Corresponding Author: José Pedraza-Chaverri
Facultad de Química, Departamento de Biología, Universidad Nacional Autónoma de México, Ciudad Universitaria, Coyoacán, México City, 4510 (México)
Tel. +52-556223878, E-Mail pedraza@unam.mx
How Micronutrients Fuel Immune System at the Molecular Level: An Approach to the Immune Response Against Respiratory Viruses
Alexis Paulina Jiménez-Uribe Ariana Ocampo-Hernández Yalith Aranciba-Hernández José Pedraza-Chaverri
Facultad de Química, Departamento de Biología, Universidad Nacional Autónoma de México, México City, México
Introduction
Respiratory tract infections (RTI) can range from a self-limiting cold to severe pneumonia with sepsis development [1]. Although several etiologies exist, viruses have gained significant attention due to the current global sanitary situation caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Among causal viruses of RTI, besides SARS-CoV-2 and other coronaviruses, are the enterovirus, respiratory syncytial virus (RSV), metapneumovirus, rhinovirus, parainfluenza virus, influenza virus, and adenovirus [2].
Despite the causal virus and the pathogenic evasion mechanisms of each one, all of them evoke the activation of the innate and adaptive immune response, which in general involves the following steps:
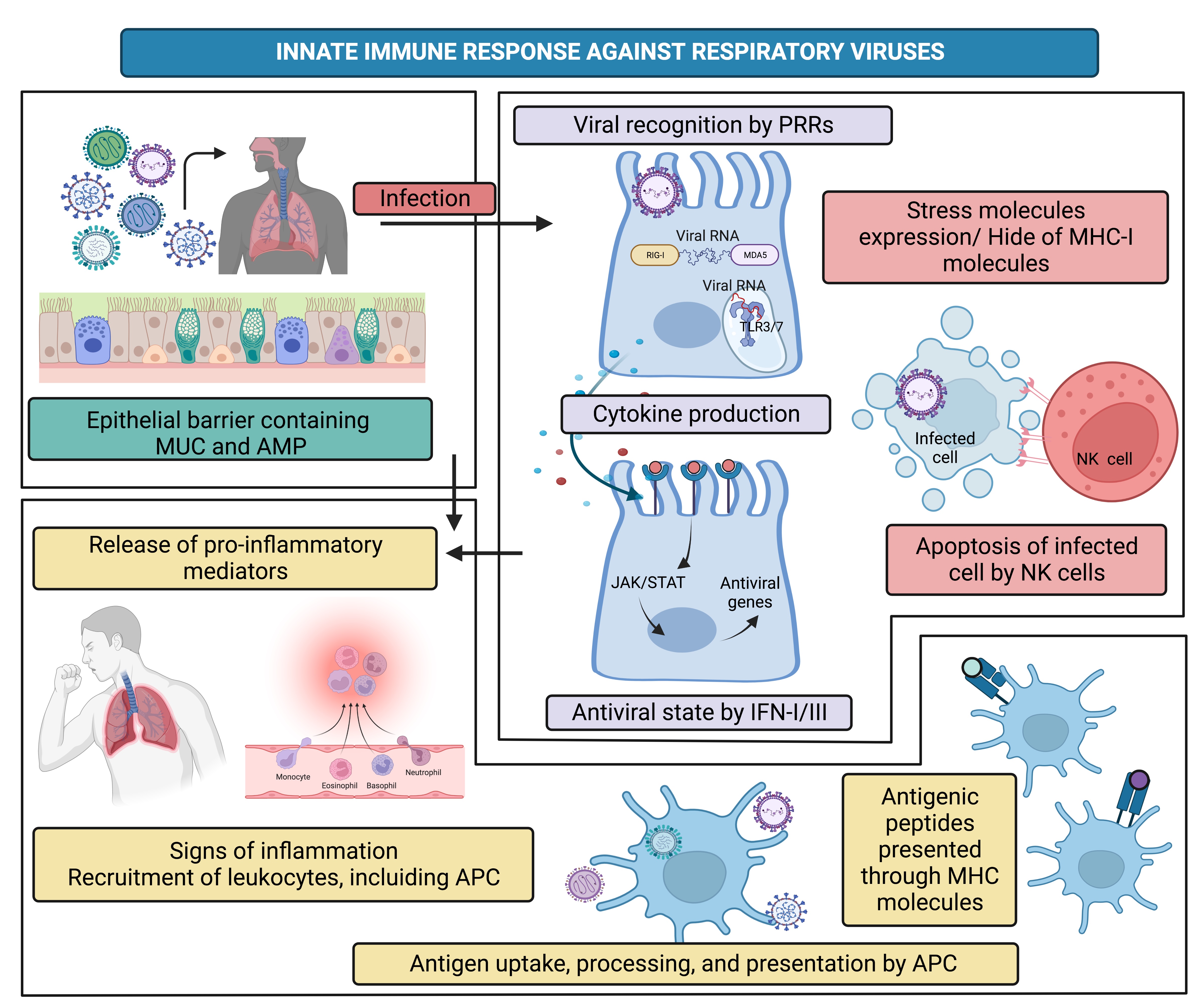
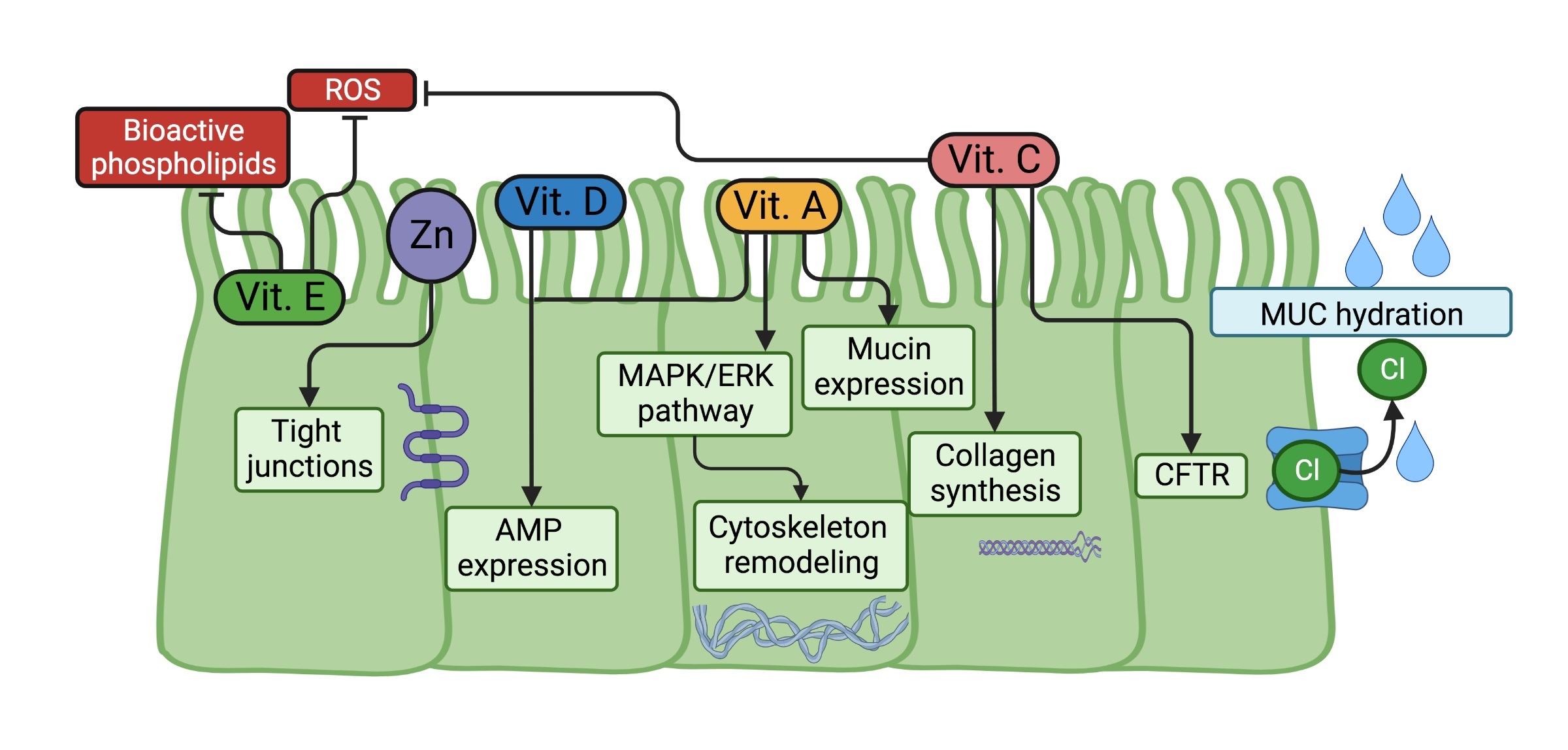
• 1.) The epithelial barrier is the first defense restricting infections; the airway epithelial barrier performs mechanical actions such as cilia movement and warming air; this also contains in the airway liquid surface (ALS) mucins (MUC) and antimicrobial peptides (AMP), molecules which help to reduce the possible infection.
• 2.) Once viruses bypass epithelial barrier actions and molecules, they infect target cells and cause cellular stress. In response, host cells will use their pattern recognition receptors (PRRs) to recognize viral pathogen-associated molecular patterns (PAMPs), triggering an antiviral alarm state in which interferons (IFN) type I and III are synthesized, natural killer (NK) cells are activated, and inflammation is generated. At this time-point viral infection might resolve, but if that is not, the antigen-presenting cells (APC) are ready to trigger the next steps.
