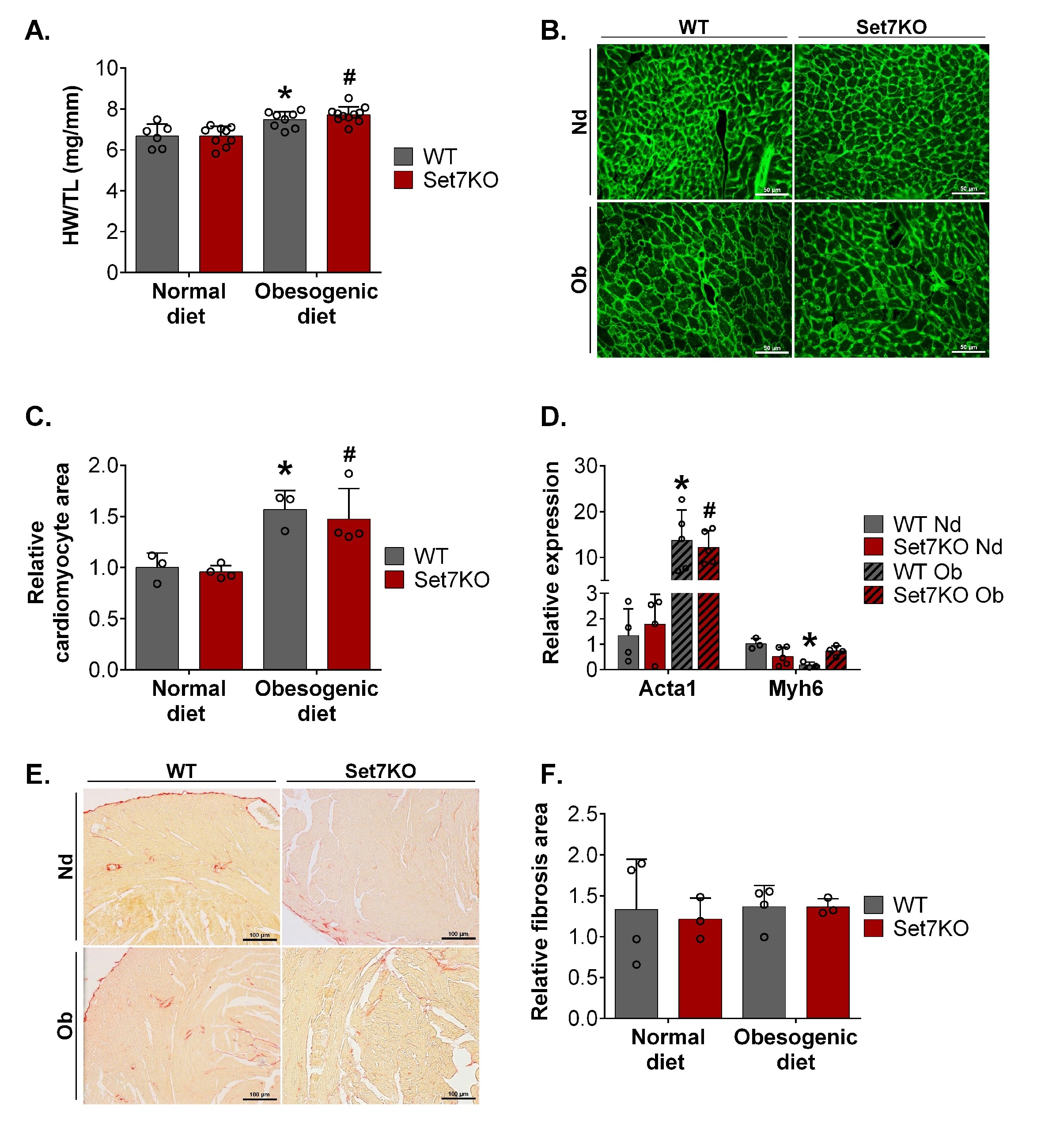
Considering that Set7 protein levels were increased in the heart of obese female mice and that this enzyme can methylate diverse proteins involved in cardiac hypertrophy [20–22, 39–41], we investigated the role of Set7 in obesogenic diet-induced cardiac hypertrophy. Both WT and Set7KO female mice fed an obesogenic diet exhibited cardiac hypertrophy, as assessed by higher HW/TL ratio (Fig. 3A). Consistent with these findings, WGA staining of transverse heart sections demonstrated that obesogenic diet increased cardiomyocyte area both in WT and Set7KO female mice compared to their respective controls (Fig. 3B, 3C). Analysis of qPCR revealed that cardiac Acta1 mRNA levels were increased in the heart of WT and Set7KO female mice fed an obesogenic diet (Fig. 3D). On the other hand, cardiac Myh6 mRNA levels were reduced in obesogenic diet-fed WT female mice compared to their respective controls (Fig. 3D). Together, these results indicate that deletion of Set7 does not influence obesogenic diet-induced cardiac hypertrophy in female mice.
Next, we examined the effect of obesogenic diet and Set7 deletion in myocardial fibrosis. No significant changes were detected in cardiac fibrosis among the groups, as assessed by picrosirius red staining (Fig. 3E, 3F), indicating that neither obesogenic diet or Set7 deletion altered myocardial collagen deposition in female mice.
