Corresponding Author: Joe G.N. Garcia
Department of Medicine, University of Arizona, 1230 N Cherry Ave, Room 441, Tucson, AZ 85721 (USA)
Tel. +1 (312) 618-7337, , E-Mail skipgarcia@email.arizona.edu
An Actin-, Cortactin- and Ena-VASP-Linked Complex Contributes to Endothelial Cell Focal Adhesion and Vascular Barrier Regulation
Joseph B. Mascarenhasa
Jin H. Songa
Amir A. Gabera
Jeffrey R. Jacobsonb
Anne E. Cressc
Sara M. Campa
Steven M. Dudekb
Joe G.N. Garciaa
aDepartment of Medicine, University of Arizona, Tucson, AZ, USA, bDepartment of Medicine, University of Illinois at Chicago, Chicago, IL, USA, cDepartment of Cellular and Molecular Medicine, University of Arizona, Tucson, AZ, USA
Introduction
The integrity of the vascular barrier is regulated by the actin cytoskeleton which dynamically balances both barrier-disrupting contractile forces [1] and barrier-protective forces that include cell-cell and cell matrix interactions [2]. Endothelial cell (EC) barrier integrity is severely impaired during acute inflammatory conditions via processes that result in prominent vascular leak, interstitial edema and organ dysfunction [3]. Perturbation of EC junctional and focal adhesion (FA) [4] protein complexes and dysregulation of the actin cytoskeleton [5] mediates this event. The dynamic processes of actin polymerization and stress fiber formation leading to paracellular gap formation involve actin-binding proteins such as cortactin, non-muscle myosin light chain kinase (nmMLCK), Arp2/3, p21-activated kinase and other effectors [6, 7]. These proteins (cortactin and MLCK) directly bind actin or regulate actin-related polymerization processes (Arp2/3) with protein interactions highly stimulus-specific. We demonstrated [6-8] that EC challenge with the vascular barrier-enhancing S1PR1 agonist, sphingosine-1-phosphate (S1P), induces EC nmMLCK activation and nmMLCK-mediated myosin light chain (MLC) phosphorylation, in conjunction with cortactin binding to nmMLCK [7, 9, 10]. Both nmMLCK and cortactin promote EC cytoskeletal remodeling in peripheral regions, including lamellipodia, processes critical for closure of intercellular gaps and restoration of vascular barrier integrity [7, 11]. The tight interactions of cortactin with actin and Arp2/3 [12] are facilitated by actin-binding domains in cortactin and the distribution of cortactin in EC cortical actin rings, membrane ruffles and lamellipodia [13, 14] enabling participation in critical vascular barrier-regulatory processes. In contrast, vascular barrier disruption by the PAR1-receptor activating procoagulant, thrombin, causes nmMLCK association with actin, disruption of nmMLCK-cortactin interaction [9], and centrally-distributed MLC phosphorylation and actin stress fiber formation that results in cell contraction and loss of vascular barrier integrity [15]. Thus, actin protein-protein interactions regulate EC barrier-regulatory responses.
The actin cytoskeleton is connected to the cell exterior environment via FA complexes that include integrins, paxillin, vinculin, focal adhesion kinase (FAK) and several other proteins [16, 17]. FAK is a non-receptor kinase that is involved in lamellipodia dynamics and assembly and disassembly of FAs [2, 18, 19] and FAK-deleted ECs show reduced tubulogenesis, proliferation and migration [20]. We have demonstrated important differential roles for FAK-mediated protein phosphorylation in both S1P-induced EC barrier enhancement and thrombin-mediated barrier disruption [18, 19]. Recently, utilizing measurements of trans-endothelial electrical resistance (TEER) and total internal reflection fluorescence (TIRF) microscopy [21], we identified Ena-VASP-like (EVL), a member of the Ena/VASP family of proteins, to be critically involved in integrity of the vasculature contributing to the number of FAs formed as well as lamellipodia responses to S1P and thrombin challenge. EVL over-expression enhanced EC barrier protective responses to S1P and reduced thrombin-induced EC barrier disruption [21]. TIRF studies demonstrated the presence of EVL in EC FAs and impacting FA size, distribution, and the number of FAs generated in response to S1P and thrombin challenge [21]. FAK plays as a key contributor in S1P-stimulated FA rearrangement in EVL-transduced EC but a limited role in thrombin-induced FA rearrangements.
As EVL is a novel regulator of vascular barrier integrity via interactions with FAK and cytoskeletal regulatory proteins, we sought to further interrogate EVL regulation of cytoskeletal and focal adhesion dynamics with a focus on the interactions between EVL and the key barrier-regulatory protein, EVL and cortactin. Deletion mapping studies identified cortactin domains that are critical for EVL binding and verified the essential role of actin in promoting the interaction of EVL and cortactin. In addition, we demonstrated that profilins, actin-binding proteins that regulate actin polymerization, are directly involved in facilitating EVL-FA binding.
Materials and Methods
Endothelial cell culture
Human pulmonary artery endothelial cells (HPAEC; Lonza, Walkersville, MD) were cultured in EGM2 (Lonza, Walkersville, MD) and used between passages 6 and 8 [22].
siRNA transfection
Non-targeting control (cat# D-001810-10-05) and EVL (cat# L-020877-00-0010) on-target plus smart pool siRNAs were obtained from Dharmacon/Horizon (Lafayette, CO, USA). HPAEC cells were transfected using siPORTamine (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s protocol. After 48 hours of transfection, EVL silencing in ECs was evaluated by Western blotting.
