Corresponding Author: Suhail Al-Salam
Department of Pathology, College of Medicine and Health Sciences, United Arab Emirates University, Al-Ain, PO Box 17666 (UAE)
Tel. +97137137464, Fax +97137671966, E-Mail suhaila@uaeu.ac.ae
Nootkatone Ameliorates Doxorubicin Induced Myocardial Injury through Modulation of NF-κB Signals and Oxidative Stress
Suhail Al-Salama Karthishwaran Kandhana Manjusha Sudhadevia Saeed Tariqb
aDepartment of Pathology, College of Medicine & Health Sciences, United Arab Emirates University, Al-Ain, United Arab Emirates, bDepartment of Anatomy, College of Medicine & Health Sciences, United Arab Emirates University, Al-Ain, United Arab Emirates
Introduction
Doxorubicin (DOXO) is a potent chemotherapeutic drug that is used in the treatment of a large number of cancers [1]. Despite its important chemotherapeutic characteristics, its usage is limited because of the serious side effects; the most noticeable is cardiotoxicity which can manifest acutely or years after completion of treatment leading to left ventricular dysfunction, dilated cardiomyopathy and heart failure [2]. The most important toxic effects of DOXO are cardiomyocyte injury and apoptotic and necrotic cell death [3]. The severity of heart disease correlates well with the cumulative dose of DOXO during the course of the cancer therapy [4]. Cardiac myocytes require optimally functioning mitochondria to produce enough ATP needed for maintaining their contractile function. Mitochondrial injury is crucial to DOXO-induced cardiac dysfunction and cell death [5]. The major cause of DOXO -induced mitochondrial injury is over-production of reactive oxygen (ROS) and nitrogen species (RNS) such as superoxide, hydrogen peroxide, hydroxyl radical and peroxynitrite [6], which lead to DNA damage, oxidation and nitrosylation of proteins and peroxidation of lipids. DOXO binds to mitochondrial DNA and impairs the electron transport chain resulting in production of ROS and decreased ATP [7]. The DNA damage stimulates the ataxia telangiectasia mutated protein which upregulates and activates p53 [8]. P53 upregulates expression of pro-apoptotic members Bax and Bad as well as increases expression of Bcl2/adenovirus E1B 19 KDa protein-interacting protein 3 (Bnip3), which can cause mitochondrial damage and necrotic cell death, as well as initiate mitophagy [9]. DNA damage and increased levels of ROS lead to downregulation of the transcription factor GATA-4 which decreases expression of the anti-apoptotic, and anti-autophagy-initiation protein, Bcl-2 [10]. The cardiac response to long term myocardial injury includes fibrotic and hypertrophic processes and a key mediator in this response is transforming growth factor-β1 (TGF-β1) [11]. Increased cardiac fibrosis is an important late outcome of doxorubicin therapy and it is associated with increased TGF-B level [12].
Nootkatone (NK), a recognized bioactive compound, is isolated for the first time from the of Cupressus nootkatensis (Alaska cedar). NK is also isolated from grapefruit and pummel [13]. It has been reported that NK has several pharmacological properties such as antimicrobial, antioxidant, antifibrotic and anti- allergic activities [14-20].
Since cardiac toxicity related to DOXO involves oxidative stress and apoptosis at early events and fibrosis and cardiac dysfunction as late events and NK has potential anti-oxidant activities at early events as well as has palliative effects against some experimental diseases involving inflammation and oxidative stress [6, 10, 14-20], we considered that it was relevant to assess the possible protective effects of NK on early DOXO-induced cardiac toxicity and the mechanisms underlying these effects in mice.
Materials and Methods
Mouse model of doxorubicin cardiac toxicity
We used mouse experimental models of acute doxorubicin cardiac toxicity which has been extensively described in literature [9].
C57BL/6 mice weighing 25-30 g were used. Mice were maintained on a standard diet. Mice were housed five per cage under a 12-h light and dark schedule for at least 1 week before DOXO administration. DOXO (Sigma-Aldrich, USA) was freshly prepared on the day of administration in sterile normal saline at a concentration of 0.5 mg/ml. DOXO was given intraperitoneally with a final cumulative dose of 20mg/kg. NK (Sigma-Aldrich, USA) was administered by gavage with a dose of 90 mg/kg. Mice were given either DOXO or NK or DOXO+NK or vehicle (normal saline) after which the mice again had free access to food and water. The method of euthanasia started with intraperitoneal injection of aesthetic drugs, which included a combination of Ketamine (100 mg/kg) and Xylazine (10 mg/kg). When the mice were completely anesthetized, blood was collected in EDTA vacutainers and hearts were resected, washed in ice cold phosphate buffered saline (PBS), and immediately frozen in liquid nitrogen and later stored in −80°C freezer. Collected blood was centrifuged at 3000 RPM for 15 minutes. The plasma was collected, alliquoted and stored at −80°C until further analysis. In addition, heart samples from the same groups were also fixed in 10% buffered formal-saline for 24 hours.
Experimental groups
Group A: C57BL/6 (n = 10), DOXO 20 mg/kg, was administered once intraperitoneally, for a total cumulative dose of 20 mg/kg. Animals were sacrificed after 5 days of the administrative dose.
Group B: C57BL/6 (n = 10), (90 mg/kg) of Nootkatone was administered by gavage one hour prior to the intraperitoneal administration of DOXO 20 mg/kg, administered once intraperitoneally, for a total cumulative dose of 20 mg/kg. Then NK was administered on daily bases for 5 days. Animals were sacrificed after 5 days of the administrative dose.
Group C: C57BL/6 (n = 10), normal saline was administered once intraperitoneally. Animals were sacrificed after 5 days of the administrative dose.
Group D: C57BL/6 (n = 10), (90 mg/kg) of Nootkatone was administered by gavage daily for 5 days. Animals were sacrificed after 5 days of the administrative dose.
Protein extraction from samples
Total protein was extracted from heart samples by homogenizing with lysis buffer and collecting the supernatant after centrifugation. For total tissue homogenate, the heart samples were thawed, weighed and put in cold lysis buffer containing 50mM Tris, 300mM NaCl, 1mM MgCl2, 3mM EDTA, 20mM β-glycerophosphate, 25mM NaF, 1% Triton X-100, 10%w/v Glycerol and protease inhibitor tablet (Roche Complete protease inhibitor cocktail tablets). The hearts were homogenized on ice by a homogenizer (IKA T25 Ultra Turrax). The samples were then centrifuged at 14000 RPM for 15 minutes at 4o C, supernatant collected, alliquoted and stored at -80 °C until further analysis. Total protein concentration was determined by BCA protein assay method (Thermo Scientific Pierce BCA Protein Assay Kit).
Tissue Processing
Hearts were excised, washed with ice-cold PBS, blotted with filter paper and weighed. Each heart was sectioned into coronal slices of 2mm thickness then cassetted and fixed directly in 10% neutral formalin for 24 hours, which was followed by dehydration in increasing concentrations of ethanol, clearing with xylene and embedding with paraffin.
Modified Gomori trichrome staining of mitochondria
Sections were stained with modified Gomori trichrome method to demonstrate the mitochondria using standard procedures. Five-µm sections were deparaffinized with xylene and rehydrated with graded alcohol. Sections were immersed in Harris Hematoxylin for 5 minutes then wash with tap water until the water is clear. Sections were then immersed in Gomori trichrome stain for 10 minutes. Sections were then differentiated with few dips of 0.2% acetic acid. Sections were then immersed directly into 95 % alcohol and dehydrated in ascending alcohol solutions. Sections were then cleared with xylene and mounted with DPX.
Immunohistochemistry
Five-µm sections were deparaffinized with xylene and rehydrated with graded alcohol. Sections were then placed in EnVisionTM FLEX Target Retrieval Solution with a high PH (PH 9) (DAKO Agilent, USA) in a water bath at 95°C for 30 minutes. Sections were then washed with distilled water for 5 minutes followed by PBS for 5 minutes. Sections were then treated with peroxidase block for 15 minutes. Sections were then incubated for one hour at room temperature with anti-Dystrophin antibody (rabbit monoclonal antibody 1:100, Thermo Fischer, USA). After conjugation with primary antibodies, sections were incubated with secondary antibody (EnVisionTM Detection System, DAKO, Agilent, USA) for 20 minutes at room temperature followed by addition of DAB chromogen (EnVisionTM Detection System, DAKO, Agilent, USA) and counter staining done with haematoxylin. Appropriate positive controls were used. For negative control, the primary antibody was not added to sections. Positive and negative controls were used in every batch of stained slides (not shown in figures).
Electron microscopic study
Samples were immediately immersed in McDowell and Trump fixative for 3 h at 25°C. Tissues were then rinsed with ethanol and propylene oxide, infiltrated, embedded in Agar-100 epoxy resin, and polymerized at 65°C for 24 h. Blocks were then trimmed and semithin and ultrathin sections were cut with Reichert Ultracuts, ultramicrotome. The semithin sections (1 µm) were stained with 1% aqueous toluidine blue on glass slides. The ultrathin sections (95 nm) on 200 mesh Cu grids were contrasted with uranyl acetate followed by lead citrate double stain. The grids were examined and photographed under a Philips CM10 transmission electron microscope.
Enzyme linked immunosorbent assay
Heart myocardial concentration of GAL-3, cleaved caspase-3, Phospho-NF kappa-B, CRP, IL1-B, IL6, and lipocalin-2 were determined by using enzyme linked immunosorbent assay (ELISA) development kits according to manufacturer instructions: [mouse galectin-3 (DY1197) R&D Systems, Minneapolis, MN, USA], mouse cleaved caspase-3 [(DYC 835), R&D Systems, Minneapolis, MN, USA], Phospho-NF kappa-B [(ab176663), Abcam, USA], [mouse CRP (DY1829) R&D Systems, Minneapolis, MN, USA], [mouse IL1-B (DY401) R&D Systems, Minneapolis, MN, USA], [mouse IL6 (DY406) R&D Systems, Minneapolis, MN, USA], [mouse lipocalin-2 (DY1857) R&D Systems, Minneapolis, MN, USA]. Heart GSH, SOD, Catalase, TBARS, and DNA/RNA oxidative damage concentrations were determined using Cayman detection kits according to manufacturer instructions [GSH (703002), Cayman Chemical, Michigan, USA], [SOD (706002), Cayman Chemical, Michigan, USA], [Catalase (707002), Cayman Chemical, Michigan, USA], [TBARS (10009055), Cayman Chemical, Michigan, USA], and [DNA/RNA oxidative damage (75817-288), Cayman Chemical, Michigan, USA].
Plasma cardiac Troponin I was determined by Elisa kit (2010-1-HSP, Life Diagnostics, Inc.), for sandwich ELISA, using standard procedure according to the manufacturer’s instructions.
The concentrations were normalized to total protein concentrations.
Nootkatone Scavenging activity
Hydroxyl Radical Scavenging activity. According to the updated method of Halliwell et al. [21], the scavenging potential for hydroxyl radical (OH•) was calculated. The incubation mixture contained 0.1 ml of 100 mM potassium phosphate buffer (pH 7.9) and varying concentrations of Nootkatone (20- 100 mg Gallic acid equivalent (GAE)) for a total volume of 1 ml. The assay was performed in distilled deionized water by adding 0.1ml EDTA (1 mM), 0.2 ml FeCl3 (10 mM), 0.1 ml Ascorbic Acid (1 mM), 0.1 ml H2O2 (10 mM) and 0.2 ml 2-Deoxyribose (10 mM). The mixture was then incubated at 37ºC for 1 hour. 1.0 ml portion of the incubated mixture was mixed with 1.0 ml of 10 % TCA and 1.0 ml of 0.5 % TBA (in 0.025 M NaOH containing 0.025 % BHA) to develop the pink chromogen measured at 532 nm. Decreased reaction mixture absorption implies increased scavenging activity of OH•. The compound’s hydroxyl radical scavenging activity is stated as percent deoxyribose degradation inhibition is measured using the following equation:
Hydroxyl radical scavenging activity = (control OD − sample OD/control OD) × 100.
Superoxide radicals assay. Superoxide radicals are produced by oxidation of NADH and assayed by the reduction of NBT in PMS-NADH systems. Superoxide anion (O2•-) scavenging activity of Nootkatone was determined by the method of Nishimiki et al. [22] with slight modifications. In these experiments, 3 ml of Tris-HCl buffer (16 mM, pH 8.0) containing 1 ml of NBT (50 mM) solution, 1 ml of NADH (78 mM) solution and varying concentrations of Nootkatone (20-100 µg GAE) were used to produce superoxide radicals. The reaction began by adding to the mixture 1 ml of PMS solution (10 mM, pH 7.4). The reaction mixture was incubated for 15 minutes at 30°C, forming a violet color complex indicating superoxide anion production, which was read with the reagent blank at 560 nm. For comparison, Gallic Acid has been used as a reference standard. The increase in superoxide anion scavenging activity was demonstrated by reduced absorption of the reaction mixture. The percent inhibition of the generation of superoxide anion was determined using the following formula:
% inhibition = (control OD − sample OD/control OD) × 100.
ABTS scavenging assay. ABTS●+ (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical cation decolorization assay by the Wolfenden and Willson [23] method determined the total antioxidant activity of Nootkatone. ABTS●+ was developed by reacting with 2.4 mM potassium persulfate to a 7 mM ABTS aqueous solution. Before used for incomplete oxidation of ABTS●+, the mixture was permitted to stand at room temperature for 16 hours in the dark. The incubation mixture contained 0.54 mL of ABTS●+, 0.5 mL of phosphate buffer and varying concentrations of Nootkatone (20-100 μg GAE) for a total volume of 5 mL. The absorption was read at 734 nm in the spectrophotometer and the results were expressed as the equivalent of gallic acid used as a standard. The activity of radical scavenging was expressed as the percentage of free radical inhibition by the sample and measured using the formula:
% ABTS radical scavenging activity = (control OD − sample OD/control OD) × 100.