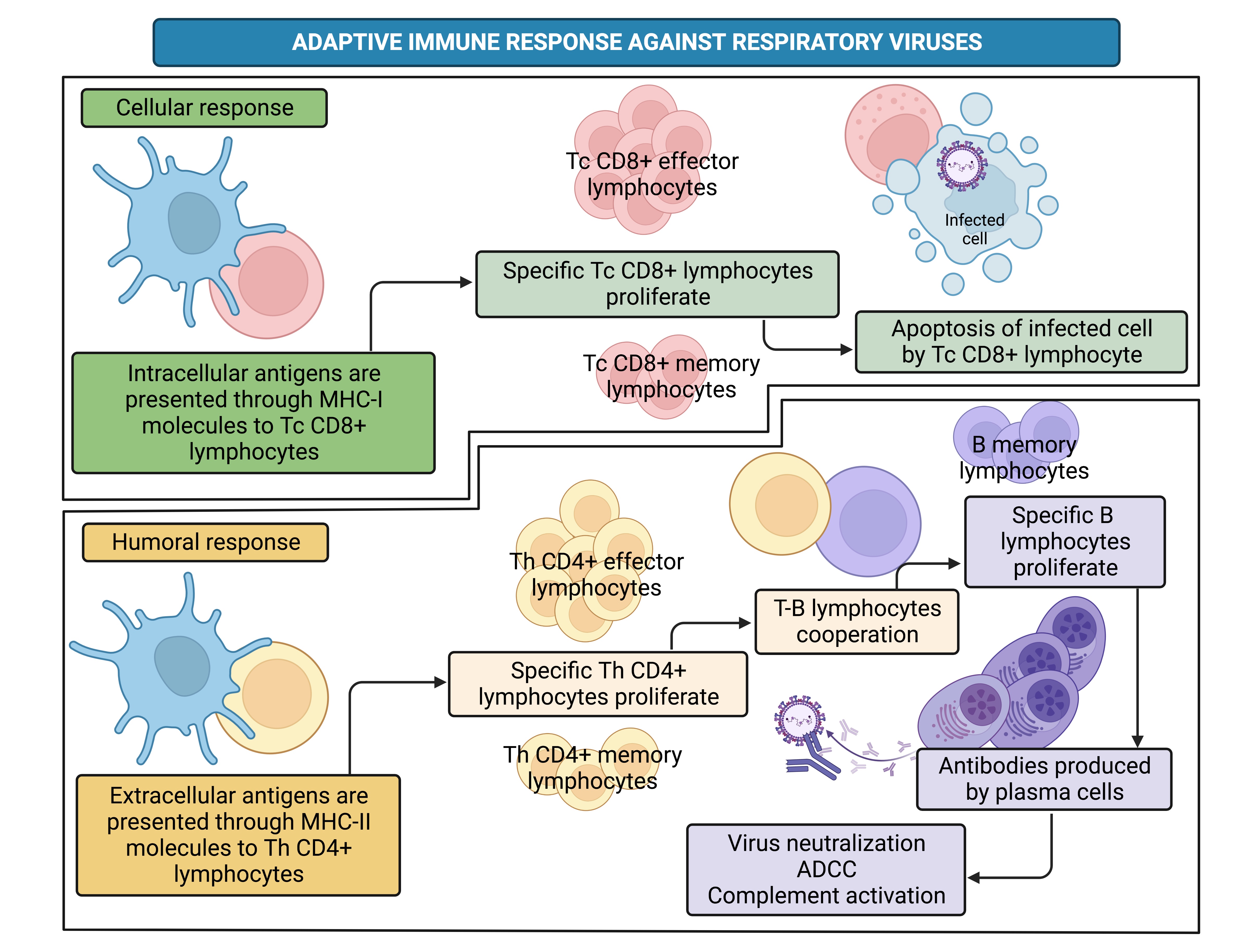
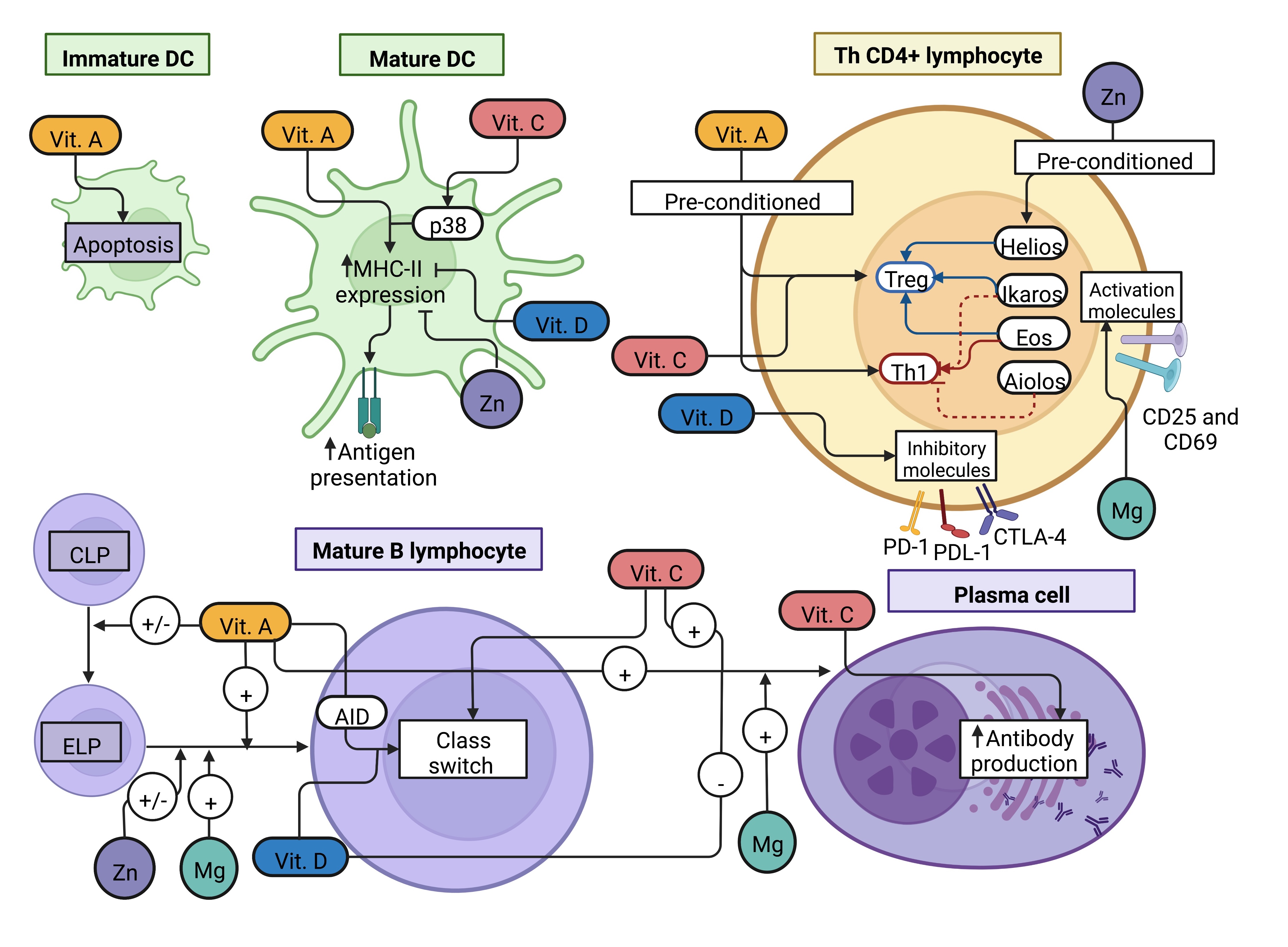
• 3.) APC such as macrophages and dendritic cells (DC) process endogenous and exogenous antigens to further present them on their major histocompatibility complex (MHC) molecules class I or II, respectively. Antigens loaded on MHC-I molecules are presented to the T cell receptor (TCR) of T cytotoxic (Tc) CD8+ lymphocytes, whereas antigens loaded on MHC-II are presented to the TCR of T helper (Th) CD4+ lymphocytes, giving rise to the adaptive immune response.
• 4.) Tc CD8+ lymphocytes are the major players in the adaptive cellular response that aims to kill infected cells. On the other hand, Th CD4+ lymphocytes could also participate in cellular response; however, they mainly cooperate with B lymphocytes to elicit the adaptive humoral response characterized by antibody production [3].
The expected result of the antiviral immune response is the control and elimination of the pathogen (Fig. 1 and 2); however, some factors could affect the infection resolution, such as evasion mechanisms of the viruses, stress, environmental pollution, hormonal status, comorbidities, and nutrition [4-10].
In this regard, SARS-CoV-2 infection causes a severe inflammatory response in patients with comorbidities such as diabetes, hypertension, and obesity [11], pathologies tightly related to metabolic and nutritional alterations.
Indeed, micronutrient imbalance is associated with the risk of complicated respiratory tract infections, as has been reported for vitamins A, D, E, and C and the trace elements zinc and magnesium [12-20]; therefore, their supplementation improves the effector function of the immune system, as has been excellently reviewed elsewhere [21, 22]. Here we will focus on how those micronutrients mentioned above, in which deficiency or supplementation impacts immune function, participate at the molecular level in each step of the immune response against respiratory viruses. Considering their three main action mechanisms as antioxidants, gene-expression regulators, and structural components of proteins, we will revise their functions as immuno-stimulators, immuno-regulators, or even both.
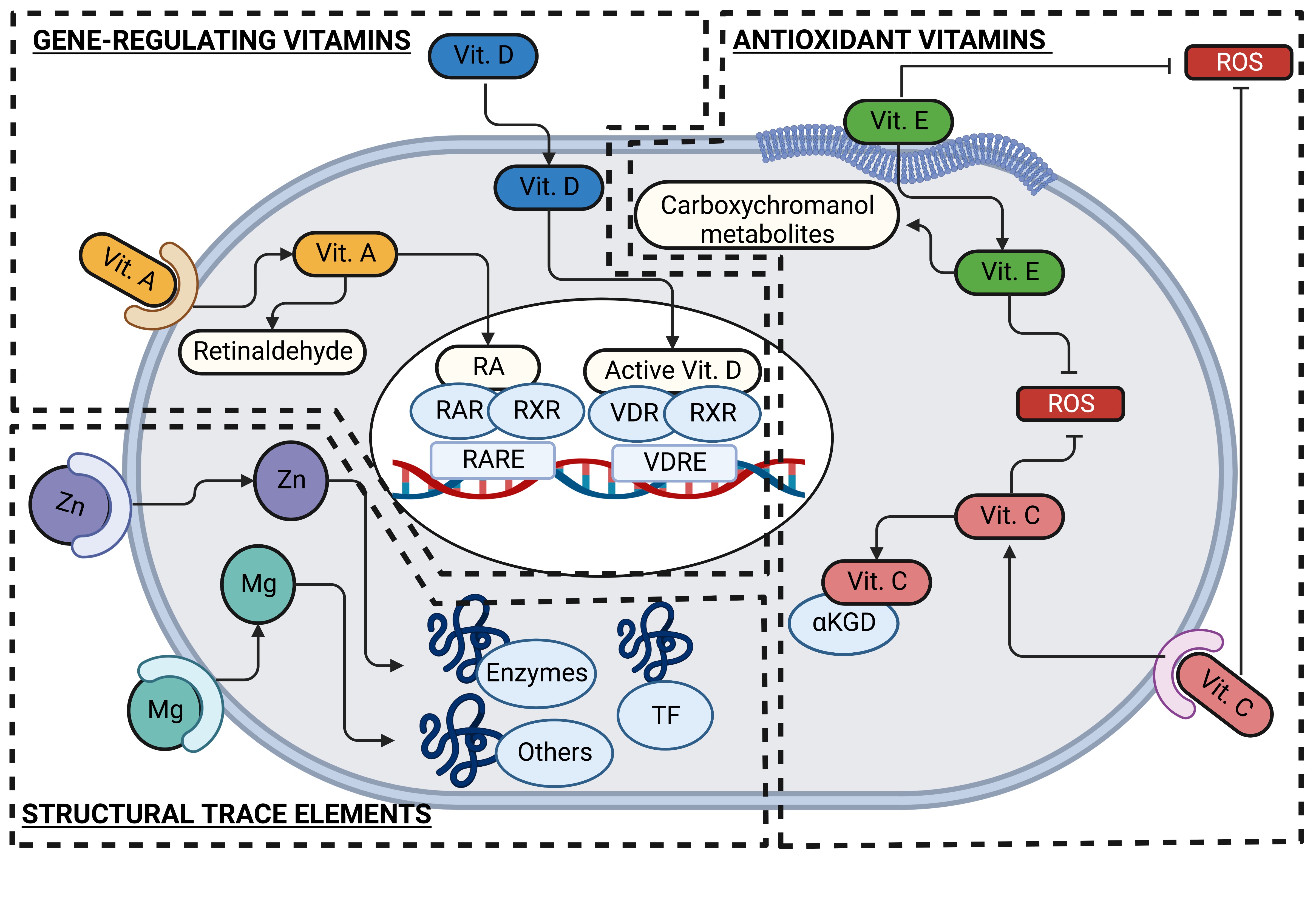
• 1.) Antioxidant vitamins: Oxidative stress is a typical process occurring during respiratory viral infections that requires to be tightly regulated to avoid its contribution to the pathology progression [23]. The two vitamins, well known for their antioxidant properties, vitamins C and E, are also involved in each step of the immune response through their antioxidant and other mechanisms.
