Corresponding Author: Sawsan Kreydiyyeh
Department of Biology, Faculty of Arts & Sciences, American University of Beirut, Beirut (Lebanon)
Tel. +961-1-350000, Fax +961-1-744461, E-Mail Sawkreyd@aub.edu.lb
Signaling Cascade Mediating the Effect of FTY720P on the Na+/K+ ATPase in LLC-PK1
Christine Khalila Rawad Hodeifyb Sawsan Kreydiyyeha
aDepartment of Biology, Faculty of Arts & Sciences, American University of Beirut, Beirut, Lebanon, bDepartment of Biotechnology, School of Arts and Sciences, American University of Ras Al Khaimah, Ras Al Khaimah, United Arab Emirates
Introduction
The kidneys play a pivotal role in the regulation of blood composition, blood volume and blood pressure. Through selective reabsorption of metabolites and electrolytes, they maintain homeostasis and the constancy of the internal milieu. Many of the transport processes across the membrane of tubular cells are geared by the sodium gradient established by the Na+/K+ ATPase, known also as the Na+/K+ pump, which resides normally in the basolateral membranes. Renal ischemia impairs kidney functions, leading to acute kidney injury (AKI), and resulting in morbidity and mortality [1, 2]. When renal epithelial cells are energy-deprived, they lose their polarity [3-5], and the Na+/K+ ATPase translocates to intracellular compartments [6]. Regain of cell polarity and relocalization of the Na+/K+ ATPase in the plasma membrane are signs of recovery from renal ischemia [7].
Sphingosine-1-phosphate (S1P), a pleiotrophic lipid mediator, was found to play a protective role against ischemic renal injury IRI [8-11] and FTY720P an analogue of S1P, reduced renal injury in diabetic nephropathy [12].
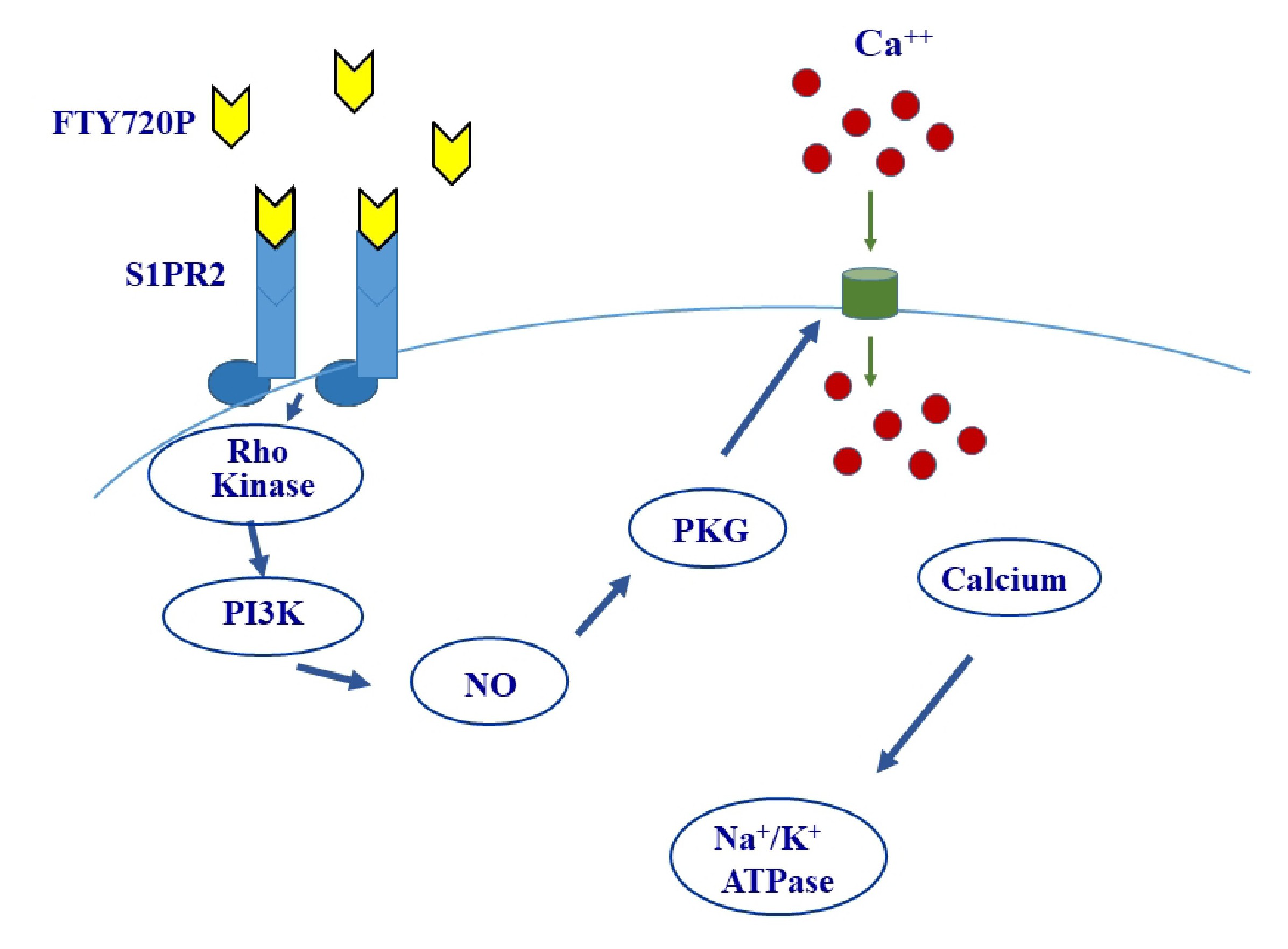
Because S1P and the Na+/K+ ATPase are both implicated in renal ischemic injury, a cause effect relationship was suspected to exist between the sphingolipid and the ATPase. The current study was undertaken to test this hypothesis using the porcine kidney proximal tubule cell line LLC-PK1 as a model, and FTY720P as an analogue of S1P. The results revealed an FTY720P-induced increase in the activity of the ATPase via S1PR2, which leads to sequential activation of Rho kinase and PI3K, followed by NO release and PKG activation. Finally, we demonstrate that these changes are dependent on intracellular calcium.
Materials and Methods
Materials
FTY720P, anti-p-Akt1/2/3 (Ser473)-R rabbit polyclonal antibody, Akt1/2/3 (H-136) rabbit polyclonal antibody, goat anti-mouse horseradish peroxidase (HRP) conjugated IgG, anti-GAPDH mouse monoclonal antibody, KT5823, carboxy-PTIO, glycol-SNAP-1,8-bromo-cGMP were purchased from Santa Cruz Biotechnology, CA, USA. Goat anti-rabbit horseradish peroxidase (HRP) conjugated IgG and the protein ladder were purchased from Abcam, Cambridge, UK. (R)-3-Amino-(3-heylphenylamino)-4-oxobutylphosphonic acid (TFA salt, W146) was purchased from Avanti Polar Lipids, Inc.,Alabaster, Alabama. SEW 2871, CYM 5520, CYM 5541, JTE-013 and sterile dimethyl sulfoxide were obtained from TOCRIS Bioscience, Bristol, UK. CA10444 was obtained from Cayman Chemical Company, Michigan, USA. Y-27632 was procured from Cell Signaling Technology, Danvers, USA. Phorbol-12-myristate-13-acetate (PMA), Rp-Adenosine 3’,5’-cyclic monophosphorothioate triethylammonium salt (RpcAMP), Calphostin C, Wortmannin, and BAPTA/AM were procured from Calbiochem, San Diego, USA. Biorad assay and protein reagent, nitrocellulose membranes, clarity western ECL substrate, were purchased from Bio-rad, California, USA. Porcine kidney cells, LLC-PK1 were purchased from American Type Culture Collection (ATCC). Anti-Na+/K+ ATPase α-1 Antibody, 2-Aminoethyldiphenyl borate (2-APB), and all other chemicals were obtained from Sigma, Chemical Co, St Louis Missouri, USA.
Culture and treatment of LLC-PK1 cells
LLC-PK1 cells were grown in DMEM supplemented with 10% FBS and 1% penicillin, in a humidified incubator (95% O2, 5% CO2) at 37 °C. Cells were treated with FTY720P at 85%-90% confluence after an overnight starvation. An equal amount of the vehicle was always added to the control group in each treatment.
Dose and Time response study on the effect of FTY720P on the activity of the Na+ /K+ ATPase
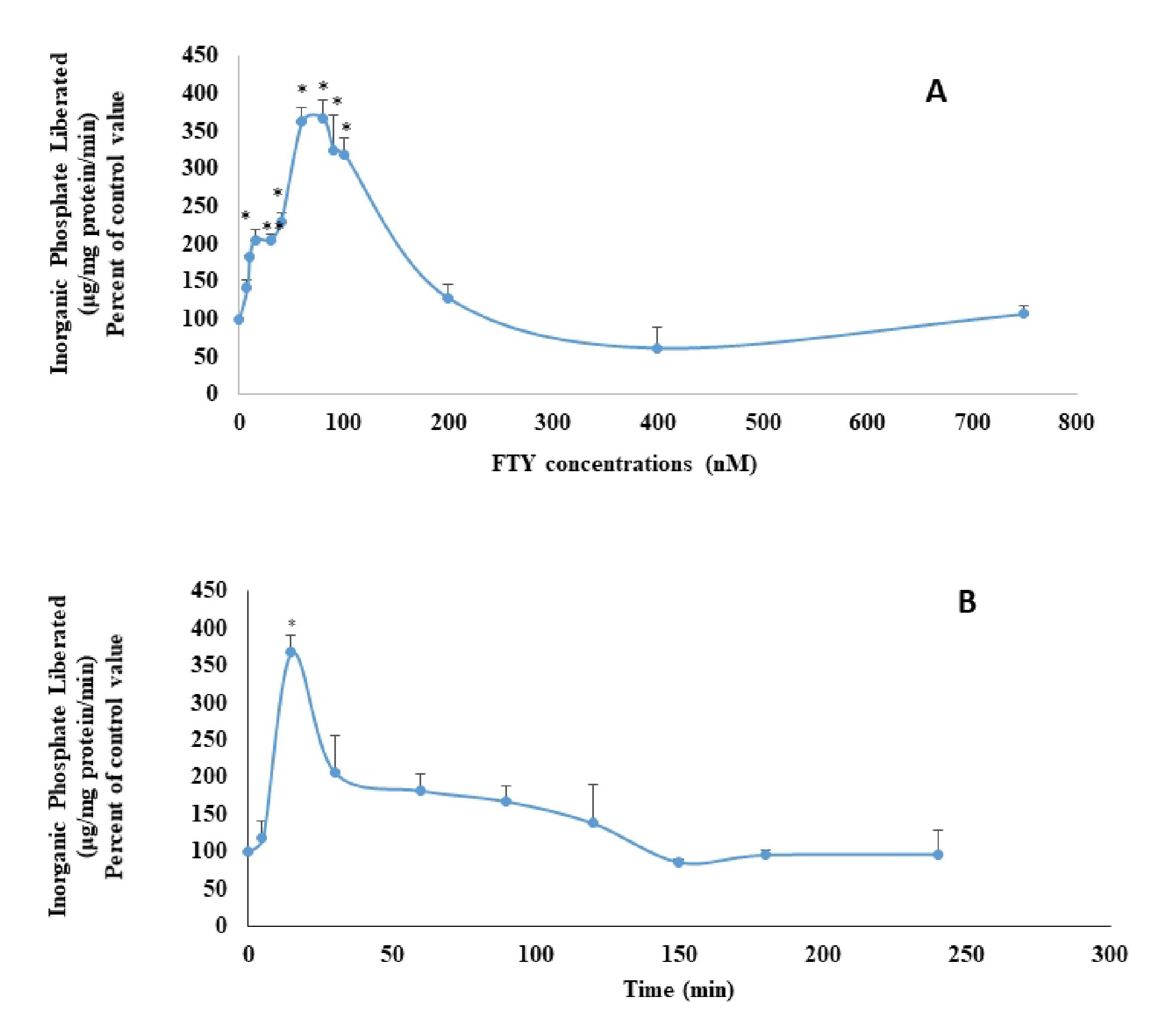
LLC-PK1 cells were treated for15 minutes with different concentrations of FTY720P ranging from 0 to 750 nM. An equal amount of the vehicle DMSO was added to the control group. The cells were then washed with PBS buffer, lysed, homogenized, and spun for 30 minutes at 35000 g and 4 °C. The supernatant was collected and used to assay for the Na+/K+ ATPase activity or for western blot analysis. Proteins in the supernatant were quantified according to the Bradford method.
