Corresponding Author: Thamer A. Hamdan
Department of Hematology and Stem Cell Transplantation, University of Duisburg-Essen, Hufelandstrasse 55, 45147 Essen (Germany)
E-Mail thamer.hamdan@uk-essen.de
Insights into Virus-Induced Immune Mediated Liver Pathology
Thamer A. Hamdana Fayiqa Ashrafb Hilal Bhatc
aDepartment of Hematology and Stem Cell Transplantation, University of Duisburg-Essen, Essen, Germany, bDepartment of Biochemistry, University of Kashmir, Kashmir, India, cCenter for Molecular Medicine Cologne (CMMC), University Hospital Cologne, Cologne, Germany
Introduction
The liver is a unique solid organ in mammals with many endocrine, metabolic, and secretory functions [1, 2]. Under certain circumstances, the liver can be a paramount hub for T cell activation and is deemed as an immune synapse skewed toward tolerance [3]. The liver cell populations are dissected into two types of cells: parenchymal and non-parenchymal. Parenchymal cells comprise hepatocytes and represent 60–70 % of total liver cells, or 90 % of the total liver mass. The remaining non-parenchymal fraction is responsible for the tolerogenic properties of the liver and is diverse and encompasses cholangiocytes (epithelial cells lining the bile ducts), liver sinusoidal epithelial cells (LSEC), Kupffer cells and hepatic stellate cells (HSC), which is fat-storing cells also known as Ito cells, and intrahepatic immune cells [4-7].
The liver harbors a wide range of innate and adaptive immune cells. Innate immune cells are scattered in the parenchyma and portal tracts and are composed of Kupffer cells and lymphocytes that constitute around 20% and 25% of the non-hepatocyte populations, respectively. Liver-resident lymphocytes include B cells, conventional T cells, unconventional T cells (NKT and TCR γδ T cells), NK cells, eosinophils, neutrophils, and resident hepatic DC as professional APC along with Kupffer cells [6, 8, 9].
Strategically located in the abdominal cavity and exposed to blood circulation, the liver is vulnerable to microbial and metabolic insults culminating in liver injury. In murine models, liver pathology could be mirrored by signs of illness such as; cachexia, ataxia, hunched posture, ruffled fur, and a moribund state. Furthermore, the liver might be diffused with necrotic spots, that appear as white or hemorrhagic areas [10]. Quantitatively, the liver dysfunction can be monitored by analyzing liver enzymes levels as functional readout of liver pathology such as; alanine aminotransferase (ALT), a liver enzyme indicating the site of liver damage [11, 12], aspartate aminotransferase (AST), which is less specific than ALT [13],
lactate dehydrogenase (LDH), and glutamate dehydrogenase (GDH), a mitochondrial hepatic enzyme [13].
Infection with hepatotropic viruses can result in serious damage to hepatocytes [14]. Injury to liver tissue can be induced by the virus itself if the virus is cytolytic, causing direct harm to the hepatocytes and pathogen-driven liver injury. Immune activation within the liver can also damage the liver. The virus-induced hepatocyte damage is virus and species specific and therefore difficult to model in animal experiments. In contrast, some hepatocyte-toxic immune mechanisms are comparable between humans and mice. Therefore, the identification of virus-induced immunopathology in mice might be applicable in human virus-induced hepatitis.
The immune mediated liver pathology after virus infection could be limited or overt depending on many factors such as; genetic predisposition factors, the age of the host upon infection, the dose and route of infection [15]. Furthermore, the balance between the immunity and immunopathology is further determined by the levels of proinflammatory and anti-inflammatory factors [16]. The state in which the proinflammatory factors outpace the anti-inflammatory, viral clearance ,and tissue damage ensued [15].