Vitamin C, also known as ascorbate or its oxidized form dehydroascorbate (DHA), is taken up by cells via sodium-dependent vitamin C transporters (SVCT) and glucose transporters (GLUT) [24]; in immune cells, SVCT2 and GLUT3 seem to be especially relevant for vitamin C uptake, and the contribution of each one depends on the lineage and differentiation status [25-27].
The antioxidant function of vitamin C relies on its electron donor capacity but also functions as a metabolic and epigenetic modulator through the alpha-ketoglutarate-dependent dioxygenases (αKGD), enzymes that use this vitamin as a cofactor [28-30].
On the other hand, vitamin E embraces eight lipophilic molecules sharing a chromanol ring structure, four tocopherols isomers (α, β, γ, δ) and four tocotrienols isomers (α, β, γ, δ); these can be distinguished among them by the number of bonds of the side chain and by the methyl groups in the chromanol ring structure. Due to its chemical nature, vitamin E could easily conjugate with lipophilic compounds such as bile acids, cholesterol, and other lipids and is taken up by cells mainly through the scavenger receptor B type I (SR-BI) but also by the cluster of differentiation (CD)36 [31-33]. Interestingly, CD36 is a molecule highly expressed on phagocytic cells such as neutrophils, monocytes, and macrophages; and its expression is affected by respiratory viruses, as has been reported for influenza virus and RSV infections in vitro [34, 35]. Once inside cells, vitamin E is transformed into carboxychromanol (COOH) metabolites through different steps of oxidation and shortening the side chain length [31, 32].
Besides its chromanol ring structure-dependent antioxidant function, other functions of this vitamin are to regulate plasma membrane curvature under stress conditions, modulate the inflammatory process, and even suggest controlling gene expression through the pregnane X receptor (PXR) [36-40].
• 2.) Gene-regulating vitamins: The liposoluble vitamins A and D can bind to their receptors to activate their transcriptional factor function and promote the expression of several genes, including some involved in the innate and adaptive immune responses.
Vitamin A, also referred to as retinol, requires to be transported by the retinol-binding protein (RBP) to enter cells by passive diffusion or through the receptors SR-BI, adenosine triphosphate (ATP)-binding cassette transporter (ABCA4), and stimulated by retinoic acid gene 6 (STRA6) [41, 42]. Inside cells, retinol is metabolized to retinaldehyde and retinoic acid (RA) through retinol dehydrogenase and retinaldehyde dehydrogenase, respectively. While retinaldehyde is involved in the visual cycle, RA regulates the expression of several genes through its recognition by the RA receptor (RAR). RAR, together with the retinoid X receptor (RXR), functions as a transcriptional factor when bound to hundreds of genes that contain RA response elements (RARE) [41]; among these are immune response genes, such as the RA inducible gene-I (RIG-I), a PRR that recognizes viral RNA; and the 2’-5’-oligoadenylate synthase 1 (OAS1), a protein involved in the viral RNA degradation [43, 44].
Vitamin D can be acquired from dietary sources as vitamin D2 (ergocalciferol) from plants or as vitamin D3 (cholecalciferol) from animals; however, its primary source is the 7-dehydrocholesterol in the skin, which is converted to vitamin D3 by the action of ultraviolet light. Vitamin D is transported by the vitamin D binding protein (DBP) to the liver, where hepatocytes convert it to 25-dihydroxy vitamin D3 (25(OH)D3 to then reach the kidneys, where tubular cells transform it into the active form of vitamin D, the 1,25-dihydroxy vitamin D3 (1,25(OH)2D3). Interestingly, some immune cells, such as macrophages, dendritic cells, and T cells, can also produce the active form of vitamin D [45-47].
The active form of vitamin D acquired or synthesized by cells is recognized by the vitamin D receptor (VDR), which also interacts with RXR to function as a transcriptional factor that binds several genes containing vitamin D response elements (VDRE) to regulate their expression [48]. The most know function of vitamin D is the induction of the expression of the transient potential vanilloid type 6 (TRPV6) required for the promotion of calcium absorption; however, it also promotes the expression of several genes associated with immunoregulatory functions [49].
• 3.) Structural trace elements: Zinc and magnesium are two metals that stabilize the structure of hundreds of biomolecules, mainly proteins, and therefore allow them to function.
Zinc is one of the most relevant metals in the organism; estimating that it interacts with near of 10% of the human proteome, mainly enzymes and transcriptional factors [50, 51]. Zinc is taken up by cells through the Zrt/Irt-like proteins (ZIP), intracellularly this mineral is found inside organelles and vesicles or bound to proteins named metallothioneins (MT); and its concentration is regulated by the ZIP-dependent uptake, as well by its release through the zinc transporters (ZnT) [52]. A relevant finding from nearly two decades ago is that zinc deficiency affects immune system development, causing thymic atrophy in rodents [53, 54] since the zinc-dependent hormone thymulin produced by thymic epithelial cells is necessary for proper T lymphocyte development [55, 56].
On the other hand, magnesium is well known for its participation in stabilizing DNA, its requirement for DNA polymerase reactions, and for being bound to ATP, facilitating the phosphate group transference; however, as occurs with zinc, it participates in several cellular processes due to its interaction with hundreds of proteins. Different transporters take up this metal in immune cells, such as the transient receptor potential cation channel subfamily M
(TRPM)6 and 7, the solute carrier family 41 members 1 and 2 (SLC41A1/A2), and the magnesium transporter 1 (MAGT1) [57, 58]. Among its different functions, the involvement of magnesium in immune response was initially discovered because a defect in its transport due to a mutation of the MAGT1 gene in humans causes a combined immunodeficiency mainly affecting T lymphocytes response [59].
Author Contributions
A.P.J.U., conceptualization, literature searching, manuscript preparation; A.O.H., literature searching; Y.A.H, literature searching; J.P.C., conceptualization, manuscript revision, funding.
Funding
This research was funded by Consejo Nacional de Ciencia y Tecnología (CONACYT) México, Grants Numbers A1-S-7495; by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT), Grant Number IN200922 of the Universidad Nacional Autónoma de México (UNAM); by Programa de Apoyo a la Investigación y el Posgrado (PAIP), Grant Number 5000-9105.
The authors declare that no conflict of interests exists.
| 1 Gu X, Zhou F, Wang Y, Fan G, Cao B: Respiratory viral sepsis: epidemiology, pathophysiology, diagnosis and treatment. Eur Respir Rev 2020;29:200038. https://doi.org/10.1183/16000617.0038-2020 |
||||