For the time-dependent study, LLC-PK1 cells were treated with 80 nM FTY720P for different time periods (0-4 hrs). The cells were then collected, treated as described above, and assayed for the Na+/K+ ATPase activity.
Type of S1PR involved in the signaling pathway of FTY720P
To determine the type of S1PR mediating FTY720P’s effect on the Na+/K+ ATPase, S1PR1, S1PR2 and S1PR3 were individually blocked with their respective antagonists: W146 (10 µM), JTE-013 (1 µM) and CAY-10444 (17.4 µM). The blockers were added 30 minutes before FTY720P (80 nM, 15 min).
To confirm the type of S1PR involved, cells were also treated for 15 minutes with the respective agonists of S1PR1, S1PR2 and S1PR3: SEW2871 (100 nM [13]), CYM5520 (2.5 µM [14]), and CYM5541 (2 µM [14]).
Determination of the G protein coupled to the S1PR
S1P receptors are coupled to Gi, Gq or G12,13 [15]. Gi is known to inhibit PKA. To investigate any role of Gi, cells were treated with a PKA inhibitor, RpcAMP (30 µM, [16]), 30 minutes prior to the addition of FTY720P or with a cell permeable cAMP analogue, dibutyryl-cAMP (dbcAMP: 10 µM [16]) for 15 minutes.
On the other hand, Gq activates PKC. The involvement of PKC was tested by treating the cells for 30 minutes, prior to FTY720P, with a PKC inhibitor, namely calphostin C (50 nM, [14]). In addition, the effect of phorbol 12-myristate 13-acetate (PMA: 100 nM, [14], 15 min), a PKC activator was investigated.
Finally, G12/13 has Rho kinase as a downstream effector. To test for the involvement of G12,13, cells were treated with a Rho kinase inhibitor, Y-27632 (10 µM, [17]) for 3 hours, prior to the addition of FTY720P.
Involvement of Rho kinase/PI3K pathway
Rho kinase was reported to activate PI3K [18], which in turn modulates the activity of the Na+/K+ ATPase [19]. The role of PI3K as a mediator was investigated by treating the cells, prior to FTY720P, with a PI3K inhibitor, wortmannin (100 nM, [20], 30min).
Cross talks between Rho kinases and PI3K have been recognized [21]. To position Rho kinase relative to PI3K, the protein expression of p-Akt, a downstream effector of PI3K, was determined by western blot analysis in cells in which Rho kinase was inhibited with Y-27632 (10 µM, 3hrs), before treatment with FTY720P.
Involvement of nitric oxide and its position relative to PI3K
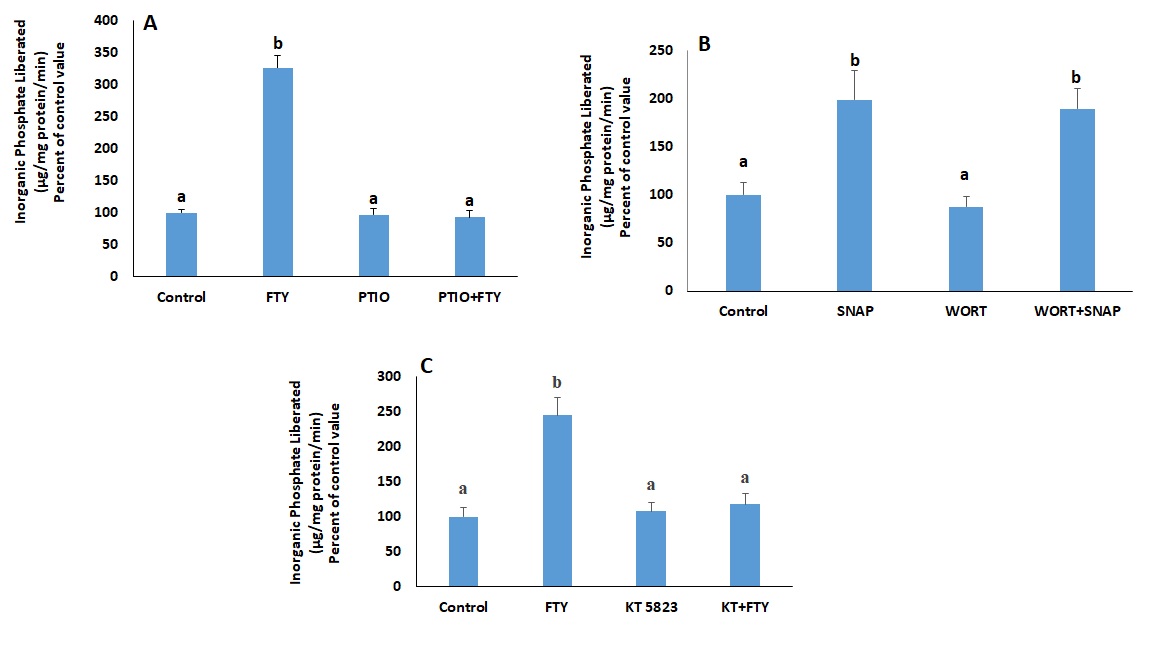
The involvement of NO was investigated by treating the cells with carboxy-PTIO, a nitric oxide scavenger (30µM, [22]) for 30 minutes before treatment with FTY720P.
To locate NO relative to PI3K, the cells were treated for 15 minutes with glyco-SNAP-1 (4 µM, [22]), a nitric oxide donor, in the presence or absence of wortmannin (100 nM), a PI3K inhibitor added 30 minutes before glyco-SNAP-1.
Involvement of PKG
Guanylate cyclase is activated by NO and induces the production of cGMP, which in turn activates PKG. The involvement of the latter was investigated by determining the effect of FTY720P in cells, pretreated with KT-5823 (2.34 µM, [22]), a PKG inhibitor, 30 minutes prior to FTY720P.
Involvement of calcium
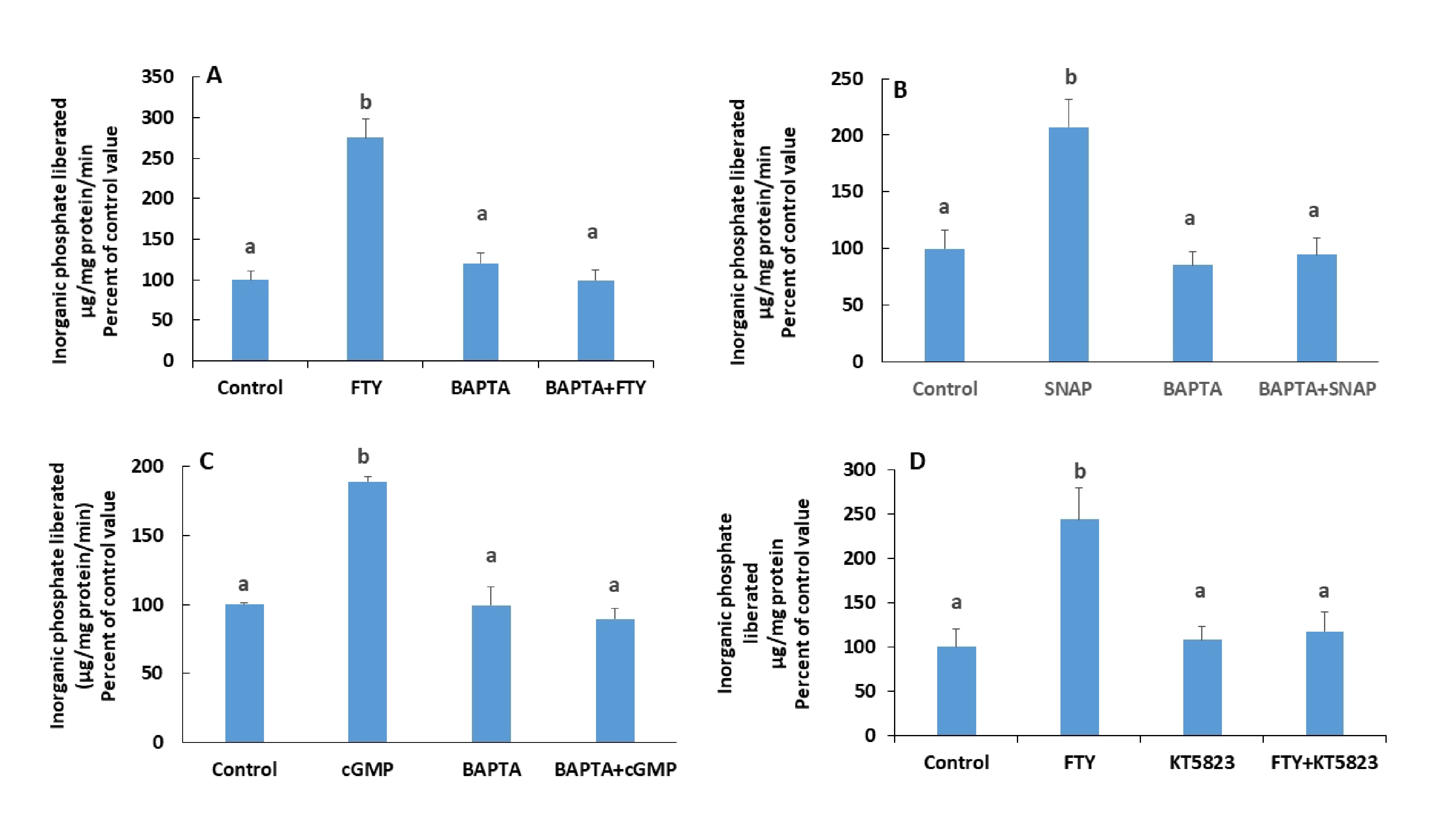
Nitric oxide and cGMP are suspected to play a role in calcium regulation. This hypothesis was tested by incubating the cells with a Ca2+ chelator, BAPTA-AM (20 nM, [15]) for 30 minutes prior to treatment with FTY720P.