Deletion of Set7 does not affect hemodynamic and echocardiographic profile in female mice
We verified whether obesogenic diet and Set7 deletion could modulate hemodynamic and echocardiographic parameters in female mice. SBP and HR (Table 2) were not affected either by obesogenic diet or by deletion of Set7, as assessed by tail cuff plethysmography. Echocardiography revealed that the IVS;d was enhanced in Set7KO female mice fed an obesogenic diet compared to their respective controls (Table 2). The LVPW;d, LVID;d, EF, FS, IVRT, IVCT, E/A ratio, and E/E’ ratio were similar between WT and Set7KO female mice fed both diets (Table 2). These findings suggest that hemodynamic parameters and cardiac performance were unchanged by obesogenic diet or by deletion of Set7.
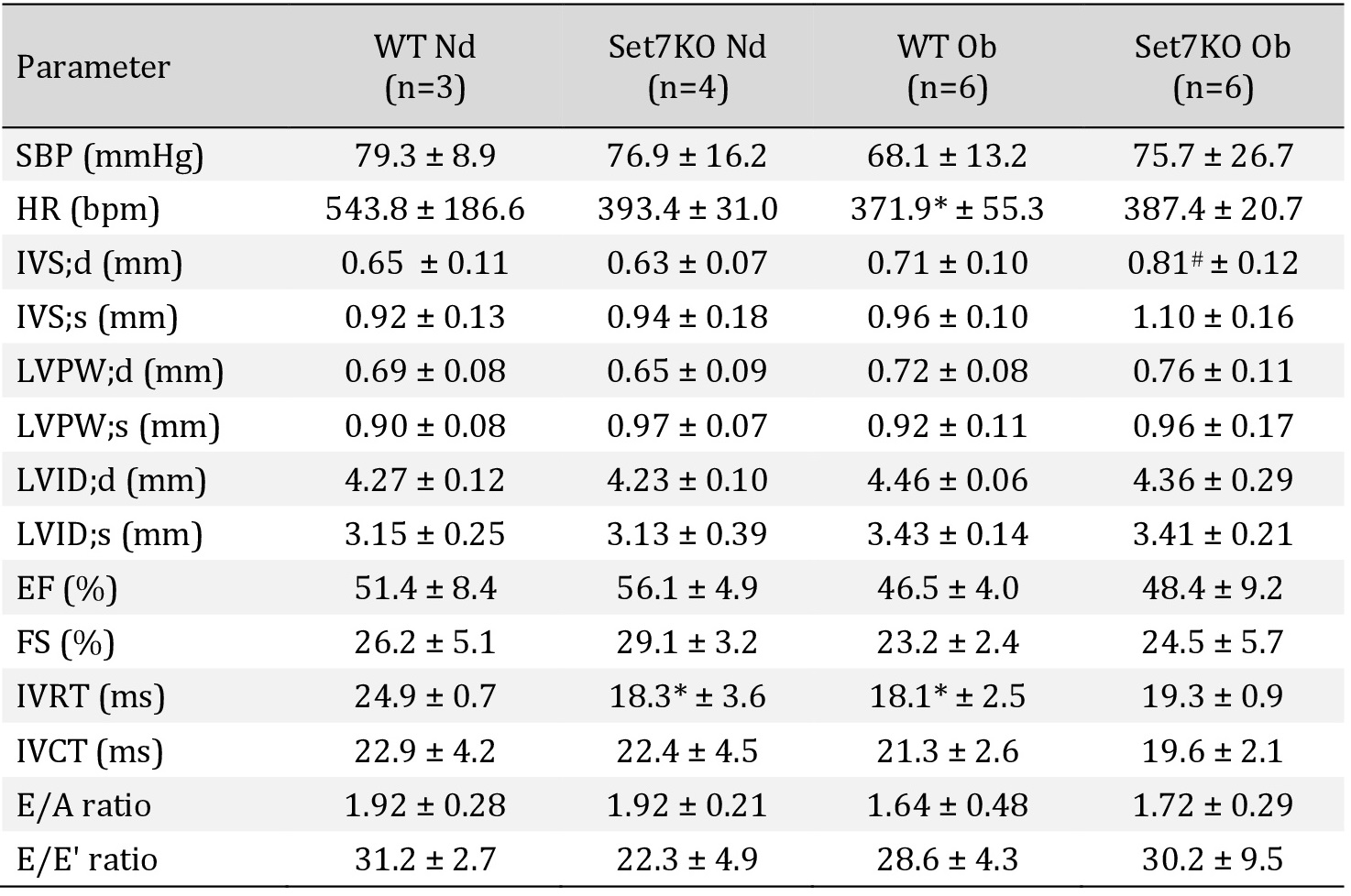
Deletion of Set7 prevents cardiac functional deterioration after I/R injury in obese female mice
Given that obesogenic diet increased Set7 protein levels in the heart of female mice, and that Set7 contributes to ischemia/hypoxia injury [27, 28, 42, 43], we investigated whether Set7 could affect cardiac function following I/R injury in obese female mice. First, baseline cardiac function was evaluated at the stabilization period using the ex vivo Langendorff perfused heart model. WT and Set7KO female mice fed an obesogenic diet displayed reduced LVDP (Fig. 4A) and −dP/dT (Fig. 4B) at the stabilization period in relation to their respective controls. The +dP/dT was reduced in WT female mice fed an obesogenic diet compared to those fed a normal diet (Fig. 4C), while no difference was observed between Set7KO female mice fed both diets.
