Corresponding Author: Mandana AmeliMojarad
Department of Biotechnology, Tehran University of Medical Science, Tehran (Iran)
E-Mail Mandanalee13@gmail.com
Simvastatin Therapy Increased miR-150-5p Expression in the Patients with Type 2 Diabetes and COVID-19
Mandana AmeliMojarad Melika AmeliMojarad Alireza Pourmadian
Department of Biotechnology, Tehran University of Medical Science, Tehran, Iran
Introduction
The corona virus disease 2019 (COVID-19) pandemic was caused by severe acute respiratory syndrome coronavirus2 (SARS-CoV-2) infections which have globally spread since 2019 and have been considered the greatest major threat worldwide [1]. The mortality rate of COVID-19 is higher, particularly in the elderly population and patients with other malignancy such as hypertension, diabetes mellitus, and cardiovascular disease, among them, Type 2 diabetes mellitus (T2DM) have a higher risk of development in COVID-19 patient increasing the risk of severe outcome and mortality [2, 3]. T2DM is one of the common chronic conditions and a public health problem, with increasing prevalence over the past decades [4]. Although the exact mechanism of Insulin resistance (IR) in T2DM is not clearly understood, dyslipidemia is closely considered to relate to IR in diabetes. In addition, serum lipid profiles were found to deregulated in diabetics more than in non-diabetic individuals from different ethnic groups [4]. mentioning the association between hyperlipidemia and increased risk of developing abnormal glucose metabolism in patients [5, 6]. Statins are lipid-lowering drugs, that reduce circulating lipids, and most specifically cholesterol packaged in low-density lipoprotein (LDL) particles while increasing the HDL cholesterol levels [6, 7]. Statins are also a candidate for treatment of cancer with their inhibitory effects on pro-inflammatory cytokines [8, 9]. Moreover, statins disturb viral binding by decreasing cholesterol as an essential component of lipid rafts in cellular plasma membranes and disrupting virus entry. There is a previous study that statins therapy reduced mortality in influenza viral infections [10]. It has also been proved that statins can modulate expressions of microRNAs previously [11]. For instance, statins could inhibit miR-133a expression which was robustly induced by cytokines/oxidants, and prevent endothelial dysfunction [12]. MicroRNAs (miRNAs) which are classified as short non-coding RNAs and they can regulate gene expression, metabolism, inflammation, and inhibit the viral translation with the ability to suppress the expression of targeted genes through post-transcriptional mechanisms [13–15]. Therefore, determining miRNAs as crucial factor in a diverse biological processes that are modified in COVID-19 patients significantly and targeting them might be an effective and highly promising tool for patients who are susceptible for T2DM and COVID-19 [16]. miR-150 is a regulator that suppresses inflammation and is considered as a novel biomarker based on its high expression in different main tissues, such as liver, adipose tissue and skeletal muscle [14, 17, 18]. More interestingly, the higher risk of T2DM was associated with lower levels of miR-150 and its down regulated observed in cases with obesity plus T1D, or T2D [18, 19]. Therefore, in this study, we study the statins effects on circulating levels of miR-150 in patients with T2DM and COVID-19 and evaluating its status as a biomarker to estimate the risk of developing the diseases.
Materials and Methods
Patient recruitment
In the present observational, pilot study, a group of 30 plasma samples of subjects with positive COVID-19 and T2DM compared with patients only with COVID-19 before and after treatment with 10 mg/day of statins (simvastatin) for 2 months. Between (March 1st and Feb 30th, 2021) were compared with COVID-19 patients who did not exhibit T2DM or other respiratory problems was obtained. We age-matched the groups by choosing ages between 30–66 years of age. The criteria for inclusion were PCR confirmation of SARS-CoV-2 and less than 48 hours hospitalization of each case. For exclusion were: suspected to any other infections besides COVID-19; patients with neoplastic or autoimmune diseases. Written consent was received from all patients. Our study was based on Helsinki Declaration and the ethical committee of Tehran University and the registry code (IR.IUMS.REC.1399.9223497212) The patient details are provided in Table 1.