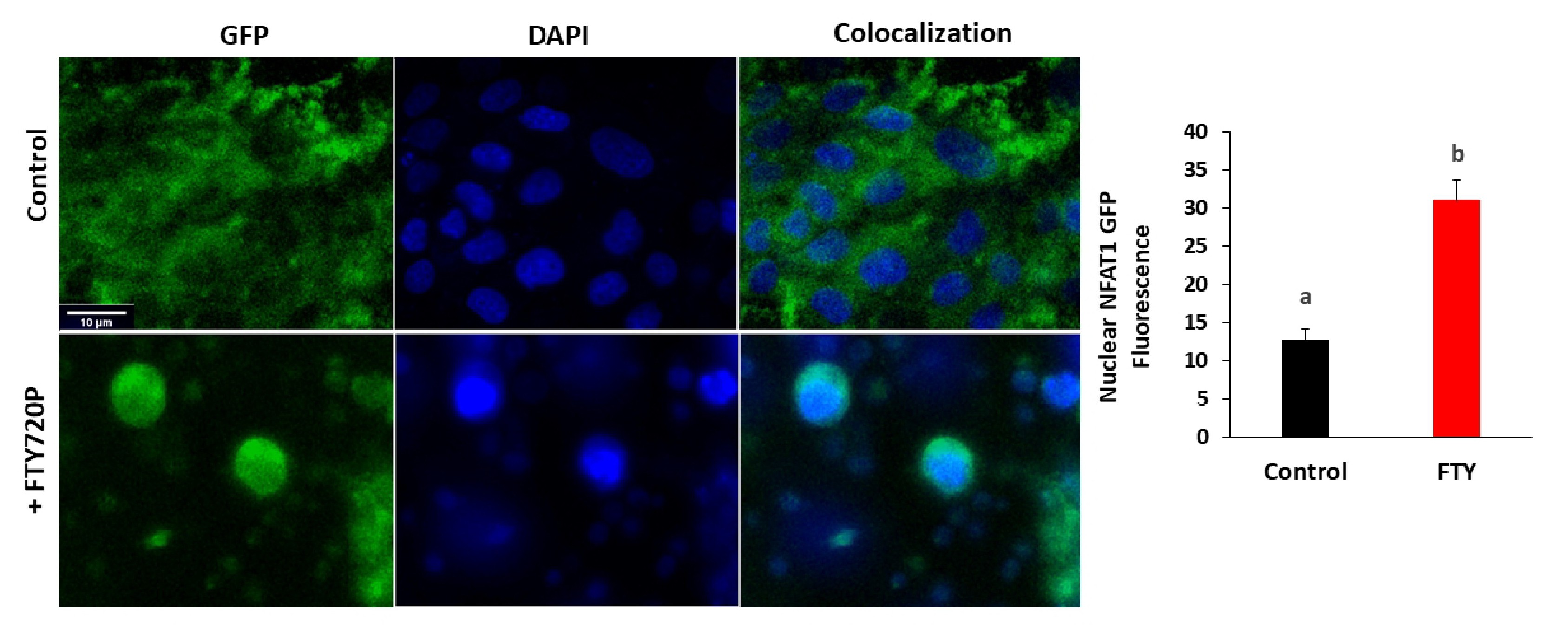
NFAT1-GFP translocation was studied using a GFP-tagged NFAT1-GFP (Addgene #11107) [23]. LLCPK1 cells were plated on Poly-D-Lysine coated glass-bottomed dishes (MatTek) one day before co-transfection with1 μg NFAT1-GFP plasmid DNA using ViaFect™ Transfection Reagent. After 24 hours of transfection, cells were treated for 15 minutes with 80 nM FTY720P before fixation with 4% paraformaldehyde and then imaging with confocal microscope Zeiss LSM710. Nuclear GFP signal was quantified in individual cells using ImageJ (National Institutes of Health).
NFAT is a transcription factor that translocates from the cytoplasm to the nucleus when activated by dephosphorylation by the Ca2+-dependent phosphatase calcineurin. The involvement of calcium was investigated further by studying NFAT1-GFP nuclear translocation after FTY720P treatment.
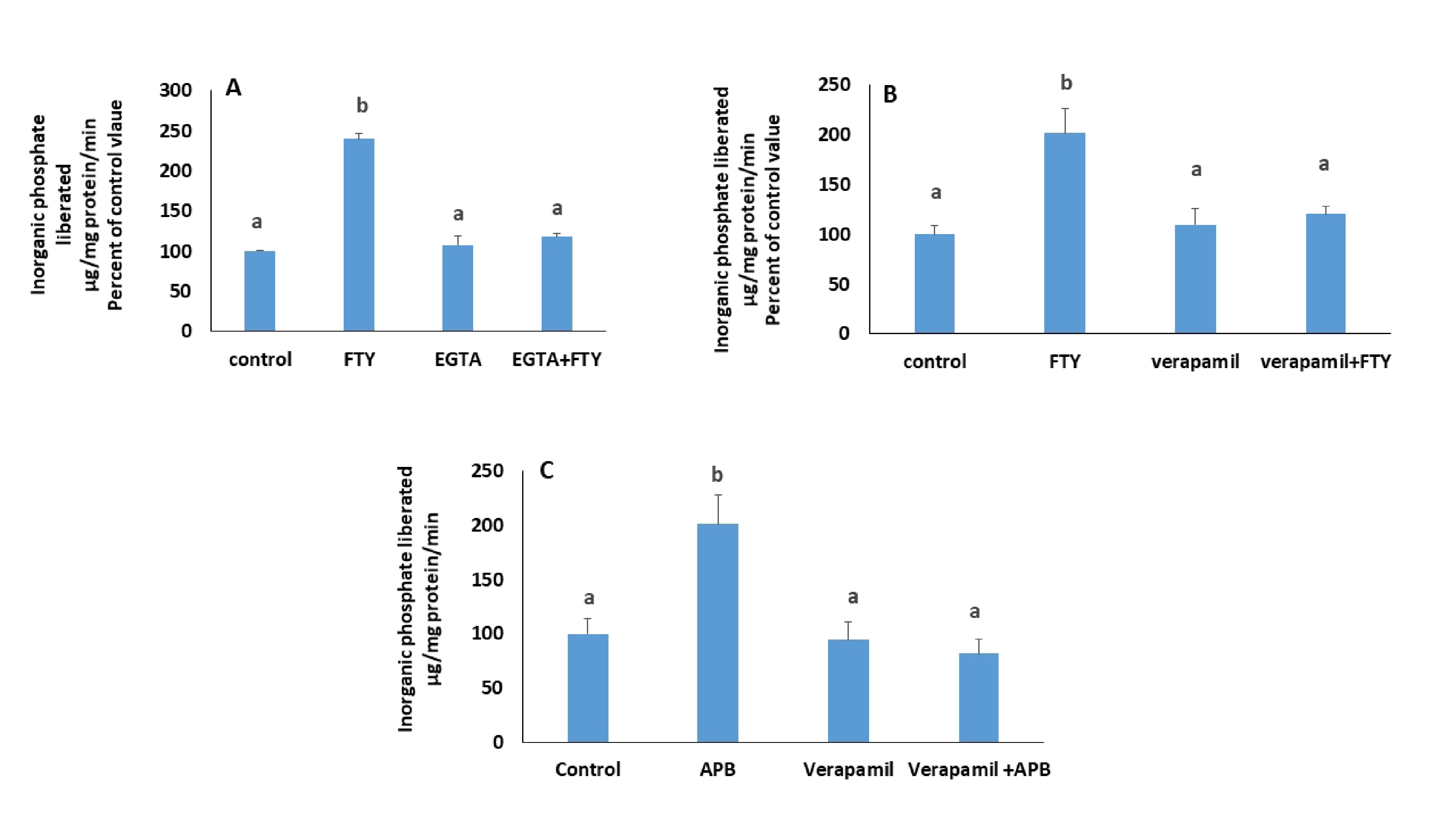
To confirm that changes in intracellular calcium are induced by NO and PKG, cells were treated for 15 min with glyco-SNAP-1 (4 µM), a nitric oxide donor, or with 8-bromo-cGMP (0.5 mM) a cell permeable cGMP analogue, in presence of a Ca2+ chelator, BAPTA-AM (20 nM). The source of calcium was determined by blocking its release from intracellular stores with 2-aminoethoxydiphenyl Borate (2-APB) (60 µM, [15]), a blocker of IP3 channels [24] or by blocking its entry from the extracellular medium with verapamil (10 µM, [25]), an L-type calcium channel blocker, or with EGTA (0.5 mM) a calcium chelator.
Since APB may induce sometimes-extracellular calcium entry, its effect on the ATPase activity was investigated in the simultaneous presence of verapamil. Verapamil (10 µM) was added 30 minutes before APB, which was then applied for 15 minutes.