After I/R, obese WT female mice displayed reduced LVDP (Fig. 4D), −dP/dT (Fig. 4E), and +dP/dT (Fig. 4F) compared to their respective controls, suggesting that obesogenic diet impaired cardiac functional recovery following I/R injury. In contrast, Set7KO female mice fed an obesogenic diet exhibited a LVDP (Fig. 4D), −dP/dT (Fig. 4E), and +dP/dT (Fig. 4F) similar to those found in their respective controls, suggesting that deletion of Set7 prevents the impaired recovery of cardiac function in response to an obesogenic diet after I/R injury.
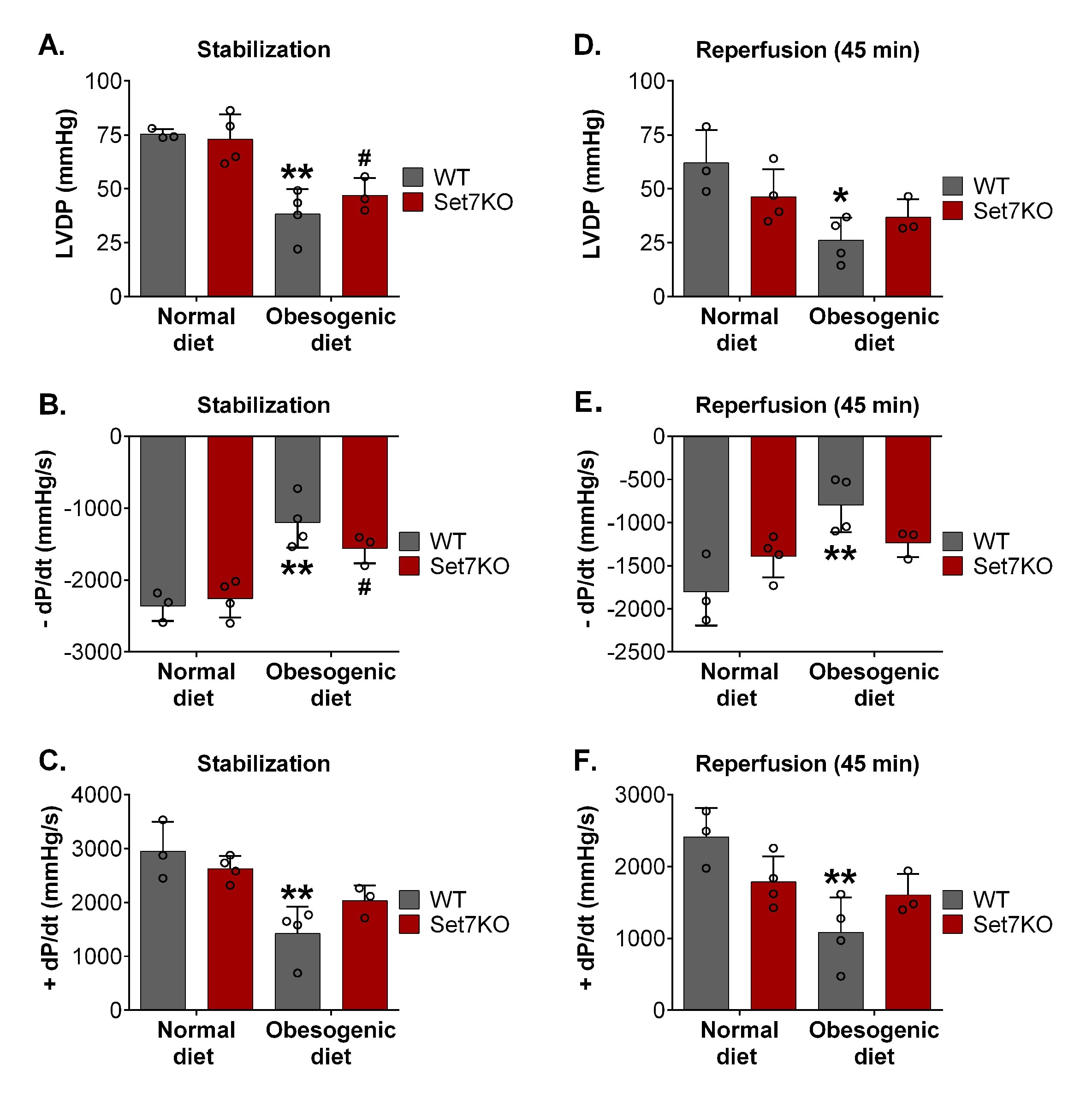
Considering that Set7 suppression modulates calcium handling in adult cardiomyocytes [44], we evaluated the protein levels of Serca2 in the hearts after I/R injury. Western blotting analysis showed that Serca2 protein levels were similar among the groups (Fig. 5A, 5B).