| 2 Moriyama M, Hugentobler WJ, Iwasaki A: Seasonality of Respiratory Viral Infections. Annu Rev Virol 2020;7:83-101. https://doi.org/10.1146/annurev-virology-012420-022445 |
||||
| 3 Mettelman RC, Allen EK, Thomas PG: Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity 2022;55:749-780. https://doi.org/10.1016/j.immuni.2022.04.013 |
||||
| 4 Kikkert M: Innate Immune Evasion by Human Respiratory RNA Viruses. J Innate Immun 2020;12:4-20. https://doi.org/10.1159/000503030 |
||||
| 5 Dhabhar FS: Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res 2014;58:193-210. https://doi.org/10.1007/s12026-014-8517-0 |
||||
| 6 Glencross DA, Ho TR, Camina N, Hawrylowicz CM, Pfeffer PE: Air pollution and its effects on the immune system. Free Radic Biol Med 2020;151:56-68. https://doi.org/10.1016/j.freeradbiomed.2020.01.179 |
||||
| 7 Klein SL, Flanagan KL: Sex differences in immune responses. Nat Rev Immunol 2016;16:626-638. https://doi.org/10.1038/nri.2016.90 |
||||
| 8 Kreutmair S, Kauffmann M, Unger S, Ingelfinger F, Nunez NG, Alberti C, De Feo D, Krishnarajah S, Friebel E, Ulutekin C, Babaei S, Gaborit B, Lutz M, Jurado NP, Malek NP, Gopel S, Rosenberger P, Haberle HA, Ayoub I, Al-Hajj S, et al.: Preexisting comorbidities shape the immune response associated with severe COVID-19. J Allergy Clin Immunol 2022;150:312-324. https://doi.org/10.1016/j.jaci.2022.05.019 |
||||
| 9 Nobs SP, Zmora N, Elinav E: Nutrition Regulates Innate Immunity in Health and Disease. Annu Rev Nutr 2020;40:189-219. https://doi.org/10.1146/annurev-nutr-120919-094440 |
||||
| 10 Calder PC: Feeding the immune system. Proc Nutr Soc 2013;72:299-309. https://doi.org/10.1017/S0029665113001286 |
||||
| 11 Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, Hosein Z, Padda I, Mangat J, Altaf M: Comorbidity and its Impact on Patients with COVID-19. SN Compr Clin Med 2020:2:1069-1076. https://doi.org/10.1007/s42399-020-00363-4 |
||||
| 12 Wang X, Li X, Jin C, Bai X, Qi X, Wang J, Zhang L, Li N, Jin N, Song W, Gao H, Gao B, Zhang Y, Wang L: Association Between Serum Vitamin A Levels and Recurrent Respiratory Tract Infections in Children. Front Pediatr 2021;9:756217. https://doi.org/10.3389/fped.2021.756217 |
||||
| 13 Zhang X, Ding F, Li H, Zhao W, Jing H, Yan Y, Chen Y: Low Serum Levels of Vitamins A, D, and E Are Associated with Recurrent Respiratory Tract Infections in Children Living in Northern China: A Case Control Study. PLoS One 2016;11:e0167689. https://doi.org/10.1371/journal.pone.0167689 |
||||
| 14 Qi YJ, Niu QL, Zhu XL, Zhao XZ, Yang WW, Wang XJ: Relationship between deficiencies in vitamin A and E and occurrence of infectious diseases among children. Eur Rev Med Pharmacol Sci 2016;20:5009-5012. | ||||
| 15 McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM: Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol 2009;44:981-988. https://doi.org/10.1002/ppul.21089 |
||||
| 16 Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A: Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr 2009;63:473-477. https://doi.org/10.1038/sj.ejcn.1602960 |
||||
| 17 Kuwabara A, Tsugawa N, Ao M, Ohta J, Tanaka K: Vitamin D deficiency as the risk of respiratory tract infections in the institutionalized elderly: A prospective 1-year cohort study. Clin Nutr ESPEN 2020;40:309-313. https://doi.org/10.1016/j.clnesp.2020.08.012 |
||||
| 18 Myint PK, Wilson AM, Clark AB, Luben RN, Wareham NJ, Khaw KT: Plasma vitamin C concentrations and risk of incident respiratory diseases and mortality in the European Prospective Investigation into Cancer-Norfolk population-based cohort study. Eur J Clin Nutr 2019;73:1492-1500. https://doi.org/10.1038/s41430-019-0393-1 |
||||
| 19 Khera D, Singh S, Purohit P, Sharma P, Singh K: Prevalence of Zinc Deficiency and the Effect of Zinc Supplementation on the Prevention of Acute Respiratory Infections. Turk Thorac J 2020;21:371-376. https://doi.org/10.5152/TurkThoracJ.2019.19020 |
||||
| 20 Nasser R, Naffaa ME, Mashiach T, Azzam ZS, Braun E: The association between serum magnesium levels and community-acquired pneumonia 30-day mortality. BMC Infect Dis 2018;18:698. https://doi.org/10.1186/s12879-018-3627-2 |
||||
| 21 Pecora F, Persico F, Argentiero A, Neglia C, Esposito S: The Role of Micronutrients in Support of the Immune Response against Viral Infections. Nutrients 2020;12:3198. https://doi.org/10.3390/nu12103198 |
||||
| 22 Junaid K, Ejaz H, Abdalla AE, Abosalif KOA, Ullah MI, Yasmeen H, Younas S, Hamam SSM, Rehman A: Effective Immune Functions of Micronutrients against SARS-CoV-2. Nutrients 2020;12:2992. https://doi.org/10.3390/nu12102992 |
||||
| 23 Fernandes IG, de Brito CA, Dos Reis VMS, Sato MN, Pereira NZ: SARS-CoV-2 and Other Respiratory Viruses: What Does Oxidative Stress Have to Do with It? Oxid Med Cell Longev 2020;2020:8844280. https://doi.org/10.1155/2020/8844280 |
||||
| 24 Li Y, Schellhorn HE: New developments and novel therapeutic perspectives for vitamin C. J Nutr 2007;137:2171-2184. https://doi.org/10.1093/jn/137.10.2171 |
||||
| 25 Liu J, Hong J, Han H, Park J, Kim D, Park H, Ko M, Koh Y, Shin DY, Yoon SS: Decreased vitamin C uptake mediated by SLC2A3 promotes leukaemia progression and impedes TET2 restoration. Br J Cancer 2020;122:1445-1452. https://doi.org/10.1038/s41416-020-0788-8 |
||||
| 26 Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, Crane GM, Cowin BL, Bruner E, Murphy MM, Chen W, Spangrude GJ, Hu Z, DeBerardinis RJ, Morrison SJ: Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 2017;549:476-481. https://doi.org/10.1038/nature23876 |
||||
| 27 Hong JM, Kim JH, Kang JS, Lee WJ, Hwang YI: Vitamin C is taken up by human T cells via sodium-dependent vitamin C transporter 2 (SVCT2) and exerts inhibitory effects on the activation of these cells in vitro . Anat Cell Biol 2016;49:88-98. https://doi.org/10.5115/acb.2016.49.2.88 |
||||
| 28 Padayatty SJ, Levine M: Vitamin C: the known and the unknown and Goldilocks. Oral Dis 2016;22:463-493. https://doi.org/10.1111/odi.12446 |
||||
| 29 Levine M, Padayatty SJ, Espey MG: Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr 2011;2:78-88. https://doi.org/10.3945/an.110.000109 |
||||
| 30 Monfort A, Wutz A: Breathing-in epigenetic change with vitamin C. EMBO Rep 2013;14:337-346. https://doi.org/10.1038/embor.2013.29 |
||||
| 31 Schmolz L, Birringer M, Lorkowski S, Wallert M: Complexity of vitamin E metabolism. World J Biol Chem 2016;7:14-43. | ||||
| 32 Schubert M, Kluge S, Schmolz L, Wallert M, Galli F, Birringer M, Lorkowski S: Long-Chain Metabolites of Vitamin E: Metabolic Activation as a General Concept for Lipid-Soluble Vitamins? Antioxidants 2018;7:10. https://doi.org/10.3390/antiox7010010 |
||||
| 33 Goncalves A, Roi S, Nowicki M, Niot I, Reboul E: Cluster-determinant 36 (CD36) impacts on vitamin E postprandial response. Mol Nutr Food Res 2014;58:2297-2306. https://doi.org/10.1002/mnfr.201400339 |
||||
| 34 Cooper GE, Pounce ZC, Wallington JC, Bastidas-Legarda LY, Nicholas B, Chidomere C, Robinson EC, Martin K, Tocheva AS, Christodoulides M, Djukanovic R, Wilkinson TM, Staples KJ: Viral Inhibition of Bacterial Phagocytosis by Human Macrophages: Redundant Role of CD36. PLoS One 2016;11:e0163889. https://doi.org/10.1371/journal.pone.0163889 |