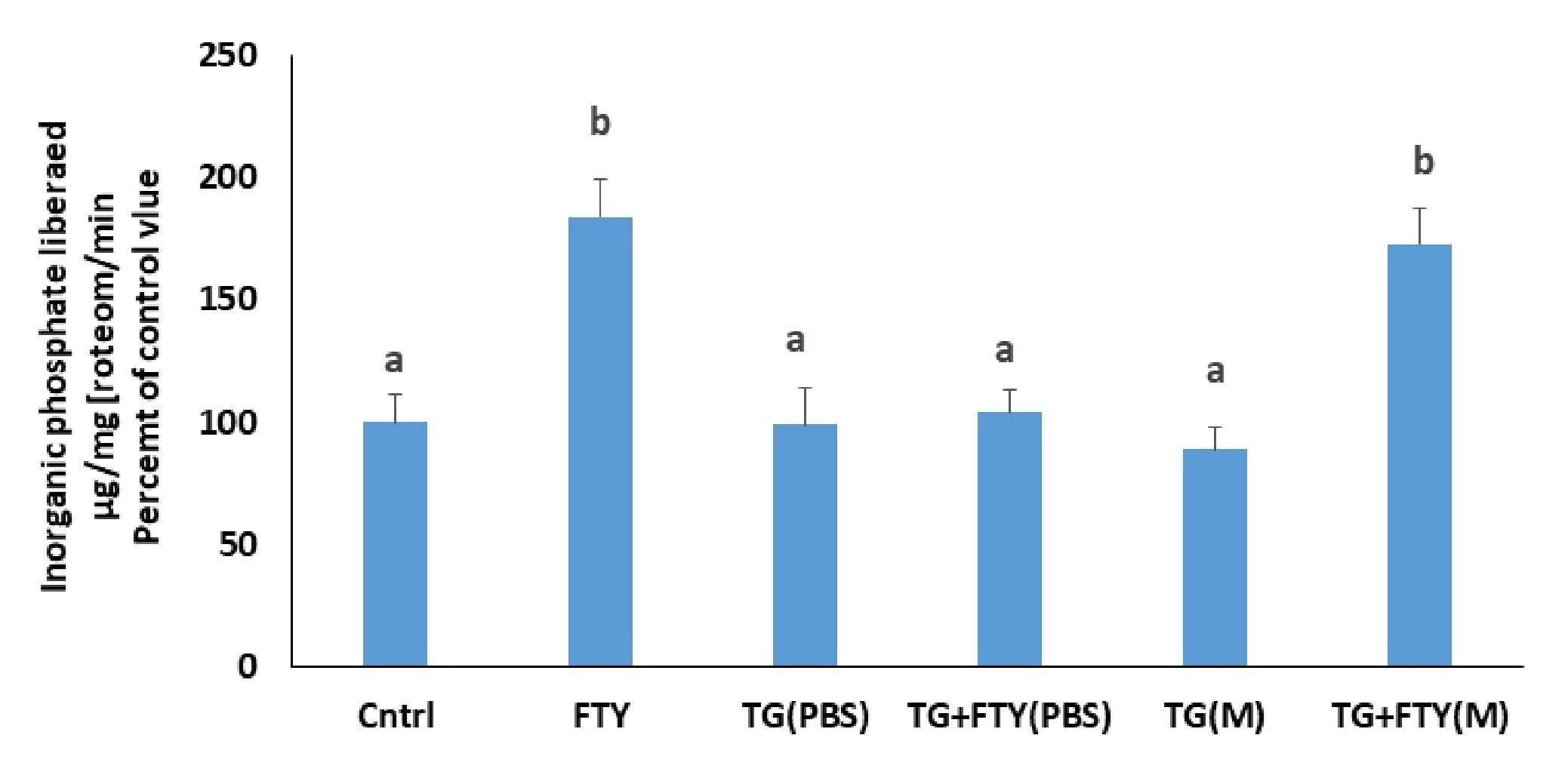
Finally, the involvement of calcium from intracellular stores in the FTY720P effect on the Na+/K+ ATPase was studied by inhibiting the sarcoplasmic Ca2+ ATPase (SERCA) with thapsigargin, blocking thus the pumping of calcium into the lumen of the endoplasmic reticulum (ER), inducing an increase in intracellular calcium levels, and eventually depletion of ER stores. Thapsigargin (1 µM, [26]) was applied 10 min before FTY720P.
The Na+ /K+ ATPase activity assay
Treated cells were collected, washed, homogenized and spun as described before. The supernatant obtained was used to assay for the Na+/K+ ATPase activity. The protein concentration of each sample was adjusted to 0.5 µg/µL by addition of histidine buffer (150 mM, pH 7.4). Samples were incubated for 15 min at room temperature with 1% saponin added at a ratio of 1:4, followed by another incubation for 15 minutes with phosphatase inhibitors (2.7 mM pyrophosphate, 2.7 mM glycerophosphate). Aliquots were then taken from each sample and incubated for 15min at 37 °C in histidine buffer containing NaCl (121.5mM), KCl (19.6 mM), MgCl2 (3.92 mM), adenosine tri-phosphate (2.94 mM), in presence or absence of ouabain (1.47 mM), a specific inhibitor of the ATPase. When ouabain was absent, it was replaced with water. The reaction was stopped by addition of 50% trichloroacetic acid at a ratio of 1:10 (v/v) and the samples were spun at 3000g for 5 min. The amount of inorganic phosphate liberated in the supernatant was measured colorimetrically at 750 nM according to the method of Taussky H, Shorr [27].
Membrane fractionation
Treated cells were washed with PBS scraped, passed through a 26-gauge needle 10 times, then through a 27-gauge needle for an additional ten times, and left on ice for 20min. The lysed cells were then spun at 720g for 5 min. The obtained supernatant was subjected to an additional 5 min centrifugation at 720g. The new supernatant obtained was spun for 10min at 10,000g. The supernatant was collected and centrifuged at 25,000g for 20min. The resultant pellet containing a crude membrane homogenate was used to assay for the protein expression of the Na+/K+ ATPase by western blot analysis. All centrifugation steps were conducted at 4 °C.
Western Blot analysis
Forty micrograms proteins from each sample homogenate were loaded and resolved on a 10% SDS polyacrylamide gel, transferred to a nitrocellulose membrane, and incubated overnight at 4 °C with a specific primary antibody for the Na+/K+ ATPase, p-Akt1/2/3 (Ser473)-R, Akt1/2/3 (H-136), or GAPDH. The membranes were then incubated with Goat anti-rabbit HRP conjugated secondary antibodies for 1 hour at room temperature. The signal was detected by chemiluminescence using Clarity ECL Substrate and its intensity was determined using a ChemiDocTM imaging system. GAPDH expression was used to check for equal loading. The bands were quantified and normalized to GAPDH using Image lab software. To assess for changes in the protein expression of the Na+/K+ ATPase in the membrane, the same steps were followed except that thirty microgram protein samples were taken from the crude membrane homogenate prepared as described before.
Statistical analysis
The data are reported as mean ± SEM, and tested for statistical significance using a one-way analysis of variance followed by a Tukey-Kramer multiple comparison test using GraphPad InStat 3.
Results
Dose and time response study
LLC-PK1 cells treated for 15 minutes with different concentrations of FTY720P showed an increase in the activity of the Na+/K+ ATPase with a highest effect observed at 80 nM (Fig. 1A). FTY720P (80nM) applied for different time periods, exerted a maximal activation of the Na+/K+ ATPase at 15 minutes (Fig. 1B).
Protein expression of the Na+ /K+ ATPase
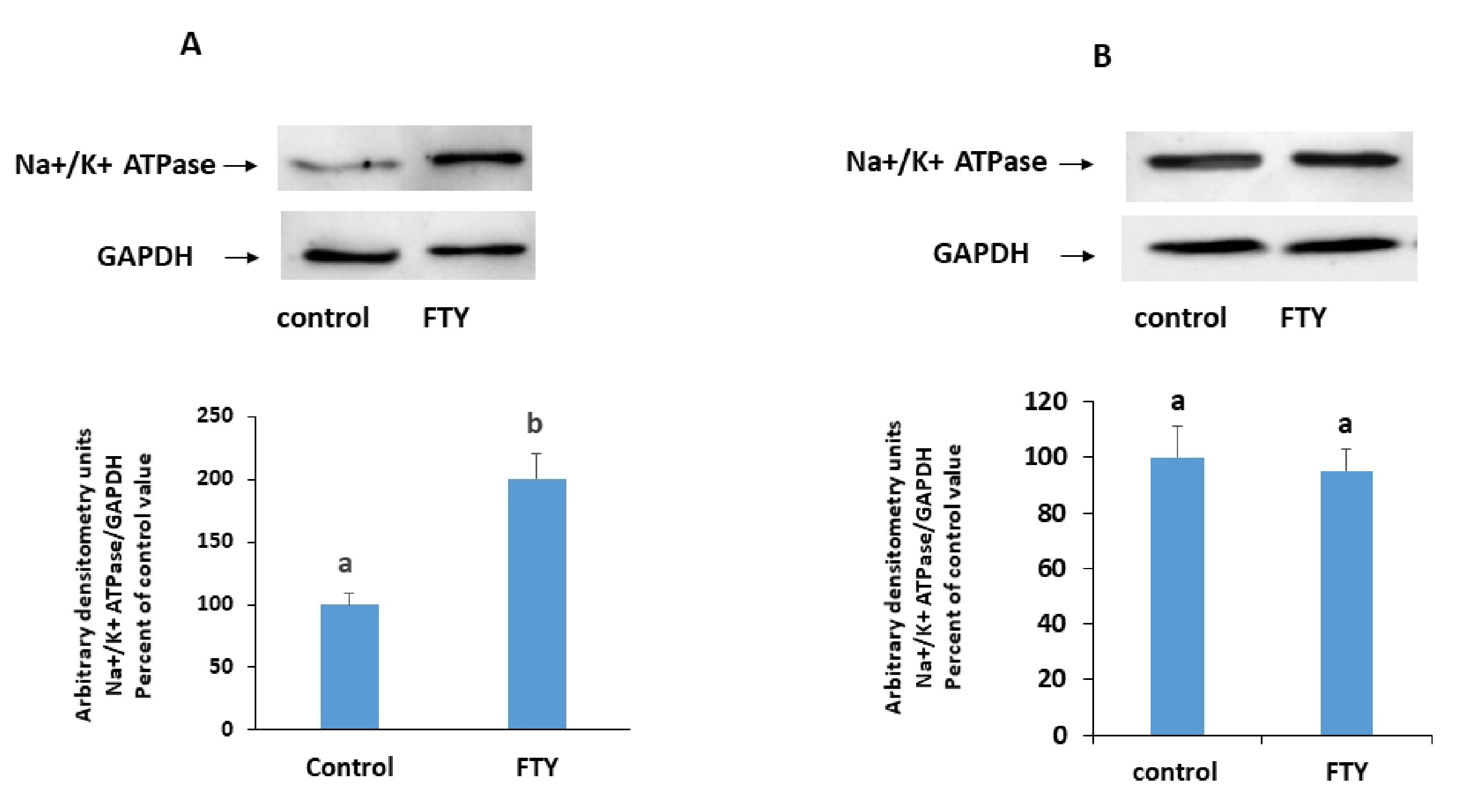
FTY720P (80 nM) applied for 15 min increased the protein expression of the Na+/K+ ATPase in the membrane. This increase was almost of the same order of magnitude as the increase in the activity of the ATPase (Fig. 2).