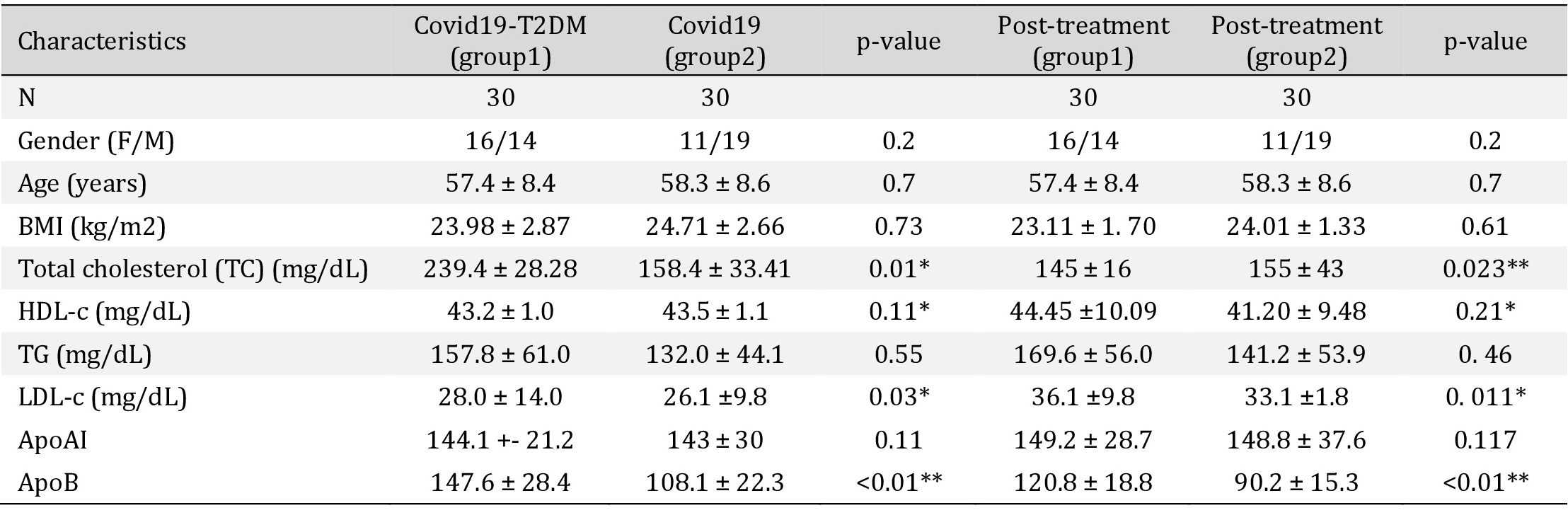
Lipid profile before and after a 2-month treatment with simvastatin. TC: total cholesterol. LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; VLDL-C: very low-density lipoprotein cholesterol; TG: Triglycerides; ApoAI: apolipoprotein AI; ApoB: apolipoprotein B (*p < 0.05, **p < 0.01, ***p < 0. 001)
Measurements of Biochemical parameters
Venous blood was collected in EDTA tubes after a 12-h overnight fast. Plasma were separated immediately afterward in a refrigerated centrifuge to avoid any loss of analytics. Modular analyzer DDPPII Hitachi (Roche, Switzerland) was used for lipid variables and colorimetric enzymatic methods for assessing total cholesterol (TC), high-density lipoprotein (HDL-C), and low-density lipoprotein (LDL-C) and triglyceride (TG) and nephelometry was used for measuring Apolipoproteins AI (apoAI) and B (apo B).
Extraction of RNA and cDNA synthesis
Anticoagulant EDTA tubes were used for blood samples collection and peripheral blood mononuclear cells (PBMCs) were obtained with centrifugation.RNA was extracted using RNX™-plus reagent (SinaClon, Iran). The cDNA was synthesized by the advanced miRNA cDNA Synthesis Kit (TaqMan). RT-qPCR with the SYBR green was used to evaluate the expression levels of selected miRNA, the reaction was set as 5 min at 95 °C, 10 s at 95 °C for 45 cycles, and 5 min at 95 °C, with specific forward primer and the universal adaptor reverse primer. The relative expression was calculated with 2-∆∆Ct method, and U6 was used as internal controls, respectively [20].