||||
| 35 Wang J, Nikrad MP, Travanty EA, Zhou B, Phang T, Gao B, Alford T, Ito Y, Nahreini P, Hartshorn K, Wentworth D, Dinarello CA, Mason RJ: Innate immune response of human alveolar macrophages during influenza A infection. PLoS One 2012;7:e29879. https://doi.org/10.1371/journal.pone.0029879 |
||||
| 36 Bradford A, Atkinson J, Fuller N, Rand RP: The effect of vitamin E on the structure of membrane lipid assemblies. J Lipid Res 2003;44:1940-1945. https://doi.org/10.1194/jlr.M300146-JLR200 |
||||
| 37 Jiang Z, Yin X, Jiang Q: Natural forms of vitamin E and 13'-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J Immunol 2011;186:1173-1179. https://doi.org/10.4049/jimmunol.1002342 |
||||
| 38 Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J: Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc Natl Acad Sci U S A 2008;105:20464-20469. https://doi.org/10.1073/pnas.0810962106 |
||||
| 39 Quinn PJ: Molecular associations of vitamin E. Vitam Horm 2007;76:67-98. https://doi.org/10.1016/S0083-6729(07)76004-1 |
||||
| 40 Landes N, Pfluger P, Kluth D, Birringer M, Ruhl R, Bol GF, Glatt H, Brigelius-Flohe R: Vitamin E activates gene expression via the pregnane X receptor. Biochem Pharmacol 2003;65:269-273. https://doi.org/10.1016/S0006-2952(02)01520-4 |
||||
| 41 Ghyselinck NB, Duester G: Retinoic acid signaling pathways. Development 2019;146:dev167502. https://doi.org/10.1242/dev.167502 |
||||
| 42 Kelly M, von Lintig J: STRA6: role in cellular retinol uptake and efflux. Hepatobiliary Surg Nutr 2015;4:229-242. | ||||
| 43 Lee KH, Chang MY, Ahn JI, Yu DH, Jung SS, Choi JH, Noh YH, Lee YS, Ahn MJ: Differential gene expression in retinoic acid-induced differentiation of acute promyelocytic leukemia cells, NB4 and HL-60 cells. Biochem Biophys Res Commun 2002;296:1125-1133. https://doi.org/10.1016/S0006-291X(02)02043-0 |
||||
| 44 Liu TX, Zhang JW, Tao J, Zhang RB, Zhang QH, Zhao CJ, Tong JH, Lanotte M, Waxman S, Chen SJ, Mao M, Hu GX, Zhu L, Chen Z: Gene expression networks underlying retinoic acid-induced differentiation of acute promyelocytic leukemia cells. Blood 2000;96:1496-1504. https://doi.org/10.1182/blood.V96.4.1496.h8001496_1496_1504 |
||||
| 45 Kundu R, Chain BM, Coussens AK, Khoo B, Noursadeghi M: Regulation of CYP27B1 and CYP24A1 hydroxylases limits cell-autonomous activation of vitamin D in dendritic cells. Eur J Immunol 2014;44:1781-1790. https://doi.org/10.1002/eji.201344157 |
||||
| 46 Lopez DV, Al-Jaberi FAH, Woetmann A, Odum N, Bonefeld CM, Kongsbak-Wismann M, Geisler C: Macrophages Control the Bioavailability of Vitamin D and Vitamin D-Regulated T Cell Responses. Front Immunol 2021;12:722806. https://doi.org/10.3389/fimmu.2021.722806 |
||||
| 47 Kongsbak M, von Essen MR, Boding L, Levring TB, Schjerling P, Lauritsen JP, Woetmann A, Odum N, Bonefeld CM, Geisler C: Vitamin D up-regulates the vitamin D receptor by protecting it from proteasomal degradation in human CD4+ T cells. PLoS One 2014;9:e96695. https://doi.org/10.1371/journal.pone.0096695 |
||||
| 48 Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G: Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev 2016;96:365-408. https://doi.org/10.1152/physrev.00014.2015 |
||||
| 49 Koivisto O, Hanel A, Carlberg C: Key Vitamin D Target Genes with Functions in the Immune System. Nutrients 2020;12:1140. https://doi.org/10.3390/nu12041140 |
||||
| 50 Ireland SM, Martin ACR: ZincBind-the database of zinc binding sites. Database (Oxford) 2019;2019:baz006. https://doi.org/10.1093/database/baz006 |
||||
| 51 Kaur K, Gupta R, Saraf SA, Saraf SK: Zinc: The Metal of Life. Compr Rev Food Sci Food Saf 2014;13:358-376. https://doi.org/10.1111/1541-4337.12067 |
||||
| 52 Maares M, Haase H: A Guide to Human Zinc Absorption: General Overview and Recent Advances of In vitro Intestinal Models. Nutrients 2020;12:762. https://doi.org/10.3390/nu12030762 |
||||
| 53 Nodera M, Yanagisawa H, Wada O: Increased apoptosis in a variety of tissues of zinc-deficient rats. Life Sci 2001;69:1639-1649. https://doi.org/10.1016/S0024-3205(01)01252-8 |
||||
| 54 King LE, Frentzel JW, Mann JJ, Fraker PJ: Chronic zinc deficiency in mice disrupted T cell lymphopoiesis and erythropoiesis while B cell lymphopoiesis and myelopoiesis were maintained. J Am Coll Nutr 2005;24:494-502. https://doi.org/10.1080/07315724.2005.10719495 |
||||
| 55 Coto JA, Hadden EM, Sauro M, Zorn N, Hadden JW: Interleukin 1 regulates secretion of zinc-thymulin by human thymic epithelial cells and its action on T-lymphocyte proliferation and nuclear protein kinase C. Proc Natl Acad Sci U S A 1992;89:7752-7756. https://doi.org/10.1073/pnas.89.16.7752 |
||||
| 56 Saha AR, Hadden EM, Hadden JW: Zinc induces thymulin secretion from human thymic epithelial cells in vitro and augments splenocyte and thymocyte responses in vivo . Int J Immunopharmacol 1995;17:729-733. https://doi.org/10.1016/0192-0561(95)00061-6 |
||||
| 57 Fiorentini D, Cappadone C, Farruggia G, Prata C: Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021;13:1136. https://doi.org/10.3390/nu13041136 |
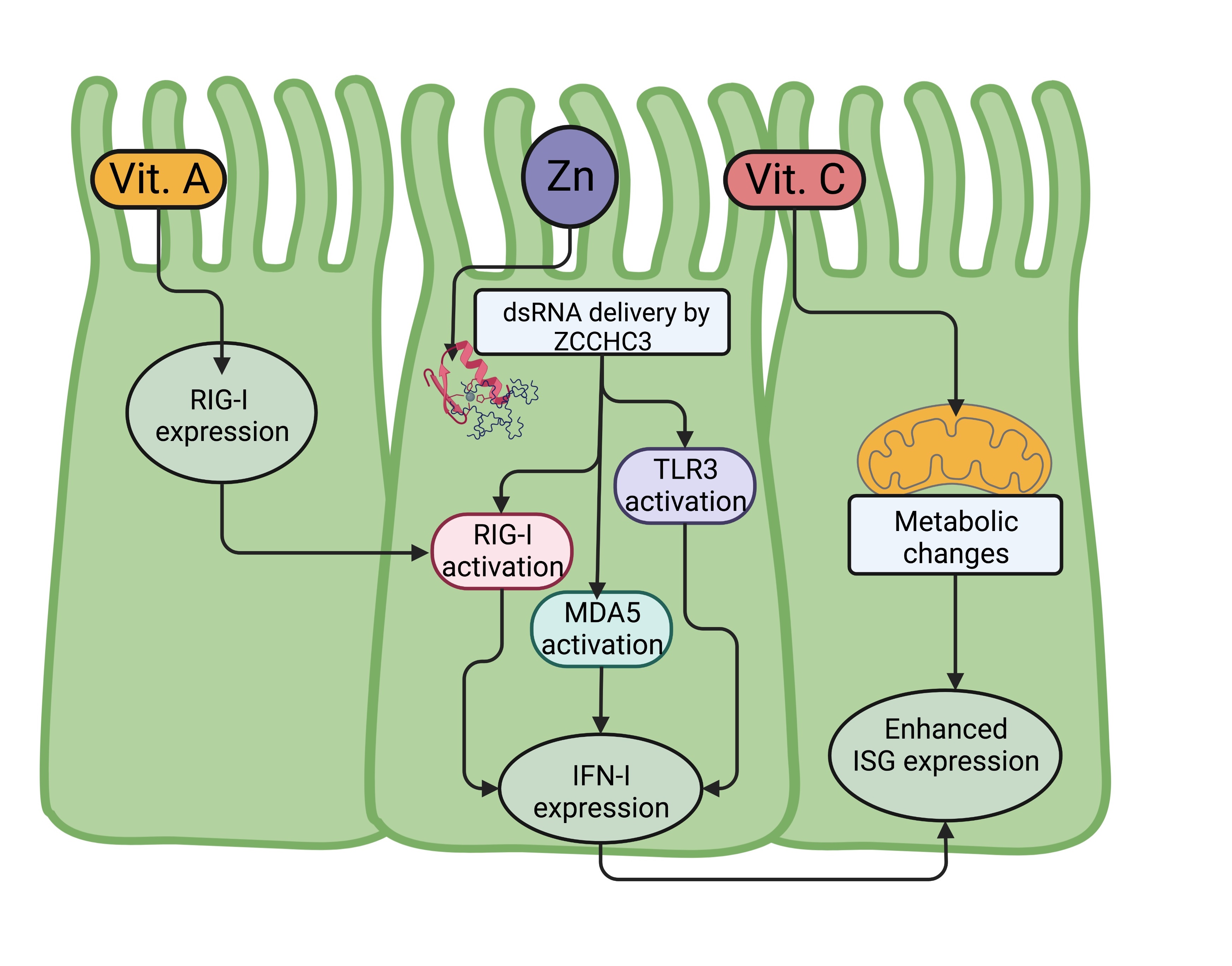
||||
| 58 Brandao K, Deason-Towne F, Perraud AL, Schmitz C: The role of Mg2+ in immune cells. Immunol Res 2013;55:261-269. https://doi.org/10.1007/s12026-012-8371-x |
||||
| 59 Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ: Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011;475:471-476. https://doi.org/10.1038/nature10246 |
||||
| 60 Lieleg O, Lieleg C, Bloom J, Buck CB, Ribbeck K: Mucin biopolymers as broad-spectrum antiviral agents. Biomacromolecules 2012;13:1724-1732. https://doi.org/10.1021/bm3001292 |
||||
| 61 McAuley JL, Corcilius L, Tan HX, Payne RJ, McGuckin MA, Brown LE: The cell surface mucin MUC1 limits the severity of influenza A virus infection. Mucosal Immunol 2017;10:1581-1593. https://doi.org/10.1038/mi.2017.16 |
||||
| 62 Li Y, Dinwiddie DL, Harrod KS, Jiang Y, Kim KC: Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro . Am J Physiol Lung Cell Mol Physiol 2010;298:L558-563. https://doi.org/10.1152/ajplung.00225.2009 |
||||
| 63 Lu W, Liu X, Wang T, Liu F, Zhu A, Lin Y, Luo J, Ye F, He J, Zhao J, Li Y, Zhong N: Elevated MUC1 and MUC5AC mucin protein levels in airway mucus of critical ill COVID-19 patients. J Med Virol 2021;93:582-584. https://doi.org/10.1002/jmv.26406 |
||||
| 64 Jiang Y, Yang D, Li W, Wang B, Jiang Z, Li M: Antiviral activity of recombinant mouse beta-defensin 3 against influenza A virus in vitro and in vivo . Antivir Chem Chemother 2012;22:255-262. https://doi.org/10.3851/IMP2077 |
||||
| 65 LeMessurier KS, Lin Y, McCullers JA, Samarasinghe AE: Antimicrobial peptides alter early immune response to influenza A virus infection in C57BL/6 mice. Antiviral Res 2016;133:208-217. https://doi.org/10.1016/j.antiviral.2016.08.013 |
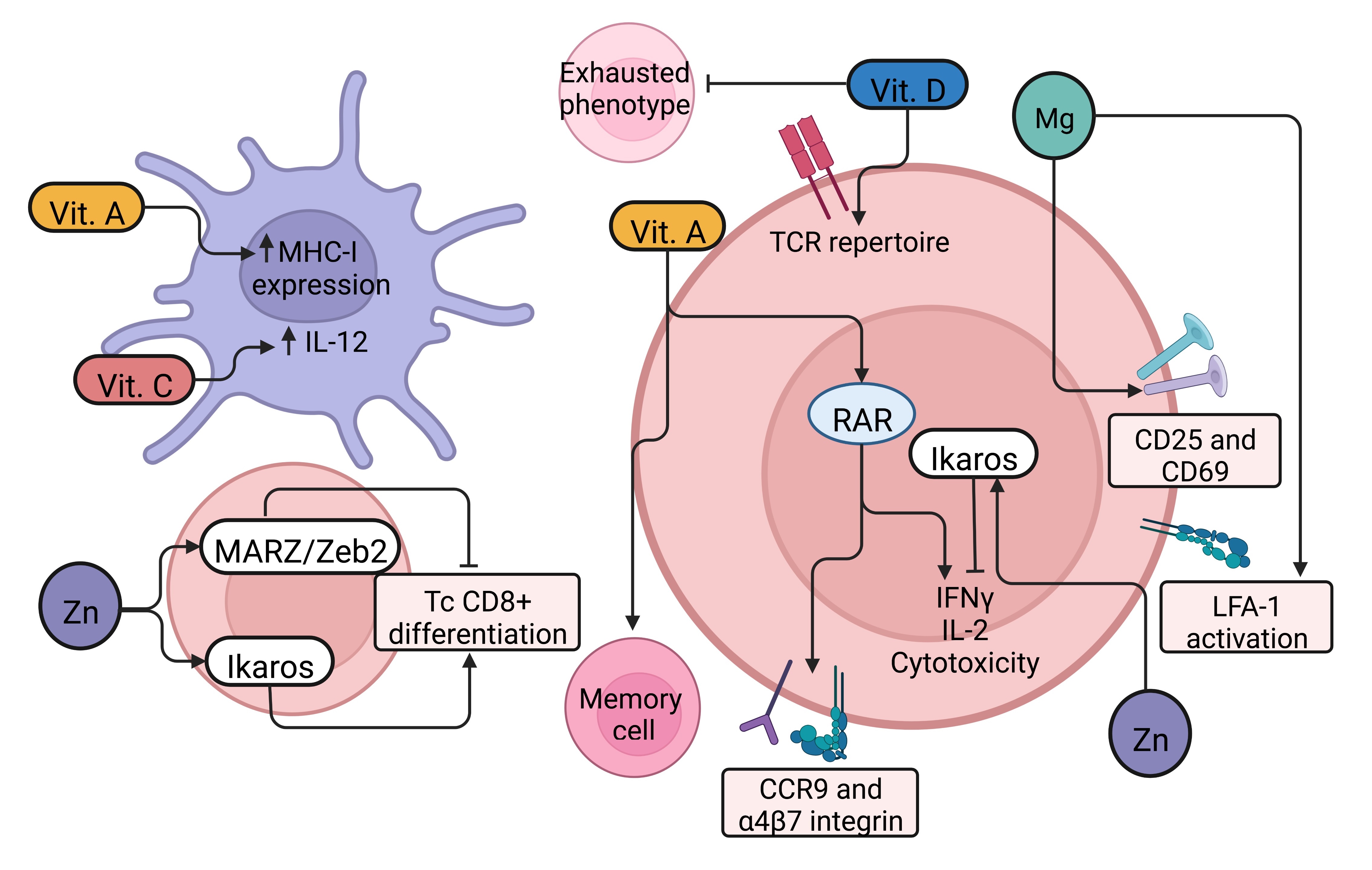
||||
| 66 Li W, Feng Y, Kuang Y, Zeng W, Yang Y, Li H, Jiang Z, Li M: Construction of eukaryotic expression vector with mBD1-mBD3 fusion genes and exploring its activity against influenza A virus. Viruses 2014;6:1237-1252. https://doi.org/10.3390/v6031237 |