Transendothelial electrical resistance
Transendothelial electrical resistance (TEER) measurements of HPAEC cells plated in 96-well TEER plates (Applied Biophysics, Troy, NY) were performed using an electrical cell–substrate impedance sensing system (ECIS) (Applied Biophysics, Troy, NY). As reported [21], S1P (Sigma, St Louis, MO) was used to simulate vascular protection in cells transfected with either control siRNA or EVL siRNA.
Immunoprecipitation from endothelial cells
ECs were transduced with lenti-viruses of myc-tagged EVL as previously described [21]. ECs overexpressing myc-EVL were treated with sphingosine-1-phosphate 1 uM or thrombin 0.5 U/ml for 0, 5 or 10 min. Cells were lysed in NP40-CHAPS buffer supplemented with inhibitors of protease (Thermo Fisher Scientific, Waltham, USA) and phosphatase (Sigma, St Louis, MO, USA). Equal amounts of protein were taken for immunoprecipitation and incubated with myc- tagged magnetic beads (Thermo Fisher Scientific, Waltham, USA). Protein complexes were isolated by incubating for 1.5 hours at room temperature, followed by washing with the same buffer and eluted in LDS sample buffer (Thermo Fisher Scientific, Waltham, USA).
Immunoprecipitation from HEK-293 cells
HEK 293 cells were transfected using Xfect (Takara Bio, Mountain View, CA) to express HA-EVL and myc-cortactin. 48 hours post-transfection cells were prepared with NP40-CHAPS buffer. Immunoprecipitation was performed using Pierce anti-HA magnetic beads (Thermo Fisher Scientific, Waltham, USA). Proteins immunoprecipitates were eluted in LDS buffer (Thermo Fisher Scientific, Waltham, USA) and analyzed using antibodies against HA- and Myc-tag (Cell Signaling Technology, Danvers, USA), profilin-1 (Abcam, Cambridge, MA, USA) and profilin-2 (MilliporeSigma, St. Louis, MO, USA).
Analysis of mouse lung tissues
A well-established preclinical mouse model of ARDS/VILI was utilized and lung tissue samples obtained from mice received either LPS alone or combination of LPS and mechanical ventilation (LPS/VILI) and control mice [23]. Immunoprecipitation and Western blotting was carried out.
Western blotting
Western blotting of lung tissue and cell extract proteins was performed with densitometric analysis normalized to β-actin expression as previously reported [21]. Primary antibodies used in this study include EVL and cortactin (Santa Cruz Biotechnology, Dallas, USA), MLC2 and p-MLC2-T18/S19 (CST, Danvers, MA, USA), and imaging was carried out using a ChemiDoc MP imaging system (Bio-Rad, Hercules, USA). β-actin was visualized using HRP-conjugated antibodies (MilliporeSigma, St. Louis, MO, USA).