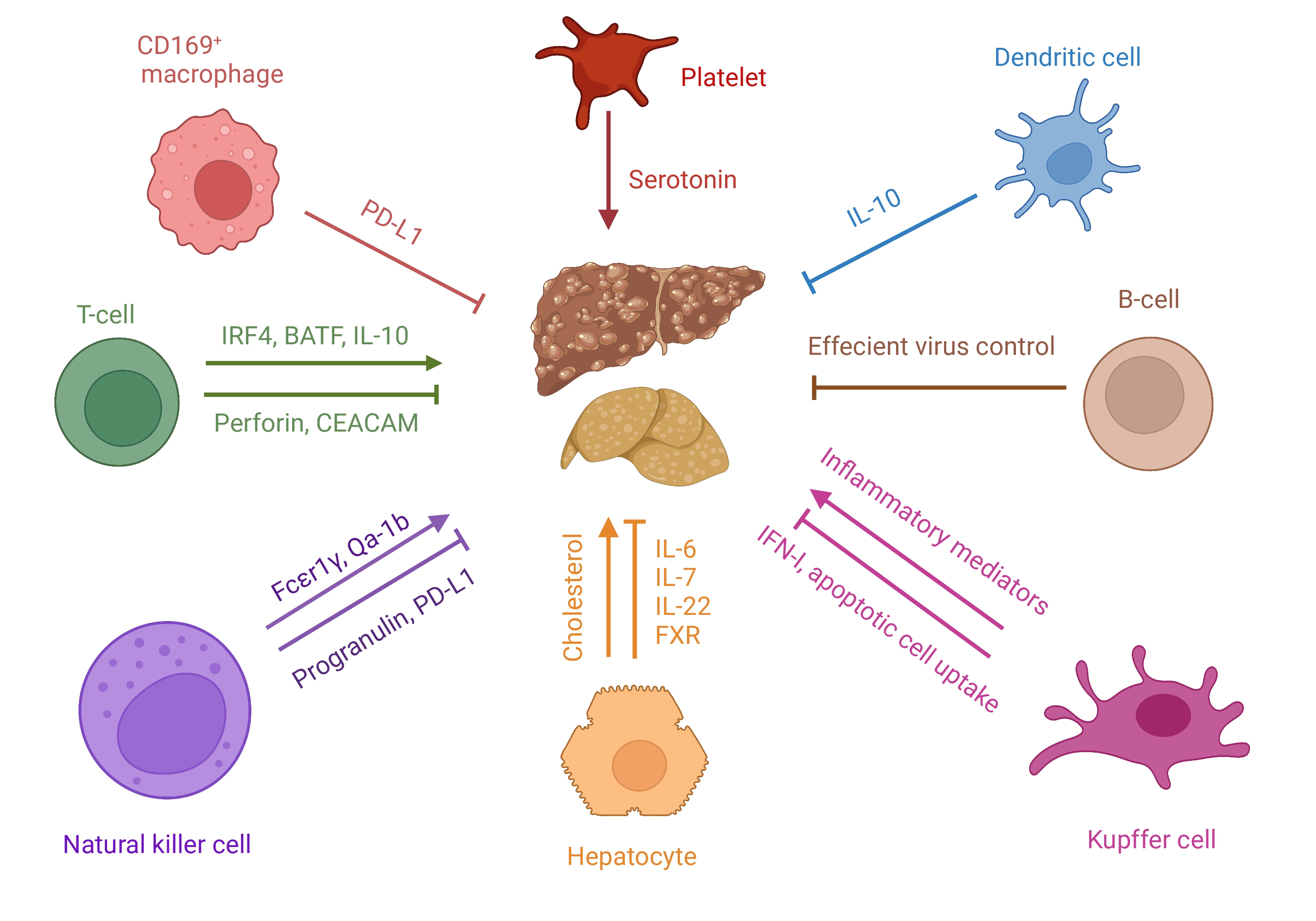
In this review we discuss immune-mediated mechanisms, that were recently described in the context of liver pathology induced during LCMV and Hepatitis virus infections as well as the roles of different immune cells and intrinsic factors (Table 1 and Fig. 1).