Next, we investigated the levels of proteins related to apoptosis, such as Casp1 (proapoptotic protein) and Bcl2 (anti-apoptotic protein) in the heart after I/R injury. The Casp1 protein levels (Fig. 5A, 5C) were similar in the hearts of WT and Set7KO female mice fed both diets after I/R. WT female mice fed an obesogenic diet displayed reduced levels of Bcl2 in the heart compared to their respective controls (Fig. 5A, 5D); however, Bcl2 levels were unchanged in the heart of Set7KO female mice fed an obesogenic diet compared to their respective controls. Together, these findings suggest that Set7 deletion prevents the decrease of cardiac Bcl2 levels induced by obesogenic diet in response to I/R.
A previous study showed that Stat3, a transcriptional factor with cardioprotective effect [45], is phosphorylated, methylated, and inhibited by Set7 [46]. To determine whether Stat3 might be involved in the beneficial effects of Set7 deletion in obese female mice after I/R injury, we evaluated the phospho-Stat3 levels. Western blotting analysis revealed that phospho-Stat3 levels were similar among the groups (Fig. 5A, 5E), suggesting that Stat3 might not be involved in the beneficial effects of Set7 deletion in obese female mice after I/R injury.
The data of this study are available upon reasonable request to the corresponding author.
Author Contributions
Juliane Miranda: Performed experiments, data analysis and writing - draft. Guilherme Lunardon: Performed experiments, data analysis and writing - review & editing. Vanessa Lima: Performed experiments, data analysis and writing - review & editing. Tábatha de Oliveira Silva: Performed experiments, data acquisition and writing - review & editing. Caroline A. Lino: Performed experiments, data acquisition and writing - review & editing. Leonardo Jensen: Performed experiments and data acquisition. Maria Cláudia Irigoyen: Methodology and writing - review & editing. Ivson Bezerra da Silva: methodology, data acquisition and writing - review & editing. Yao Wei Lu: Resources, methodology and writing - review & editing. Jianming Liu: Resources, methodology and writing - review & editing. Jose Donato Júnior: Resources, methodology and writing - review & editing. Maria Luiza Barreto-Chaves: Resources, methodology and writing - review & editing. Da-Zhi Wang: Resources, methodology and writing - review & editing. Gabriela Diniz: conceptualization, resources, project administration, supervision, writing - review & editing, and funding acquisition.
Funding
This work was supported by São Paulo Research Foundation (FAPESP, Grant numbers #2018/10338-2, 2018/25814-4 and 2020/13211-3) and by the Coordenação de Aperfeiçoamento de Pessoa de Nível Superior – Brasil (CAPES).
The authors declare that they have no conflicts of interest.
| 1 Blüher M: Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 2019;15:288-298. https://doi.org/10.1038/s41574-019-0176-8 |
||||
| 2 Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K: Sex Hormones and Sex Chromosomes Cause Sex Differences in the Development of Cardiovascular Diseases. Arterioscler Thromb Vasc Biol 2017;37:746-756. https://doi.org/10.1161/ATVBAHA.116.307301 |
||||
| 3 Halland H, Lønnebakken MT, Pristaj N, Saeed S, Midtbø H, Einarsen E, Gerdts E: Sex differences in subclinical cardiac disease in overweight and obesity (the FATCOR study). Nutr Metab Cardiovasc Dis 2018;28:1054-1060. https://doi.org/10.1016/j.numecd.2018.06.014 |
||||
| 4 Manrique-Acevedo C, Chinnakotla B, Padilla J, Martinez-Lemus LA, Gozal D: Obesity and cardiovascular disease in women. Int J Obes 2020;44:1210-1226. https://doi.org/10.1038/s41366-020-0548-0 |
||||
| 5 Toedebusch R, Belenchia A, Pulakat L: Diabetic Cardiomyopathy: Impact of Biological Sex on Disease Development and Molecular Signatures. Front Physiol 2018;9:453. https://doi.org/10.3389/fphys.2018.00453 |
||||
| 6 Bortolin RC, Vargas AR, Gasparotto J, Chaves PR, Schnorr CE, Martinello KB, Silveira AK, Rabelo TK, Gelain DP, Moreira JCF: A new animal diet based on human Western diet is a robust diet-induced obesity model: comparison to high-fat and cafeteria diets in term of metabolic and gut microbiota disruption. Int J Obes 2018;42:525-534. https://doi.org/10.1038/ijo.2017.225 |