Statistical analysis
Western blot images were analyzed using ImageJ software. All images were analyzed in triplicate. All data were analyzed using Student›s t test and the statistically significant threshold was at p < 0.05.
Results
The EVL-cortactin interaction is influenced by vascular-regulatory agents
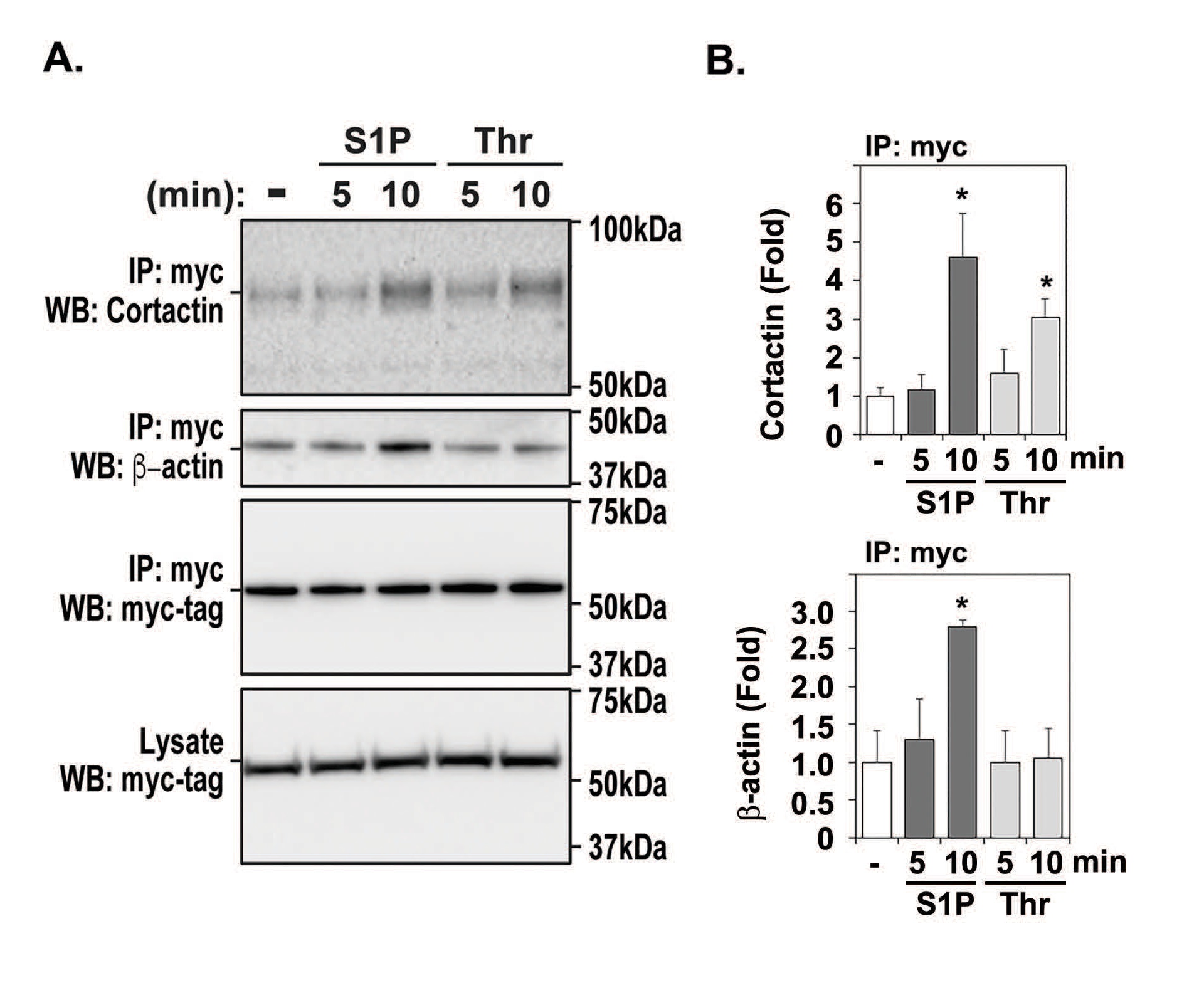
As we have previously shown that cortactin is centrally involved in vascular EC barrier regulation [8], to mechanistically determine the basis for EVL influences on vascular barrier integrity, we sought to investigate if EVL interacts with cortactin to stabilize the vascular barrier. HPAEC cells transduced with EVL-myc were stimulated with S1P or thrombin and cell lysates obtained for comparison to untreated EC. Upon immunoprecipitation (IP) with myc-tagged magnetic beads, Western blotting was performed to detect EVL and cortactin binding. While EVL over-expression was detected at unchanged level by exposures of S1P or thrombin in both cell lysates (Fig. 1A, blot 4 and 1B) and IP samples (Fig. 1A, blot 3 and 1B), the interaction of cortactin and EVL was strongly increased by S1P and thrombin following 10 minute challenge (Fig. 1A, blot 1). Co-immunoprecipitation of β-actin with EVL was also elevated in S1P-treated IPs but not to the same extent as thrombin challenge (Fig. 1A, blot 2). Because EVL is involved in EC focal adhesions in response to S1P and thrombin challenge, these data suggest that EVL-cortactin interaction may be an important event in vascular barrier regulation.
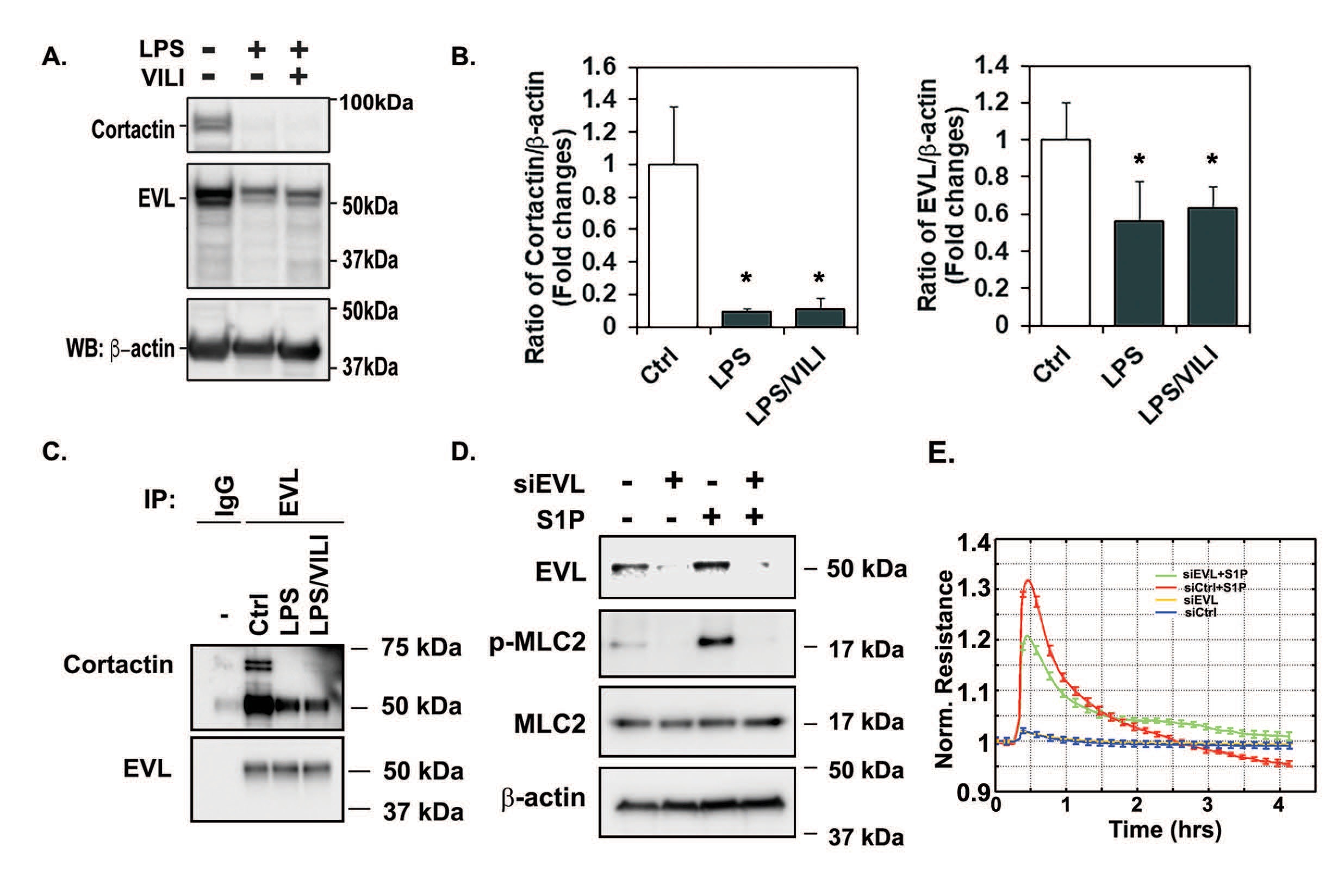
To further translate this important finding, we analyzed EVL and cortactin levels in lysates obtained from murine lungs obtained from PBS-, LPS- and LPS-VILI-challenged mice. EVL levels in lung tissue homogenates were slightly reduced across all treatments (Fig. 2A, blot 2 and 2B), however, cortactin levels were greatly decreased in both LPS and LPS-VILI treated animals compared to control animals which maintained cortactin levels (Fig. 2A, blot 1 and 2B). Protein expression of β-actin (Fig. 2A, blot 3) was used as a loading control in these experiments to verify equal protein loading. To see effects of LPS or LPS/EVL challenges on the EVL-cortactin interaction, binding assay was performed in mouse lung tissue homogenates. Immunoprecipitation with EVL conjugated beads demonstrate that the interaction of EVL and cortactin