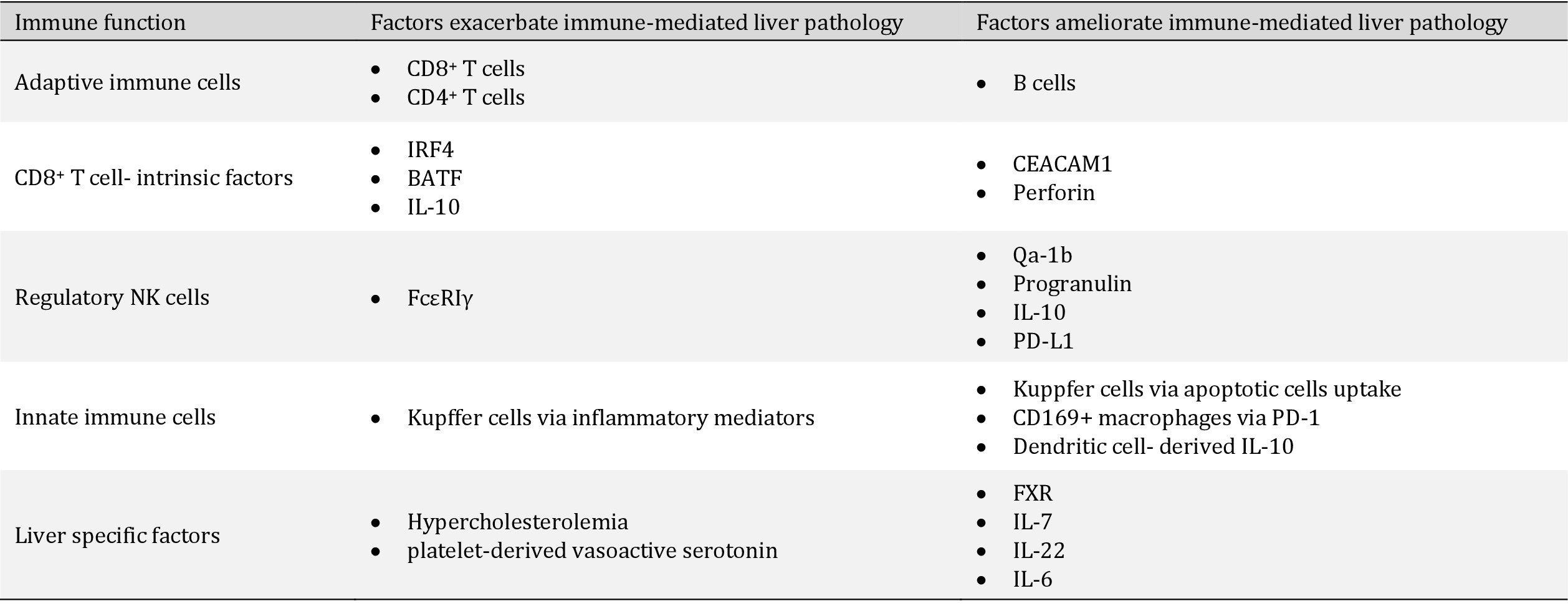
The cellular and soluble factors that provoke or curb the virus-induced immune mediated liver pathology
Author Contributions
Conceptualization, T.A.H; resources, T.A.H.; writing—original draft preparation, T.A.H.; writing—review and editing, T.A.H., F.A. and H.B.. All authors have read and agreed to the published version of the manuscript.
The authors have no conflicts of interest to declare.
| 1 Gordillo M, Evans T, Gouon-Evans V: Orchestrating liver development. Development 2015;142:2094-2108. https://doi.org/10.1242/dev.114215 |
||||
| 2 Gruppuso PA, Sanders JA: Regulation of liver development: implications for liver biology across the lifespan. J Mol Endocrinol 2016;56:R115-125. https://doi.org/10.1530/JME-15-0313 |
||||
| 3 Crispe IN: The liver as a lymphoid organ. Annu Rev Immunol 2009;27:147-163. https://doi.org/10.1146/annurev.immunol.021908.132629 |
||||
| 4 Alpini G, Phillips JO, Vroman B, LaRusso NF: Recent advances in the isolation of liver cells. Hepatology 1994;20:494-514. https://doi.org/10.1002/hep.1840200231 |
||||
| 5 Damm G, Pfeiffer E, Burkhardt B, Vermehren J, Nussler AK, Weiss TS: Human parenchymal and non-parenchymal liver cell isolation, culture and characterization. Hepatol Int 2013;7:951-958. https://doi.org/10.1007/s12072-013-9475-7 |
||||
| 6 Racanelli V, Rehermann B: The liver as an immunological organ. Hepatology 2006;43:S54-62. https://doi.org/10.1002/hep.21060 |
||||
| 7 Tiegs G, Lohse AW: Immune tolerance: what is unique about the liver. J Autoimmun 2010;34:1-6. https://doi.org/10.1016/j.jaut.2009.08.008 |
||||
| 8 Gao B, Jeong WI, Tian Z: Liver: An organ with predominant innate immunity. Hepatology 2008;47:729-736. https://doi.org/10.1002/hep.22034 |
||||
| 9 Freitas-Lopes MA, Mafra K, David BA, Carvalho-Gontijo R, Menezes GB: Differential Location and Distribution of Hepatic Immune Cells. Cells 2017;6:48. https://doi.org/10.3390/cells6040048 |
||||
| 10 Roth E, Pircher H: IFN-gamma promotes Fas ligand- and perforin-mediated liver cell destruction by cytotoxic CD8 T cells. J Immunol 2004;172:1588-1594. https://doi.org/10.4049/jimmunol.172.3.1588 |
||||
| 11 Merrick BA, Bruno ME, Madenspacher JH, Wetmore BA, Foley J, Pieper R, Zhao M, Makusky AJ, McGrath AM, Zhou JX, Taylor J, Tomer KB: Alterations in the rat serum proteome during liver injury from acetaminophen exposure. J Pharmacol Exp Ther 2006;318:792-802. https://doi.org/10.1124/jpet.106.102681 |
||||
| 12 Carakostas MC, Gossett KA, Church GE, Cleghorn BL: Evaluating toxin-induced hepatic injury in rats by laboratory results and discriminant analysis. Vet Pathol 1986;23:264-269. https://doi.org/10.1177/030098588602300306 |
||||
| 13 Giannini EG, Testa R, Savarino V: Liver enzyme alteration: a guide for clinicians. CMAJ 2005;172:367-379. https://doi.org/10.1503/cmaj.1040752 |
||||
| 14 Kiyasu PK, Caldwell SH: Diagnosis and treatment of the major hepatotropic viruses. Am J Med Sci 1993;306:248-261. https://doi.org/10.1097/00000441-199310000-00008 |
||||
| 15 Rouse BT, Sehrawat S: Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol 2010;10:514-526. https://doi.org/10.1038/nri2802 |
||||
| 16 Cicchese JM, Evans S, Hult C, Joslyn LR, Wessler T, Millar JA, Marino S, Cilfone NA, Mattila JT, Linderman JJ, Kirschner DE: Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol Rev 2018;285:147-167. https://doi.org/10.1111/imr.12671 |
||||