||||
| 7 Bagchi RA, Weeks KL: Histone deacetylases in cardiovascular and metabolic diseases. J Mol Cell Cardiol 2019;130:151-159. https://doi.org/10.1016/j.yjmcc.2019.04.003 |
||||
| 8 Deiuliis JA: MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics. Int J Obes 2016;40:88-101. https://doi.org/10.1038/ijo.2015.170 |
||||
| 9 Loh M, Zhou L, Ng HK, Chambers JC: Epigenetic disturbances in obesity and diabetes: Epidemiological and functional insights. Mol Metab 2019;27:S33-41. https://doi.org/10.1016/j.molmet.2019.06.011 |
||||
| 10 Ouni M, Schürmann A: Epigenetic contribution to obesity. Mamm Genome 2020;31:134-145. https://doi.org/10.1007/s00335-020-09835-3 |
||||
| 11 Wang H, Cao R, Xia L, Erdjument-Bromage H, Borchers C, Tempst P, Zhang Y: Purification and Functional Characterization of a Histone H3-Lysine 4-Specific Methyltransferase. Mol Cell 2001;8:1207-1217. https://doi.org/10.1016/S1097-2765(01)00405-1 |
||||
| 12 Nishioka K: Set9, a novel histone H3 methyltransferase that facilitates transcription by precluding histone tail modifications required for heterochromatin formation. Genes Dev 2002;16:479-489. https://doi.org/10.1101/gad.967202 |
||||
| 13 Tao Y, Neppl RL, Huang ZP, Chen J, Tang RH, Cao R, Zhang Y, Jin SW, Wang DZ: The histone methyltransferase Set7/9 promotes myoblast differentiation and myofibril assembly. J Cell Biol 2011;194:551-565. https://doi.org/10.1083/jcb.201010090 |
||||
| 14 He S, Owen DR, Jelinsky SA, Lin LL: Lysine Methyltransferase SETD7 (SET7/9) Regulates ROS Signaling through mitochondria and NFE2L2/ARE pathway. Sci Rep 2015;5:14368. https://doi.org/10.1038/srep14368 |
||||
| 15 Evans-Molina C, Robbins RD, Kono T, Tersey SA, Vestermark GL, Nunemaker CS, Garmey JC, Deering TG, Keller SR, Maier B, Mirmira RG: Peroxisome Proliferator-Activated Receptor γ Activation Restores Islet Function in Diabetic Mice through Reduction of Endoplasmic Reticulum Stress and Maintenance of Euchromatin Structure. Mol Cell Biol 2009;29:2053-2067. https://doi.org/10.1128/MCB.01179-08 |
||||
| 16 Sun G, Reddy MA, Yuan H, Lanting L, Kato M, Natarajan R: Epigenetic Histone Methylation Modulates Fibrotic Gene Expression. J Am Soc Nephrol 2010;21:2069-2080. https://doi.org/10.1681/ASN.2010060633 |
||||
| 17 Batista I de AA, Helguero LA: Biological processes and signal transduction pathways regulated by the protein methyltransferase SETD7 and their significance in cancer. Signal Transduct Target Ther 2018;3:19. https://doi.org/10.1038/s41392-018-0017-6 |
||||
| 18 Subramanian K, Jia D, Kapoor-Vazirani P, Powell DR, Collins RE, Sharma D, Peng J, Cheng X, Vertino PM: Regulation of Estrogen Receptor α by the SET7 Lysine Methyltransferase. Mol Cell 2008;30:336-347. https://doi.org/10.1016/j.molcel.2008.03.022 |
||||
| 19 Chuikov S, Kurash JK, Wilson JR, Xiao B, Justin N, Ivanov GS, McKinney K, Tempst P, Prives C, Gamblin SJ, Barlev NA, Reinberg D: Regulation of p53 activity through lysine methylation. Nature 2004;432:353-360. https://doi.org/10.1038/nature03117 |
||||
| 20 Calnan DR, Webb AE, White JL, Stowe TR, Goswami T, Shi X, Espejo A, Bedford MT, Gozani O, Gygi SP, Brunet A: Methylation by Set9 modulates FoxO3 stability and transcriptional activity. Aging (Albany NY) 2012;4:462-479. https://doi.org/10.18632/aging.100471 |
||||
| 21 Dhayalan A, Kudithipudi S, Rathert P, Jeltsch A: Specificity Analysis-Based Identification of New Methylation Targets of the SET7/9 Protein Lysine Methyltransferase. Chem Biol 2011;18:111-120. https://doi.org/10.1016/j.chembiol.2010.11.014 |
||||
| 22 Shen C, Wang D, Liu X, Gu B, Du Y, Wei F, Cao L, Song B, Lu X, Yang Q, Zhu Q, Hou T, Li M, Wang L, Wang H, Zhao Y, Yang Y, Zhu W: SET7/9 regulates cancer cell proliferation by influencing β‐catenin stability. FASEB J 2015;29:4313-4323. https://doi.org/10.1096/fj.15-273540 |
||||