Type of S1PR involved in the signaling pathway of FTY720P
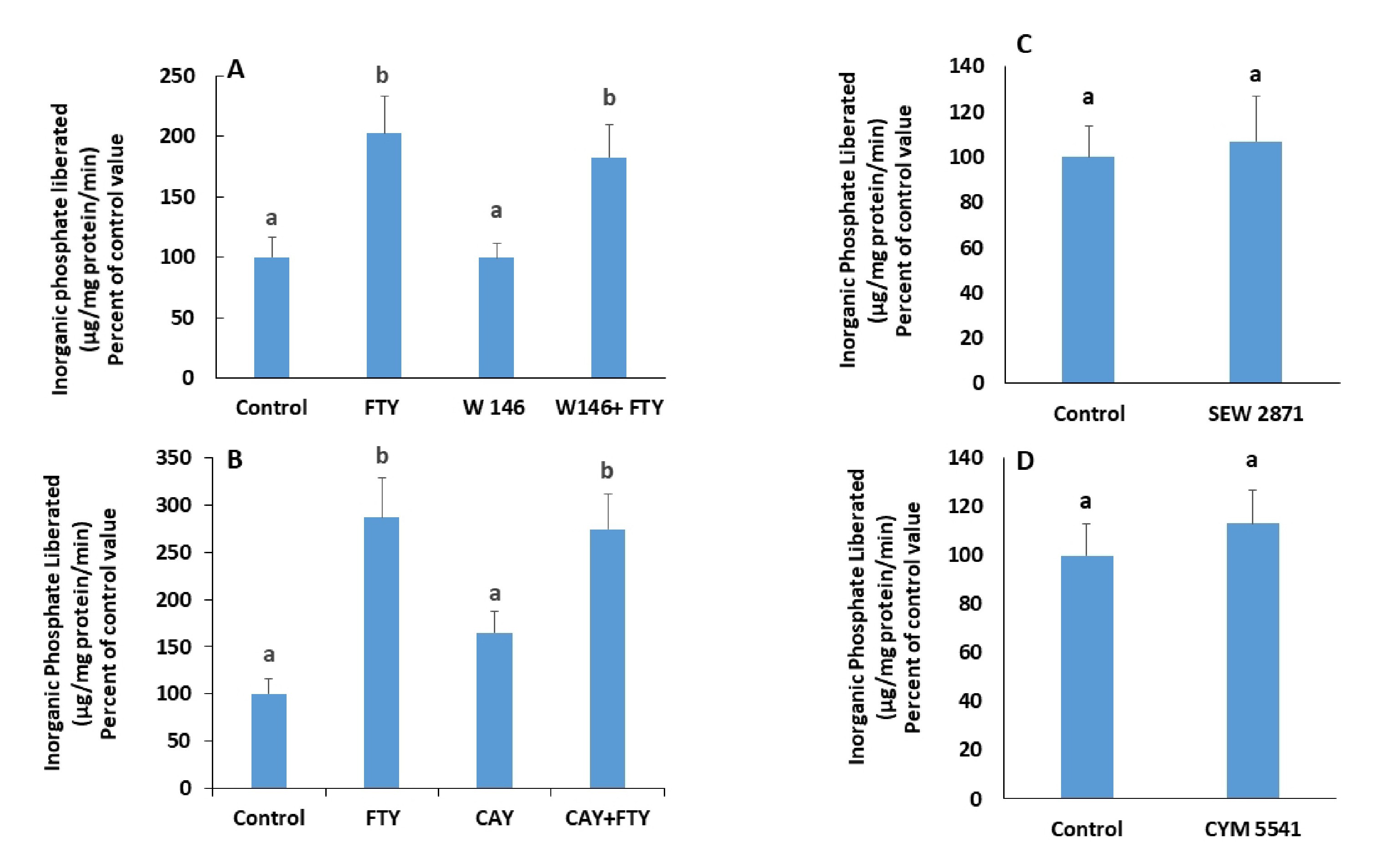
Blocking S1PR1 with W146 (Fig. 3A), and S1PR3 with CAY-10444 (Fig. 3B), did not eliminate FTY720P’s effect on the Na+/K+ ATPase. In addition, neither SEW2871, a S1PR1 agonist (Fig. 3C) nor CYM5541, a S1PR3 agonist (Fig. 3D) exerted any effect on the activity of the ATPase.
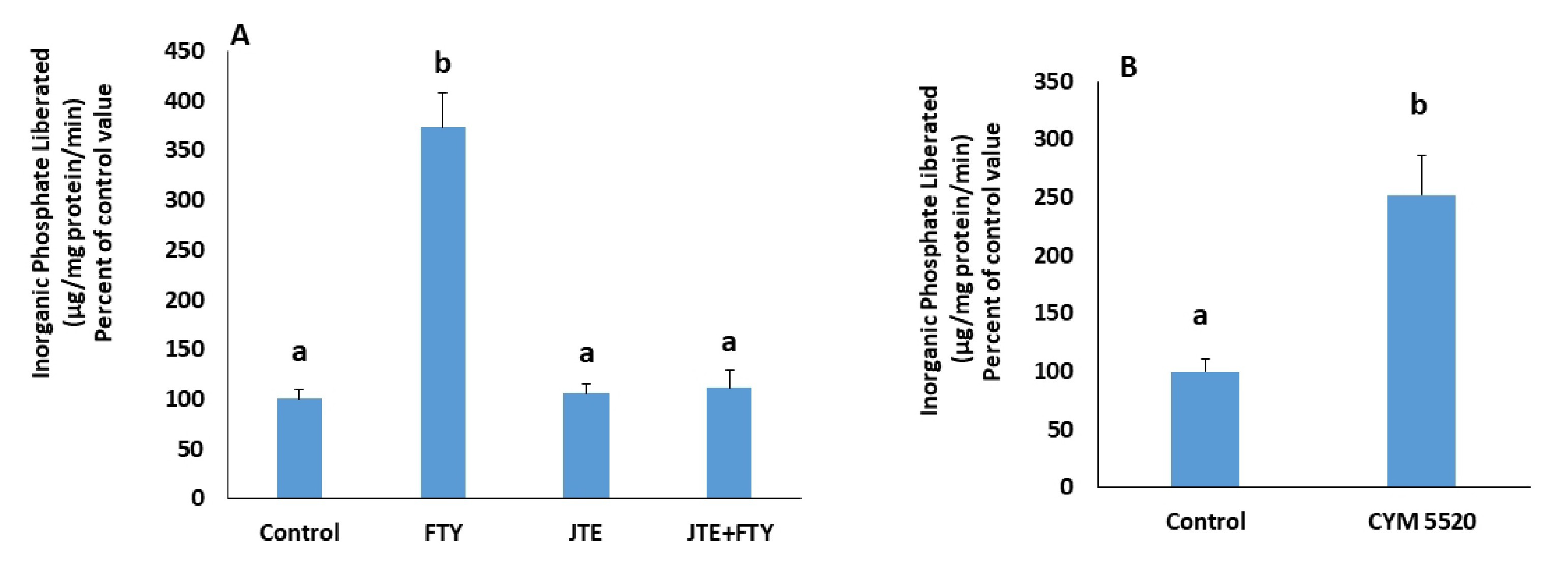
In presence of JTE-013, a S1PR2 antagonist, FTY720P’s activation of the pump was not observed anymore (Fig. 4A), while CYM5520, a S1PR2 agonist induced a significant increase in the pump’s activity (Fig. 4B).
Mediators of FTY720P’s effect on the Na+ /K+ ATPase
S1PR2 is coupled to Gi/o, Gq and G12/13.
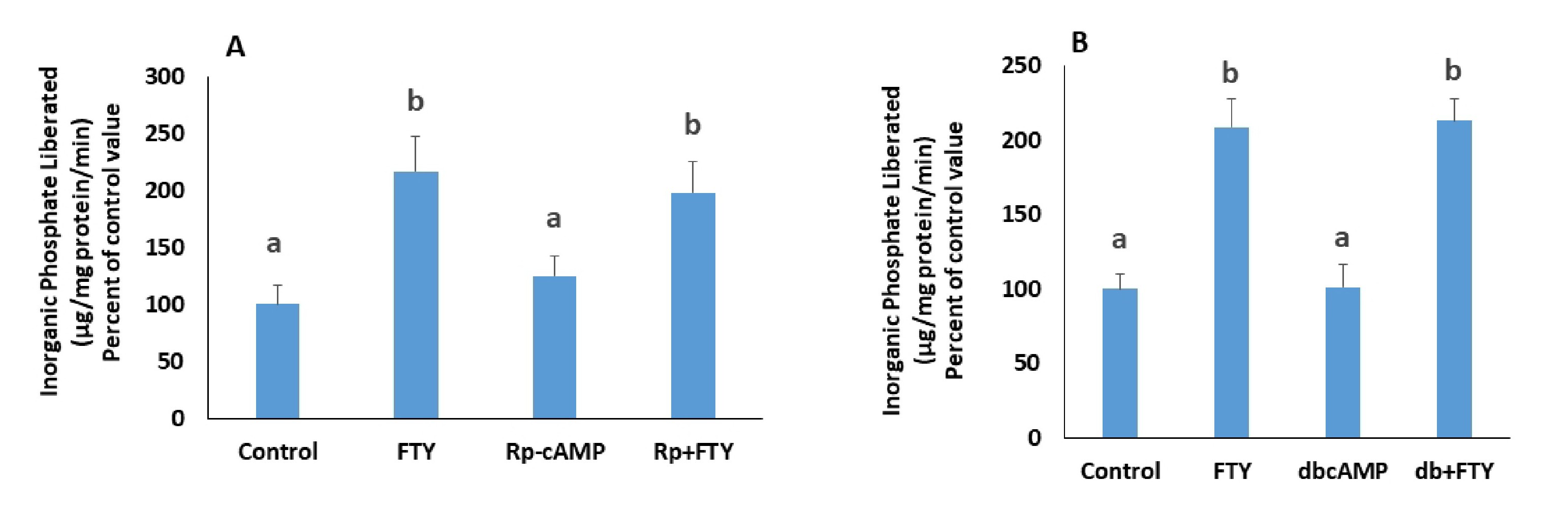
Gi/o inhibits adenylyl cyclase, decreases the level of cAMP and consequently inhibits PKA. On the other hand, Gq activates PKC while G12/13 acts via Rho kinase. Inhibition or activation of these kinases should respectively mimic or cancel the effect of FTY720P. The involvement of PKA was studied using a PKA inhibitor, RpcAMP, and a cell permeable analogue of cAMP, dibutyryl-cAMP (dbcAMP). RpcAMP did not mimic the effect of FTY720P on the pump (Fig. 5A) nor did dbcAMP abolish its stimulatory effect on the pump (Fig. 5B).