| 17 Guidotti LG, Chisari FV: Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol 2006;1:23-61. https://doi.org/10.1146/annurev.pathol.1.110304.100230 |
||||
| 18 Shin EC, Sung PS, Park SH: Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol 2016;16:509-523. https://doi.org/10.1038/nri.2016.69 |
||||
| 19 Chisari FV, Ferrari C: Hepatitis B virus immunopathogenesis. Annu Rev Immunol 1995;13:29-60. https://doi.org/10.1146/annurev.iy.13.040195.000333 |
||||
| 20 Asabe S, Wieland SF, Chattopadhyay PK, Roederer M, Engle RE, Purcell RH, Chisari FV: The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol 2009;83:9652-9662. https://doi.org/10.1128/JVI.00867-09 |
||||
| 21 Zhao J, Zhang Z, Luan Y, Zou Z, Sun Y, Li Y, Jin L, Zhou C, Fu J, Gao B, Fu Y, Wang FS: Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology 2014;59:1331-1342. https://doi.org/10.1002/hep.26916 |
||||
| 22 Sun HQ, Zhang JY, Zhang H, Zou ZS, Wang FS, Jia JH: Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J Viral Hepat 2012;19:396-403. https://doi.org/10.1111/j.1365-2893.2011.01561.x |
||||
| 23 Brady MT, MacDonald AJ, Rowan AG, Mills KH: Hepatitis C virus non-structural protein 4 suppresses Th1 responses by stimulating IL-10 production from monocytes. Eur J Immunol 2003;33:3448-3457. https://doi.org/10.1002/eji.200324251 |
||||
| 24 Chang Q, Wang YK, Zhao Q, Wang CZ, Hu YZ, Wu BY: Th17 cells are increased with severity of liver inflammation in patients with chronic hepatitis C. J Gastroenterol Hepatol 2012;27:273-278. https://doi.org/10.1111/j.1440-1746.2011.06782.x |
||||
| 25 Lee HC, Sung SS, Krueger PD, Jo YA, Rosen HR, Ziegler SF, Hahn YS: Hepatitis C virus promotes T-helper (Th)17 responses through thymic stromal lymphopoietin production by infected hepatocytes. Hepatology 2013;57:1314-1324. https://doi.org/10.1002/hep.26128 |
||||
| 26 Choi YS, Lee J, Lee HW, Chang DY, Sung PS, Jung MK, Park JY, Kim JK, Lee JI, Park H, Cheong JY, Suh KS, Kim HJ, Lee JS, Kim KA, Shin EC: Liver injury in acute hepatitis A is associated with decreased frequency of regulatory T cells caused by Fas-mediated apoptosis. Gut 2015;64:1303-1313. https://doi.org/10.1136/gutjnl-2013-306213 |
||||
| 27 Manangeeswaran M, Jacques J, Tami C, Konduru K, Amharref N, Perrella O, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ, Perrella A, Kaplan GG: Binding of hepatitis A virus to its cellular receptor 1 inhibits T-regulatory cell functions in humans. Gastroenterology 2012;142:1516-1525 e1513. https://doi.org/10.1053/j.gastro.2012.02.039 |
||||
| 28 Moriyama T, Guilhot S, Klopchin K, Moss B, Pinkert CA, Palmiter RD, Brinster RL, Kanagawa O, Chisari FV: Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science 1990;248:361-364. https://doi.org/10.1126/science.1691527 |
||||
| 29 Iannacone M, Sitia G, Ruggeri ZM, Guidotti LG: HBV pathogenesis in animal models: recent advances on the role of platelets. J Hepatol 2007;46:719-726. https://doi.org/10.1016/j.jhep.2007.01.007 |
||||
| 30 Rehermann B: Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest 2009;119:1745-1754. https://doi.org/10.1172/JCI39133 |
||||
| 31 Shin EC, Park SH, Demino M, Nascimbeni M, Mihalik K, Major M, Veerapu NS, Heller T, Feinstone SM, Rice CM, Rehermann B: Delayed induction, not impaired recruitment, of specific CD8(+) T cells causes the late onset of acute hepatitis C. Gastroenterology 2011;141:686-695, 695.e1. https://doi.org/10.1053/j.gastro.2011.05.006 |