DPPH radical scavenging assay
The free radical scavenging activity of Nootkatone was calculated by a radical scavenging assay of 1,1-diphenyl-2-picryl-hydrazil (DPPH) using the Brand-Williams et al. [24] method. Based on the bleaching of the purple colored DPPH methanolic solution, the hydrogen atom or electron donation potential of the sample was measured. To the varying concentrations of Nootkatone (20-100 μg) dissolved in 0.5 percent DMSO and making up to 3 ml of water, one milliliter of the reaction solution containing 0.1 mmol/l DPPH in methanol was added. The mixture was vigorously shaken and allowed to stand at room temperature for 30 minutes and the absorption of the resulting solution using a spectrophotometer was measured at 517 nm (GE UltroSpec 7000 UV-Vis, USA). As a reference standard for comparison, gallic acid was used. The following equation measures the inhibition percentage of DPPH radical scavenging activity:
% DPPH radical scavenging activity= (control OD − sample OD/control OD) × 100.
Statistical analysis
All statistical analyses were done using GraphPad Prism Software version 5. Comparisons between the various groups were achieved by one-way analysis of variance (ANOVA), followed by Newman-Keuls multiple range tests. Data are presented in mean ± standard error (S.E). P values < 0.05 are considered significant.
Results
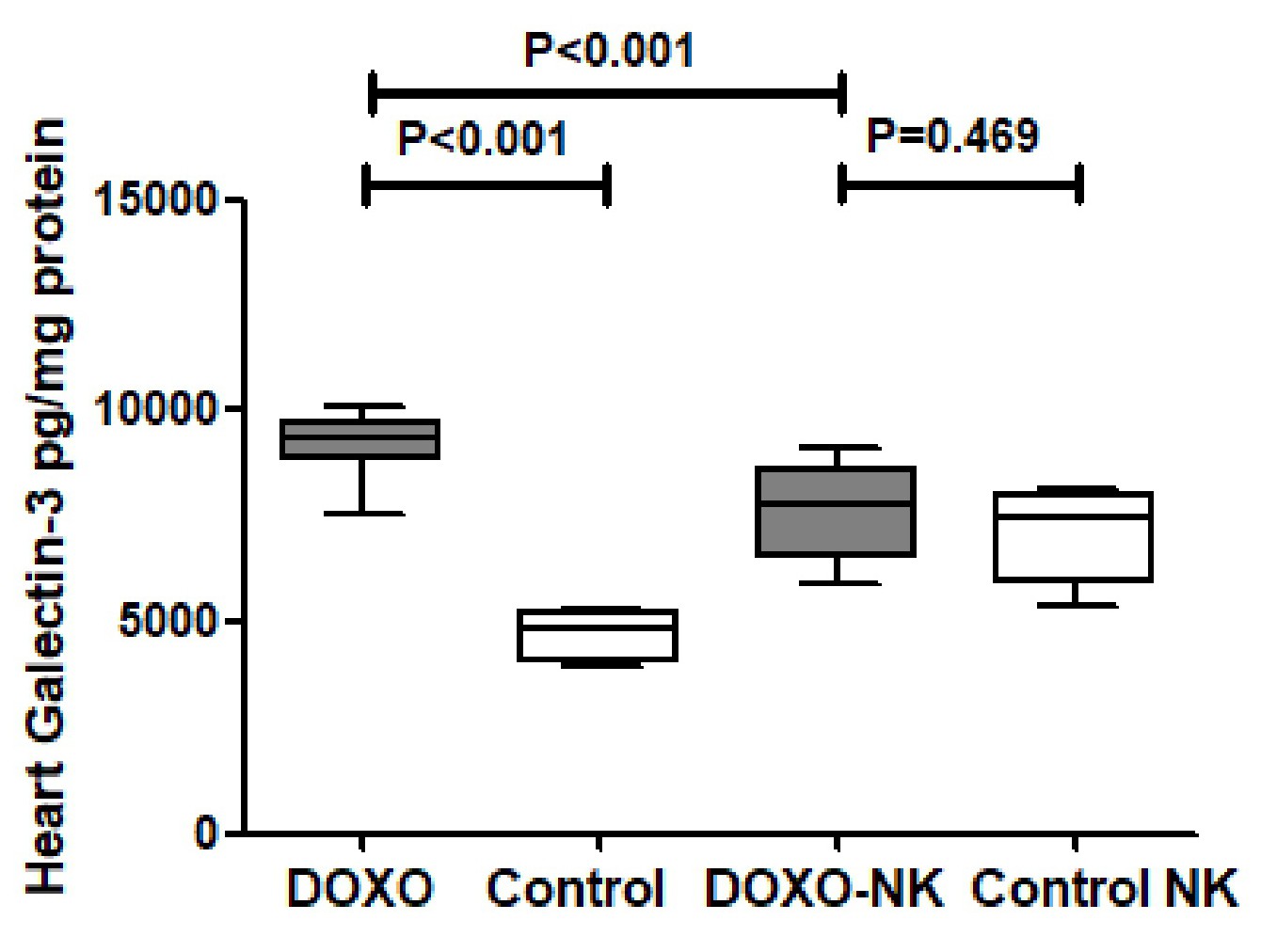
Nootkatone decreases heart GAL-3 in DOXO-induced cardiac injury
Galectin-3 was measured by ELISA in tissue homogenate of hearts in C57BL/6J mice treated with DOXO and DOXO + NK compared to their corresponding sham controls. Our results show GAL-3 level is significantly higher in DOXO-treated mice group than sham control group (9125 ± 313.2 vs 4701 ± 262.8 pg/mg, p<0.001 ), while there was no significant difference of GAL-3 levels between DOXO-NK treated mice and their sham control group (7563 ± 434.2 vs 7085 ± 467.3 pg/mg, p=0.46 ) (Fig. 1). There were significant higher levels of GAL-3 in hearts of DOXO-treated mice than DOXO-NK-treated group (9125 ± 313.2 vs 7563 ± 434.2 pg/mg, p<0.001 ) (Fig. 1).
Nootkatone possesses anti-inflammatory effects in DOXO-induced cardiac injury
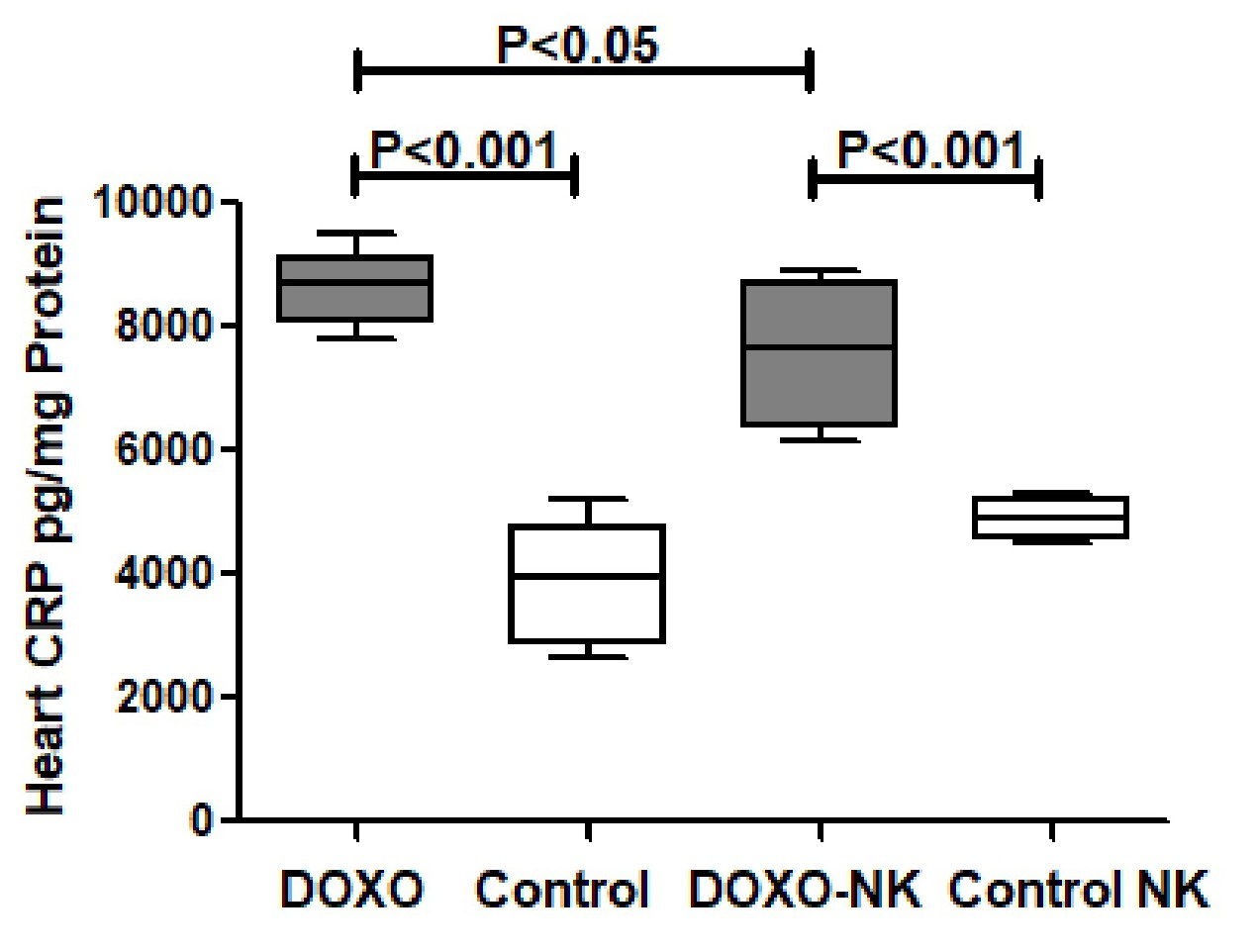
Nootkatone decreases heart CRP in DOXO-induced cardiac injury. C-reactive protein (CRP) was measured by ELISA in tissue homogenate of hearts in C57BL/6J mice treated with DOXO and DOXO + NK compared to their corresponding sham controls. Our results show CRP level is significantly higher in DOXO-treated mice group than sham control group (8597 ± 281.7 vs 4701 ± 3894 pg/mg, p<0.001) (Fig. 2). There was a significant higher level of CRP in DOXO-NK treated mice than their sham control mice (7483 ± 428.7 vs 4895 ± 132.4 pg/mg, p<0.001) (Fig. 2). There were significant higher levels of CRP in hearts of DOXO-treated mice than DOXO-NK-treated group (8597 ± 281.7 vs 7483 ± 428.7 pg/mg, p<0.05) (Fig. 2).