| 23 Maganti A V., Maier B, Tersey SA, Sampley ML, Mosley AL, Özcan S, Pachaiyappan B, Woster PM, Hunter CS, Stein R, Mirmira RG: Transcriptional Activity of the Islet β Cell Factor Pdx1 Is Augmented by Lysine Methylation Catalyzed by the Methyltransferase Set7/9. J Biol Chem 2015;290:9812-9822. https://doi.org/10.1074/jbc.M114.616219 |
||||
| 24 Aguilo F, Li S, Balasubramaniyan N, Sancho A, Benko S, Zhang F, Vashisht A, Rengasamy M, Andino B, Chen C, Zhou F, Qian C, Zhou MM, Wohlschlegel JA, Zhang W, Suchy FJ, Walsh MJ: Deposition of 5-Methylcytosine on Enhancer RNAs Enables the Coactivator Function of PGC-1α. Cell Rep 2016;14:479-492. https://doi.org/10.1016/j.celrep.2015.12.043 |
||||
| 25 Liu X, Wang D, Zhao Y, Tu B, Zheng Z, Wang L, Wang H, Gu W, Roeder RG, Zhu WG: Methyltransferase Set7/9 regulates p53 activity by interacting with Sirtuin 1 (SIRT1). Proc Natl Acad Sci 2011;108:1925-1930. https://doi.org/10.1073/pnas.1019619108 |
||||
| 26 Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, Ren B, Natarajan R: Role of the Histone H3 Lysine 4 Methyltransferase, SET7/9, in the Regulation of NF-κB-dependent Inflammatory Genes. J Biol Chem 2008;283:26771-26781. https://doi.org/10.1074/jbc.M802800200 |
||||
| 27 Liu X, Chen Z, Xu C, Leng X, Cao H, Ouyang G, Xiao W: Repression of hypoxia-inducible factor α signaling by Set7-mediated methylation. Nucleic Acids Res 2015;43:5081-5098. https://doi.org/10.1093/nar/gkv379 |
||||
| 28 Sharma N, Sankrityayan H, Kale A, Gaikwad AB: Role of SET7/9 in the progression of ischemic renal injury in diabetic and non-diabetic rats. Biochem Biophys Res Commun 2020;528:14-20. https://doi.org/10.1016/j.bbrc.2020.05.075 |
||||
| 29 Malek V, Sharma N, Gaikwad AB: Simultaneous inhibition of neprilysin and activation of ACE2 prevented diabetic cardiomyopathy. Pharmacol Reports 2019;71:958-967. https://doi.org/10.1016/j.pharep.2019.05.008 |
||||
| 30 Kurash JK, Lei H, Shen Q, Marston WL, Granda BW, Fan H, Wall D, Li E, Gaudet F: Methylation of p53 by Set7/9 Mediates p53 Acetylation and Activity In Vivo. Mol Cell 2008;29:392-400. https://doi.org/10.1016/j.molcel.2007.12.025 |
||||
| 31 de Oliveira Silva T, Lino CA, Buzatto VC, Fontes Asprino P, Lu YW, Lima VM, Fonseca RIB, Jensen L, Murata GM, Filho SV, Ribeiro MAC, Donato Jr. J, Ferreira JCB, Rodrigues AC, Irigoyen MC, Barreto-Chaves MLM, Huang ZP, Favoretto Galante PA, Wang DZ, Diniz GP: Deletion of miRNA-22 Induces Cardiac Hypertrophy in Females but Attenuates Obesogenic Diet-Mediated Metabolic Disorders. Cell Physiol Biochem 2020;54:1199-1217. https://doi.org/10.33594/000000309 |
||||
| 32 Festuccia WT, Blanchard PG, Belchior T, Chimin P, Paschoal VA, Magdalon J, Hirabara SM, Simões D, St-Pierre P, Carpinelli A, Marette A, Deshaies Y: PPARγ activation attenuates glucose intolerance induced by mTOR inhibition with rapamycin in rats. Am J Physiol Metab 2014;306:E1046-1054. https://doi.org/10.1152/ajpendo.00683.2013 |
||||
| 33 da Silva IB, Gomes DA, Alenina N, Bader M, dos Santos RA, Barreto-Chaves MLM: Cardioprotective effect of thyroid hormone is mediated by AT2 receptor and involves nitric oxide production via Akt activation in mice. Heart Vessels 2018;33:671-681. https://doi.org/10.1007/s00380-017-1101-5 |
||||
| 34 Guedes EC, da Silva IB, Lima VM, Miranda JB, Albuquerque RP, Ferreira JCB, Barreto‐Chaves MLM, Diniz GP: High fat diet reduces the expression of miRNA‐29b in heart and increases susceptibility of myocardium to ischemia/reperfusion injury. J Cell Physiol 2019;234:9399-9407. https://doi.org/10.1002/jcp.27624 |
||||
| 35 Lima VM, Liu J, Brandão BB, Lino CA, Balbino Silva CS, Ribeiro MAC, Oliveira TE, Real CC, de Paula Faria D, Cederquist C, Huang Z-P, Hu X, Barreto-Chaves ML, Ferreira JCB, Festuccia WT, Mori MA, Kahn CR, Wang DZ, Diniz GP: miRNA-22 deletion limits white adipose expansion and activates brown fat to attenuate high-fat diet-induced fat mass accumulation. Metabolism 2021;117:154723. https://doi.org/10.1016/j.metabol.2021.154723 |
||||