||||
| 32 Heydtmann M: Macrophages in hepatitis B and hepatitis C virus infections. J Virol 2009;83:2796-2802. https://doi.org/10.1128/JVI.00996-08 |
||||
| 33 Wu Z, Han M, Chen T, Yan W, Ning Q: Acute liver failure: mechanisms of immune-mediated liver injury. Liver Int 2010;30:782-794. https://doi.org/10.1111/j.1478-3231.2010.02262.x |
||||
| 34 Kolios G, Valatas V, Kouroumalis E: Role of Kupffer cells in the pathogenesis of liver disease. World J Gastroenterol 2006;12:7413-7420. https://doi.org/10.3748/wjg.v12.i46.7413 |
||||
| 35 Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE: Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci 2007;96:2-15. https://doi.org/10.1093/toxsci/kfl173 |
||||
| 36 Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ: Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology 2003;38:1188-1198. https://doi.org/10.1053/jhep.2003.50472 |
||||
| 37 Sitia G, Iannacone M, Aiolfi R, Isogawa M, van Rooijen N, Scozzesi C, Bianchi ME, von Andrian UH, Chisari FV, Guidotti LG: Kupffer cells hasten resolution of liver immunopathology in mouse models of viral hepatitis. PLoS Pathog 2011;7:e1002061. https://doi.org/10.1371/journal.ppat.1002061 |
||||
| 38 Saraiva M, O'Garra A: The regulation of IL-10 production by immune cells. Nat Rev Immunol 2010;10:170-181. https://doi.org/10.1038/nri2711 |
||||
| 39 Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A: In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol 1996;157:798-805. | ||||
| 40 Couper KN, Blount DG, Riley EM: IL-10: the master regulator of immunity to infection. J Immunol 2008;180:5771-5777. https://doi.org/10.4049/jimmunol.180.9.5771 |
||||
| 41 Wilson EB, Brooks DG: The role of IL-10 in regulating immunity to persistent viral infections. Curr Top Microbiol Immunol 2011;350:39-65. https://doi.org/10.1007/82_2010_96 |
||||
| 42 Groux H, Bigler M, de Vries JE, Roncarolo MG: Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol 1998;160:3188-3193. | ||||
| 43 Fioravanti J, Di Lucia P, Magini D, Moalli F, Boni C, Benechet AP, Fumagalli V, Inverso D, Vecchi A, Fiocchi A, Wieland S, Purcell R, Ferrari C, Chisari FV, Guidotti LG, Iannacone M: Effector CD8(+) T cell-derived interleukin-10 enhances acute liver immunopathology. J Hepatol 2017;67:543-548. https://doi.org/10.1016/j.jhep.2017.04.020 |
||||
| 44 Napoli J, Bishop GA, McGuinness PH, Painter DM, McCaughan GW: Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology 1996;24:759-765. https://doi.org/10.1002/hep.510240402 |
||||
| 45 Asano MS, Ahmed R: Immune conflicts in lymphocytic choriomeningitis virus. Springer Semin Immunopathol 1995;17:247-259. https://doi.org/10.1007/BF00196168 |
||||
| 46 Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A, Williams R, Vergani D, Naoumov NV, Ferrari C, Bertoletti A: The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 2000;191:1269-1280. https://doi.org/10.1084/jem.191.8.1269 |
||||
| 47 Zinkernagel RM, Haenseler E, Leist T, Cerny A, Hengartner H, Althage A: T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J Exp Med 1986;164:1075-1092. https://doi.org/10.1084/jem.164.4.1075 |
||||
| 48 Yi JS, Cox MA, Zajac AJ: T-cell exhaustion: characteristics, causes and conversion. Immunology 2010;129:474-481. https://doi.org/10.1111/j.1365-2567.2010.03255.x |
||||