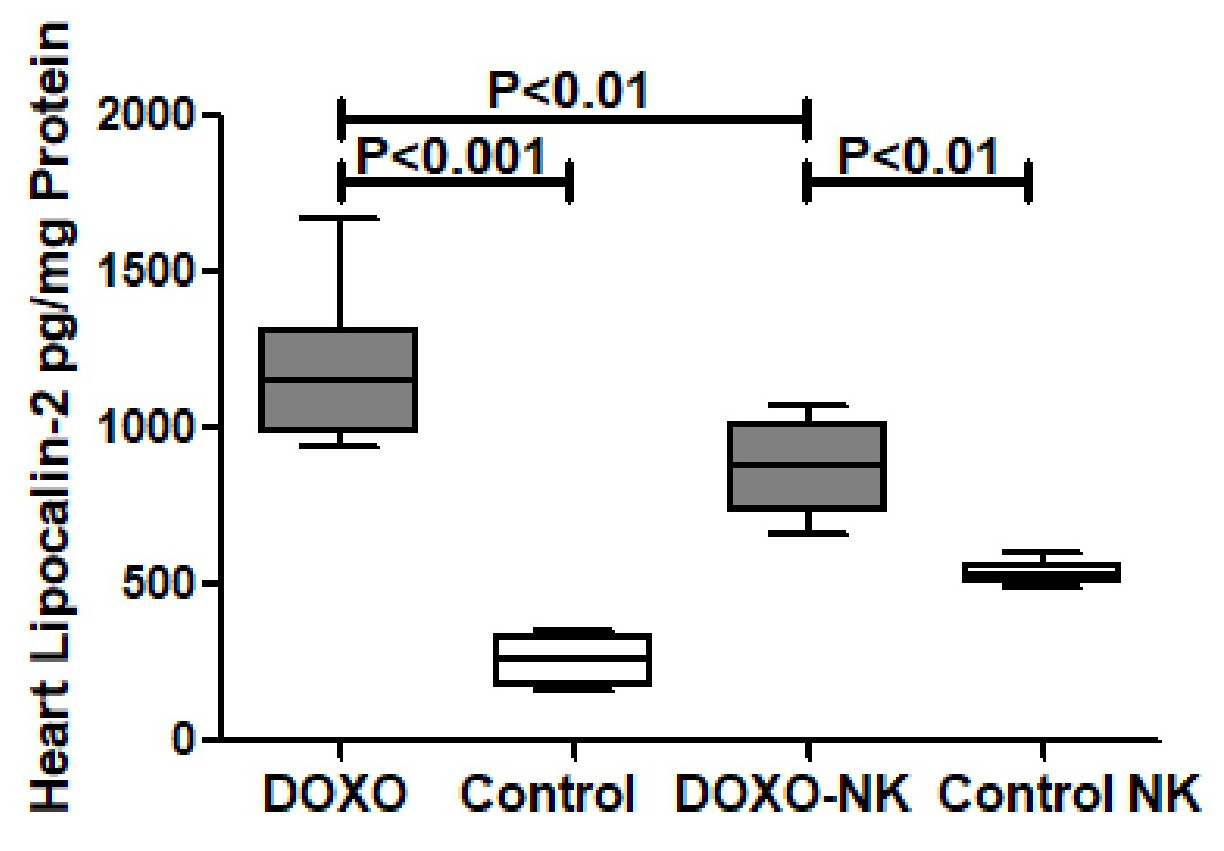
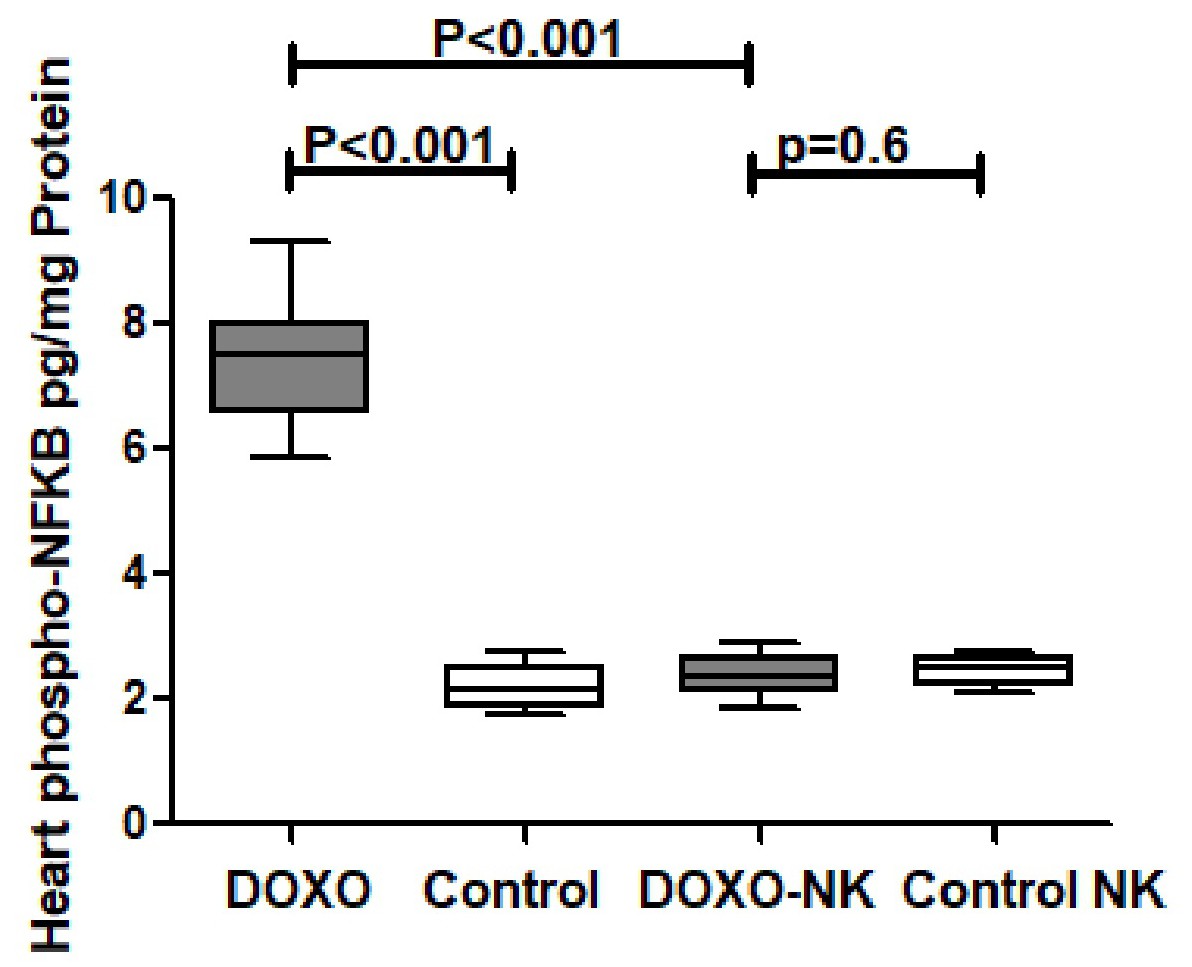
Nootkatone possesses anti-oxidant effects in DOXO-induced cardiac injury
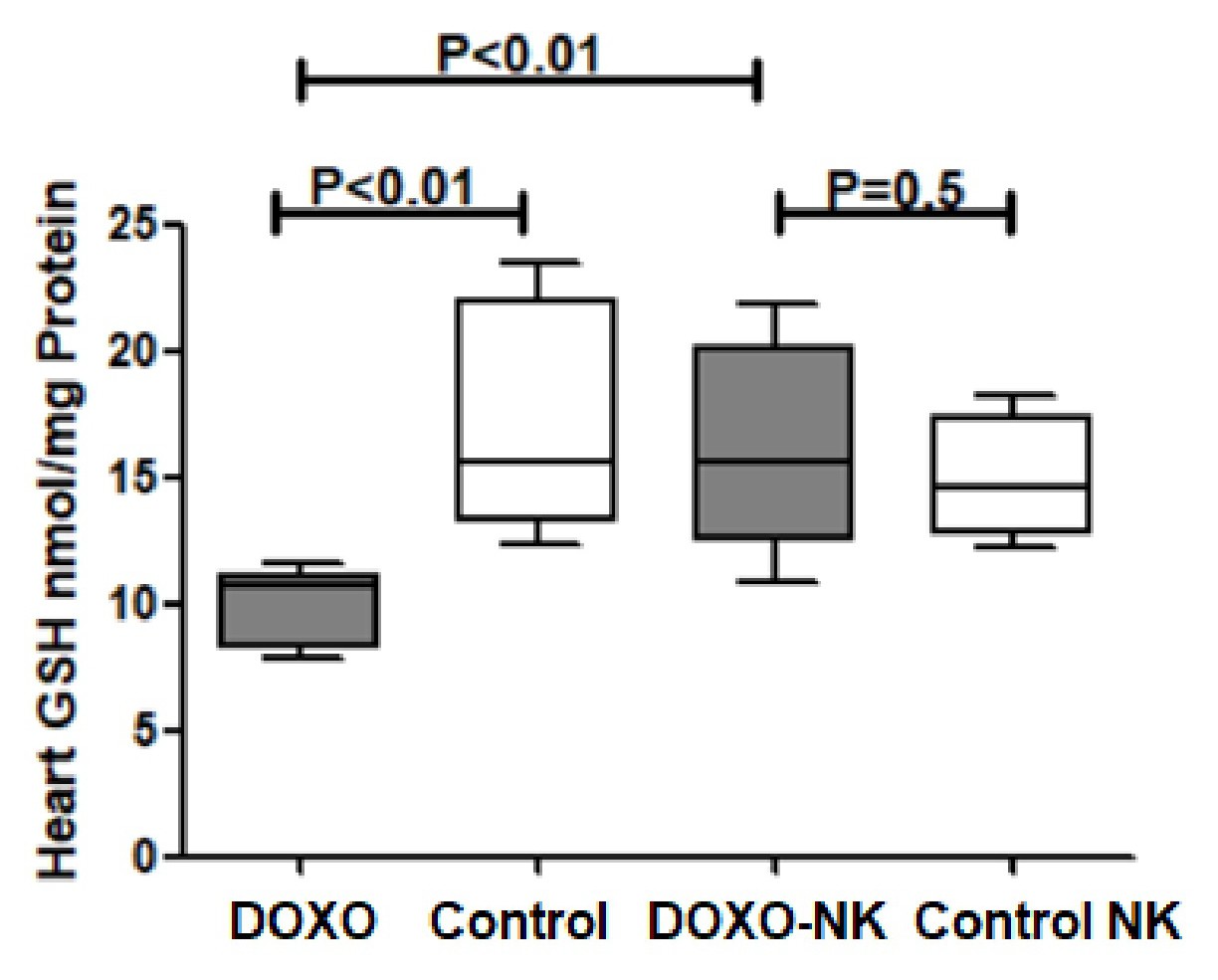
Nootkatone increases heart GSH in DOXO-induced cardiac injury. GSH was measured in tissue homogenate of hearts in C57BL/6J mice treated with DOXO and DOXO + NK compared to their corresponding sham controls. Our results show GSH level is significantly decreased in DOXO-treated mice group when compared with sham control group (10.12 ± 0.5585 vs 17.00 ± 1.826 nmol/mg, p<0.1
) (Fig. 7). There was a non-significant higher level of GSH in DOXO-NK treated mice than their sham control mice (16.16 ± 1.542 vs 14.90 ± 1.072 nmol /mg, p=0.5) (Fig. 7). There were significant higher levels of GSH in hearts of DOXO-NK treated mice than DOXO- treated group (16.16 ± 1.542 vs 10.12 ± 0.5585 pg/mg, p<0.01) (Fig. 7).
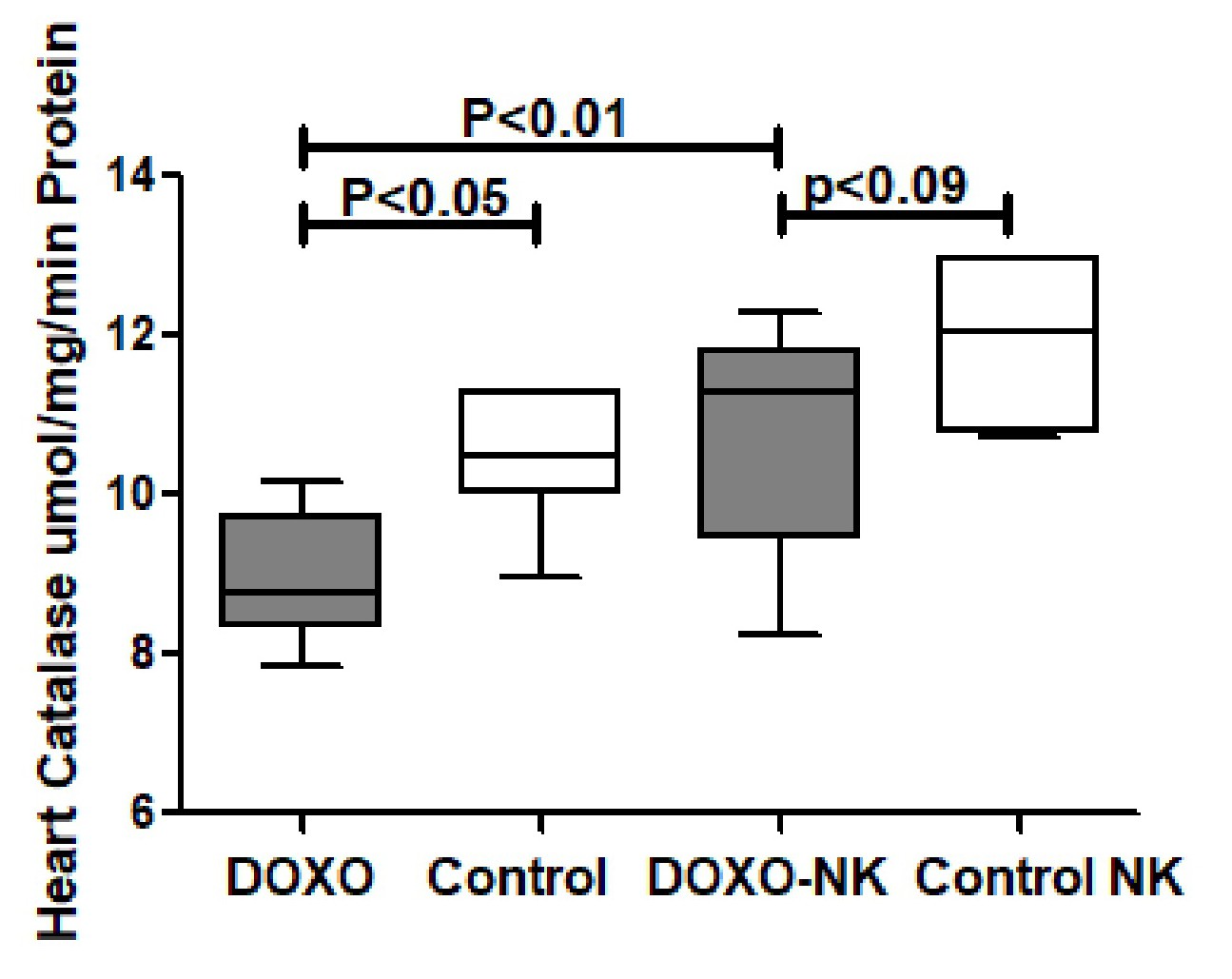