| 36 Barsyte-Lovejoy D, Li F, Oudhoff MJ, Tatlock JH, Dong A, Zeng H, Wu H, Freeman SA, Schapira M, Senisterra GA, Kuznetsova E, Marcellus R, Allali-Hassani A, Kennedy S, Lambert JP, Couzens AL, Aman A, Gingras AC, Al-Awar R, Fish PV, et al.: (R)-PFI-2 is a potent and selective inhibitor of SETD7 methyltransferase activity in cells. Proc Natl Acad Sci 2014;111:12853-12858. https://doi.org/10.1073/pnas.1407358111 |
||||
| 37 Wakabayashi K, Okamura M, Tsutsumi S, Nishikawa NS, Tanaka T, Sakakibara I, Kitakami J, Ihara S, Hashimoto Y, Hamakubo T, Kodama T, Aburatani H, Sakai J: The Peroxisome Proliferator-Activated Receptor γ/Retinoid X Receptor α Heterodimer Targets the Histone Modification Enzyme PR-Set7/Setd8 Gene and Regulates Adipogenesis through a Positive Feedback Loop. Mol Cell Biol 2009;29:3544-3555. https://doi.org/10.1128/MCB.01856-08 |
||||
| 38 Hamidi T, Singh AK, Veland N, Vemulapalli V, Chen J, Hardikar S, Bao J, Fry CJ, Yang V, Lee KA, Guo A, Arrowsmith CH, Bedford MT, Chen T: Identification of Rpl29 as a major substrate of the lysine methyltransferase Set7/9. J Biol Chem 2018;293:12770-12780. https://doi.org/10.1074/jbc.RA118.002890 |
||||
| 39 Blankesteijn W, Vandeschans V, Terhorst P, Smits J: The Wnt/frizzled/GSK-3β pathway: a novel therapeutic target for cardiac hypertrophy. Trends Pharmacol Sci 2008;29:175-180. https://doi.org/10.1016/j.tips.2008.01.003 |
||||
| 40 Passariello CL, Li J, Dodge-Kafka K, Kapiloff MS: mAKAP-A Master Scaffold for Cardiac Remodeling. J Cardiovasc Pharmacol 2015;65:218-225. https://doi.org/10.1097/FJC.0000000000000206 |
||||
| 41 Tremblay ML, Giguère V: Phosphatases at the Heart of FoxO Metabolic Control. Cell Metab 2008;7:101-103. https://doi.org/10.1016/j.cmet.2008.01.004 |
||||
| 42 Song H, Feng X, Zhang M, Jin X, Xu X, Wang L, Ding X, Luo Y, Lin F, Wu Q, Liang G, Yu T, Liu Q, Zhang Z: Crosstalk between lysine methylation and phosphorylation of ATG16L1 dictates the apoptosis of hypoxia/reoxygenation-induced cardiomyocytes. Autophagy 2018;14:825-844. https://doi.org/10.1080/15548627.2017.1389357 |
||||
| 43 Dang Y, Ma X, Li Y, Hao Q, Xie Y, Zhang Q, Zhang F, Qi X: Inhibition of SETD7 protects cardiomyocytes against hypoxia/reoxygenation-induced injury through regulating Keap1/Nrf2 signaling. Biomed Pharmacother 2018;106:842-849. https://doi.org/10.1016/j.biopha.2018.07.007 |
||||
| 44 Lee J, Shao N, Paik DT, Wu H, Guo H, Termglinchan V, Churko JM, Kim Y, Kitani T, Zhao MT, Zhang Y, Wilson KD, Karakikes I, Snyder MP, Wu JC: SETD7 Drives Cardiac Lineage Commitment through Stage-Specific Transcriptional Activation. Cell Stem Cell 2018;22:428-444.e5. https://doi.org/10.1016/j.stem.2018.02.005 |
||||
| 45 O'Sullivan KE, Breen EP, Gallagher HC, Buggy DJ, Hurley JP: Understanding STAT3 signaling in cardiac ischemia. Basic Res Cardiol 2016;111:27. https://doi.org/10.1007/s00395-016-0543-8 |
||||
| 46 Yang J, Huang J, Dasgupta M, Sears N, Miyagi M, Wang B, Chance MR, Chen X, Du Y, Wang Y, An L, Wang Q, Lu T, Zhang X, Wang Z, Stark GR: Reversible methylation of promoter-bound STAT3 by histone-modifying enzymes. Proc Natl Acad Sci 2010;107:21499-21504. https://doi.org/10.1073/pnas.1016147107 |
||||
| 47 de Souza GO, Wasinski F, Donato J: Characterization of the metabolic differences between male and female C57BL/6 mice. Life Sci 2022;301:120636. https://doi.org/10.1016/j.lfs.2022.120636 |
||||
| 48 Casimiro I, Stull ND, Tersey SA, Mirmira RG: Phenotypic sexual dimorphism in response to dietary fat manipulation in C57BL/6J mice. J Diabetes Complications 2021;35:107795. https://doi.org/10.1016/j.jdiacomp.2020.107795 |
||||
| 49 Griffin C, Lanzetta N, Eter L, Singer K: Sexually dimorphic myeloid inflammatory and metabolic responses to diet-induced obesity. Am J Physiol Integr Comp Physiol 2016;311:R211-216. https://doi.org/10.1152/ajpregu.00136.2016 |
||||
| 50 Macotela Y, Boucher J, Tran TT, Kahn CR: Sex and Depot Differences in Adipocyte Insulin Sensitivity and Glucose Metabolism. Diabetes 2009;58:803-812. https://doi.org/10.2337/db08-1054 |
||||
| 51 Winn NC, Vieira-Potter VJ, Gastecki ML, Welly RJ, Scroggins RJ, Zidon TM, Gaines TL, Woodford ML, Karasseva NG, Kanaley JA, Sacks HS, Padilla J: Loss of UCP1 exacerbates Western diet-induced glycemic dysregulation independent of changes in body weight in female mice. Am J Physiol Integr Comp Physiol 2017;312:R74-84. https://doi.org/10.1152/ajpregu.00425.2016 |