was remarkably reduced by LPS and LPS/VILI challenges (Fig. 2C). These results indicate that disruptive stimuli such as LPS and LPS-VILI decrease cortactin levels, thereby disrupting EVL-cortactin interactions which we speculate this interaction is involved in inflammation-induced vascular permeability and lung injury. To test this hypothesis, we silenced EVL expression in HPAECs using EVL siRNA to disrupt the interaction between EVL and cortactin. Then, ECs transfected with control siRNA or EVL siRNA. were exposed to S1P. S1P activates MLC2 by phosphirylation at threonine-18/serine-19 residues. As expected S1P exposure of control cells resulted in increased phosphorylation of MLC2 (Fig. 2D). In contrast, S1P exposure of EVL siRNA cells did not show increased phospho-MLC2 level. Changes in barrier integrity assessed by ECIS also demonstrated that EVL siRNA inhibited the barrier protective effect of S1P (Fig. 2E). These data suggest that EVL-cortactin interaction is required to maintain vascular integrity.
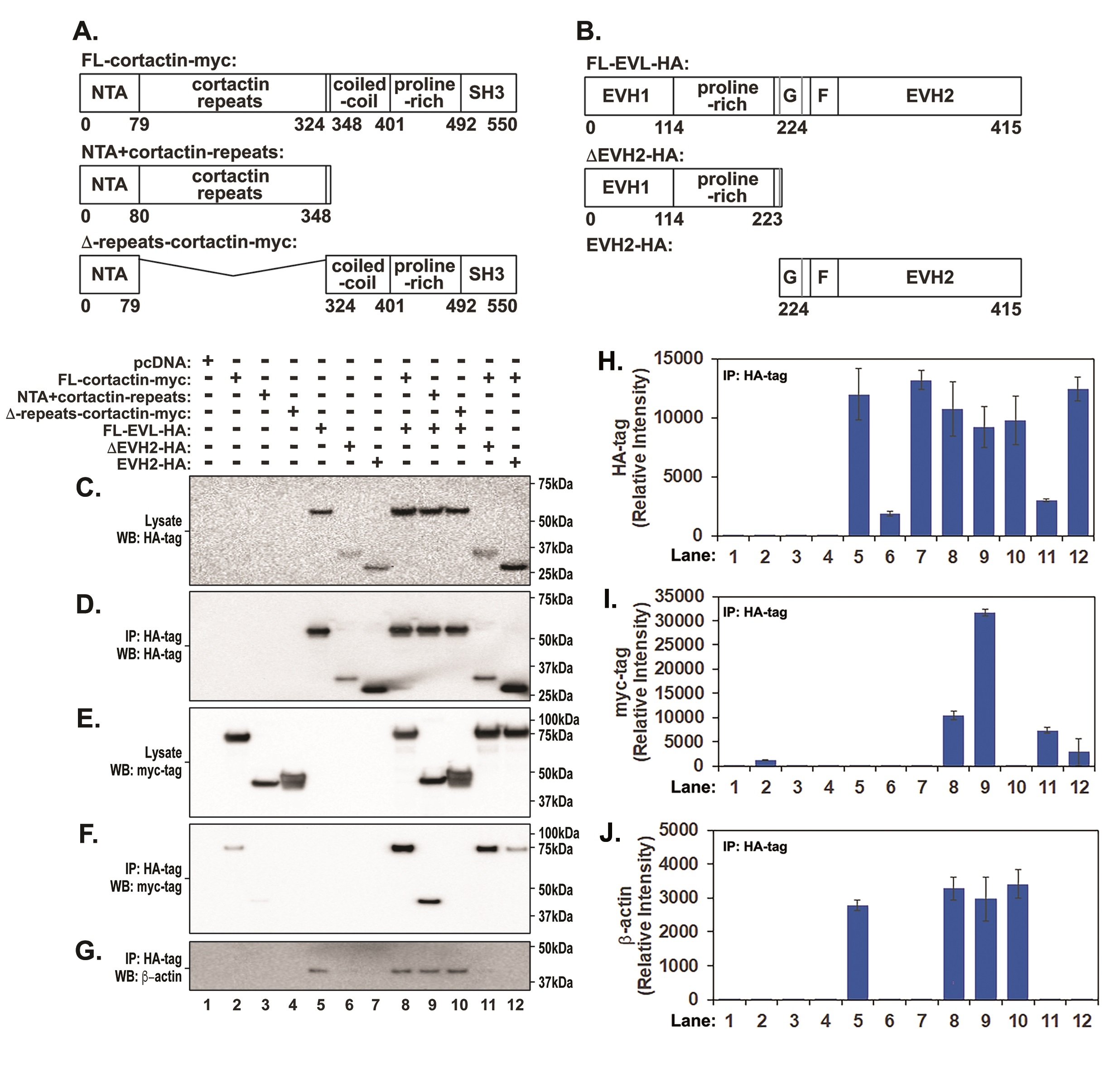
Polymerized actin drives EVL-cortactin interactions
To detail the EVL-cortactin interaction, HEK-293T cells were co-transfected with full length EVL fused to a HA tag (FL-EVL-HA) and full length cortactin fused to a myc tag (FL-cortactin-myc) (Fig. 3A and B). EVL protein was immunoprecipitated using anti-HA agarose beads and immunoprecipitated EVL was detected by Western blot using anti-HA antibody. Co-immunoprecipitated cortactin was detected by Western blot analysis with an anti-myc antibody (Fig. 3C, D, E, F and I). To validate this protein-protein interaction, we generated cortactin mutants lacking specific regions (Fig. 3A). These include a cortactin mutant lacking actin repeat region known to be important for binding to actin (∆-repeats cortactin-myc) [24] and a construct that included the NTA domain and the cortactin repeats (NTA + cortactin repeats). As expected, we detected full length EVL immunoprecipitates in cell lysates transfected with the full-length protein (Fig. 3D and H, Lanes 5, 8, 9 and 10) that were accompanied by the presence of β-actin in immunoprecipitates. To analyze the interaction, we next performed Western blots using myc-tag specific antibodies detected full-length cortactin in co-immunoprecipitates (Fig. 3F and I, Lane 8) and the cortactin deletion containing only the NTA and cortactin repeats (Fig. 3F and I, Lane 9). Interestingly, deletion of the cortactin repeats (∆-repeats cortactin-MYC) that was previously