Target Prediction of miR-150-5p and Functional Enrichment Analysis
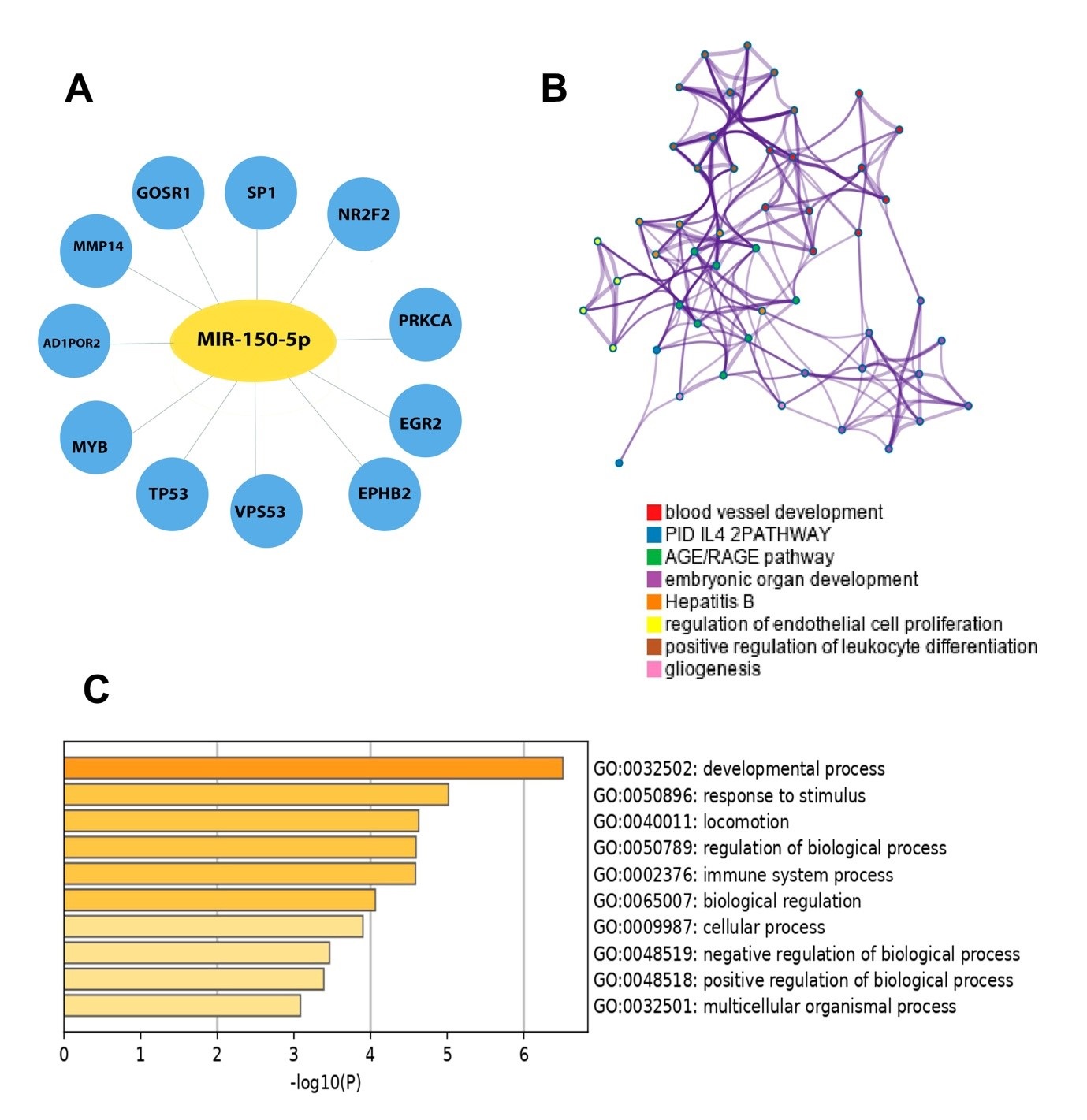
We analyzed the potential miR-150-5p target genes with MiRWalk 3.0 (http://mirwalk.umm.uni-heidelberg.de) and evaluated the other overlapping genes with three different highly recognizable miRNA-target prediction tools miRDB: (http://mirdb.org), and Targetscan7.2: http://www.targetscan.org/vert_72/) and Miranda (http://www.microrna.org) for accurate results. 11 selected overlapping genes were obtained. Then we used Metascape (metascape.org/gp/index.html) for further GO annotation analysis. P < 0.05 was considered as significant. Data was analysied with Graph Pad Prism 6 (Graph Pad Software, USA) and are shown as mean ± standard deviation (SD). To examine the normal distribution of the variables Kolmogorov–Smirnov test was used and Mann–Whitney U test. Student’s t-test was used to evaluate the differences between the two groups. P < 0.05 was considered as statistically significant.