| 49 Waggoner SN, Cornberg M, Selin LK, Welsh RM: Natural killer cells act as rheostats modulating antiviral T cells. Nature 2011;481:394-398. https://doi.org/10.1038/nature10624 |
||||
| 50 Badovinac VP, Hamilton SE, Harty JT: Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity 2003;18:463-474. https://doi.org/10.1016/S1074-7613(03)00079-7 |
||||
| 51 Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H: Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 1994;369:31-37. https://doi.org/10.1038/369031a0 |
||||
| 52 Matloubian M, Suresh M, Glass A, Galvan M, Chow K, Whitmire JK, Walsh CM, Clark WR, Ahmed R: A role for perforin in downregulating T-cell responses during chronic viral infection. J Virol 1999;73:2527-2536. https://doi.org/10.1128/JVI.73.3.2527-2536.1999 |
||||
| 53 Straub T, Pircher H: Enhancing immunity prevents virus-induced T-cell-mediated immunopathology in B cell-deficient mice. Eur J Immunol 2019;49:782-789. https://doi.org/10.1002/eji.201847962 |
||||
| 54 Grusdat M, McIlwain DR, Xu HC, Pozdeev VI, Knievel J, Crome SQ, Robert-Tissot C, Dress RJ, Pandyra AA, Speiser DE, Lang E, Maney SK, Elford AR, Hamilton SR, Scheu S, Pfeffer K, Bode J, Mittrucker HW, Lohoff M, Huber M, et al.: IRF4 and BATF are critical for CD8(+) T-cell function following infection with LCMV. Cell Death Differ 2014;21:1050-1060. https://doi.org/10.1038/cdd.2014.19 |
||||
| 55 Khairnar V, Duhan V, Patil AM, Zhou F, Bhat H, Thoens C, Sharma P, Adomati T, Friendrich SK, Bezgovsek J, Dreesen JD, Wennemuth G, Westendorf AM, Zelinskyy G, Dittmer U, Hardt C, Timm J, Gothert JR, Lang PA, Singer BB, et al.: CEACAM1 promotes CD8(+) T cell responses and improves control of a chronic viral infection. Nat Commun 2018;9:2561. https://doi.org/10.1038/s41467-018-04832-2 |
||||
| 56 Lang PA, Recher M, Honke N, Scheu S, Borkens S, Gailus N, Krings C, Meryk A, Kulawik A, Cervantes-Barragan L, Van Rooijen N, Kalinke U, Ludewig B, Hengartner H, Harris N, Haussinger D, Ohashi PS, Zinkernagel RM, Lang KS: Tissue macrophages suppress viral replication and prevent severe immunopathology in an interferon-I-dependent manner in mice. Hepatology 2010;52:25-32. https://doi.org/10.1002/hep.23640 |
||||
| 57 Shaabani N, Duhan V, Khairnar V, Gassa A, Ferrer-Tur R, Haussinger D, Recher M, Zelinskyy G, Liu J, Dittmer U, Trilling M, Scheu S, Hardt C, Lang PA, Honke N, Lang KS: CD169(+) macrophages regulate PD-L1 expression via type I interferon and thereby prevent severe immunopathology after LCMV infection. Cell Death Dis 2016;7:e2446. https://doi.org/10.1038/cddis.2016.350 |
||||
| 58 Frebel H, Nindl V, Schuepbach RA, Braunschweiler T, Richter K, Vogel J, Wagner CA, Loffing-Cueni D, Kurrer M, Ludewig B, Oxenius A: Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med 2012;209:2485-2499. https://doi.org/10.1084/jem.20121015 |
||||
| 59 Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H: Mechanisms of immune-mediated liver injury. Toxicol Sci 2010;115:307-321. https://doi.org/10.1093/toxsci/kfq009 |
||||
| 60 Swain MG: Hepatic NKT cells: friend or foe? Clin Sci (Lond) 2008;114:457-466. https://doi.org/10.1042/CS20070328 |
||||
| 61 Muhlen KA, Schumann J, Wittke F, Stenger S, Van Rooijen N, Van Kaer L, Tiegs G: NK cells, but not NKT cells, are involved in Pseudomonas aeruginosa exotoxin A-induced hepatotoxicity in mice. J Immunol 2004;172:3034-3041. https://doi.org/10.4049/jimmunol.172.5.3034 |
||||