shown to bind actin, abolished cortactin binding to full length EVL (Fig. 3F and I, lane 10). The presence of β-actin in these immunoprecipitates (Fig. 3G and J, Lane 10) indicated that EVL is required for actin and cortactin assembly. To further identify regions involved in EVL-cortactin interaction, two additional constructs were generated that included the EVH2 domain that possesses the F- and G-actin binding regions (EVH2-HA) and a construct that lacks the EVH2 (∆-EVH2-HA) (Fig. 3B, C and D, lanes 5-7, lanes 10-12). Deletion of the EVH2 domain did not significantly alter EVL binding to full length cortactin (Fig. 3F and I, lane 11). In contrast, cortactin interaction with the EVH2-containing fragment was greatly decreased (Fig. 3F and I, lane 12). Both deletion fragments of EVL (EVH2-HA) and (∆-EVH2-HA) markedly lost β-actin binding (Fig. 3G and J, lanes 6 and 11, lanes 7 and 12). In summary, these data indicate that the repeat region of cortactin is critical in mediating the EVL-cortactin interaction. In EVL deletion of EVH1 and proline-rich domains resulted in decreased cortactin binding, suggesting that the EVH1 domain located at the N-terminus is preferentially involved in mediating EVL interaction with cortactin.
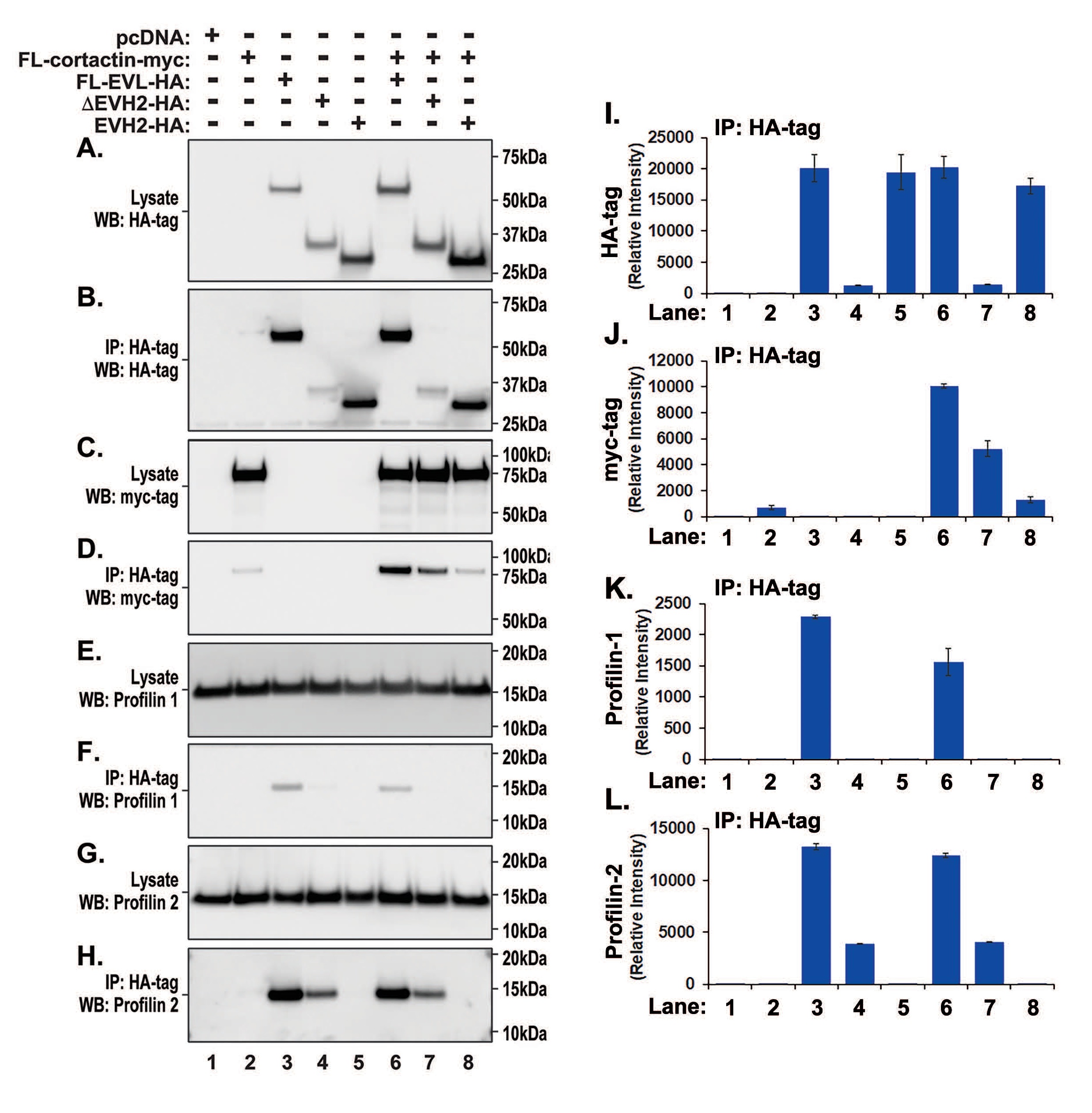
Profilin-2 is involved in EVL-cortactin interactions
We have demonstrated that only full length EVL efficiently binds to cortactin and actin. Although the actin-binding region of EVL resides within the EVH2 domain [25], immunoprecipitate analysis of cells transfected with EVH2 alone indicates that the EVH2 domain did not bind actin. In contrast, actin was detected in EVH1 domain-transfected cell immunoprecipitates suggesting the involvement of other actin-binding proteins. To further investigate this possibility, we performed western blotting for profilins, proteins capable of binding to both actin and EVL, and that promote EVL-induced actin polymerization [26]. Transfected HA- and myc-tagged proteins corresponding to EVL and cortactin respectively were identified in cell lysates (Fig. 4A, B, C, D, I and J) with abundant endogenous expression of both profilin-1 (Fig. 4E) and -2 (Fig. 4G). Abundant levels of profilin-2 were observed in immunoprecipitates of full-length EVL (Fig. 4H and L, lanes 3, 6) while profilin-2 levels were reduced in immunoprecipitates of ∆-EVH2-HA transfections reflecting weaker transduction of ∆-EVH2-HA in cell lysates. Detectable levels of profilin-2 were not observed in the EVH2 immunoprecipitates indicating preferential binding of profilin-2 to the EVH1 domain. Similarly, EVH1 domain was required for the interaction with profilin-1 (Fig. 4F and K). These data suggest that the EVL-cortactin interaction occurs via a complex involving both actin and profilin-2.