Results
Effects of Statins supplementation on lipid levels and miR-150 expression
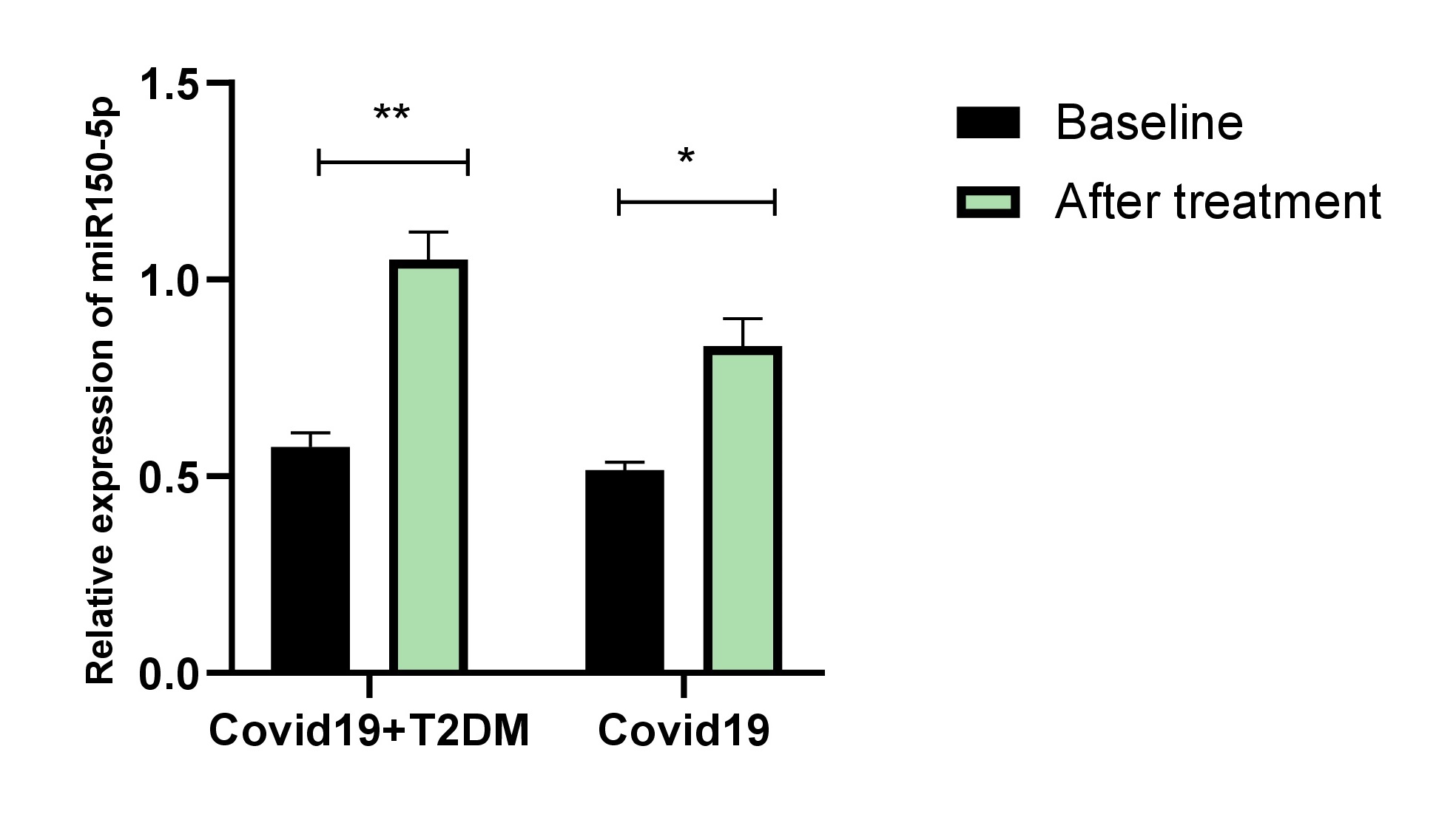
Lipid levels before and after simvastatin treatments are presented as mean ± SD in Table 1. Treatments significantly improved the lipid profile in both groups. Simvastatin treatments significantly improved the LDL-C and Apo B. There were no significant differences in weight, BMI TG, total cholesterol, HDL-C, or ApoA1. Next to verifying the statin effects on miR-150-5p, miR-150 expression levels were measured before and after simvastatin treatments. our data revealed that plasma levels of miR-150-5p were lower in both groups and the expression level of miR-150-5p, was significantly increased after two months of treatment with simvastatin, especially in patients with COVID-19 and T2DM (Fig. 1).