||||
| 67 Kota S, Sabbah A, Chang TH, Harnack R, Xiang Y, Meng X, Bose S: Role of human beta-defensin-2 during tumor necrosis factor-alpha/NF-kappaB-mediated innate antiviral response against human respiratory syncytial virus. J Biol Chem 2008;283:22417-22429. https://doi.org/10.1074/jbc.M710415200 |
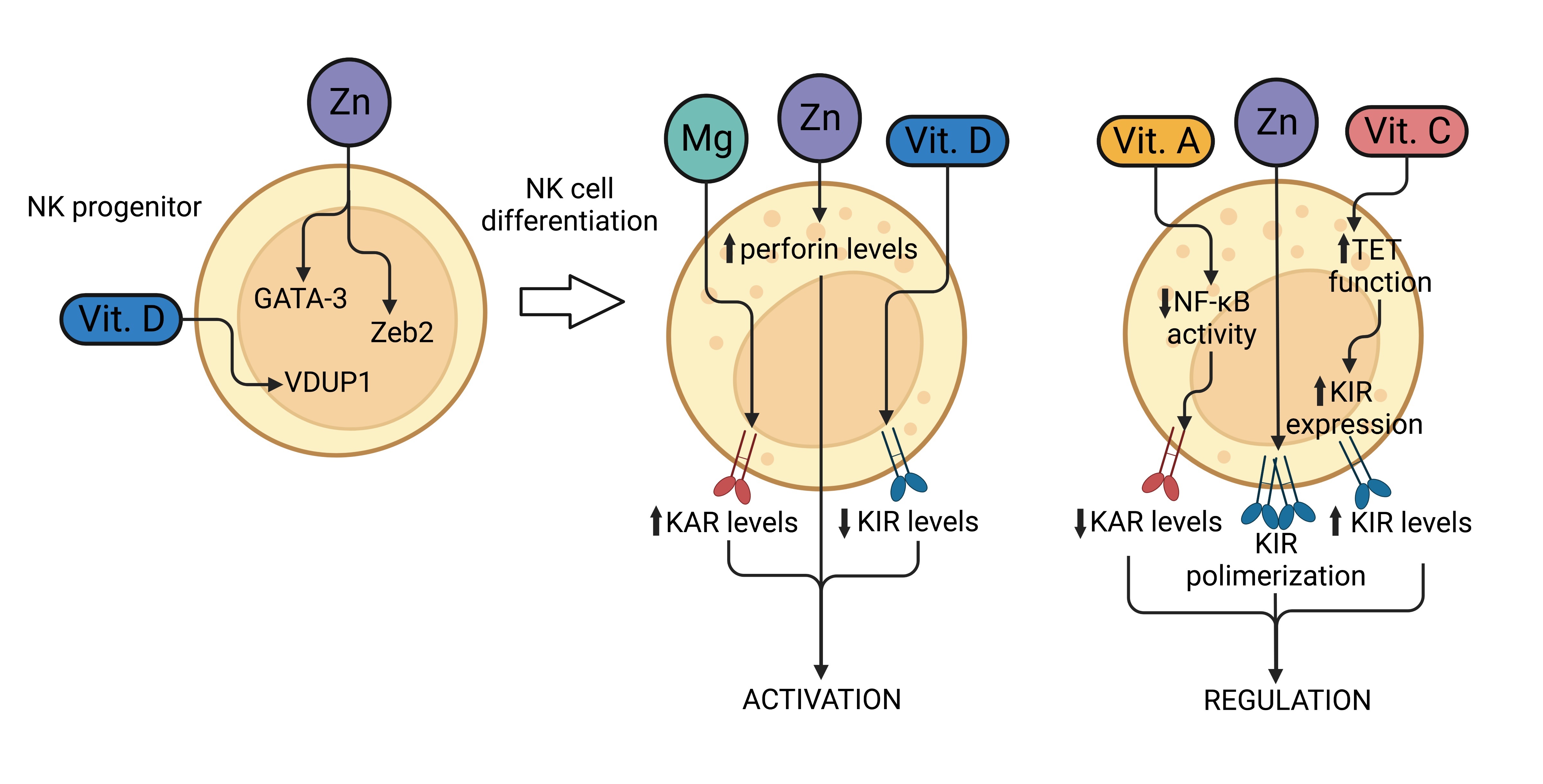
||||
| 68 Zhao H, Zhou J, Zhang K, Chu H, Liu D, Poon VK, Chan CC, Leung HC, Fai N, Lin YP, Zhang AJ, Jin DY, Yuen KY, Zheng BJ: A novel peptide with potent and broad-spectrum antiviral activities against multiple respiratory viruses. Sci Rep 2016;6:22008. https://doi.org/10.1038/srep22008 |
||||
| 69 Kim J, Yang YL, Jang SH, Jang YS: Human beta-defensin 2 plays a regulatory role in innate antiviral immunity and is capable of potentiating the induction of antigen-specific immunity. Virol J 2018;15:124. https://doi.org/10.1186/s12985-018-1035-2 |
||||
| 70 Vemula SV, Amen O, Katz JM, Donis R, Sambhara S, Mittal SK: Beta-defensin 2 enhances immunogenicity and protection of an adenovirus-based H5N1 influenza vaccine at an early time. Virus Res 2013;178:398-403. https://doi.org/10.1016/j.virusres.2013.09.013 |
||||
| 71 Xu C, Wang A, Marin M, Honnen W, Ramasamy S, Porter E, Subbian S, Pinter A, Melikyan GB, Lu W, Chang TL: Human Defensins Inhibit SARS-CoV-2 Infection by Blocking Viral Entry. Viruses 2021;13:1246. https://doi.org/10.3390/v13071246 |
||||
| 72 Faurschou M, Sorensen OE, Johnsen AH, Askaa J, Borregaard N: Defensin-rich granules of human neutrophils: characterization of secretory properties. Biochim Biophys Acta 2002;1591:29-35. https://doi.org/10.1016/S0167-4889(02)00243-4 |
||||
| 73 Abdeen S, Bdeir K, Abu-Fanne R, Maraga E, Higazi M, Khurram N, Feldman M, Deshpande C, Litzky LA, Heyman SN, Montone KT, Cines DB, Higazi AA: Alpha-defensins: risk factor for thrombosis in COVID-19 infection. Br J Haematol 2021;194:44-52. https://doi.org/10.1111/bjh.17503 |
||||
| 74 Currie SM, Gwyer Findlay E, McFarlane AJ, Fitch PM, Bottcher B, Colegrave N, Paras A, Jozwik A, Chiu C, Schwarze J, Davidson DJ: Cathelicidins Have Direct Antiviral Activity against Respiratory Syncytial Virus In vitro and Protective Function In vivo in Mice and Humans. J Immunol 2016;196:2699-2710. https://doi.org/10.4049/jimmunol.1502478 |
||||
| 75 Harcourt JL, McDonald M, Svoboda P, Pohl J, Tatti K, Haynes LM: Human cathelicidin, LL-37, inhibits respiratory syncytial virus infection in polarized airway epithelial cells. BMC Res Notes 2016;9:11. https://doi.org/10.1186/s13104-015-1836-y |
||||
| 76 Tripathi S, Tecle T, Verma A, Crouch E, White M, Hartshorn KL: The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J Gen Virol 2013;94:40-49. https://doi.org/10.1099/vir.0.045013-0 |
||||
| 77 Barlow PG, Svoboda P, Mackellar A, Nash AA, York IA, Pohl J, Davidson DJ, Donis RO: Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS One 2011;6:e25333. https://doi.org/10.1371/journal.pone.0025333 |
||||
| 78 Sousa FH, Casanova V, Findlay F, Stevens C, Svoboda P, Pohl J, Proudfoot L, Barlow PG: Cathelicidins display conserved direct antiviral activity towards rhinovirus. Peptides 2017;95:76-83. https://doi.org/10.1016/j.peptides.2017.07.013 |
||||
| 79 Casanova V, Sousa FH, Shakamuri P, Svoboda P, Buch C, D'Acremont M, Christophorou MA, Pohl J, Stevens C, Barlow PG: Citrullination Alters the Antiviral and Immunomodulatory Activities of the Human Cathelicidin LL-37 During Rhinovirus Infection. Front Immunol 2020;11:85. https://doi.org/10.3389/fimmu.2020.00085 |
||||
| 80 Wang C, Wang S, Li D, Chen P, Han S, Zhao G, Chen Y, Zhao J, Xiong J, Qiu J, Wei DQ, Zhao J, Wang J: Human Cathelicidin Inhibits SARS-CoV-2 Infection: Killing Two Birds with One Stone. ACS Infect Dis 2021;7:1545-1554. https://doi.org/10.1021/acsinfecdis.1c00096 |
||||
| 81 Marquez HA, Chen F: Retinoic Acid Signaling and Development of the Respiratory System. Subcell Biochem 2020;95:151-174. https://doi.org/10.1007/978-3-030-42282-0_6 |
||||
| 82 Niu C, Liu N, Liu J, Zhang M, Ying L, Wang L, Tian D, Dai J, Luo Z, Liu E, Zou L, Fu Z: Vitamin A maintains the airway epithelium in a murine model of asthma by suppressing glucocorticoid-induced leucine zipper. Clin Exp Allergy 2016;46:848-860. https://doi.org/10.1111/cea.12646 |
||||
| 83 Liu J, Zhang M, Niu C, Luo Z, Dai J, Wang L, Liu E, Fu Z: Dexamethasone inhibits repair of human airway epithelial cells mediated by glucocorticoid-induced leucine zipper (GILZ). PLoS One 2013;8:e60705. https://doi.org/10.1371/journal.pone.0060705 |
||||
| 84 Esteban-Pretel G, Marin MP, Renau-Piqueras J, Sado Y, Barber T, Timoneda J: Vitamin A deficiency disturbs collagen IV and laminin composition and decreases matrix metalloproteinase concentrations in rat lung. Partial reversibility by retinoic acid. J Nutr Biochem 2013;24:137-145. https://doi.org/10.1016/j.jnutbio.2012.03.010 |
||||
| 85 Koo JS, Jetten AM, Belloni P, Yoon JH, Kim YD, Nettesheim P: Role of retinoid receptors in the regulation of mucin gene expression by retinoic acid in human tracheobronchial epithelial cells. Biochem J 1999;338:351-357. https://doi.org/10.1042/bj3380351 |
||||
| 86 Kim SW, Hong JS, Ryu SH, Chung WC, Yoon JH, Koo JS: Regulation of mucin gene expression by CREB via a nonclassical retinoic acid signaling pathway. Mol Cell Biol 2007;27:6933-6947. https://doi.org/10.1128/MCB.02385-06 |
||||
| 87 Jacobo-Delgado YM, Torres-Juarez F, Rodriguez-Carlos A, Santos-Mena A, Enciso-Moreno JE, Rivas-Santiago C, Diamond G, Rivas-Santiago B: Retinoic acid induces antimicrobial peptides and cytokines leading to Mycobacterium tuberculosis elimination in airway epithelial cells. Peptides 2021;142:170580. https://doi.org/10.1016/j.peptides.2021.170580 |
||||
| 88 Schrumpf JA, Amatngalim GD, Veldkamp JB, Verhoosel RM, Ninaber DK, Ordonez SR, van der Does AM, Haagsman HP, Hiemstra PS: Proinflammatory Cytokines Impair Vitamin D-Induced Host Defense in Cultured Airway Epithelial Cells. Am J Respir Cell Mol Biol 2017;56:749-761. https://doi.org/10.1165/rcmb.2016-0289OC |
||||
| 89 Brockman-Schneider RA, Pickles RJ, Gern JE: Effects of vitamin D on airway epithelial cell morphology and rhinovirus replication. PLoS One 2014;9:e86755. https://doi.org/10.1371/journal.pone.0086755 |
||||
| 90 Merriman KE, Kweh MF, Powell JL, Lippolis JD, Nelson CD: Multiple beta-defensin genes are upregulated by the vitamin D pathway in cattle. J Steroid Biochem Mol Biol 2015;154:120-129. https://doi.org/10.1016/j.jsbmb.2015.08.002 |
||||
| 91 Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH: Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 2004;173:2909-2912. https://doi.org/10.4049/jimmunol.173.5.2909 |
||||
| 92 Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW: Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 2008;181:7090-7099. https://doi.org/10.4049/jimmunol.181.10.7090 |
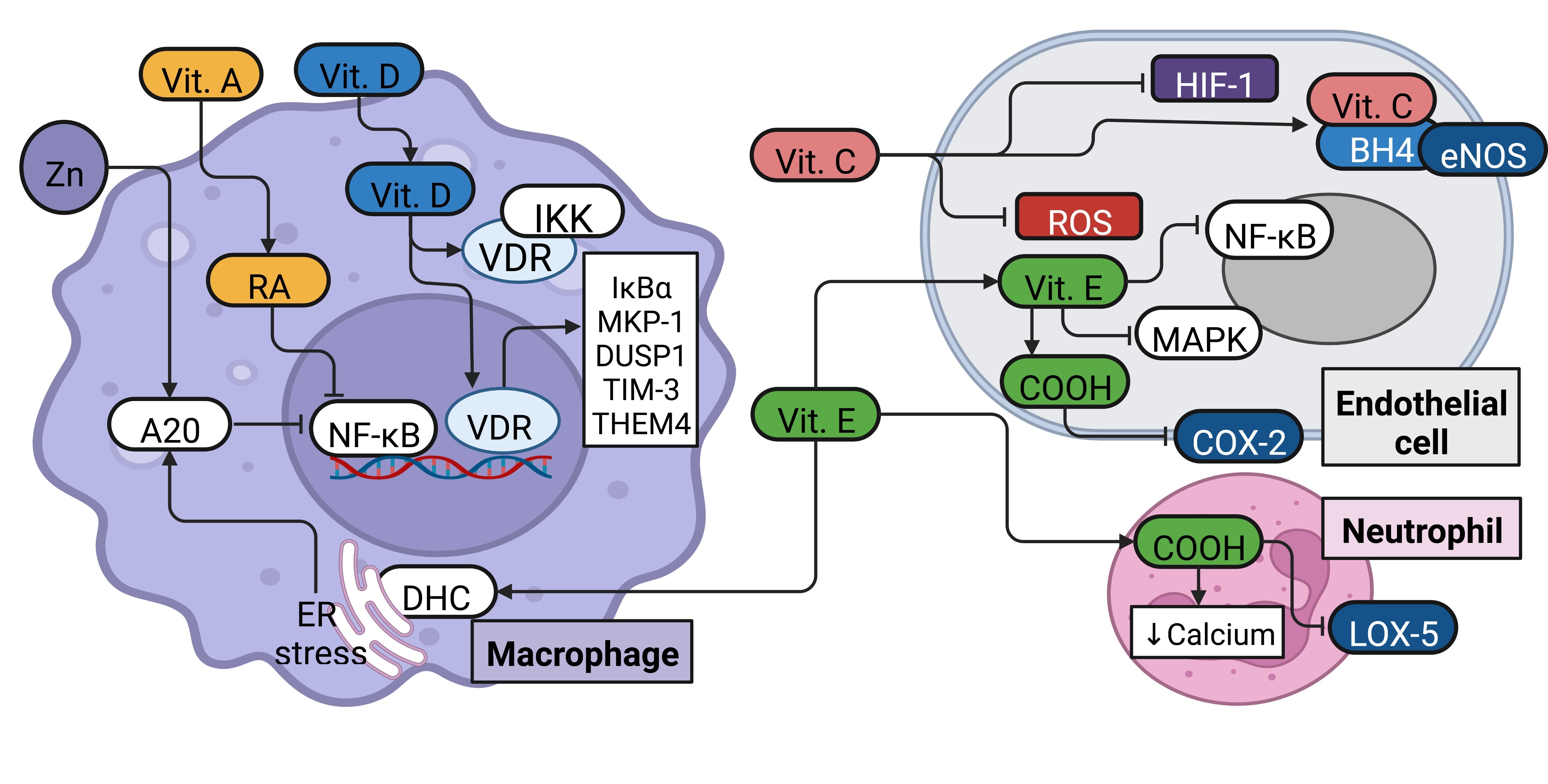
||||
| 93 Ekstrand-Hammarstrom B, Osterlund C, Lilliehook B, Bucht A: Vitamin E down-modulates mitogen-activated protein kinases, nuclear factor-kappaB and inflammatory responses in lung epithelial cells. Clin Exp Immunol 2007;147:359-369. https://doi.org/10.1111/j.1365-2249.2006.03285.x |