Ena VASP-like protein or EVL, regulates peripheral cytoskeletal remodeling and lamelli-podial activity to restore EC barrier integrity. EVL aggregates in EC focal adhesions after S1P presumably increasing FA strength during cytoskeletal remodeling and lamellipodial dynamics. The C-terminal EVH2 domain of EVL is involved in G-/F-actin binding and tetramerization via the oligomerization domain. The EVH1 domain, located at the N-terminus, mediates interaction with other proteins and FA targeting [4]. EVL interaction via the SH3 domain of alpha-spectrins establishes a spectrin-actin network and facilitates dynamic actin reorganization at filopodia and lamellipodia while its interaction with CRMP-1 enhances actin polymerization in the protruding lamellipodia [4, 27].
As we demonstrated that EVL promotes vascular FA remodeling involved in maintaining EC barrier integrity [21], we examined whether EVL participates in cytoskeletal rearrangements and explored EVL-cortactin interactions. EVL exerts a protective role in thrombin-induced vascular disruption and enhances EC responses to the vascular barrier enhancing agonist, S1P [21]. In the current study, we demonstrated that EVL-cortactin interaction is also directly influenced by EC challenge with either S1P or thrombin. The coimmunoprecipitation of EVL and cortactin suggests that EVL promotes the cortactin-mediated assembly of the actin polymerization proteins during the process of recovery from thrombin-induced vascular disruption. However, lung tissues obtained from mice exhibiting acute inflammatory lung injury showed loss of cortactin expression but not EVL expression. This result suggests the potential for EVL-mediated EC barrier protection to be dependent on an interaction with cortactin. Reduced cortactin expression in the lung tissues of LPS-treated mice could be explained by the reports demonstrating that LPS exposure promotes degradation of cortactin via ubiquitination and ERK-mediated phosphorylation [28]. Sarcomeric myosin heavy chain protein was also reduced in acute inflammatory lung injury models upon LPS instillation with concordant upregulation of Trim63 (MuRF1), a ubiquitin E3-ligase that tags proteins for degradation via the ubiquitin proteasomal pathway.