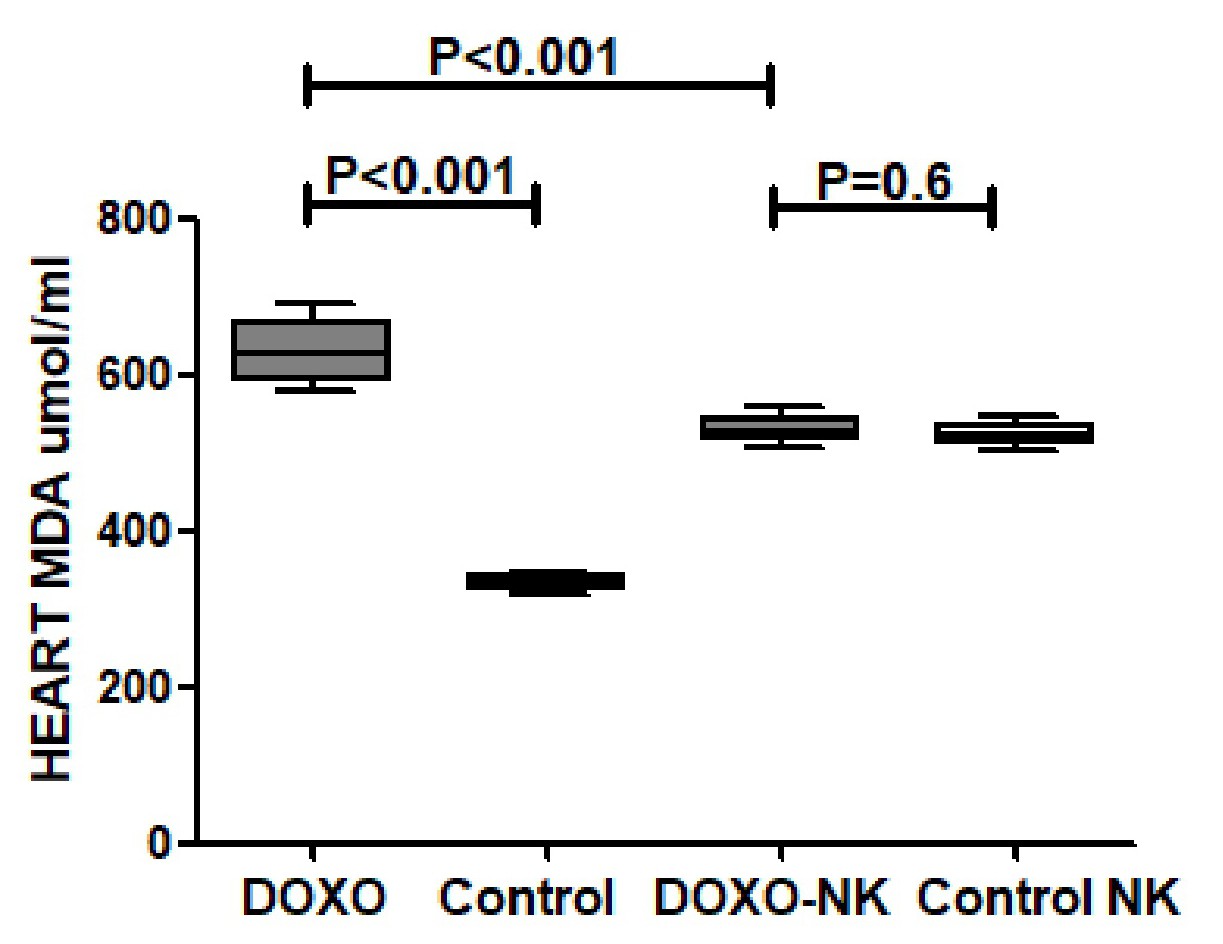
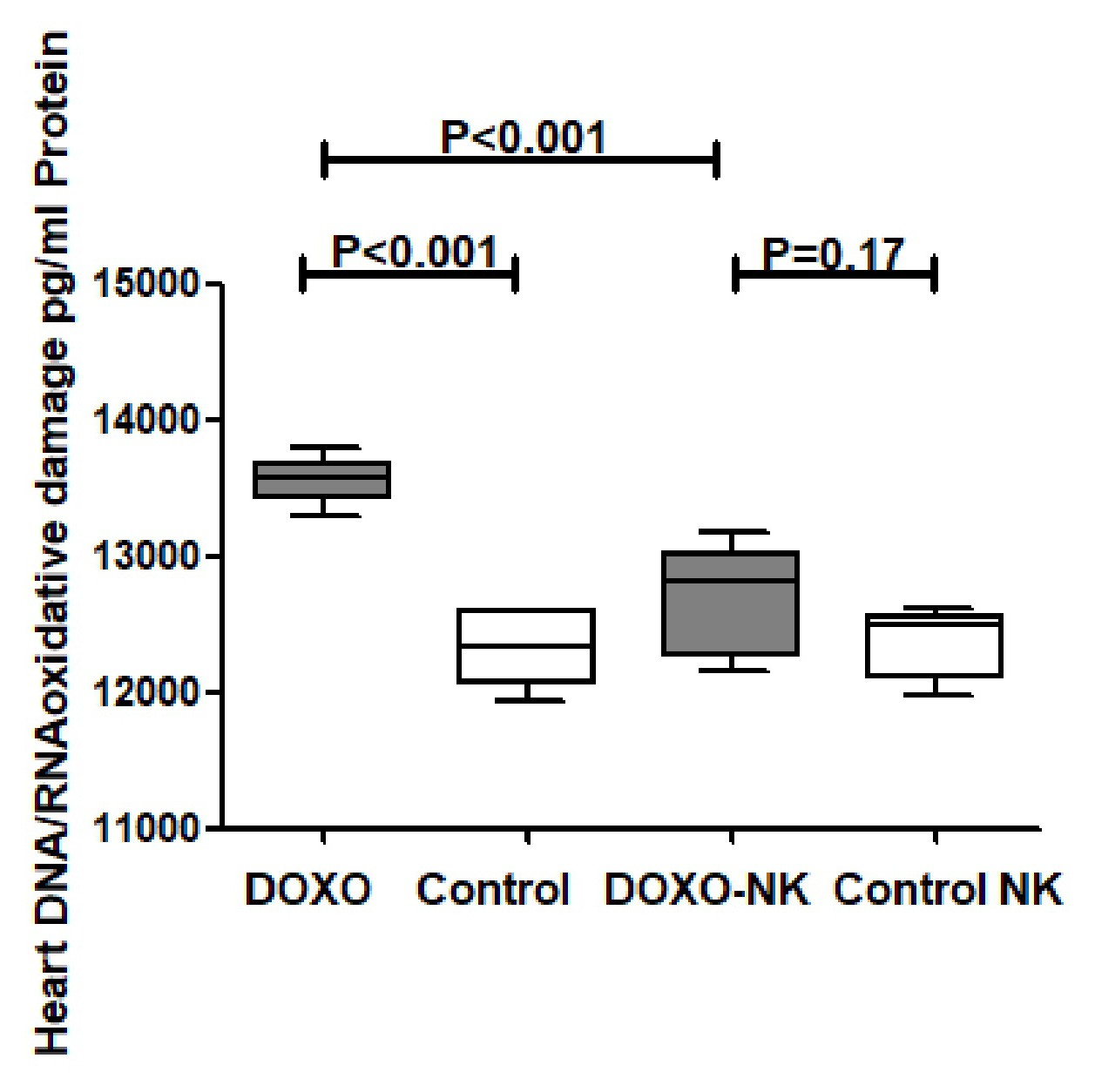
In vitro antioxidant activity of Nootkatone
NK has shown a strong antioxidant activity when compared to Gallic acid in the following assays. NK has shown dose dependent inhibition of free radical.
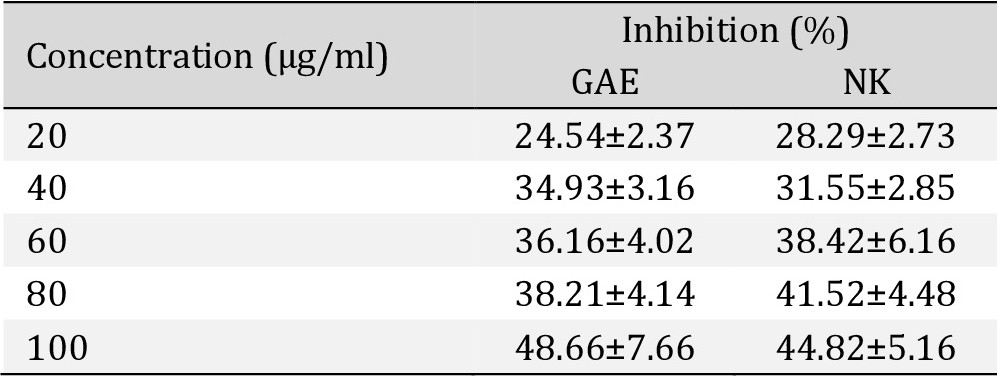
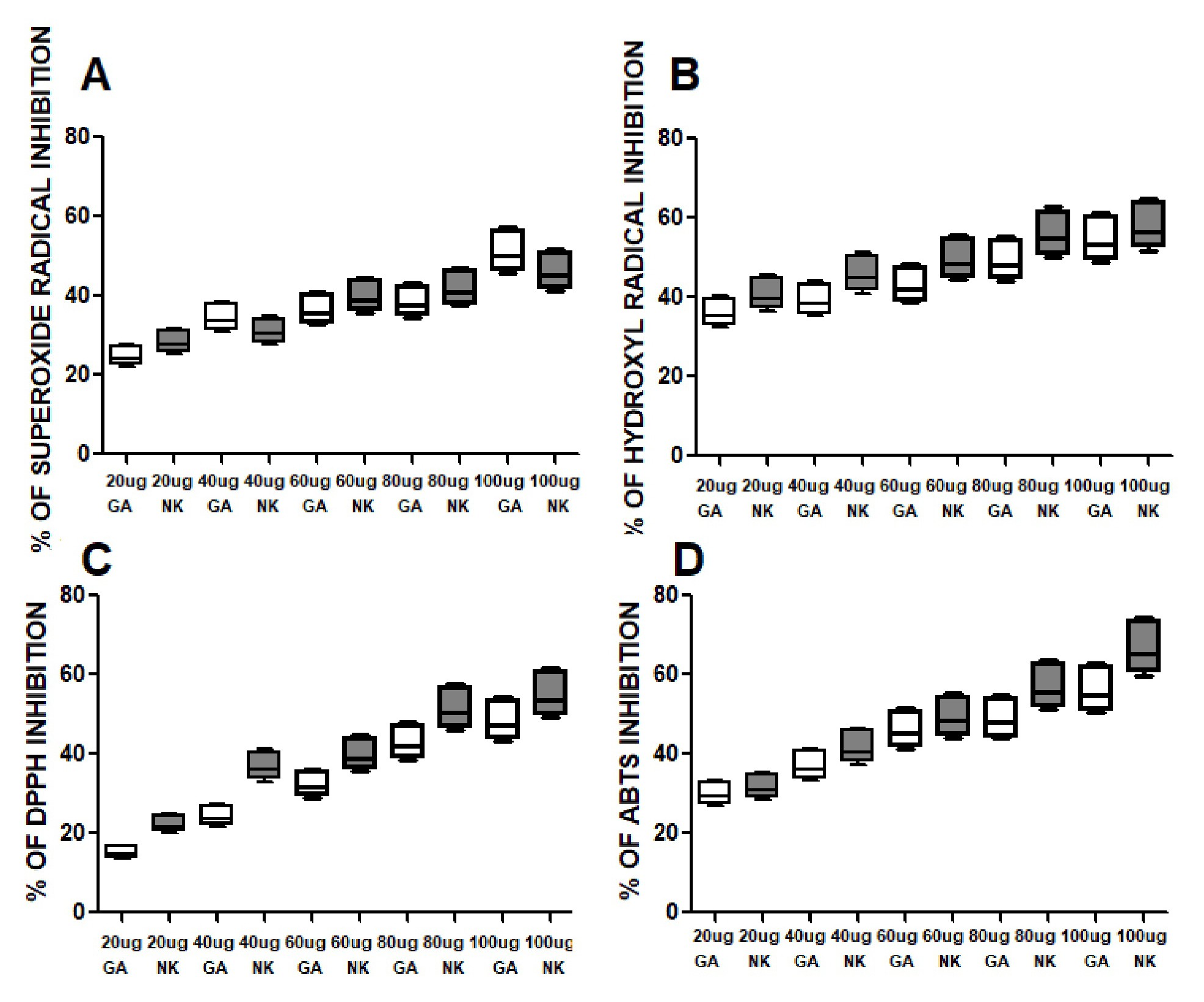
Superoxide radicals scavenging assay. There was a dose-response strong antioxidant activity of NK when compared with Gallic acid (Table 1), Fig. 12.
Hydroxyl Radical scavenging activity. There was a dose-response strong antioxidant activity of NK when compared with Gallic acid (Table 2), Fig. 12.
ABTS radical scavenging activity. There was a dose-response strong antioxidant activity of NK when compared with Gallic acid (Table 3), Fig. 12.
DPPH radical scavenging assay. There was a dose-response strong antioxidant activity of NK when compared with Gallic acid (Table 4), Fig. 12.