Author Contributions
M.A and M.A contributed equally to writing and drafting. A.P worked on editing all authors and approved the final submitted version.
Statement of Ethics
This research was confirmed by the Tehran College of Therapeutic Sciences with the code of ethics IR.IUMS.REC.1399.9223497212. Written informed consent was obtained from all patients.
The authors declare that no conflict of interests exists.
| 1 Kandeel M, Ibrahim A, Fayez M, Al-Nazawi M: From SARS and MERS CoVs to SARS-CoV-2: Moving toward more biased codon usage in viral structural and nonstructural genes. J Med Virol 2020;92:660-666. https://doi.org/10.1002/jmv.25754 |
||||
| 2 Alberca GGF, Solis-Castro RL, Solis-Castro ME, Alberca RW: Coronavirus disease-2019 and the intestinal tract: An overview. World J Gastroenterol 2021;27:1255-1266. https://doi.org/10.3748/wjg.v27.i13.1255 |
||||
| 3 Fehr AR, Perlman S: Coronaviruses: An Overview of Their Replication and Pathogenesis. Methods Mol Biol 2015;1282:1-23. https://doi.org/10.1007/978-1-4939-2438-7_1 |
||||
| 4 Pitso L, Mofokeng TRP, Nel R: Dyslipidaemia pattern and prevalence among type 2 diabetes mellitus patients on lipid-lowering therapy at a tertiary hospital in central South Africa. BMC Endocr Disord 2021;21:159. https://doi.org/10.1186/s12902-021-00813-7 |
||||
| 5 Chen GY, Li L, Dai F, Li XJ, Xu XX, Fan JG: Prevalence of and risk factors for type 2 diabetes mellitus in hyperlipidemia in China. Med Sci Monit 2015;21:2476-2484. https://doi.org/10.12659/MSM.894246 |
||||
| 6 Sen S, Chakraborty R, Kalita P, Pathak MP: Diabetes mellitus and COVID-19: Understanding the association in light of current evidence. World J Clin Cases 2021;9:8327-8339. https://doi.org/10.12998/wjcc.v9.i28.8327 |
||||
| 7 Kritchevsky SB, Kritchevsky D: Serum cholesterol and cancer risk: An epidemiologic perspective. Annu Rev Nutr 1992;12:391-416. https://doi.org/10.1146/annurev.nu.12.070192.002135 |
||||
| 8 Albert MA, Danielson E, Rifai N, Ridker PM: Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. J Am Med Assoc 2001;286:64-70. https://doi.org/10.1001/jama.286.1.64 |
||||
| 9 Beckwitt CH, Brufsky A, Oltvai ZN, Wells A: Statin drugs to reduce breast cancer recurrence and mortality 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. Breast Cancer Res 2018;20:1-11. https://doi.org/10.1186/s13058-018-1066-z |
||||
| 10 Vandermeer ML, Thomas AR, Kamimoto L, Reingold A, Gershman K, Meek J, Farley MM, Ryan P, Lynfield R, Baumbach J, Schaffner W, Bennett N, Zansky S: Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: A multistate study. J Infect Dis 2012;205:13-19. https://doi.org/10.1093/infdis/jir695 |
||||
| 11 Zambrano T, Hirata RDC, Hirata MH, Cerda Á, Salazar LA: Statins differentially modulate microRNAs expression in peripheral cells of hyperlipidemic subjects: A pilot study. Eur J Pharm Sci 2018;117:55-61. https://doi.org/10.1016/j.ejps.2018.02.007 |
||||
| 12 Yang H, Zhang H, Yang Y, Wang X, Deng T, Liu R, Ning T, Bai M, Li H, Zhu K, Li J, Fan Q, Ying G, Ba Y: Hypoxia induced exosomal circRNA promotes metastasis of colorectal cancer via targeting GEF-H1/RhoA axis. Theranostics 2020;10:8211-8226. https://doi.org/10.7150/thno.44419 |