We have identified that the EVL-cortactin interaction occurs through a complex of proteins including actin and profilin-2 to regulate lamellipodia dynamics and actin polymerization. As mentioned above, protein complexes containing cortactin serve as a bridge between the Arp2/3 and actin to regulate lamellipodia persistence [29]. This lamellipodia persistence is highly dependent on actin polymerization and the presence of profilin to inhibit hydrolysis of actin-bound ATP favoring monomeric actin that is competent for the addition to barbed ends of actin filaments. It has also been observed that profilin-2 showed a preferential binding to EVL in breast cancer [26] and the related member, VASP, can recruit profilin to lamellipodia regions involved in actin polymerization [30]. These data indicate that localization of EVL to the lamellipodia with other proteins including cortactin and profilin enhances the rate of polymerization of actin leading to lamellipodia and filopodia extension. Moreover, disrupting EVL-cortactin interaction by silencing EVL expression increased MLC2 phsophorylation and supressed S1P-mediated barrier protection. In summary, EVL plays a significant role in regulating EC barrier integrity and vascular permeability via protein interactions with specific cortactin domains. By examining this protein-protein interaction during vascular barrier changes, we clarified EVL participation in the regulation of vascular barrier integrity and in the highly choreographed interactions between key FA and cytoskeletal partners, including cortactin. These studies inform the novel participation of the member of the Ena-VASP family known as Ena-VASP-like (EVL) in promoting vascular focal adhesion (FA) remodeling and endothelial cell (EC) barrier restoration/preservation. Using in vitro and in vivo strategies, both cortactin and profilins were found to participate in EVL-mediated EC barrier regulation. These studies further substantiate EVL participation in regulation of vascular barrier integrity and in the highly choreographed cytoskeletal interactions between key FA and cytoskeletal partners.
Author Contributions
JBM (Conceptualization; Investigation; Data curation; Formal analysis; Roles/Writing - original draft). JHS (Investigation; Data curation; Formal analysis; Roles/Writing - original draft). AAG (Investigation; Data curation; Formal analysis). SMC (Formal analysis; Visualization; Roles/Writing - original draft; Writing - review & editing). JRJ, AEC, SMD (Conceptualization; Writing - review & editing). JGNG (Conceptualization; Investigation; Funding acquisition; Resources; Roles/Writing - original draft; Writing - review & editing).
Funding
This work was supported by the NIH/ NHLBI grants R01 HL91889, P01 HL058064, and P01 HL126609 (JGNG).
Statement of Ethics
For any in vivo samples used in this study, all animal care procedures, methods, animal randomization, and experiments were performed in accordance to relevant ethical and ARRIVE guidelines/regulations. In addition, all experiments were approved by and performed in accordance with relevant institutional licensing committee guidelines and regulations (Institutional Animal Care and Use Committee (IACUC), University of Arizona, protocol # 13-490).
Joe G.N. Garcia, MD is CEO and Founder of Aqualung Therapeutics Corporation. All other authors declare no competing interests.
| 1 Dudek SM, Garcia JG: Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol (1985) 2001;91:1487-1500. https://doi.org/10.1152/jappl.2001.91.4.1487 |
||||
| 2 Romer LH, Birukov KG, Garcia JG: Focal adhesions: paradigm for a signaling nexus. Circ Res 2006;98:606-616. https://doi.org/10.1161/01.RES.0000207408.31270.db |
||||
| 3 Fan E, Brodie D, Slutsky AS: Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018;319:698-710. https://doi.org/10.1001/jama.2017.21907 |
||||
| 4 Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB: Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol 2003;19:541-564. https://doi.org/10.1146/annurev.cellbio.19.050103.103356 |
||||
| 5 Komarova YA, Kruse K, Mehta D, Malik AB: Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ Res 2017;120:179-206. https://doi.org/10.1161/CIRCRESAHA.116.306534 |
||||
| 6 Adyshev DM, Dudek SM, Moldobaeva N, Kim KM, Ma SF, Kasa A, Garcia JG, Verin AD: Ezrin/radixin/moesin proteins differentially regulate endothelial hyperpermeability after thrombin. Am J Physiol Lung Cell Mol Physiol 2013;305:L240-255. https://doi.org/10.1152/ajplung.00355.2012 |
||||
| 7 Dudek SM, Chiang ET, Camp SM, Guo Y, Zhao J, Brown ME, Singleton PA, Wang L, Desai A, Arce FT, Lal R, Van Eyk JE, Imam SZ, Garcia JG: Abl tyrosine kinase phosphorylates nonmuscle Myosin light chain kinase to regulate endothelial barrier function. Mol Biol Cell 2010;21:4042-4056. https://doi.org/10.1091/mbc.e09-10-0876 |
||||
| 8 Dudek SM, Birukov KG, Zhan X, Garcia JG: Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem Biophys Res Commun 2002;298:511-519. https://doi.org/10.1016/S0006-291X(02)02492-0 |
||||
| 9 Brown M, Adyshev D, Bindokas V, Moitra J, Garcia JG, Dudek SM: Quantitative distribution and colocalization of non-muscle myosin light chain kinase isoforms and cortactin in human lung endothelium. Microvasc Res 2010;80:75-88. https://doi.org/10.1016/j.mvr.2009.12.010 |