In vitro Superoxide Radical Scavenging activity assay shows percentage of Superoxide Radical inhibition activity of Nootkatone
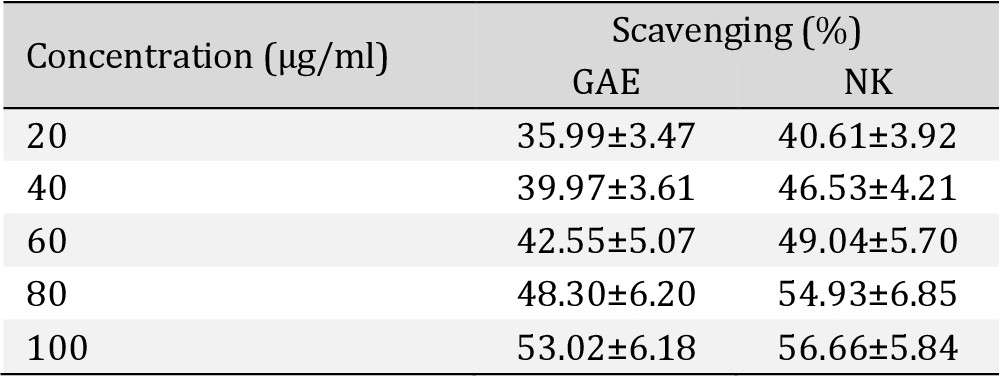
In vitro Hydroxyl Radical Scavenging activity assay shows percentage of Hydroxyl Radical inhibition activity of Nootkatone
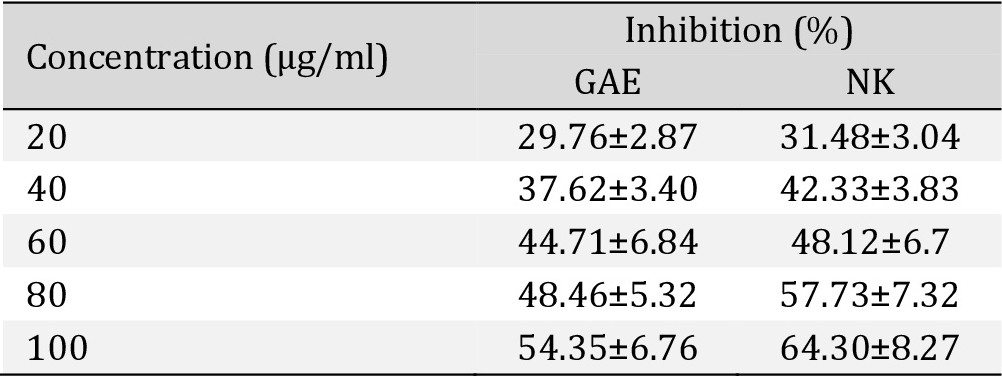
In vitro ABTS scavenging assay shows percentage of free radical inhibition activity of Nootkatone
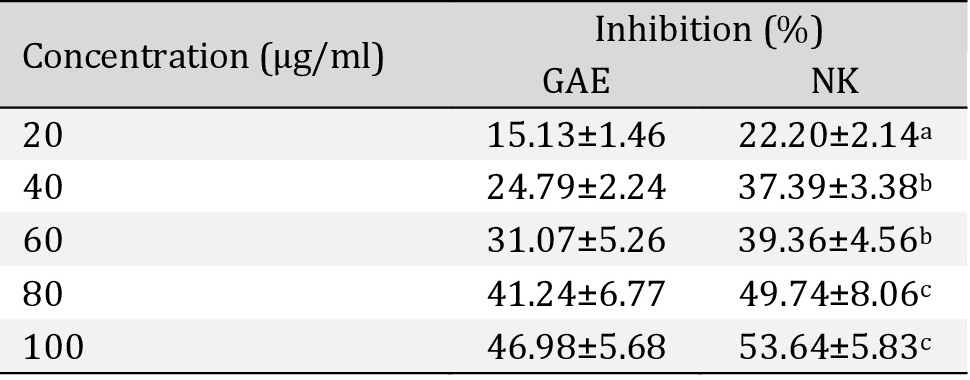
In vitro DPPH radical scavenging assay shows percentage of free radical inhibition activity of Nootkatone
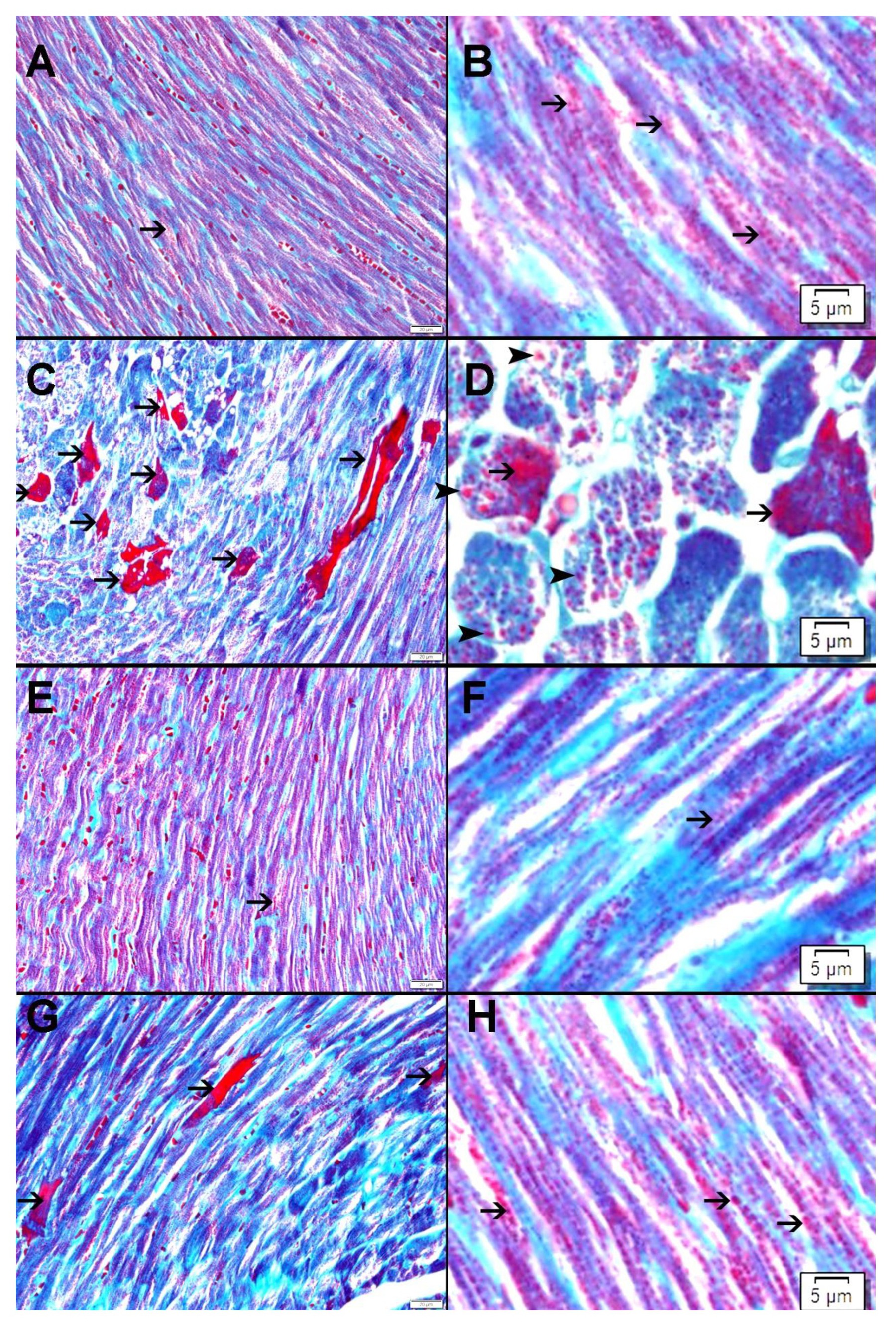
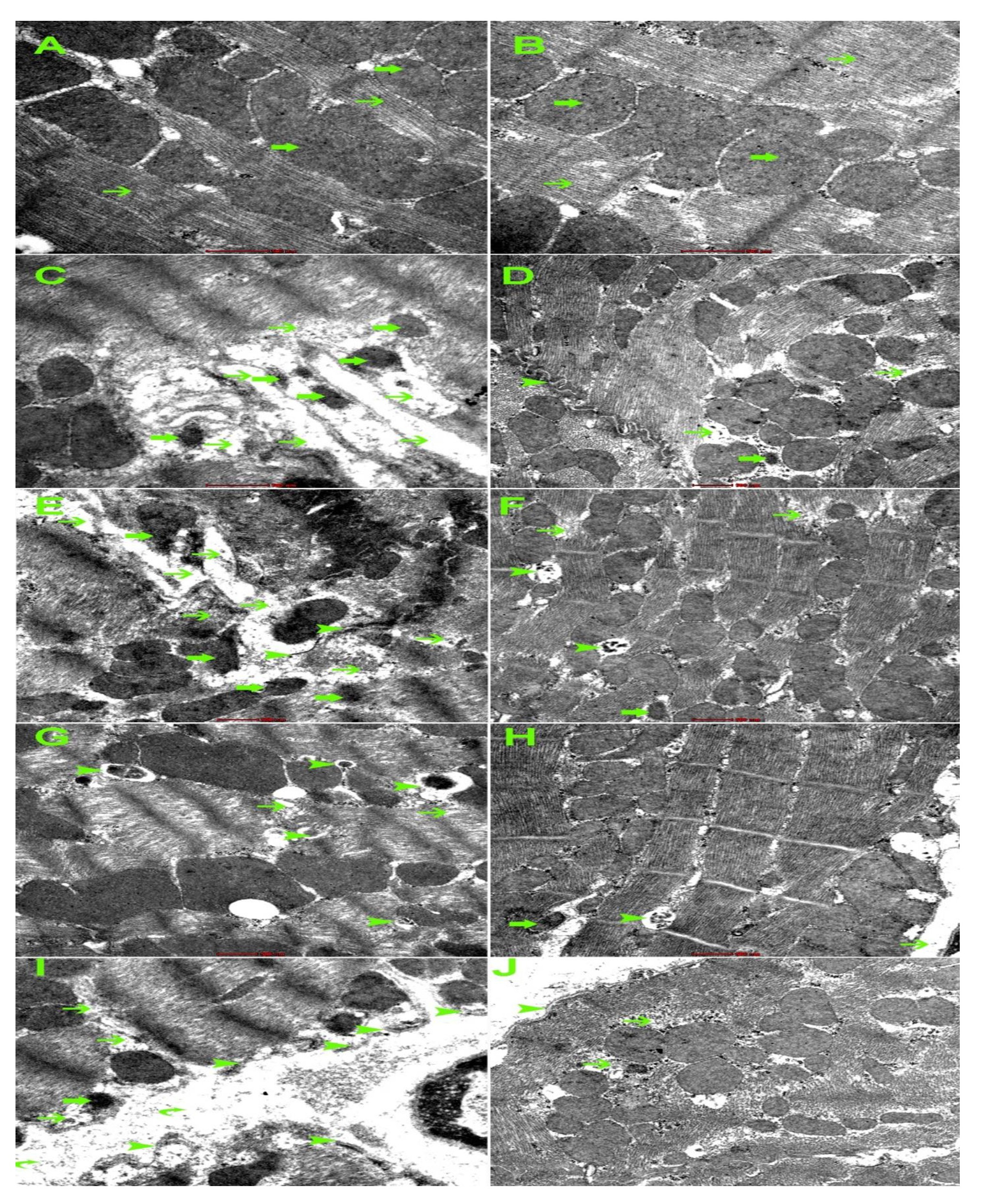
Electron microscopic changes
DOXO has shown ultrastructural changes in cardiomyocytes including myofibril damage, mitochondrial damage, intercalating disc damage, increased mitophagy, sarcoplasmic damage. The intensity of the damage is reduced in DOXO-NK treated nice (Fig. 17).
We have studied the effect of NK on DOXO-induced cardiac toxicity. It has been reported that NK has antioxidant, and anti-inflammatory properties [14-20], and since the early events of DOXO-induced cardiac toxicity encompasses oxidative stress and apoptosis [6-10], and NK has potential anti-oxidant activities [14-20], we think that it will be appropriate to assess the possible protective effects of NK on early DOXO-induced cardiac toxicity and the mechanisms underlying these effects in mice.