||||
| 13 Mojarad MA, Mojarad MA, Nourbakhsh M: Circulating circular RNA ADAM9 a potential biomarker for human colorectal cancer. Gene Reports 2022;101516. https://doi.org/10.1016/j.genrep.2022.101516 |
||||
| 14 Jiménez-Lucena R, Camargo A, Alcalá-Diaz JF, Romero-Baldonado C, Luque RM, van Ommen B, Delgado-Lista J, Ordovás JM, Pérez-Martínez P, Rangel-Zúniga OA, López-Miranda J: A plasma circulating miRNAs profile predicts type 2 diabetes mellitus and prediabetes: from the CORDIOPREV study. Exp Mol Med 2018;50:1-12. https://doi.org/10.1038/s12276-018-0194-y |
||||
| 15 AmeliMojarad M, Amelimojarad M: piRNAs and PIWI proteins as potential biomarkers in Breast cancer. Mol Biol Rep 2022;49:9855-9862. https://doi.org/10.1007/s11033-022-07506-x |
||||
| 16 Arghiani N, Nissan T, Matin MM: Role of microRNAs in COVID-19 with implications for therapeutics. Biomed Pharmacother 2021;144:112247. https://doi.org/10.1016/j.biopha.2021.112247 |
||||
| 17 Chen S, Zhu H, Sun J, Zhu L, Qin L, Wan J: Anti‑inflammatory effects of miR‑150 are associated with the downregulation of STAT1 in macrophages following lipopolysaccharide treatment. Exp Ther Med 2021;22:1049. https://doi.org/10.3892/etm.2021.10483 |
||||
| 18 Dong W, Zhang H, Zhao C, Luo Y, Chen Y: Silencing of miR-150-5p Ameliorates Diabetic Nephropathy by Targeting SIRT1/p53/AMPK Pathway. Front Physiol 2021;12:356. https://doi.org/10.3389/fphys.2021.624989 |
||||
| 19 Yu F, Chapman S, Pham DL, Ko ML, Zhou B, Ko GYP: Decreased miR-150 in obesity-associated type 2 diabetic mice increases intraocular inflammation and exacerbates retinal dysfunction. BMJ Open Diabetes Res Care 2020;8:e001446. https://doi.org/10.1136/bmjdrc-2020-001446 |
||||
| 20 AmeliMojarad M, AmeliMojarad M, Pourmahdian A: The inhibitory role of stigmasterol on tumor growth by inducing apoptosis in Balb/c mouse with spontaneous breast tumor (SMMT). BMC Pharmacol Toxicol 2022;23:42 https://doi.org/10.1186/s40360-022-00578-2 |
||||
| 21 Chen GY, Li L, Dai F, Li XJ, Xu XX, Fan JG: Prevalence of and Risk Factors for Type 2 Diabetes Mellitus in Hyperlipidemia in China. Med Sci Monit 2015;21:2476. https://doi.org/10.12659/MSM.894246 |
||||
| 22 Lohia P, Kapur S, Benjaram S, Cantor Z, Mahabadi N, Mir T, Badr MS: Statins and clinical outcomes in hospitalized COVID-19 patients with and without Diabetes Mellitus: a retrospective cohort study with propensity score matching. Cardiovasc Diabetol 2021;20:140. https://doi.org/10.1186/s12933-021-01336-0 |
||||
| 23 Ho P, Zheng JQ, Wu CC, Hou YC, Liu WC, Lu CL, Zheng CM, Lu KC, Chao YC: Perspective adjunctive therapies for COVID-19: Beyond antiviral therapy. Int J Med Sci 2021;18:314-324. https://doi.org/10.7150/ijms.51935 |
||||
| 24 Peymani P, Dehesh T, Aligolighasemabadi F, Sadeghdoust M, Kotfis K, Ahmadi M, Mehrbod P, Iranpour P, Dastghaib S, Nasimian A, Ravandi A, Kidane B, Ahmed N, Sharma P, Shojaei S, Lankarani KB, Medej A, Rezaei N, Madrakian T, Los MJ, et al.: Statins in patients with COVID-19: a retrospective cohort study in Iranian COVID-19 patients. Transl Med Commun 2021;6:3. https://doi.org/10.1186/s41231-021-00082-5 |
||||