||||
| 10 Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG: Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem 2004;279:24692-24700. https://doi.org/10.1074/jbc.M313969200 |
||||
| 11 Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D: Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 2001;108:689-701. https://doi.org/10.1172/JCI12450 |
||||
| 12 Li Y, Uruno T, Haudenschild C, Dudek SM, Garcia JG, Zhan X: Interaction of cortactin and Arp2/3 complex is required for sphingosine-1-phosphate-induced endothelial cell remodeling. Exp Cell Res 2004;298:107-121. https://doi.org/10.1016/j.yexcr.2004.03.023 |
||||
| 13 Dudek SM, Camp SM, Chiang ET, Singleton PA, Usatyuk PV, Zhao Y, Natarajan V, Garcia JG: Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell Signal 2007;19:1754-1764. https://doi.org/10.1016/j.cellsig.2007.03.011 |
||||
| 14 Arce FT, Whitlock JL, Birukova AA, Birukov KG, Arnsdorf MF, Lal R, Garcia JG, Dudek SM: Regulation of the micromechanical properties of pulmonary endothelium by S1P and thrombin: role of cortactin. Biophys J 2008;95:886-894. https://doi.org/10.1529/biophysj.107.127167 |
||||
| 15 Xie L, Chiang ET, Wu X, Kelly GT, Kanteti P, Singleton PA, Camp SM, Zhou T, Dudek SM, Natarajan V, Wang T, Black SM, Garcia JG, Jacobson JR: Regulation of Thrombin-Induced Lung Endothelial Cell Barrier Disruption by Protein Kinase C Delta. PLoS One 2016;11:e0158865. https://doi.org/10.1371/journal.pone.0158865 |
||||
| 16 Hu YL, Lu S, Szeto KW, Sun J, Wang Y, Lasheras JC, Chien S: FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Sci Rep 2014;4:6024. https://doi.org/10.1038/srep06024 |
||||
| 17 Young LE, Higgs HN: Focal Adhesions Undergo Longitudinal Splitting into Fixed-Width Units. Curr Biol 2018;28:2033-2045 e2035. https://doi.org/10.1016/j.cub.2018.04.073 |
||||
| 18 Shikata Y, Birukov KG, Birukova AA, Verin A, Garcia JG: Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J 2003;17:2240-2249. https://doi.org/10.1096/fj.03-0198com |
||||
| 19 Shikata Y, Birukov KG, Garcia JG: S1P induces FA remodeling in human pulmonary endothelial cells: role of Rac, GIT1, FAK, and paxillin. J Appl Physiol (1985) 2003;94:1193-1203. https://doi.org/10.1152/japplphysiol.00690.2002 |
||||
| 20 Shen TL, Park AY, Alcaraz A, Peng X, Jang I, Koni P, Flavell RA, Gu H, Guan JL: Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol 2005;169:941-952. https://doi.org/10.1083/jcb.200411155 |
||||
| 21 Mascarenhas JB, Gaber AA, Larrinaga TM, Mayfield R, Novak S, Camp SM, Gregorio C, Jacobson JR, Cress AE, Dudek SM, Garcia JGN: EVL is a novel focal adhesion protein involved in the regulation of cytoskeletal dynamics and vascular permeability. Pulm Circ 2021;11:20458940211049002. https://doi.org/10.1177/20458940211049002 |
||||
| 22 Sun X, Shikata Y, Wang L, Ohmori K, Watanabe N, Wada J, Shikata K, Birukov KG, Makino H, Jacobson JR, Dudek SM, Garcia JG: Enhanced interaction between focal adhesion and adherens junction proteins: involvement in sphingosine 1-phosphate-induced endothelial barrier enhancement. Microvasc Res 2009;77:304-313. https://doi.org/10.1016/j.mvr.2008.12.004 |
||||
| 23 Quijada H, Bermudez T, Kempf CL, Valera DG, Garcia AN, Camp SM, Song JH, Franco E, Burt JK, Sun B, Mascarenhas JB, Burns K, Gaber A, Oita RC, Reyes Hernon V, Barber C, Moreno-Vinasco L, Sun X, Cress AE, Martin D, et al.: Endothelial eNAMPT Amplifies Preclinical Acute Lung Injury: Efficacy of an eNAMPT-Neutralising mAb. Eur Respir J 2021;57:2002536. https://doi.org/10.1183/13993003.02536-2020 |
||||
| 24 Weed SA, Karginov AV, Schafer DA, Weaver AM, Kinley AW, Cooper JA, Parsons JT: Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J Cell Biol 2000;151:29-40. https://doi.org/10.1083/jcb.151.1.29 |
||||
| 25 Sechi AS, Wehland J: ENA/VASP proteins: multifunctional regulators of actin cytoskeleton dynamics. Front Biosci 2004;9:1294-1310. https://doi.org/10.2741/1324 |
||||
| 26 Mouneimne G, Hansen SD, Selfors LM, Petrak L, Hickey MM, Gallegos LL, Simpson KJ, Lim J, Gertler FB, Hartwig JH, Mullins RD, Brugge JS: Differential remodeling of actin cytoskeleton architecture by profilin isoforms leads to distinct effects on cell migration and invasion. Cancer Cell 2012;22:615-630. https://doi.org/10.1016/j.ccr.2012.09.027 |
||||
| 27 Lambrechts A, Kwiatkowski AV, Lanier LM, Bear JE, Vandekerckhove J, Ampe C, Gertler FB: cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J Biol Chem 2000;275:36143-36151. https://doi.org/10.1074/jbc.M006274200 |
||||
| 28 Zhao J, Wei J, Mialki R, Zou C, Mallampalli RK, Zhao Y: Extracellular signal-regulated kinase (ERK) regulates cortactin ubiquitination and degradation in lung epithelial cells. J Biol Chem 2012;287:19105-19114. https://doi.org/10.1074/jbc.M112.339507 |
||||
| 29 Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, Weaver AM: Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr Biol 2005;15:1276-1285. https://doi.org/10.1016/j.cub.2005.06.043 |
||||
| 30 Kang F, Laine RO, Bubb MR, Southwick FS, Purich DL: Profilin interacts with the Gly-Pro-Pro-Pro-Pro-Pro sequences of vasodilator-stimulated phosphoprotein (VASP): implications for actin-based Listeria motility. Biochemistry 1997;36:8384-8392. https://doi.org/10.1021/bi970065n |
||||