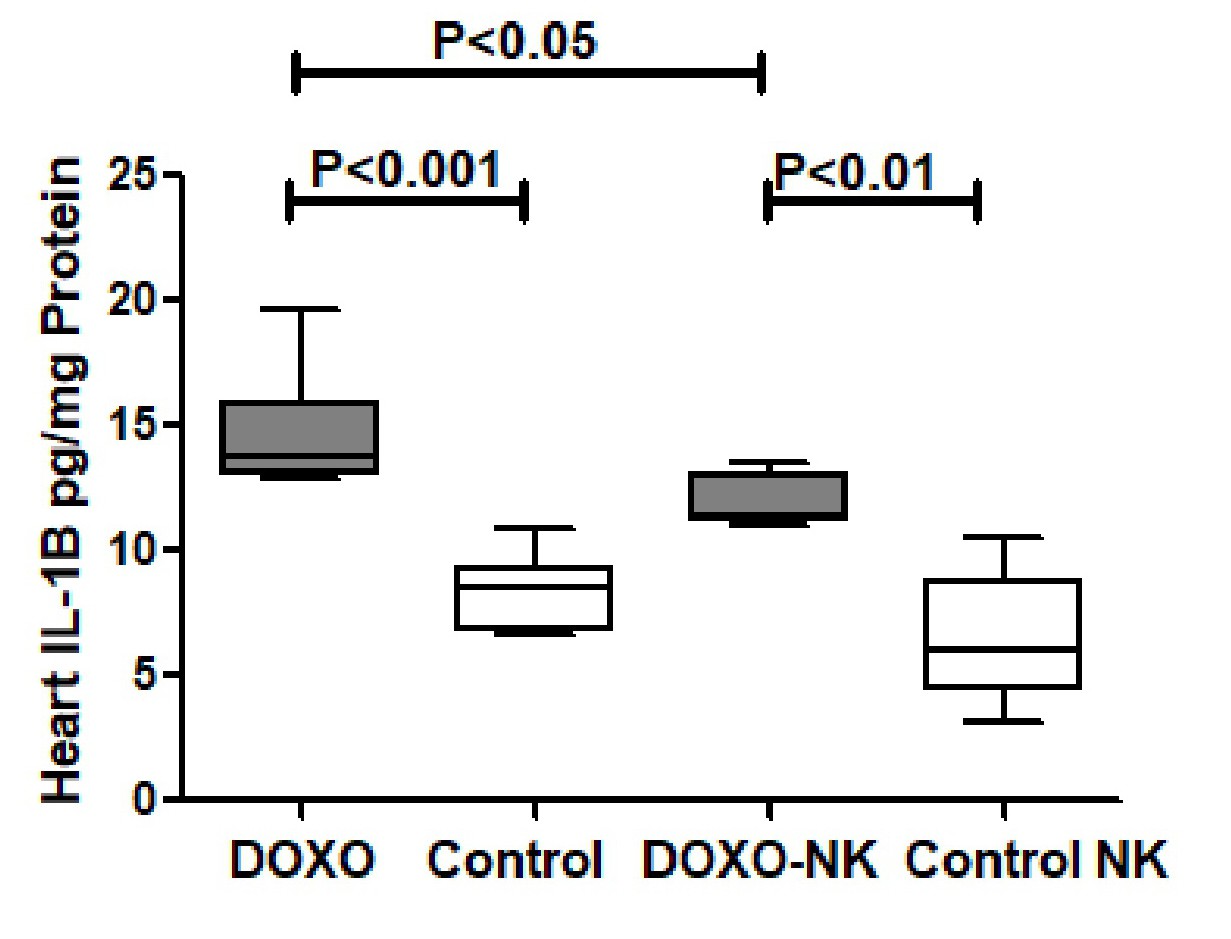
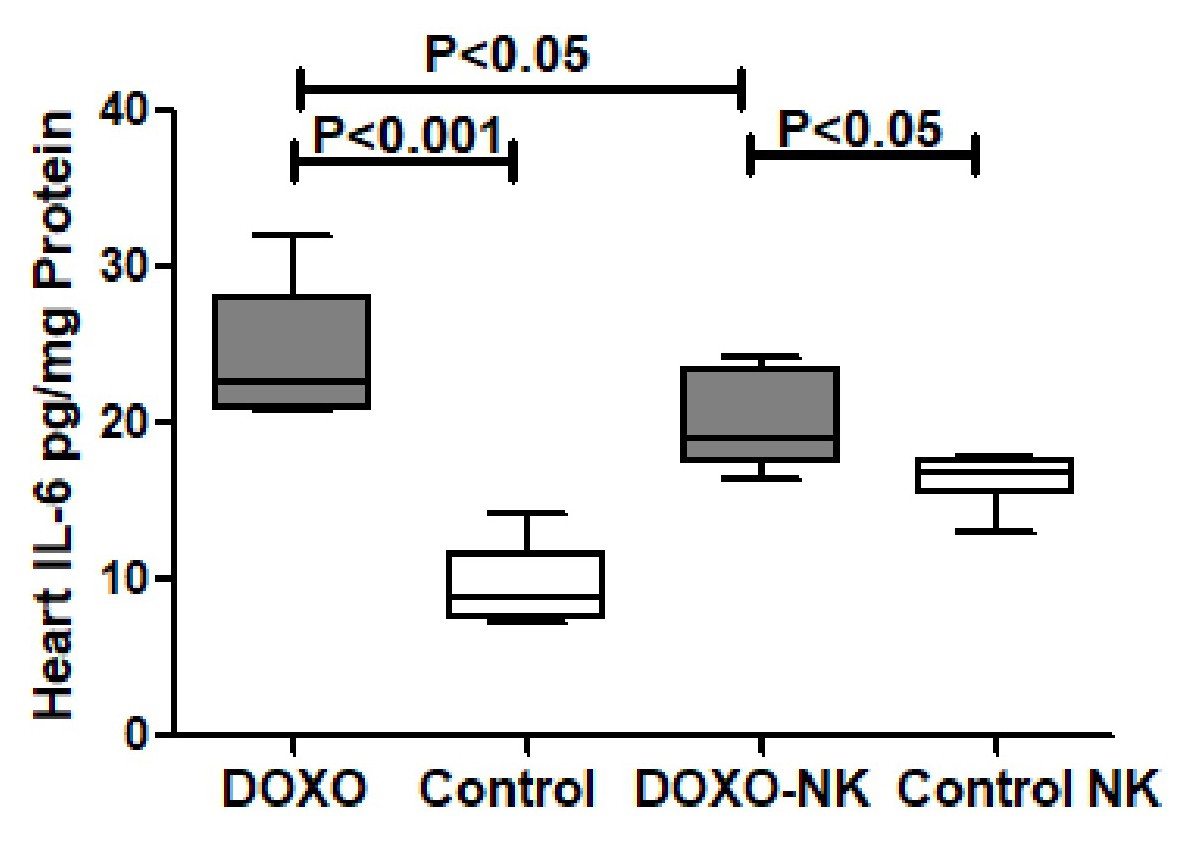
Our results have shown a significant increase in cardiac GAL-3 concentration in DOXO-treated mice when compared with sham control mice. Whereas cardiac GAL-3 concentration is significantly reduced in DOXO-NK-treated mice when compared with DOXO-treated mice; suggesting a potential effect of NK on intracellular concentration of GAL-3. GAL-3 is a proinflammatory protein [28-31], therefor, the reduction of GAL-3 following the use of NK supports anti-inflammatory role of NK. This is the first time of reporting inhibiting effect of NK on myocardial GAL-3. In support of that, we have identified a significantly lower concentrations of myocardial inflammatory markers CRP, IL-1B, IL-6 and lipocalin-2 in DOXO-NK-treated mice than DOXO-treated mice confirming anti-inflammatory role of NK. To understand the mechanism of anti-inflammatory role of NK in DOXO-induced cardiac injury, we have measured myocardial phospho-NF-κB concentration which shows a significantly lower concentration of phospho- NF-κB in the myocardium of DOXO-NK- treated mice than DOXO-treated mice, suggesting that NK plays a role in the reduction of phospho-NF-κB. NF-κB induces the expression of various pro-inflammatory genes, including those encoding cytokines and chemokines, and also participates in inflammasome regulation [32, 33]. In addition, NF-κB plays a critical role in regulating the survival, activation and differentiation of innate immune cells and inflammatory T cells [34]. Studies have shown NF-κB to be involved in the induction of GAL-3 [35] as well as GAL-3 is involved in the activation of NF-κB [36]. This indicates that there is a cross talk between NF-κB and GAL-3.
It is possible that NK exerts its anti-inflammatory action through inhibition of NF-κB or GAL-3 or both. Studies are supporting our finding of the inhibitory actions of NK on NF-κB [37, 38].
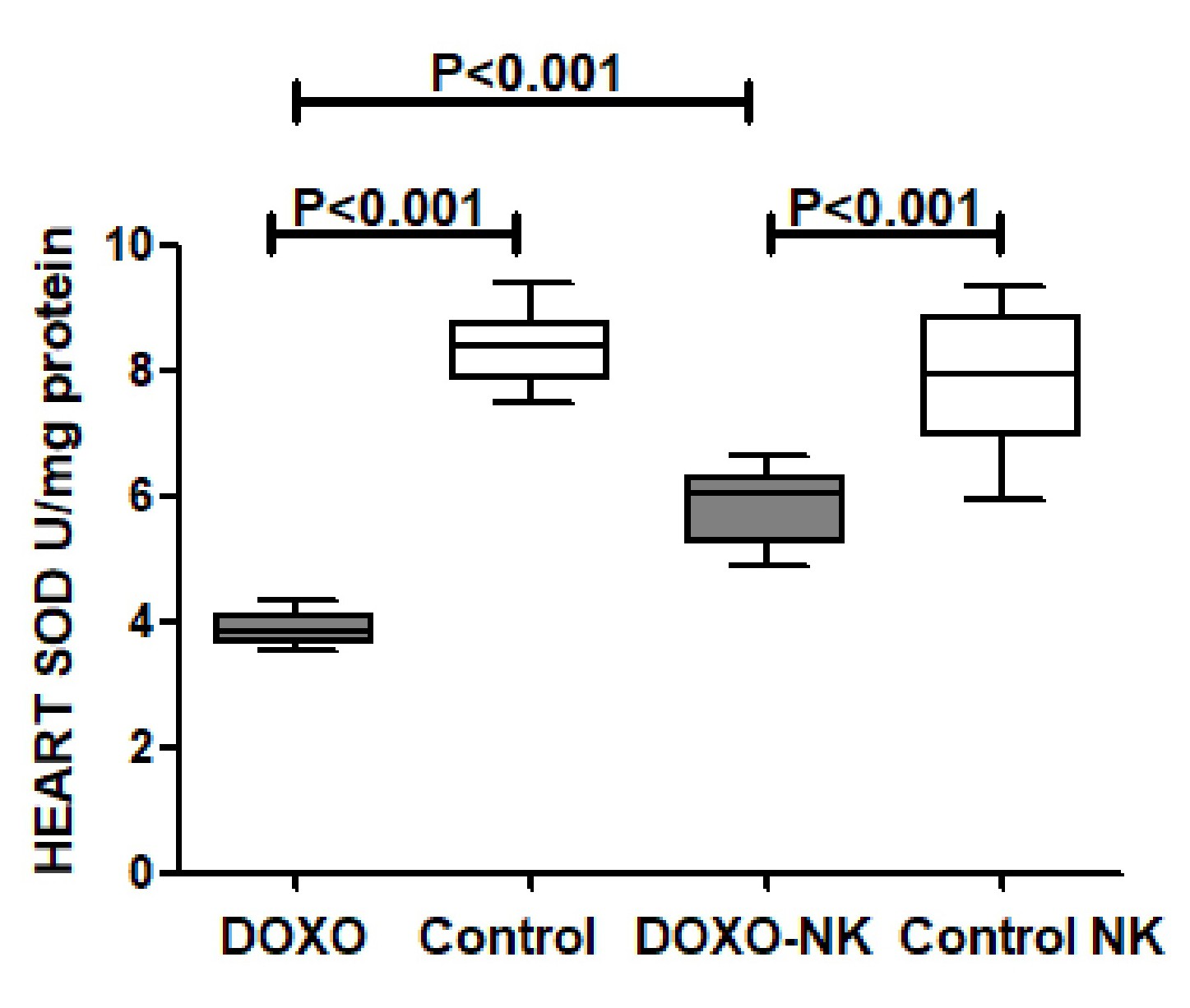
We have also shown a significantly higher concentrations of myocardial anti-oxidants GSH, catalase, and SOD in DOXO-NK-treated mice than DOXO-treated mice supporting the anti-oxidative role of NK. We also have identified a lower level of oxidative stress, MDA and DNA/RNA oxidative damage, in the myocardium of DOXO-NK-treated mice than DOXO-treated mice which also support the antioxidant activity of NK. There is a high possibility that the higher antioxidant activity of NK is mainly related to its anti-inflammatory effects. There is cross reaction between inflammation and oxidative stress as increased oxidative stress leads to inflammation and inflammation increases oxidative stress [39]. There is also a cross talk between oxidative stress and DNA damage [39].
Cardiomyocytes are loaded with mitochondria, which are the major target of DOXO. Mitochondria within cardiomyocytes are found to be 30-40% higher than other types of cells [40]. Mitochondria are the major source of ROS production. DOXO increases ROS through reduction of the redox cycle at complex I of the electron transport chain, leading to the disruption in ATP synthesis [41].
Mitochondrial ROS producing enzymes converts DOXO into semiquinone which reacts with oxygen to create superoxide anions that are neutralized into relatively stable and low-toxic hydrogen peroxide by SOD, or further transformed to ROS or RNS in a sequence of reactions known as the redox cycle [42]. Superoxide anions and hydrogen peroxide may also produce highly reactive and toxic hydroxyl radicals [42]. Iron also plays an important role in DOXO-induced cardiotoxicity through the Fenton reaction as an electron donor to hydrogen peroxide with the subsequent production of a hydroxyl radical [43]. The generated ROS then reacts with mitochondrial lipids, proteins, and nucleic acids. DOXO is known to react with mitochondrial DNA, forming adducts that interrupt normal mitochondrial function, protein expression, and lipid oxidation [44]. Our results show clear reduction of DOXO induced oxidative stress when we use NK in combination with DOXO through the significant reduction of cardiac MDA and DNA/RNA oxidative damage as well as significant increase of cardiac GSH, SOD and catalase. Moreover, we have also confirmed a dose-response antioxidant activity of NK through in vitro analysis of antioxidant activity of NK in comparison with Gallic acid; which is, to best of our knowledge, the first time to be reported in the literature.