||||
| 52 Jetton TL, Flores-Bringas P, Leahy JL, Gupta D: SetD7 (Set7/9) is a novel target of PPARγ that promotes the adaptive pancreatic β-cell glycemic response. J Biol Chem 2021;297:101250. https://doi.org/10.1016/j.jbc.2021.101250 |
||||
| 53 Son MJ, Kim WK, Oh KJ, Park A, Lee DS, Han BS, Lee SC, Bae KH: Methyltransferase and demethylase profiling studies during brown adipocyte differentiation. BMB Rep 2016;49:388-393. https://doi.org/10.5483/BMBRep.2016.49.7.062 |
||||
| 54 Son MJ, Kim WK, Park A, Oh KJ, Kim JH, Han BS, Kim IC, Chi SW, Park SG, Lee SC, Bae KH: Set7/9, a methyltransferase, regulates the thermogenic program during brown adipocyte differentiation through the modulation of p53 acetylation. Mol Cell Endocrinol 2016;431:46-53. https://doi.org/10.1016/j.mce.2016.04.022 |
||||
| 55 Elkouris M, Kontaki H, Stavropoulos A, Antonoglou A, Nikolaou KC, Samiotaki M, Szantai E, Saviolaki D, Brown PJ, Sideras P, Panayotou G, Talianidis I: SET9-Mediated Regulation of TGF-β Signaling Links Protein Methylation to Pulmonary Fibrosis. Cell Rep 2016;15:2733-2744. https://doi.org/10.1016/j.celrep.2016.05.051 |
||||
| 56 Sasaki K, Doi S, Nakashima A, Irifuku T, Yamada K, Kokoroishi K, Ueno T, Doi T, Hida E, Arihiro K, Kohno N, Masaki T: Inhibition of SET Domain-Containing Lysine Methyltransferase 7/9 Ameliorates Renal Fibrosis. J Am Soc Nephrol 2016;27:203-215. https://doi.org/10.1681/ASN.2014090850 |
||||
| 57 Tamura R, Doi S, Nakashima A, Sasaki K, Maeda K, Ueno T, Masaki T: Inhibition of the H3K4 methyltransferase SET7/9 ameliorates peritoneal fibrosis. PLoS One 2018;13:e0196844. https://doi.org/10.1371/journal.pone.0196844 |
||||
| 58 Aoyagi T, Higa JK, Aoyagi H, Yorichika N, Shimada BK, Matsui T: Cardiac mTOR rescues the detrimental effects of diet-induced obesity in the heart after ischemia-reperfusion. Am J Physiol Circ Physiol 2015;308:H1530-1539. https://doi.org/10.1152/ajpheart.00008.2015 |
||||
| 59 Liu J, Wang P, Zou L, Qu J, Litovsky S, Umeda P, Zhou L, Chatham J, Marsh SA, Dell'Italia LJ, Lloyd SG: High-fat, low-carbohydrate diet promotes arrhythmic death and increases myocardial ischemia-reperfusion injury in rats. Am J Physiol Circ Physiol 2014;307:H598-608. https://doi.org/10.1152/ajpheart.00058.2014 |
||||
| 60 Thakker GD, Frangogiannis NG, Zymek PT, Sharma S, Raya JL, Barger PM, Taegtmeyer H, Entman ML, Ballantyne CM: Increased Myocardial Susceptibility to Repetitive Ischemia With High-fat diet-induced Obesit. Obesity 2008;16:2593-2600. https://doi.org/10.1038/oby.2008.414 |
||||
| 61 Tan Y, Mui D, Toan S, Zhu P, Li R, Zhou H: SERCA Overexpression Improves Mitochondrial Quality Control and Attenuates Cardiac Microvascular Ischemia-Reperfusion Injury. Mol Ther - Nucleic Acids 2020;22:696-707. https://doi.org/10.1016/j.omtn.2020.09.013 |
||||
| 62 Vande Walle L, Lamkanfi M: Pyroptosis. Curr Biol 2016;26:R568-572. https://doi.org/10.1016/j.cub.2016.02.019 |
||||
| 63 Shamas-Din A, Kale J, Leber B, Andrews DW: Mechanisms of Action of Bcl-2 Family Proteins. Cold Spring Harb Perspect Biol 2013;5:a008714-a008714. https://doi.org/10.1101/cshperspect.a008714 |
||||
| 64 Ballal K, Wilson CR, Harmancey R, Taegtmeyer H: Obesogenic high fat western diet induces oxidative stress and apoptosis in rat heart. Mol Cell Biochem 2010;344:221-230. https://doi.org/10.1007/s11010-010-0546-y |
||||
| 65 Zhang N, Ye F, Zhu W, Hu D, Xiao C, Nan J, Su S, Wang Y, Liu M, Gao K, Hu X, Chen J, Yu H, Xie X, Wang J: Cardiac ankyrin repeat protein attenuates cardiomyocyte apoptosis by upregulation of Bcl-2 expression. Biochim Biophys Acta - Mol Cell Res 2016;1863:3040-3049. https://doi.org/10.1016/j.bbamcr.2016.09.024 |
||||
| 66 Sepúlveda P, Encabo A, Carbonell-Uberos F, Miñana MD: BCL-2 expression is mainly regulated by JAK/STAT3 pathway in human CD34+ hematopoietic cells. Cell Death Differ 2007;14:378-380. https://doi.org/10.1038/sj.cdd.4402007 |
||||