| 62 Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, Elford AR, Dhanji S, Shaabani N, Tran CW, Dissanayake D, Rahbar R, Ghazarian M, Brustle A, Fine J, Chen P, Weaver CT, Klose C, Diefenbach A, Haussinger D, et al.: Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci U S A 2012;109:1210-1215. https://doi.org/10.1073/pnas.1118834109 |
||||
| 63 Crouse J, Xu HC, Lang PA, Oxenius A: NK cells regulating T cell responses: mechanisms and outcome. Trends Immunol 2015;36:49-58. https://doi.org/10.1016/j.it.2014.11.001 |
||||
| 64 Pallmer K, Oxenius A: Recognition and Regulation of T Cells by NK Cells. Front Immunol 2016;7:251. https://doi.org/10.3389/fimmu.2016.00251 |
||||
| 65 Pallmer K, Barnstorf I, Baumann NS, Borsa M, Jonjic S, Oxenius A: NK cells negatively regulate CD8 T cells via natural cytotoxicity receptor (NCR) 1 during LCMV infection. PLoS Pathog 2019;15:e1007725. https://doi.org/10.1371/journal.ppat.1007725 |
||||
| 66 Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, Sun R, Yokoyama WM, Tian Z: Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest 2013;123:1444-1456. https://doi.org/10.1172/JCI66381 |
||||
| 67 Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J, Riley JK, Zhu J, Tian Z, Yokoyama WM: Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife 2014;3:e01659. https://doi.org/10.7554/eLife.01659 |
||||
| 68 Zhou J, Peng H, Li K, Qu K, Wang B, Wu Y, Ye L, Dong Z, Wei H, Sun R, Tian Z: Liver-Resident NK Cells Control Antiviral Activity of Hepatic T Cells via the PD-1-PD-L1 Axis. Immunity 2019;50:403-417 e404. https://doi.org/10.1016/j.immuni.2018.12.024 |
||||
| 69 Hamdan TA, Lang PA, Lang KS: The Diverse Functions of the Ubiquitous Fcgamma Receptors and Their Unique Constituent, FcRgamma Subunit. Pathogens 2020;9:140. https://doi.org/10.3390/pathogens9020140 |
||||
| 70 Duhan V, Hamdan TA, Xu HC, Shinde P, Bhat H, Li F, Al-Matary Y, Haussinger D, Bezgovsek J, Friedrich SK, Hardt C, Lang PA, Lang KS: NK cell-intrinsic FcepsilonRIgamma limits CD8+ T-cell expansion and thereby turns an acute into a chronic viral infection. PLoS Pathog 2019;15:e1007797. https://doi.org/10.1371/journal.ppat.1007797 |
||||
| 71 Xu HC, Huang J, Pandyra AA, Lang E, Zhuang Y, Thons C, Timm J, Haussinger D, Colonna M, Cantor H, Lang KS, Lang PA: Lymphocytes Negatively Regulate NK Cell Activity via Qa-1b following Viral Infection. Cell Rep 2017;21:2528-2540. https://doi.org/10.1016/j.celrep.2017.11.001 |
||||
| 72 Huang A, Shinde PV, Huang J, Senff T, Xu HC, Margotta C, Haussinger D, Willnow TE, Zhang J, Pandyra AA, Timm J, Weggen S, Lang KS, Lang PA: Progranulin prevents regulatory NK cell cytotoxicity against antiviral T cells. JCI Insight 2019;4:e129856. https://doi.org/10.1172/jci.insight.129856 |
||||
| 73 Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, Chisari FV, Ruggeri ZM, Guidotti LG: Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med 2005;11:1167-1169. https://doi.org/10.1038/nm1317 |
||||
| 74 Benechet AP, Iannacone M: Determinants of hepatic effector CD8(+) T cell dynamics. J Hepatol 2017;66:228-233. https://doi.org/10.1016/j.jhep.2016.07.011 |
||||
| 75 Lang PA, Contaldo C, Georgiev P, El-Badry AM, Recher M, Kurrer M, Cervantes-Barragan L, Ludewig B, Calzascia T, Bolinger B, Merkler D, Odermatt B, Bader M, Graf R, Clavien PA, Hegazy AN, Lohning M, Harris NL, Ohashi PS, Hengartner H, et al.: Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med 2008;14:756-761. https://doi.org/10.1038/nm1780 |
||||