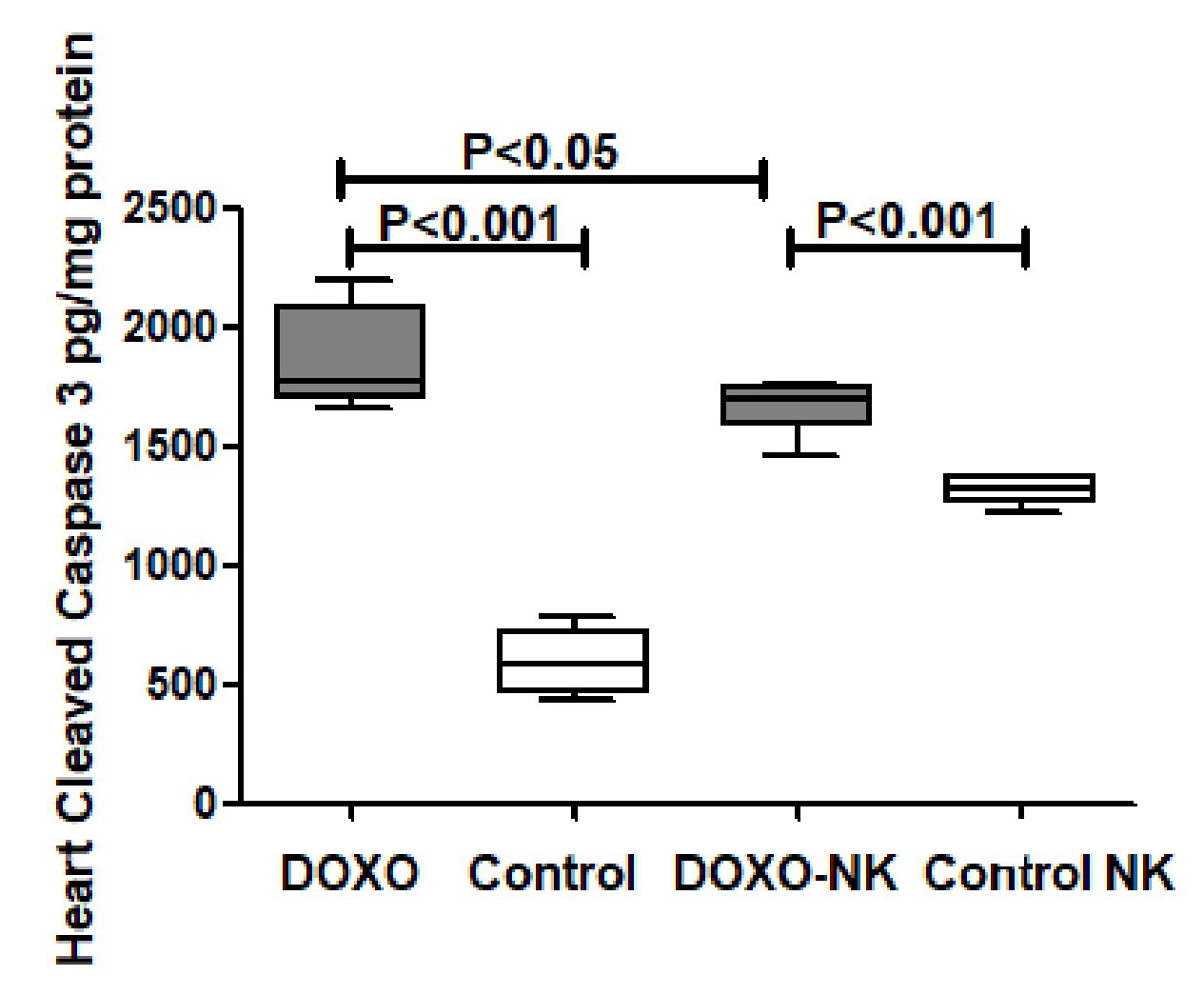
Our results have shown a significant decrease in cleaved caspase-3 concentration in the myocardium in DOXO-NK- treated mice when compared with DOXO- treated mice, suggesting less apoptotic activity and less damage to the myocardium following the use of NK in combination with DOXO. Electron microscopic study has clearly shown DOXO-induced cardiomyocyte damage through ultrastructural alterations in cardiomyocytes including myofibrils damage, mitochondrial damage, increased mitophagy, sarcoplasmic membrane damage, widening of interstitial space and damage to the intercalating disc. Interestingly, DOXO damaging effect was ameliorated when used in combination with NK. We have also used Gomori trichrome stain to show DOXO- mediated mitochondrial damage is decreased following the use of NK. Studies have shown mitochondrial damage as a cause of apoptosis, hence the decrease of mitochondrial damage following the use of NK supports lower apoptotic activity in DOXO-NK myocardium [45-47].
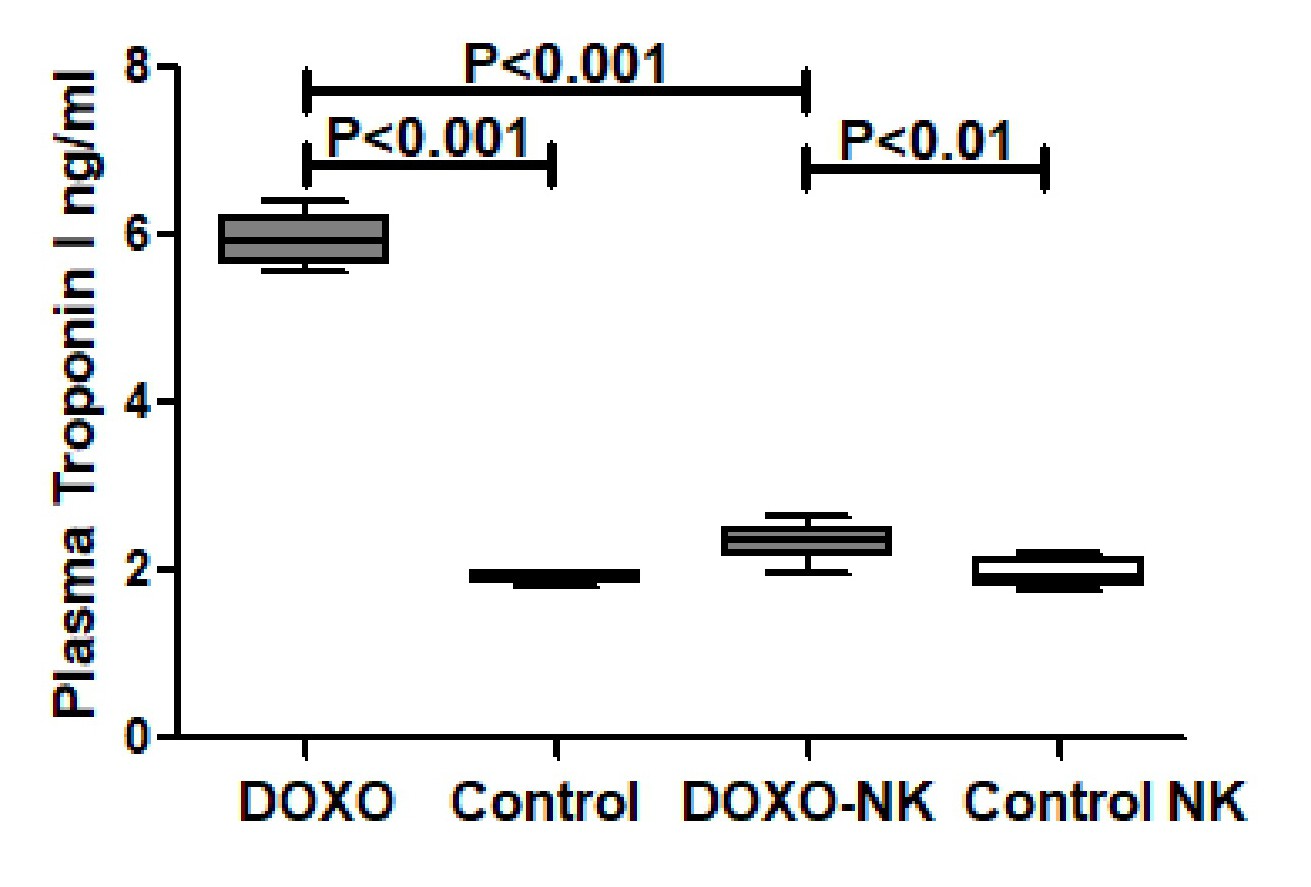
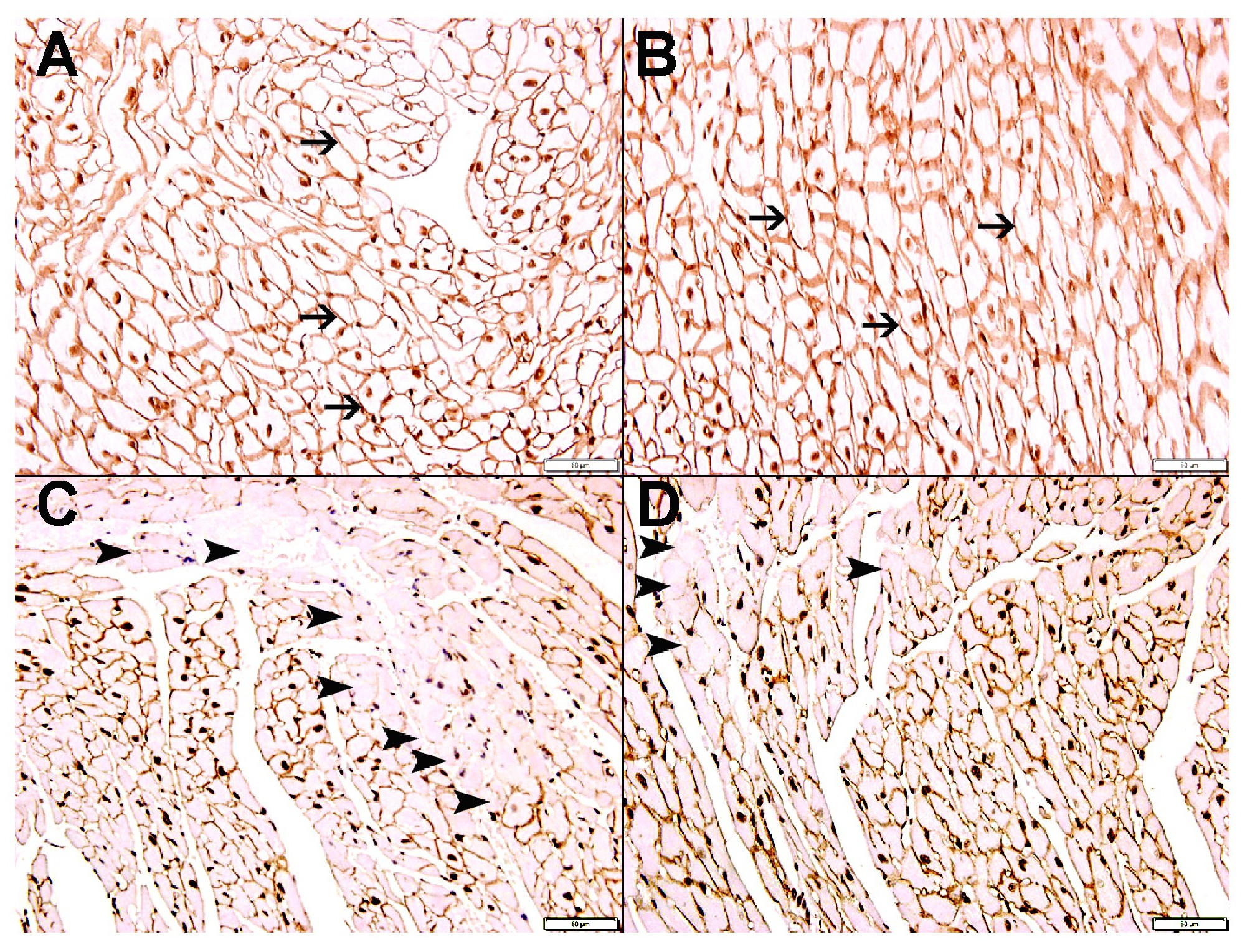
We have also shown loss of dystrophin staining in damaged cardiomyocytes in DOXO-treated mice highlighting areas of DOXO-damaged cardiomyocytes. While in DOXO-NK-treated mice there is less loss of dystrophin staining of cardiomyocytes when compared with DOXO-treated mice, indicating a lesser damaged cardiomyocytes in this group. We had reported loss of dystrophin staining in cardiomyocytes in acute myocardial infarction [25]. In support of that we have shown a significantly lower plasma troponin I concentration in DOXO-NK- treated mice than DOXO-treated mice confirming a decline in cardiac myocyte injury following the use of NK in DOXO-NK treated mice which supports the protective effect of NK on cardiac myocytes.
Troponin I is a very specific cardiomyocyte protein and can only be seen in the peripheral blood when there is injury to cardiac myocytes that allows passage of troponin I to the circulation. Studies have shown that troponin I can be taken as a marker of cardiomyocyte injury and can be indirectly linked to the infarct size [48, 49].
So it seems that NK is likely to orchestrate a response to DOXO-induced myocardial injury through modulation of NF-κB signals and reduction of oxidative stress, which is reflected on the significant reduction in apoptotic proteins and cardiomyocytes death markers. This speaks on a protective role of NK on the heart in DOXO-treated mice.
Conclusion
Nootkatone improves DOXO-induced myocardial injury through modulation of NF-κB signals and reduction of oxidative stress.
The authors would like to thank the College of Medicine & Health Sciences, United Arab Emirates University for their support of this project. In addition, we would like to thank the Zayed Bin Sultan Center for Health Sciences for support of this project.
Author Contributions
All authors reviewed and approved the submitted version of the manuscript. S.A Introduced the concept, designed the study, analyzed, and interpreted the data, designed the figures, and wrote the manuscript. K.K. performed mice experimental model of DOXO cardiac toxicity, performed protein extraction, ELISA technique, and submitted resulted data. M.S. performed tissue processing and staining. S.T. performed electron microscopy processing, staining, and submitted images.
Funding
This project is supported by CMHS grant NP-18-29 and The Zayed Bin Sultan Center for Health Sciences (ZCHS), 21RO73.
Statement of Ethics
All animal experimental procedures are approved by the Animal Research Ethics Committee of the UAE University, Protocol ERA_2017_5687.