||||
| 94 Feldman C, Anderson R, Theron AJ, Steel HC, van Rensburg CE, Cole PJ, Wilson R: Vitamin E attenuates the injurious effects of bioactive phospholipids on human ciliated epithelium in vitro . Eur Respir J 2001;18:122-129. https://doi.org/10.1183/09031936.01.00037401 |
||||
| 95 Wang X, Nijman R, Camuzeaux S, Sands C, Jackson H, Kaforou M, Emonts M, Herberg JA, Maconochie I, Carrol ED, Paulus SC, Zenz W, Van der Flier M, de Groot R, Martinon-Torres F, Schlapbach LJ, Pollard AJ, Fink C, Kuijpers TT, Anderson S, et al.: Plasma lipid profiles discriminate bacterial from viral infection in febrile children. Sci Rep 2019;9:17714. https://doi.org/10.1038/s41598-019-53721-1 |
||||
| 96 Niewoehner DE, Rice K, Sinha AA, Wangensteen D: Injurious effects of lysophosphatidylcholine on barrier properties of alveolar epithelium. J Appl Physiol (1985) 1987;63:1979-1986. https://doi.org/10.1152/jappl.1987.63.5.1979 |
||||
| 97 McManus LM, Deavers SI: Platelet activating factor in pulmonary pathobiology. Clin Chest Med 1989;10:107-118. https://doi.org/10.1016/S0272-5231(21)00608-0 |
||||
| 98 Mereness JA, Bhattacharya S, Wang Q, Ren Y, Pryhuber GS, Mariani TJ: Type VI collagen promotes lung epithelial cell spreading and wound-closure. PLoS One 2018;13:e0209095. https://doi.org/10.1371/journal.pone.0209095 |
||||
| 99 Liu C, Huang K, Li G, Wang P, Liu C, Guo C, Sun Z, Pan J: Ascorbic acid promotes 3T3-L1 cells adipogenesis by attenuating ERK signaling to upregulate the collagen VI. Nutr Metab (Lond) 2017;14:79. https://doi.org/10.1186/s12986-017-0234-y |
||||
| 100 Fischer H, Schwarzer C, Illek B: Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci U S A 2004;101:3691-3696. https://doi.org/10.1073/pnas.0308393100 |
||||
| 101 Adler KB, Tuvim MJ, Dickey BF: Regulated mucin secretion from airway epithelial cells. Front Endocrinol (Lausanne) 2013;4:129. https://doi.org/10.3389/fendo.2013.00129 |
||||
| 102 Roscioli E, Jersmann HP, Lester S, Badiei A, Fon A, Zalewski P, Hodge S: Zinc deficiency as a codeterminant for airway epithelial barrier dysfunction in an ex vivo model of COPD. Int J Chron Obstruct Pulmon Dis 2017;12:3503-3510. https://doi.org/10.2147/COPD.S149589 |
||||
| 103 Bao S, Knoell DL: Zinc modulates cytokine-induced lung epithelial cell barrier permeability. Am J Physiol Lung Cell Mol Physiol 2006;291:L1132-1141. https://doi.org/10.1152/ajplung.00207.2006 |
||||
| 104 Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, et al.: A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11:875-879. https://doi.org/10.1038/nm1267 |
||||
| 105 Anderson CS, Chu CY, Wang Q, Mereness JA, Ren Y, Donlon K, Bhattacharya S, Misra RS, Walsh EE, Pryhuber GS, Mariani TJ: CX3CR1 as a respiratory syncytial virus receptor in pediatric human lung. Pediatr Res 2020;87:862-867. https://doi.org/10.1038/s41390-019-0677-0 |
||||
| 106 Alymova IV, Portner A, Mishin VP, McCullers JA, Freiden P, Taylor GL: Receptor-binding specificity of the human parainfluenza virus type 1 hemagglutinin-neuraminidase glycoprotein. Glycobiology 2012;22:174-180. https://doi.org/10.1093/glycob/cwr112 |
||||
| 107 Nicholls JM, Bourne AJ, Chen H, Guan Y, Peiris JS: Sialic acid receptor detection in the human respiratory tract: evidence for widespread distribution of potential binding sites for human and avian influenza viruses. Respir Res 2007;8:73. https://doi.org/10.1186/1465-9921-8-73 |
||||
| 108 Baggen J, Thibaut HJ, Staring J, Jae LT, Liu Y, Guo H, Slager JJ, de Bruin JW, van Vliet AL, Blomen VA, Overduin P, Sheng J, de Haan CA, de Vries E, Meijer A, Rossmann MG, Brummelkamp TR, van Kuppeveld FJ: Enterovirus D68 receptor requirements unveiled by haploid genetics. Proc Natl Acad Sci U S A 2016;113:1399-1404. https://doi.org/10.1073/pnas.1524498113 |
||||
| 109 Cox RG, Mainou BA, Johnson M, Hastings AK, Schuster JE, Dermody TS, Williams JV: Human Metapneumovirus Is Capable of Entering Cells by Fusion with Endosomal Membranes. PLoS Pathog 2015;11:e1005303. https://doi.org/10.1371/journal.ppat.1005303 |
||||
| 110 Rankl C, Kienberger F, Wildling L, Wruss J, Gruber HJ, Blaas D, Hinterdorfer P: Multiple receptors involved in human rhinovirus attachment to live cells. Proc Natl Acad Sci U S A 2008;105:17778-17783. https://doi.org/10.1073/pnas.0806451105 |
||||
| 111 Hograindleur MA, Effantin G, Fenel D, Mas C, Lieber A, Schoehn G, Fender P, Vassal-Stermann E: Binding Mechanism Elucidation of the Acute Respiratory Disease Causing Agent Adenovirus of Serotype 7 to Desmoglein-2. Viruses 2020;12:1075. https://doi.org/10.3390/v12101075 |
||||
| 112 Bauernfried S, Scherr MJ, Pichlmair A, Duderstadt KE, Hornung V: Human NLRP1 is a sensor for double-stranded RNA. Science 2021;371:eabd0811. https://doi.org/10.1126/science.abd0811 |
||||
| 113 Zhu S, Ding S, Wang P, Wei Z, Pan W, Palm NW, Yang Y, Yu H, Li HB, Wang G, Lei X, de Zoete MR, Zhao J, Zheng Y, Chen H, Zhao Y, Jurado KA, Feng N, Shan L, Kluger Y, et al.: Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature 2017;546:667-670. https://doi.org/10.1038/nature22967 |
||||
| 114 Li D, Wu M: Pattern recognition receptors in health and diseases. Signal transduction and targeted therapy 2021;6:291. https://doi.org/10.1038/s41392-021-00687-0 |
||||
| 115 Su C, Tang YD, Zheng C: DExD/H-box helicases: multifunctional regulators in antiviral innate immunity. Cell Mol Life Sci 2021;79:2. https://doi.org/10.1007/s00018-021-04072-6 |
||||
| 116 Chiang HS, Liu HM: The Molecular Basis of Viral Inhibition of IRF- and STAT-Dependent Immune Responses. Front Immunol 2018;9:3086. https://doi.org/10.3389/fimmu.2018.03086 |
||||
| 117 Liu T, Zhang L, Joo D, Sun SC: NF-kappaB signaling in inflammation. Signal Transduct Target Ther 2017;2:17023. https://doi.org/10.1038/sigtrans.2017.23 |
||||
| 118 Guan J, Miah SM, Wilson ZS, Erick TK, Banh C, Brossay L: Role of type I interferon receptor signaling on NK cell development and functions. PLoS One 2014;9:e111302. https://doi.org/10.1371/journal.pone.0111302 |
||||
| 119 Madera S, Rapp M, Firth MA, Beilke JN, Lanier LL, Sun JC: Type I IFN promotes NK cell expansion during viral infection by protecting NK cells against fratricide. J Exp Med 2016;213:225-233. https://doi.org/10.1084/jem.20150712 |
||||
| 120 Lee AJ, Mian F, Poznanski SM, Stackaruk M, Chan T, Chew MV, Ashkar AA: Type I Interferon Receptor on NK Cells Negatively Regulates Interferon-gamma Production. Front Immunol 2019;10:1261. https://doi.org/10.3389/fimmu.2019.01261 |
||||
| 121 Simmons DP, Wearsch PA, Canaday DH, Meyerson HJ, Liu YC, Wang Y, Boom WH, Harding CV: Type I IFN drives a distinctive dendritic cell maturation phenotype that allows continued class II MHC synthesis and antigen processing. J Immunol 2012;188:3116-3126. https://doi.org/10.4049/jimmunol.1101313 |
||||
| 122 Pantel A, Teixeira A, Haddad E, Wood EG, Steinman RM, Longhi MP: Direct type I IFN but not MDA5/TLR3 activation of dendritic cells is required for maturation and metabolic shift to glycolysis after poly IC stimulation. PLoS Biol 2014;12:e1001759. https://doi.org/10.1371/journal.pbio.1001759 |