| 25 Kouhpeikar H, Khosaravizade Tabasi H, Khazir Z, Naghipour A, Mohammadi Moghadam H, Forouzanfar H, Abbasidard M, Kirichenko TV, Reiner Z, Banach M, Sahebkar A: Statin Use in COVID-19 Hospitalized Patients and Outcomes: A Retrospective Study. Front Cardiovasc Med 2022;9:820260. https://doi.org/10.3389/fcvm.2022.820260 |
||||
| 26 Ubilla CG, Prado Y, Angulo J, Obreque I, Paez I, Saavedra N, Zambrano T, Salazar LA: MicroRNA-33b is a Potential Non-Invasive Biomarker for Response to Atorvastatin Treatment in Chilean Subjects With Hypercholesterolemia: A Pilot Study. Front Pharmacol 2021;12:674252. https://doi.org/10.3389/fphar.2021.674252 |
||||
| 27 AmeliMojarad M, AmeliMojarad M, Pourmahdian A: Circular RNA circ_0051620 sponges miR-338-3p and regulates ADAM17 to promote the gastric cancer progression. Pathol Res Pract 2022 May;233:153887. https://doi.org/10.1016/j.prp.2022.153887 |
||||
| 28 de Souza Nicoletti A, Visacri MB, da Ronda CRSC, do Nascimento Silva Vasconcelos PE, Quintanilha JCF, de Souza RN, de Souza Ventura D, Eguti A, de Souza Silva LG, Perroud Junio MW, Catharino RR, Reis LO, Dos Santos LA, Durán N, Fávaro WJ, Lancellotti M, da Costa JL, Moriel P, de Carvalho Pincinato E: Differentially expressed plasmatic microRNAs in Brazilian patients with Coronavirus disease 2019 (COVID-19): preliminary results. Mol Biol Rep. 2022;49:6931-6943. https://doi.org/10.1007/s11033-022-07338-9 |
||||
| 29 Ameli Mojarad M, Ameli Mojarad M, Pourmahdian A: MicroRNA-26b Reduces Cell Viability by Inhibition of Nicotinamide Phosphoribosyltransferase in Breast Cancer Cells. DNA Cell Biol 2022;41:735-741. https://doi.org/10.1089/dna.2022.0214 |
||||
| 30 Guterres A, de Azeredo Lima CH, Miranda RL, Gadelha MR: What is the potential function of microRNAs as biomarkers and therapeutic targets in COVID-19? Infect Genet Evol 2020;85:104417. https://doi.org/10.1016/j.meegid.2020.104417 |
||||
| 31 Zambrano T, Hirata RDC, Hirata MH, Cerda Á, Salazar LA: Statins differentially modulate microRNAs expression in peripheral cells of hyperlipidemic subjects: A pilot study. Eur J Pharm Sci 2018;117:55-61. https://doi.org/10.1016/j.ejps.2018.02.007 |
||||
| 32 Akula SM, Bolin P, Cook PP: Cellular miR-150-5p may have a crucial role to play in the biology of SARS-CoV-2 infection by regulating nsp10 gene. RNA Biol 2022;19:1-11. https://doi.org/10.1080/15476286.2021.2010959 |
||||
| 33 Moles R, Bellon M, Nicot C: STAT1: A novel target of miR-150 and miR-223 is involved in the proliferation of HTLV-I-transformed and ATL cells. Neoplasia 2015;17:449-462. https://doi.org/10.1016/j.neo.2015.04.005 |
||||
| 34 Yu F, Chapman S, Pham DL, Ko ML, Zhou B, Ko GYP: Decreased miR-150 in obesity-associated type 2 diabetic mice increases intraocular inflammation and exacerbates retinal dysfunction. BMJ Open Diabetes Res Care 2020;8:e001446. https://doi.org/10.1136/bmjdrc-2020-001446 |
||||
| 35 Mazzeo A, Lopatina T, Gai C, Trento M, Porta M, Beltramo E: Functional analysis of miR-21-3p, miR-30b-5p and miR-150-5p shuttled by extracellular vesicles from diabetic subjects reveals their association with diabetic retinopathy. Exp Eye Res 2019;184:56-63. https://doi.org/10.1016/j.exer.2019.04.015 |
||||