The authors declare that no conflicts of interest exist.
| 1 Lipshultz SE, Miller TL, Lipsitz SR, Neuberg DS, Dahlberg SE, Colan SD, Silverman LB, Henkel JM, Franco VI, Cushman LL, Asselin BL, Clavell LA, Athale U, Michon B, Laverdière C, Schorin MA, Larsen E, Usmani N, Sallan SE, Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium: Continuous Versus Bolus Infusion of Doxorubicin in Children With ALL: Long-term Cardiac Outcomes. Pediatrics 2012;130:1003-1011. https://doi.org/10.1542/peds.2012-0727 |
||||
| 2 Colombo A, Cipolla C, Beggiato M, Cardinale D: Cardiac toxicity of anticancer agents. Curr Cardiol Rep 2013;15:362. https://doi.org/10.1007/s11886-013-0362-6 |
||||
| 3 Wang EY, Biala AK, Gordon JW, Kirshenbaum LA: Autophagy in the heart: too much of a good thing? J Cardiovasc Pharmacol 2012;60:110-117. https://doi.org/10.1097/FJC.0b013e31824cc427 |
||||
| 4 Zhao L, Zhang B: Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci Rep 2017;7:44735. https://doi.org/10.1038/srep44735 |
||||
| 5 Zhang YW, Shi J, Li YJ, Wei L: Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch Immunol Ther Exp (Warsz) 2009;57:435-445. https://doi.org/10.1007/s00005-009-0051-8 |
||||
| 6 Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, Mutharasan RK, Naik TJ, Ardehali H: Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 2014;124:617-630. https://doi.org/10.1172/JCI72931 |
||||
| 7 Miura T, Muraoka S, Ogiso T: Adriamycin-Fe3+-induced mitochondrial protein damage with lipid peroxidation. Biol Pharm Bull 1995;18:514-517. https://doi.org/10.1248/bpb.18.514 |
||||
| 8 Yoshida M, Shiojima I, Ikeda H, Komuro I: Chronic doxorubicin cardiotoxicity is mediated by oxidative DNA damage-ATM-p53-apoptosis pathway and attenuated by pitavastatin through the inhibition of Rac1 activity. J Mol Cell Cardiol 2009;47:698-705. https://doi.org/10.1016/j.yjmcc.2009.07.024 |
||||
| 9 Dhingra R, Margulets V, Chowdhury SR, Thliveris J, Jassal D, Fernyhough P, Dorn GW 2nd, Kirshenbaum LA: Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. Proc Natl Acad Sci U S A 2014;111:E5537-5544. https://doi.org/10.1073/pnas.1414665111 |
||||
| 10 Park AM, Nagase H, Liu L, Vinod Kumar S, Szwergold N, Wong CM, Suzuki YJ: Mechanism of anthracycline-mediated down-regulation of GATA4 in the heart. Cardiovasc Res 2011;90:97-104. https://doi.org/10.1093/cvr/cvq361 |
||||
| 11 Miyasato SK, Loeffler J, Shohet R, Zhang J, Lindsey M, Le Saux CJ: Caveolin-1 modulates TGF-β1 signaling in cardiac remodeling. Matrix Biol 2011;30:318-329. https://doi.org/10.1016/j.matbio.2011.05.003 |
||||
| 12 Arafa MH, Mohammad NS, Atteia HH, Abd-Elaziz HR: Protective effect of resveratrol against doxorubicin-induced cardiac toxicity and fibrosis in male experimental rats. J Physiol Biochem 2014;70:701-711. https://doi.org/10.1007/s13105-014-0339-y |
||||
| 13 Leonhardt RH, Berger RG: Nootkatone. Adv Biochem Eng Biotechnol 2015;148:391-404. https://doi.org/10.1007/10_2014_279 |
||||
| 14 Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Ali BH: Thrombosis and systemic and cardiac oxidative stress and DNA damage induced by pulmonary exposure to diesel exhaust particles and the effect of nootkatone thereon. Am J Physiol Heart Circ Physiol 2018;314:H917-H927. https://doi.org/10.1152/ajpheart.00313.2017 |
||||
| 15 Kurdi A, Hassan K, Venkataraman B, Rajesh M: Nootkatone confers hepatoprotective and anti-fibrotic actions in a murine model of liver fibrosis by suppressing oxidative stress, inflammation, and apoptosis. J Biochem Mol Toxicol 2018; DOI: 10.1002/jbt.22017. https://doi.org/10.1002/jbt.22017 |
||||
| 16 Ali BH, Al-Salam S, Adham SA, Al Balushi K, Al Za'abi M, Beegam S, Yuvaraju P, Manoj P, Nemmar A: Testicular Toxicity of Water Pipe Smoke Exposure in Mice and the Effect of Treatment with Nootkatone Thereon. Oxid Med Cell Longev 2019;2019:2416935. https://doi.org/10.1155/2019/2416935 |
||||
| 17 Chen CM, Lin CY, Chung YP, Liu CH, Huang KT, Guan SS, Wu CT, Liu SH: Protective Effects of Nootkatone on Renal Inflammation, Apoptosis, and Fibrosis in a Unilateral Ureteral Obstructive Mouse Model. Nutrients 2021;13:3921. https://doi.org/10.3390/nu13113921 |
||||
| 18 Jha AK, Gairola S, Kundu S, Doye P, Syed AM, Ram C, Kulhari U, Kumar N, Murty US, Sahu BD: Biological Activities, Pharmacokinetics and Toxicity of Nootkatone: A Review. Mini Rev Med Chem 2022; DOI: 10.2174/1389557522666220214092005. https://doi.org/10.2174/1389557522666220214092005 |
||||
| 19 Yao Z, Li J, Bian L, Li Q, Wang X, Yang X, Wei X, Wan G, Wang Y, Shi J, Guo J: Nootkatone alleviates rotenone-induced Parkinson's disease symptoms through activation of the PI3K/Akt signaling pathway. Phytother Res 2022; DOI: 10.1002/ptr.7552. https://doi.org/10.1002/ptr.7552 |
||||
| 20 Tsoyi K, Jang HJ, Lee YS, Kim YM, Kim HJ, Seo HG, Lee JH, Kwak JH, Lee DU, Chang KC: (+)-Nootkatone and (+)-valencene from rhizomes of Cyperus rotundus increase survival rates in septic mice due to heme oxygenase-1 induction. J Ethnopharmacol 2011;137:1311-1317. https://doi.org/10.1016/j.jep.2011.07.062 |
||||
| 21 Halliwell B, Gutteridge JM, Aruoma OI: The deoxyribose method: a simple "test-tube" assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 1987;165:215-219. https://doi.org/10.1016/0003-2697(87)90222-3 |
||||
| 22 Nishimiki M, Rao N A, Yagi K: The occurance of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophy Res Commun 1972;46:849-853. https://doi.org/10.1016/S0006-291X(72)80218-3 |
||||
| 23 Wolfenden BS, Willson RL: Radical-cations as reference chromogens in kinetic studies of one- electron transfer reactions; pulse radiolysis studies of 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate). J Chem Soc Perkin Trans 1982;2:805-812. https://doi.org/10.1039/P29820000805 |
||||
| 24 Brand-Williams W, Cuvelier ME, Berset CLWT: Use of free radical method to evaluate antioxidant activity. Food Sci Technol 1995;28:25-30. https://doi.org/10.1016/S0023-6438(95)80008-5 |
||||
| 25 Hashmi S, Al-Salam S: Loss of dystrophin staining in cardiomyocytes: a novel method for detection early myocardial infarction. Int J Clin Exp Pathol 2013;6:249-257. | ||||
| 26 Renu K, Abilash VG, Tirupathi Pichia PB, Arunachalam S: Molecular mechanism of Doxoorubicin-induced cardiomyopathy - an update. Eur J Pharmacol 2018;818:241-253. https://doi.org/10.1016/j.ejphar.2017.10.043 |
||||
| 27 Nebigil CG, Désaubry L: Updates in anthracycline-mediated cardiotoxicity. Front Pharmacol 2018;9:1262. https://doi.org/10.3389/fphar.2018.01262 |
||||
| 28 Simovic Markovic B, Nikolic A, Gazdic M, Bojic S, Vucicevic L, Kosic M, Mitrovic S, Milosavljevic M, Besra G, Trajkovic V, Arsenijevic N, Lukic ML, Volarevic V: Galectin-3 Plays an Important Pro-inflammatory Role in the Induction Phase of Acute Colitis by Promoting Activation of NLRP3 Inflammasome and Production of IL-1β in Macrophages. J Crohns Colitis 2016;10:593-606. https://doi.org/10.1093/ecco-jcc/jjw013 |
||||
| 29 Al-Salam S, Hashmi S: Myocardial Ischemia Reperfusion Injury: Apoptotic, Inflammatory and Oxidative Stress Role of Galectin-3. Cell Physiol Biochem 2018;50:1123-1139. https://doi.org/10.1159/000494539 |
||||
| 30 Colnot C, Ripoche MA, Milon G, Montagutelli X, Crocker PR, Poirier F: Maintenance of granulocyte numbers during acute peritonitis is defective in galectin-3-null mutant mice. Immunology 1998;94:290-296 https://doi.org/10.1046/j.1365-2567.1998.00517.x |
||||
| 31 Kuwabara I, Liu FT: Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol 1996;156:3939-3944. | ||||
| 32 Tak PP, Firestein GS: NF-kappaB: a key role in inflammatory diseases. J Clin Invest 2001;107:7-11. https://doi.org/10.1172/JCI11830 |
||||
| 33 Fiordelisi A, Iaccarino G, Morisco C, Coscioni E, Sorriento D: NFkappaB is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases. Int J Mol Sci 2019;20:1599. https://doi.org/10.3390/ijms20071599 |
||||
| 34 Liu T, Zhang L, Joo D, Sun SC: NF-kappaB signaling in inflammation. Signal Transduct Target Ther 2017;2:17023. https://doi.org/10.1038/sigtrans.2017.23 |
||||
| 35 Dumic J, Lauc G, Flögel M: Expression of galectin-3 in cells exposed to stress-roles of jun and NF-kappaB. Cell Physiol Biochem 2000;10:149-158. https://doi.org/10.1159/000016345 |
||||
| 36 Zhou W, Chen X, Hu Q, Chen X, Chen Y, Huang L: Galectin-3 activates TLR4/NF-κB signaling to promote lung adenocarcinoma cell proliferation through activating lncRNA-NEAT1 expression. BMC Cancer 2018;18:580. https://doi.org/10.1186/s12885-018-4461-z |
||||
| 37 Xu Y, Zhang M, Yang W, Xia B, Wang W, Pan X: Nootkatone protects cartilage against degeneration in mice by inhibiting NF-kappa B signaling pathway. Int Immunopharmacol 2021;100:108119. https://doi.org/10.1016/j.intimp.2021.108119 |
||||
| 38 Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Hamadi N, Ali BH: In vivo Protective Effects of Nootkatone against Particles-Induced Lung Injury Caused by Diesel Exhaust Is Mediated via the NF-kappaB Pathway. Nutrients 2018;10:263. https://doi.org/10.3390/nu10030263 |
||||
| 39 García N, Zazueta C, Aguilera-Aguirre L: Oxidative Stress and Inflammation in Cardiovascular Disease. Oxid Med Cell Longev 2017;2017:5853238. https://doi.org/10.1155/2017/5853238 |
||||
| 40 Steffi G, Wiesner RJ: Regulation of mitochondrial proliferation in the heart: power plant failure contributes to cardiac failure in hypertrophy. Cardiovasc Resv 2004;64:98. https://doi.org/10.1016/j.cardiores.2004.06.030 |
||||
| 41 Alexieva B, Sainova I, Pavlova V, Markova TZ, Valkova I, Nikolova E: Insights into mechanisms of doxorubicin cardiotoxicity. J Phys Pharm Adv 2014;4:342-348. | ||||
| 42 Cappetta D, De Angelis A, Sapio L, Illiano M, Quaini F, Rossi F, Berrino L, Naviglio S, Urbanek K: Oxidative stress and cellular response to doxorubicin: a common factor in the complex milieu of anthracycline cardiotoxicity. Oxid Med Cell Longev 2017;2017:1521020. https://doi.org/10.1155/2017/1521020 |
||||
| 43 Canzoneri JC, Oyelere AK: Interaction of anthracyclines with iron responsive element mRNAs: Nucleic Acids Res 2008;36:6825-6834. https://doi.org/10.1093/nar/gkn774 |
||||
| 44 Eder AR, Arriaga EA: Capillary electrophoresis monitors enhancement in subcellular reactive oxygen species production upon treatment with doxorubicin. Chem Res Toxicol 2006;19:1151. https://doi.org/10.1021/tx060083i |
||||
| 45 Gogvadze V, Orrenius S, Zhivotovsky B: Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta 2006;1757:639-647. https://doi.org/10.1016/j.bbabio.2006.03.016 |
||||
| 46 Green DR, Kroemer G: The pathophysiology of mitochondrial cell death. Science 2004;305:626-629. https://doi.org/10.1126/science.1099320 |
||||
| 47 Lemasters JJ, Qian T, He L, Kim JS, Elmore SP, Cascio WE, Brenner DA: Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid Redox Signal 2002;4:769-781. https://doi.org/10.1089/152308602760598918 |
||||
| 48 Metzler B, Hammerer-Lercher A, Jehle J, Dietrich H, Pachinger O, Xu Q, Mair J: Plasma cardiac troponin T closely correlates with infarct size in a mouse model of acute myocardial infarction. Clin Chim Acta 2002;325:87-90. https://doi.org/10.1016/S0009-8981(02)00296-6 |
||||
| 49 Hallén J: Troponin for the estimation of infarct size: what have we learned? Cardiology 2012;121:204-212. https://doi.org/10.1159/000337113 |
||||