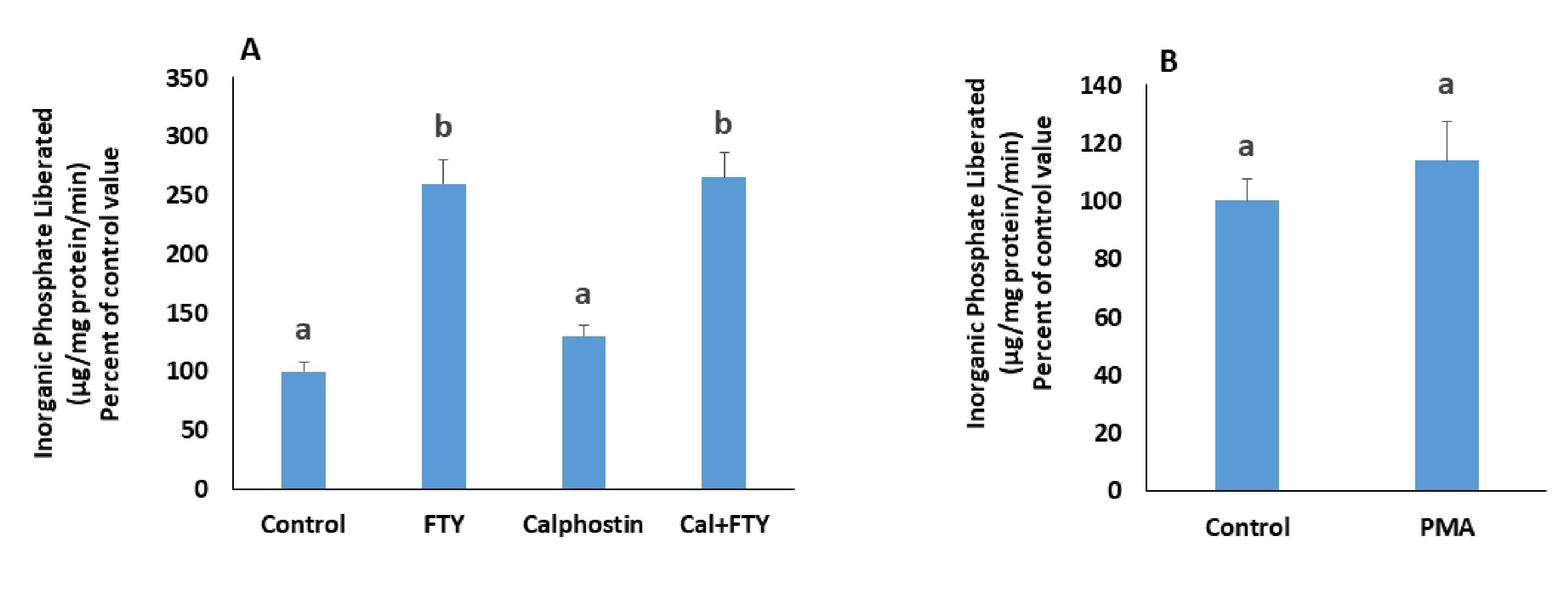
If the S1PR2s activated by FTY720P act via Gq, then they are expected to induce PKC activation. The activation of the Na+/K+ ATPase was still observed in presence of calphostin C (Fig. 6A), a PKC inhibitor, and phorbol 12-myristate 13-acetate (PMA), a PKC activator, did not exert any effect on the ATPase (Fig. 6B).
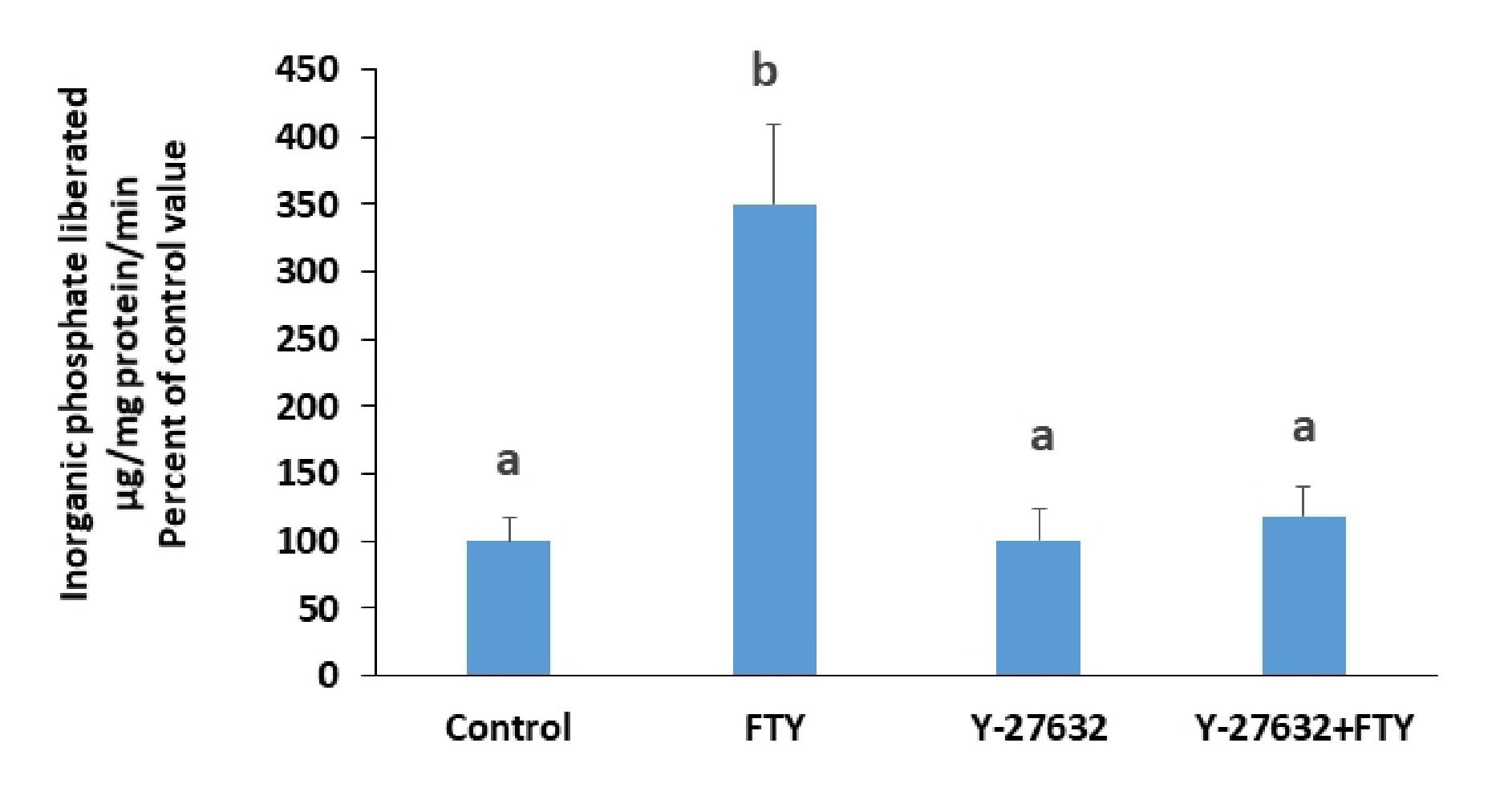
G12/13 is another G protein to which S1PR2 could be coupled, and when activated induces activation of Rho kinase. Treating the cells with a Rho kinase inhibitor, Y-27632, abolished the stimulation induced by FTY720P (Fig. 7).
Rho kinase activates PI3K
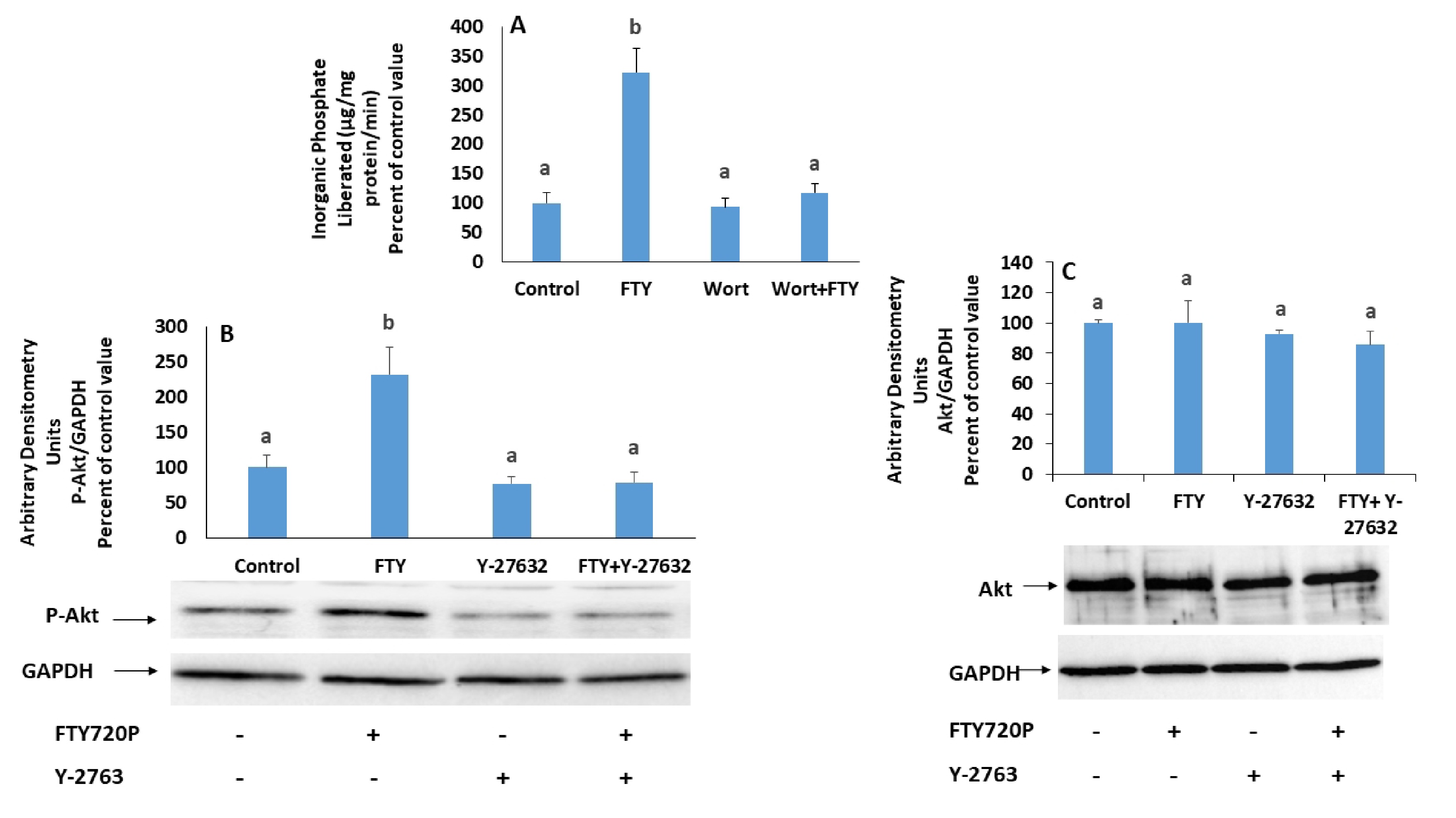
Inhibiting PI3K with wortmannin eliminated the activation of the Na+/K+ ATPase induced by FTY720P on (Fig. 8A).