| 76 Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB: Interleukin-10 determines viral clearance or persistence in vivo. Nat Med 2006;12:1301-1309. https://doi.org/10.1038/nm1492 |
||||
| 77 Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG: Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med 2006;203:2461-2472. https://doi.org/10.1084/jem.20061462 |
||||
| 78 Gassa A, Jian F, Kalkavan H, Duhan V, Honke N, Shaabani N, Friedrich SK, Dolff S, Wahlers T, Kribben A, Hardt C, Lang PA, Witzke O, Lang KS: IL-10 Induces T Cell Exhaustion During Transplantation of Virus Infected Hearts. Cell Physiol Biochem 2016;38:1171-1181. https://doi.org/10.1159/000443067 |
||||
| 79 Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R: Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 2003;4:1191-1198. https://doi.org/10.1038/ni1009 |
||||
| 80 Chazen GD, Pereira GM, LeGros G, Gillis S, Shevach EM: Interleukin 7 is a T-cell growth factor. Proc Natl Acad Sci U S A 1989;86:5923-5927. https://doi.org/10.1073/pnas.86.15.5923 |
||||
| 81 Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, Assouline B, Lahl K, Sparwasser T, Tedder TF, Paik JH, DePinho RA, Basta S, Ohashi PS, Mak TW: IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 2011;144:601-613. https://doi.org/10.1016/j.cell.2011.01.011 |
||||
| 82 Radaeva S, Sun R, Pan HN, Hong F, Gao B: Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 2004;39:1332-1342. https://doi.org/10.1002/hep.20184 |
||||
| 83 Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA: Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 2007;27:647-659. https://doi.org/10.1016/j.immuni.2007.07.023 |
||||
| 84 Klein C, Wustefeld T, Assmus U, Roskams T, Rose-John S, Muller M, Manns MP, Ernst M, Trautwein C: The IL-6-gp130-STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. J Clin Invest 2005;115:860-869. https://doi.org/10.1172/JCI23640 |
||||
| 85 Allen K, Jaeschke H, Copple BL: Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol 2011;178:175-186. https://doi.org/10.1016/j.ajpath.2010.11.026 |
||||
| 86 Honke N, Shaabani N, Hardt C, Krings C, Haussinger D, Lang PA, Lang KS, Keitel V: Farnesoid X Receptor in Mice Prevents Severe Liver Immunopathology During Lymphocytic Choriomeningitis Virus Infection. Cell Physiol Biochem 2017;41:323-338. https://doi.org/10.1159/000456168 |
||||
| 87 Ludewig B, Jaggi M, Dumrese T, Brduscha-Riem K, Odermatt B, Hengartner H, Zinkernagel RM: Hypercholesterolemia exacerbates virus-induced immunopathologic liver disease via suppression of antiviral cytotoxic T cell responses. J Immunol 2001;166:3369-3376. https://doi.org/10.4049/jimmunol.166.5.3369 |
||||
| 88 Zhou X, Paulsson G, Stemme S, Hansson GK: Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest 1998;101:1717-1725. https://doi.org/10.1172/JCI1216 |
||||
| 89 Seiler P, Aichele P, Odermatt B, Hengartner H, Zinkernagel RM, Schwendener RA: Crucial role of marginal zone macrophages and marginal zone metallophils in the clearance of lymphocytic choriomeningitis virus infection. Eur J Immunol 1997;27:2626-2633. https://doi.org/10.1002/eji.1830271023 |
||||
| 90 Simons K, Ikonen E: Functional rafts in cell membranes. Nature 1997;387:569-572. https://doi.org/10.1038/42408 |
||||
| 91 Janes PW, Ley SC, Magee AI, Kabouridis PS: The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin Immunol 2000;12:23-34. https://doi.org/10.1006/smim.2000.0204 |
||||