Corresponding Author: Lei Lang
Key Laboratory of Laboratory Medical Diagnostics, Chinese Ministry of Education,
Chongqing Medical University, No.1, Yi-Xue-Yuan Road, Chongqing 400016 (China)
E-Mail langlei@cqu.edu.cn
Erratum
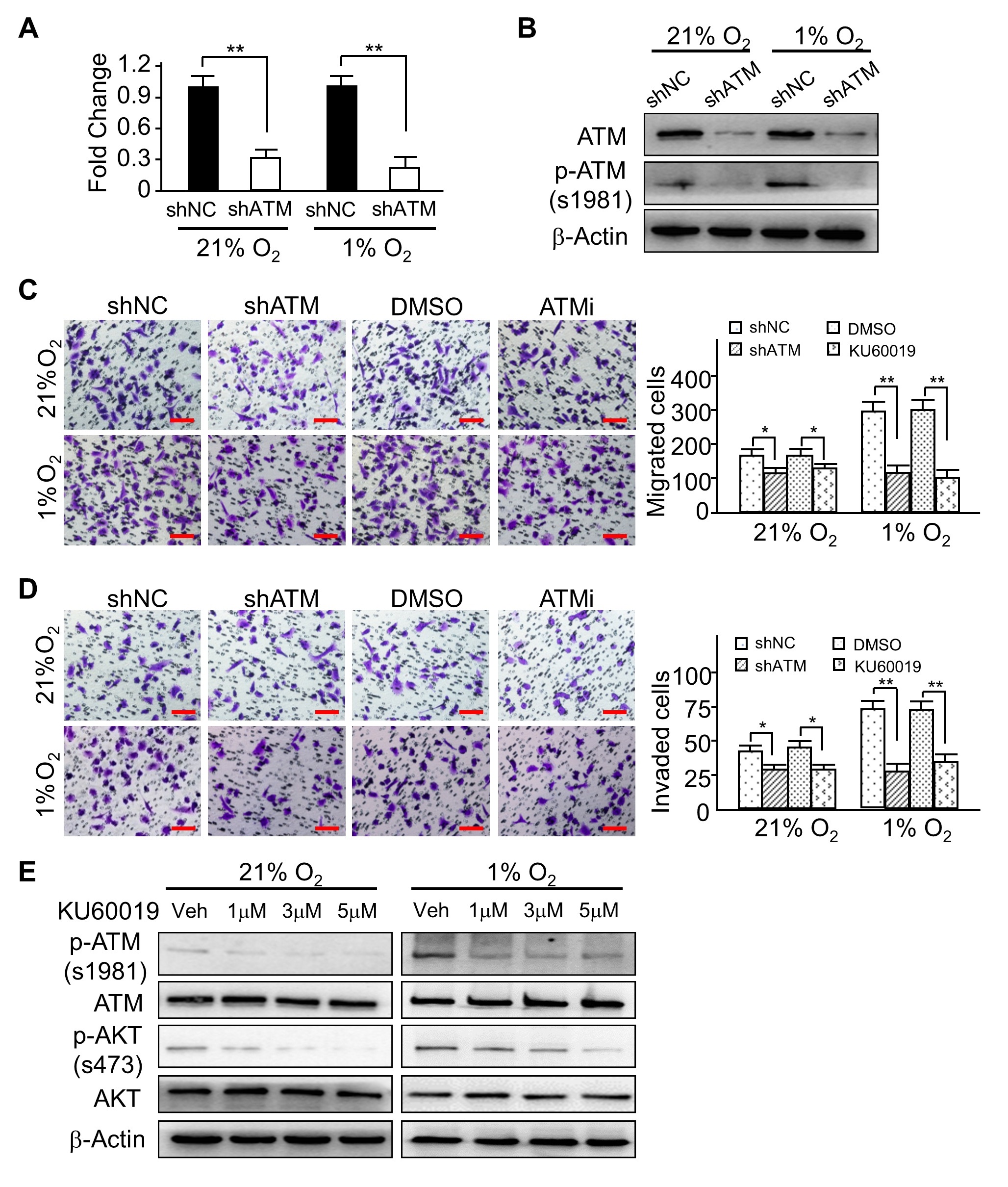
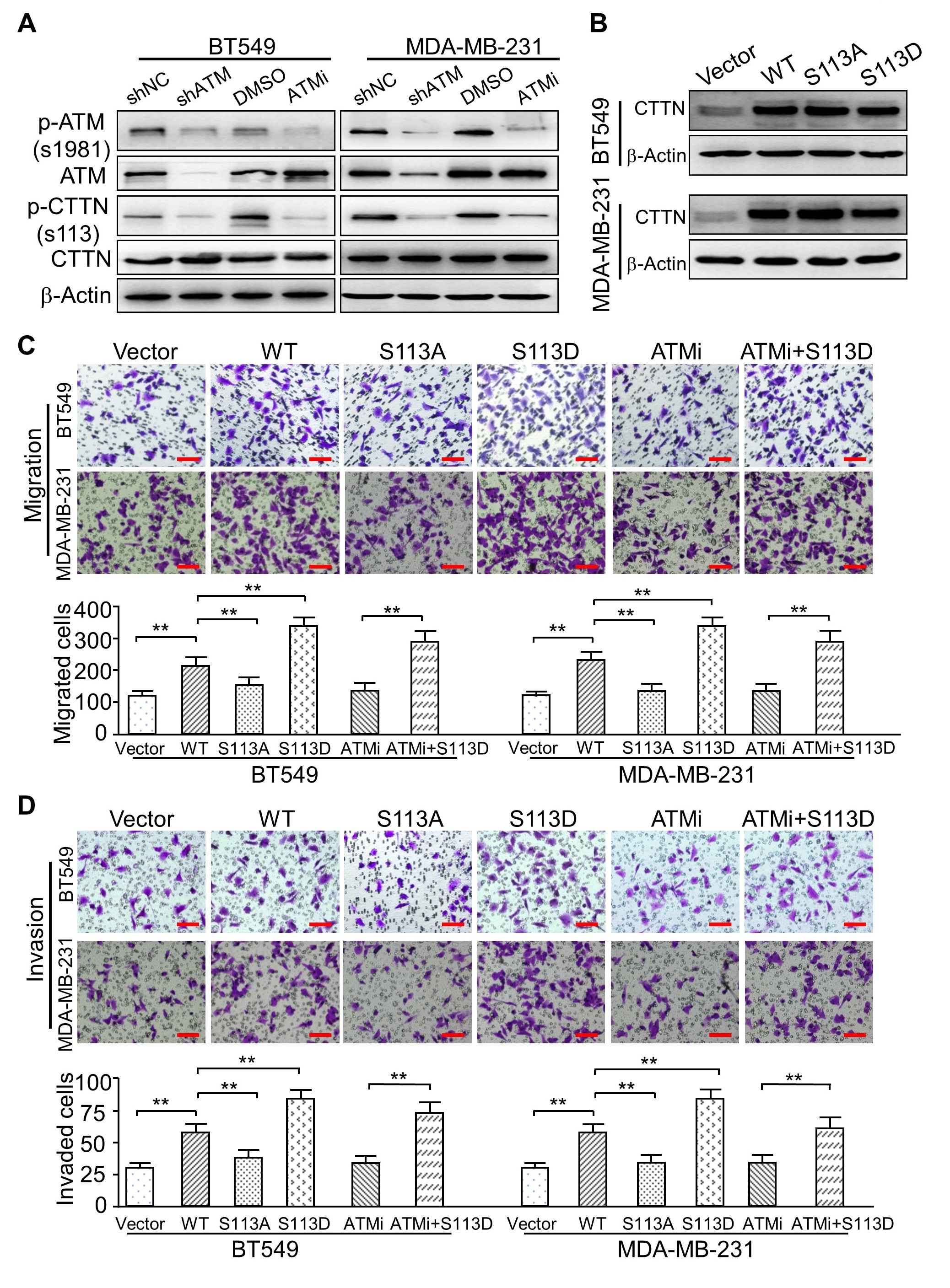
In the article “ATM-Mediated Phosphorylation of Cortactin Involved in Actin Polymerization
Promotes Breast Cancer Cells Migration and Invasion” [Cell Physiol Biochem 2018;51:2972-
2988. DOI: 10.1159/000496048] by Lang et al., the incorrect images were included in Figure 2,
Figure 6 and Figure 7 due to an error in Figure preparation.
The corrected representative images are:
1. Figure 2C 21% O2/ATMi;
2. Figure 6C BT549/WT and BT549/S113A;
3. Figure 6D MDA-MB-231/ATMi+S113D and BT549/S113A;
4. Figure 7A BT549/Extract CTTN;
5. Figure 7B MDA-MB-231/Extract CTTN and Arp3.
The corrected Figure 2, Figure 6 and Figure 7 are shown below.