The expression of p-Akt, a known target of PI3K, increased following treatment with FTY720P. However, it came back to the control value when Rho kinase was inhibited with Y-27632 (Fig. 8B). On the other hand, the expression of total Akt was not affected by FTY720P (Fig. 8C).
Calcium is involved in the signaling pathway and is downstream nitric oxide
An increase in cytosolic calcium can be the result of extracellular calcium influx through membrane channels or calcium release from the endoplasmic reticulum through IP3 receptors.
The translocation of the transcription factor NFAT from the cytosol to the nucleus is a calcium dependent process. Confocal microscopy revealed a significant increase in nuclear fluorescence in cells expressing GFP-tagged NFAT1 and treated with FTY720P (Fig. 10), suggesting involvement of calcium in the FTY720P signaling pathway.
In presence of BAPTA-AM, a Ca2+ chelator, the stimulatory effect of FTY720P on Na+/K+ ATPase disappeared (Fig. 11A). Similary, the effect of Glyco-SNAP-1 and and 8-Br-cGMP, a cell permeable analogue of cGMP, were not manifested in presence of BAPTA-AM. (Fig. 11B and C). FTY720P did not exert any effect on the ATPase when PKG, the target of cGMP, was inhibited with KT 5823 (Fig. 11D).
Source of calcium
Chelating extracellular calcium with EGTA (Fig. 12A) as well as blocking L-type calcium channels with verapamil (Fig. 12B), both abrogated the stimulatory effect of FTY720P, suggesting an extracellular source for the increase in cytosolic calcium.
Cells treated with FTY720P in presence of 2-aminoethoxydiphenyl Borate (2-APB) (60 µM), a blocker of IP3 receptors, exhibited an unexpectedly, higher activity of the Na+/K+ ATPase that disappeared in presence of verapamil (Fig. 12C), suggesting that 2-ABP at the used dose, triggered calcium entry through plasma membrane channels.
The inhibition of the sarco/endoplasmic reticulum Ca ATPase (SERCA) with thapsigargin did not have any effect on the activity of the Na+/K+ ATPase and in its presence, FTY720P exerted its full stimulatory effect when the cells were treated in a normal medium. The effect of FTY720P, however, did not appear when cells were treated in calcium-free PBS (Fig. 13).
Author Contributions
SK and RH contributed to the study conception and design. CK contributed to data collection and analysis. The manuscript was written by SK, reviewed and approved by the other authors.
Funding Sources
This work was supported by a grant from the University Research Board.
Statement of Ethics
The authors have no ethical conflicts to disclose.
The authors declare that no conflicts of interest exist.
| 1 Brodie JC, Humes HD: Stem cell approaches for the treatment of renal failure. Pharmacol Rev 2005;57:299-313. https://doi.org/10.1124/pr.57.3.3 |
||||
| 2 Schrier RW, Wang W, Poole B, Mitra A: Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 2004;114:5-14. https://doi.org/10.1172/JCI200422353 |
||||
| 3 Gaudio KM, Thulin G, Ardito T, Kashgarian M, Siegel NJ: Metabolic alterations in proximal tubule suspensions obtained from ischemic kidneys. Am J Physiol 1989;257:F383-F389. https://doi.org/10.1152/ajprenal.1989.257.3.F383 |
||||
| 4 Molitoris BA, Geerdes A, McIntosh JR: Dissociation and redistribution of Na+,K(+)-ATPase from its surface membrane actin cytoskeletal complex during cellular ATP depletion. J Clin Invest 1991;88:462-469. https://doi.org/10.1172/JCI115326 |
||||
| 5 Riordan M, Sreedharan R, Wang S, Thulin G, Mann A, Stankewich M, Van Why S, Kashgarian M, Siegel NJ: HSP70 binding modulates detachment of Na-K-ATPase following energy deprivation in renal epithelial cells. Am J Physiol Renal Physiol 2005;288:F1236-F1242. https://doi.org/10.1152/ajprenal.00438.2004 |
||||
| 6 Mandel LJ, Doctor RB, Bacallao R: ATP depletion: a novel method to study junctional properties in epithelial tissues. II. Internalization of Na+,K(+)-ATPase and E-cadherin. J Cell Sci 1994;107:3315-3324. https://doi.org/10.1242/jcs.107.12.3315 |
||||
| 7 van Why SK, Kim S, Geibel J, Seebach FA, Kashgarian M, Siegel NJ: Thresholds for cellular disruption and activation of the stress response in renal epithelia. Am J Physiol 1999;277:F227-F234. https://doi.org/10.1152/ajprenal.1999.277.2.F227 |
||||
| 8 Awad AS, Ye H, Huang L, Li L, Foss FW Jr, Macdonald TL, Lynch KR, Okusa MD: Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 2006;290:F1516-F1524. https://doi.org/10.1152/ajprenal.00311.2005 |
||||
| 9 Huwiler A, Pfeilschifter J: Sphingolipid signaling in renal fibrosis. Matrix Biol 2018;68-69:230-247. https://doi.org/10.1016/j.matbio.2018.01.006 |
||||
| 10 Park SW, Kim M, Kim JY, Brown KM, Haase VH, D'Agati VD, Lee HT: Proximal tubule sphingosine kinase-1 has a critical role in A1 adenosine receptor-mediated renal protection from ischemia. Kidney Int 2012;82:878-891. https://doi.org/10.1038/ki.2012.224 |
||||
| 11 Troncoso P, Ortíz M, Martínez L, Kahan BD: FTY 720 prevents ischemic reperfusion damage in rat kidneys. Transplant Proc 2001;33:857-859. https://doi.org/10.1016/S0041-1345(00)02349-6 |
||||
| 12 Awad AS, Rouse MD, Khutsishvili K, Huang L, Bolton WK, Lynch KR, Okusa MD: Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney Int 2011;79:1090-1098. https://doi.org/10.1038/ki.2010.544 |
||||
| 13 Mullershausen F, Craveiro LM, Shin Y, Cortes-Cros M, Bassilana F, Osinde M, Wishart WL, Guerini D, Thallmair M, Schwab ME, Sivasankaran R, Seuwen K, Dev KK: Phosphorylated FTY720 promotes astrocyte migration through sphingosine-1-phosphate receptors. J Neurochem 2007;102:1151-1161. https://doi.org/10.1111/j.1471-4159.2007.04629.x |
||||
| 14 Chakkour M, Kreydiyyeh S: FTY720P Upregulates the Na+/K+ ATPase in HepG2 Cells by Activating S1PR3 and Inducing PGE2 Release. Cell Physiol Biochem 2019;53:518-531. https://doi.org/10.33594/000000155 |
||||
| 15 Brinkmann V: Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 2007;115:84-105. https://doi.org/10.1016/j.pharmthera.2007.04.006 |
||||
| 16 Hodeify R, Chakkour M, Rida R, Kreydiyyeh S: PGE2 upregulates the Na+/K+ ATPase in HepG2 cells via EP4 receptors and intracellular calcium. PLoS One 2021;16:e0245400. https://doi.org/10.1371/journal.pone.0245400 |
||||
| 17 Tong J, Wang Y, Chang B, Zhang D, Wang B: Y-27632 inhibits ethanol-induced increase in intestinal epithelial barrier permeability. Mol Med Rep 2014;9:2357-2361. https://doi.org/10.3892/mmr.2014.2060 |
||||
| 18 Fritsch R, de Krijger I, Fritsch K, George R, Reason B, Kumar MS, Diefenbacher M, Stamp G, Downward J: RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell 2013;153:1050-1063. https://doi.org/10.1016/j.cell.2013.04.031 |
||||
| 19 Wang Q, Zheng X, Cheng Y, Zhang YL, Wen HX, Tao Z, Li H, Hao Y, Gao Y, Yang LM, Smith FG, Huang CJ, Jin SW: Resolvin D1 stimulates alveolar fluid clearance through alveolar epithelial sodium channel, Na,K-ATPase via ALX/cAMP/PI3K pathway in lipopolysaccharide-induced acute lung injury. J Immunol 2014;192:3765-3777. https://doi.org/10.4049/jimmunol.1302421 |
||||
| 20 Serhan MF, Kreydiyyeh SI: Insulin targets the Na(+)/K(+) ATPase in enterocytes via PI3K, PKC, and MAPKS. J Recept Signal Transduct Res 2011;31:299-306. https://doi.org/10.3109/10799893.2011.587821 |
||||
| 21 McCormick B, Chu JY, Vermeren S: Cross-talk between Rho GTPases and PI3K in the neutrophil. Small GTPases 2019;10:187-195. https://doi.org/10.1080/21541248.2017.1304855 |
||||
| 22 El Moussawi L, Chakkour M, Kreydiyyeh S: The epinephrine-induced PGE2 reduces Na+/K+ ATPase activity in Caco-2 cells via PKC, NF-κB and NO. PLoS One 2019;14:e0220987. https://doi.org/10.1371/journal.pone.0220987 |
||||
| 23 Aramburu J, Yaffe MB, López-Rodríguez C, Cantley LC, Hogan PG, Rao A: Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 1999;285:2129-2133. https://doi.org/10.1126/science.285.5436.2129 |
||||
| 24 Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K: 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem 1997;122:498-505. https://doi.org/10.1093/oxfordjournals.jbchem.a021780 |
||||
| 25 Bergson P, Lipkind G, Lee SP, Duban ME, Hanck DA: Verapamil block of T-type calcium channels. Mol Pharmacol 2011;79:411-419. https://doi.org/10.1124/mol.110.069492 |
||||
| 26 Hodeify R, Nandakumar M, Own M, Courjaret RJ, Graumann J, Hubrack SZ, Machaca K: The CCT chaperonin is a novel regulator of Ca2+ signaling through modulation of Orai1 trafficking. Sci Adv 2018;4:eaau1935. https://doi.org/10.1126/sciadv.aau1935 |
||||
| 27 Taussky HH, Shorr E: A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem 1953;202:675-685. https://doi.org/10.1016/S0021-9258(18)66180-0 |
||||
| 28 Jørgensen PL: Structure, function and regulation of Na,K-ATPase in the kidney. Kidney Int 1986;29:10-20. https://doi.org/10.1038/ki.1986.3 |
||||
| 29 Clausen MV, Hilbers F, Poulsen H: The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front Physiol 2017;8:371. https://doi.org/10.3389/fphys.2017.00371 |
||||
| 30 Kristensen ML, Kierulf-Lassen C, Nielsen PM, Krag S, Birn H, Nejsum LN, Nørregaard R: Remote ischemic perconditioning attenuates ischemia/reperfusion-induced downregulation of AQP2 in rat kidney. Physiol Rep 2016;4:e12865. https://doi.org/10.14814/phy2.12865 |
||||
| 31 Lai LW, Yong KC, Igarashi S, Lien YH: A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int 2007;71:1223-1231. https://doi.org/10.1038/sj.ki.5002203 |
||||
| 32 Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, Rosin DL, Haase VH, Macdonald TL, Lynch KR, Okusa MD: Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol 2010;21:955-965. https://doi.org/10.1681/ASN.2009060662 |
||||
| 33 Sykes DA, Riddy DM, Stamp C, Bradley ME, McGuiness N, Sattikar A, Guerini D, Rodrigues I, Glaenzel A, Dowling MR, Mullershausen F, Charlton SJ: Investigating the molecular mechanisms through which FTY720-P causes persistent S1P1 receptor internalization. Br J Pharmacol 2014;171:4797-4807. https://doi.org/10.1111/bph.12620 |
||||
| 34 Rida R, Kreydiyyeh S: FTY720P inhibits the Na+/K+ ATPase in Caco-2 cells via S1PR2: PGE2 and NO are along the signaling pathway. Life Sci 2018;215:198-206. https://doi.org/10.1016/j.lfs.2018.11.026 |
||||
| 35 Al Alam N, Kreydiyyeh SI: FTY720P inhibits hepatic Na(+)-K(+) ATPase via S1PR2 and PGE2. Biochem Cell Biol 2016;94:371-377. https://doi.org/10.1139/bcb-2016-0025 |
||||
| 36 Albert R, Hinterding K, Brinkmann V, Guerini D, Müller-Hartwieg C, Knecht H, Simeon C, Streiff M, Wagner T, Welzenbach K, Zécri F, Zollinger M, Cooke N, Francotte E: Novel immunomodulator FTY720 is phosphorylated in rats and humans to form a single stereoisomer. Identification, chemical proof, and biological characterization of the biologically active species and its enantiomer. J Med Chem 2005;48:5373-5377. https://doi.org/10.1021/jm050242f |
||||
| 36 Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR: The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 2002;277:21453-21457. https://doi.org/10.1074/jbc.C200176200 |
||||
| 38 Sobel K, Menyhart K, Killer N, Renault B, Bauer Y, Studer R, Steiner B, Bolli MH, Nayler O, Gatfield J: Sphingosine 1-phosphate (S1P) receptor agonists mediate pro-fibrotic responses in normal human lung fibroblasts via S1P2 and S1P3 receptors and Smad-independent signaling. J Biol Chem 2013;288:14839-14851. https://doi.org/10.1074/jbc.M112.426726 |
||||
| 39 Abraham D, Dashwood M: Endothelin--role in vascular disease. Rheumatology (Oxford) 2008;47:v23-4. https://doi.org/10.1093/rheumatology/ken282 |
||||
| 40 Chibalin AV, Ogimoto G, Pedemonte CH, Pressley TA, Katz AI, Féraille E, Berggren PO, Bertorello AM: Dopamine-induced endocytosis of Na+,K+-ATPase is initiated by phosphorylation of Ser-18 in the rat alpha subunit and Is responsible for the decreased activity in epithelial cells. J Biol Chem 1999;274:1920-1927. https://doi.org/10.1074/jbc.274.4.1920 |
||||
| 41 Chibalin AV, Pedemonte CH, Katz AI, Féraille E, Berggren PO, Bertorello AM: Phosphorylation of the catalyic alpha-subunit constitutes a triggering signal for Na+,K+-ATPase endocytosis. J Biol Chem 1998;273:8814-8819. https://doi.org/10.1074/jbc.273.15.8814 |
||||
| 42 Khundmiri SJ, Bertorello AM, Delamere NA, Lederer ED: Clathrin-mediated endocytosis of Na+,K+-ATPase in response to parathyroid hormone requires ERK-dependent phosphorylation of Ser-11 within the alpha1-subunit. J Biol Chem 2004;279:17418-17427. https://doi.org/10.1074/jbc.M311715200 |
||||
| 43 Ridge KM, Dada L, Lecuona E, Bertorello AM, Katz AI, Mochly-Rosen D, Sznajder JI: Dopamine-induced exocytosis of Na,K-ATPase is dependent on activation of protein kinase C-epsilon and -delta. Mol Biol Cell 2002;13:1381-1389. https://doi.org/10.1091/mbc.01-07-0323 |
||||
| 44 El-Zein O, Usta J, El Moussawi L, Kreydiyyeh SI: Leptin inhibits the Na(+)/K(+) ATPase in Caco-2 cells via PKC and p38MAPK. Cell Signal 2015;27:416-423. https://doi.org/10.1016/j.cellsig.2014.12.004 |
||||
| 45 Dada LA, Novoa E, Lecuona E, Sun H, Sznajder JI: Role of the small GTPase RhoA in the hypoxia-induced decrease of plasma membrane Na,K-ATPase in A549 cells. J Cell Sci 2007;120:2214-2222. https://doi.org/10.1242/jcs.003038 |
||||
| 46 Lecuona E, Ridge K, Pesce L, Batlle D, Sznajder JI: The GTP-binding protein RhoA mediates Na,K-ATPase exocytosis in alveolar epithelial cells. Mol Biol Cell 2003;14:3888-3897. https://doi.org/10.1091/mbc.e02-12-0781 |
||||
| 47 Ridley AJ: Rho proteins: linking signaling with membrane trafficking. Traffic 2001;2:303-310. https://doi.org/10.1034/j.1600-0854.2001.002005303.x |
||||
| 48 Bilanges B, Posor Y, Vanhaesebroeck B: PI3K isoforms in cell signalling and vesicle trafficking. Nat Rev Mol Cell Biol 2019;20:515-534. https://doi.org/10.1038/s41580-019-0129-z |
||||
| 49 Obradovic M, Zafirovic S, Jovanovic A, Milovanovic ES, Mousa SA, Labudovic-Borovic M, Isenovic ER: Effects of 17β-estradiol on cardiac Na(+)/K(+)-ATPase in high fat diet fed rats. Mol Cell Endocrinol 2015;416:46-56. https://doi.org/10.1016/j.mce.2015.08.020 |
||||
| 50 Manning BD, Cantley LC: AKT/PKB signaling: navigating downstream. Cell 2007;129:1261-1274. https://doi.org/10.1016/j.cell.2007.06.009 |
||||
| 51 Basile JR, Gavard J, Gutkind JS: Plexin-B1 utilizes RhoA and Rho kinase to promote the integrin-dependent activation of Akt and ERK and endothelial cell motility. J Biol Chem 2007;282:34888-34895. https://doi.org/10.1074/jbc.M705467200 |
||||
| 52 Gupta S, McArthur C, Grady C, Ruderman NB: Stimulation of vascular Na(+)-K(+)-ATPase activity by nitric oxide: a cGMP-independent effect. Am J Physiol 1994;266:H2146-H2151. https://doi.org/10.1152/ajpheart.1994.266.5.H2146 |
||||
| 53 Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC: Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 1999;399:597-601. https://doi.org/10.1038/21218 |
||||
| 54 Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM: Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999;399:601-605. https://doi.org/10.1038/21224 |
||||
| 55 Villegas SN, Gombos R, García-López L, Gutiérrez-Pérez I, García-Castillo J, Vallejo DM, Da Ros VG, Ballesta-Illán E, Mihály J, Dominguez M: PI3K/Akt Cooperates with Oncogenic Notch by Inducing Nitric Oxide-Dependent Inflammation. Cell Rep 2018;22:2541-2549. https://doi.org/10.1016/j.celrep.2018.02.049 |
||||
| 56 Liu LJ, Yu JJ, Xu XL: Kappa-opioid receptor agonist U50448H protects against renal ischemia-reperfusion injury in rats via activating the PI3K/Akt signaling pathway. Acta Pharmacol Sin 2018;3:97-106. https://doi.org/10.1038/aps.2017.51 |
||||
| 57 Denninger JW, Marletta MA: Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta 1999;1411:334-350. https://doi.org/10.1016/S0005-2728(99)00024-9 |
||||
| 58 Milbourne EA, Bygrave FL; Do nitric oxide and cGMP play a role in calcium cycling? Cell Calcium 1995;18:207-213. https://doi.org/10.1016/0143-4160(95)90065-9 |
||||
| 59 Pandol SJ, Schoeffield-Payne MS: Cyclic GMP mediates the agonist-stimulated increase in plasma membrane calcium entry in the pancreatic acinar cell. J Biol Chem 1990;265:12846-12853. https://doi.org/10.1016/S0021-9258(19)38236-5 |
||||
| 60 Wu LG, Hamid E, Shin W, Chiang HC: Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annu Rev Physiol 2014;76:301-31. https://doi.org/10.1146/annurev-physiol-021113-170305 |
||||
| 61 Okamura H, Aramburu J, García-Rodríguez C, Viola JP, Raghavan A, Tahiliani M, Zhang X, Qin J, Hogan PG, Rao A: Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol Cell 2000;6:539-550. https://doi.org/10.1016/S1097-2765(00)00053-8 |
||||
| 62 Wang Y, Wagner MB, Joyner RW, Kumar R: cGMP-dependent protein kinase mediates stimulation of L-type calcium current by cGMP in rabbit atrial cells. Cardiovasc Res 2000;48:310-322. https://doi.org/10.1016/S0008-6363(00)00178-4 |
||||
| 63 Kumar R, Namiki T, Joyner RW: Effects of cGMP on L-type calcium current of adult and newborn rabbit ventricular cells. Cardiovasc Res 1997;33:573-582. https://doi.org/10.1016/S0008-6363(96)00258-1 |
||||
| 64 Xiong Z, Sperelakis N: Regulation of L-type calcium channels of vascular smooth muscle cells. J Mol Cell Cardiol 1995;27:75-91. https://doi.org/10.1016/S0022-2828(08)80009-0 |
||||
| 65 DeHaven WI, Smyth JT, Boyles RR, Bird GS, Putney JW Jr: Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J Biol Chem 2008;283:19265-19273. https://doi.org/10.1074/jbc.M801535200 |
||||
| 66 Maeda A, Amano M, Fukata Y, Kaibuchi K: Translocation of Na(+),K(+)-ATPase is induced by Rho small GTPase in renal epithelial cells. Biochem Biophys Res Commun 2002;297:1231-1237. https://doi.org/10.1016/S0006-291X(02)02342-2 |
||||
| 67 Micaroni M: The role of calcium in intracellular trafficking. Curr Mol Med 2010;10:763-773. https://doi.org/10.2174/156652410793384204 |
||||
| 68 Sargeant J, Hay JC: Ca2+ regulation of constitutive vesicle trafficking. Fac Rev 2022;11:6. https://doi.org/10.12703/r/11-6 |
||||