||||
| 123 Lokugamage KG, Hage A, de Vries M, Valero-Jimenez AM, Schindewolf C, Dittmann M, Rajsbaum R, Menachery VD: Type I Interferon Susceptibility Distinguishes SARS-CoV-2 from SARS-CoV. J Virol 2020;94:e01410-20. https://doi.org/10.1128/JVI.01410-20 |
||||
| 124 Mibayashi M, Martinez-Sobrido L, Loo YM, Cardenas WB, Gale M, Jr., Garcia-Sastre A: Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J Virol 2007;81:514-524. https://doi.org/10.1128/JVI.01265-06 |
||||
| 125 Zhang W, Yang H, Kong X, Mohapatra S, San Juan-Vergara H, Hellermann G, Behera S, Singam R, Lockey RF, Mohapatra SS: Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nat Med 2005;11:56-62. https://doi.org/10.1038/nm1174 |
||||
| 126 Ban J, Lee NR, Lee NJ, Lee JK, Quan FS, Inn KS: Human Respiratory Syncytial Virus NS 1 Targets TRIM25 to Suppress RIG-I Ubiquitination and Subsequent RIG-I-Mediated Antiviral Signaling. Viruses 2018;10:716. https://doi.org/10.3390/v10120716 |
||||
| 127 Kumar A, Ishida R, Strilets T, Cole J, Lopez-Orozco J, Fayad N, Felix-Lopez A, Elaish M, Evseev D, Magor KE, Mahal LK, Nagata LP, Evans DH, Hobman TC: SARS-CoV-2 Nonstructural Protein 1 Inhibits the Interferon Response by Causing Depletion of Key Host Signaling Factors. J Virol 2021;95:e0026621. https://doi.org/10.1128/JVI.00266-21 |
||||
| 128 Galani IE, Rovina N, Lampropoulou V, Triantafyllia V, Manioudaki M, Pavlos E, Koukaki E, Fragkou PC, Panou V, Rapti V, Koltsida O, Mentis A, Koulouris N, Tsiodras S, Koutsoukou A, Andreakos E: Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol 2021;22:32-40. https://doi.org/10.1038/s41590-020-00840-x |
||||
| 129 Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, Pere H, Charbit B, Bondet V, Chenevier-Gobeaux C, Breillat P, Carlier N, Gauzit R, Morbieu C, Pene F, Marin N, Roche N, Szwebel TA, Merkling SH, Treluyer JM, et al.: Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020;369:718-724. https://doi.org/10.1126/science.abc6027 |
||||
| 130 Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Moller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR: Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19 Cell 2020;181:1036-1045 e1039. https://doi.org/10.1016/j.cell.2020.04.026 |
||||
| 131 Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S: Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016;19:181-193. https://doi.org/10.1016/j.chom.2016.01.007 |
||||
| 132 Soye KJ, Trottier C, Richardson CD, Ward BJ, Miller WH, Jr.: RIG-I is required for the inhibition of measles virus by retinoids. PLoS One 2011;6:e22323. https://doi.org/10.1371/journal.pone.0022323 |
||||
| 133 Liu G, Park HS, Pyo HM, Liu Q, Zhou Y: Influenza A Virus Panhandle Structure Is Directly Involved in RIG-I Activation and Interferon Induction. J Virol 2015;89:6067-6079. https://doi.org/10.1128/JVI.00232-15 |
||||
| 134 Kouwaki T, Nishimura T, Wang G, Oshiumi H: RIG-I-Like Receptor-Mediated Recognition of Viral Genomic RNA of Severe Acute Respiratory Syndrome Coronavirus-2 and Viral Escape From the Host Innate Immune Responses. Front Immunol 2021;12:700926. https://doi.org/10.3389/fimmu.2021.700926 |
||||
| 135 Goutagny N, Jiang Z, Tian J, Parroche P, Schickli J, Monks BG, Ulbrandt N, Ji H, Kiener PA, Coyle AJ, Fitzgerald KA: Cell type-specific recognition of human metapneumoviruses (HMPVs) by retinoic acid-inducible gene I (RIG-I) and TLR7 and viral interference of RIG-I ligand recognition by HMPV-B1 phosphoprotein. J Immunol 2010;184:1168-1179. https://doi.org/10.4049/jimmunol.0902750 |
||||
| 136 Sabbah A, Bose S: Retinoic acid inducible gene I activates innate antiviral response against human parainfluenza virus type 3. Virol J 2009;6:200. https://doi.org/10.1186/1743-422X-6-200 |
||||
| 137 Teafatiller T, Agrawal S, De Robles G, Rahmatpanah F, Subramanian VS, Agrawal A: Vitamin C Enhances Antiviral Functions of Lung Epithelial Cells. Biomolecules 2021;11:1148. https://doi.org/10.3390/biom11081148 |
||||
| 138 Verhelst J, Parthoens E, Schepens B, Fiers W, Saelens X: Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly. J Virol 2012;86:13445-13455. https://doi.org/10.1128/JVI.01682-12 |
||||
| 139 Verhelst J, Hulpiau P, Saelens X: Mx proteins: antiviral gatekeepers that restrain the uninvited. Microbiol Mol Biol Rev 2013;77:551-566. https://doi.org/10.1128/MMBR.00024-13 |
||||
| 140 Fujisawa K, Hara K, Takami T, Okada S, Matsumoto T, Yamamoto N, Sakaida I: Evaluation of the effects of ascorbic acid on metabolism of human mesenchymal stem cells. Stem Cell Res Ther 2018;9:93. https://doi.org/10.1186/s13287-018-0825-1 |
||||
| 141 Kc S, Carcamo JM, Golde DW: Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J 2005;19:1657-1667. https://doi.org/10.1096/fj.05-4107com |
||||
| 142 Cai Y, Li YF, Tang LP, Tsoi B, Chen M, Chen H, Chen XM, Tan RR, Kurihara H, He RR: A new mechanism of vitamin C effects on A/FM/1/47(H1N1) virus-induced pneumonia in restraint-stressed mice. Biomed Res Int 2015;2015:675149. https://doi.org/10.1155/2015/675149 |
||||
| 143 Wu B, Hur S: How RIG-I like receptors activate MAVS. Curr Opin Virol 2015;12:91-98. https://doi.org/10.1016/j.coviro.2015.04.004 |
||||
| 144 Berg K, Bolt G, Andersen H, Owen TC: Zinc potentiates the antiviral action of human IFN-alpha tenfold. J Interferon Cytokine Res 2001;21:471-474. https://doi.org/10.1089/10799900152434330 |
||||
| 145 Lian H, Wei J, Zang R, Ye W, Yang Q, Zhang XN, Chen YD, Fu YZ, Hu MM, Lei CQ, Luo WW, Li S, Shu HB: ZCCHC3 is a co-sensor of cGAS for dsDNA recognition in innate immune response. Nat Commun 2018;9:3349. https://doi.org/10.1038/s41467-018-05559-w |
||||
| 146 Lian H, Zang R, Wei J, Ye W, Hu MM, Chen YD, Zhang XN, Guo Y, Lei CQ, Yang Q, Luo WW, Li S, Shu HB: The Zinc-Finger Protein ZCCHC3 Binds RNA and Facilitates Viral RNA Sensing and Activation of the RIG-I-like Receptors. Immunity 2018;49:438-448 e435. https://doi.org/10.1016/j.immuni.2018.08.014 |
||||
| 147 Zang R, Lian H, Zhong X, Yang Q, Shu HB: ZCCHC3 modulates TLR3-mediated signaling by promoting recruitment of TRIF to TLR3 J Mol Cell Biol 2020;12:251-262. https://doi.org/10.1093/jmcb/mjaa004 |
||||
| 148 Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW: Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol 2010;184:965-974. https://doi.org/10.4049/jimmunol.0902840 |
||||
| 149 Koutsakos M, McWilliam HEG, Aktepe TE, Fritzlar S, Illing PT, Mifsud NA, Purcell AW, Rockman S, Reading PC, Vivian JP, Rossjohn J, Brooks AG, Mackenzie JM, Mintern JD, Villadangos JA, Nguyen THO, Kedzierska K: Downregulation of MHC Class I Expression by Influenza A and B Viruses. Front Immunol 2019;10:1158. https://doi.org/10.3389/fimmu.2019.01158 |
||||
| 150 Zhang Y, Chen Y, Li Y, Huang F, Luo B, Yuan Y, Xia B, Ma X, Yang T, Yu F, Liu J, Liu B, Song Z, Chen J, Yan S, Wu L, Pan T, Zhang X, Li R, Huang W, et al.: The ORF8 protein of SARS-CoV-2 mediates immune evasion through down-regulating MHC-Iota. Proc Natl Acad Sci U S A 2021;118:e2024202118. https://doi.org/10.1073/pnas.2024202118 |
||||
| 151 Perera Molligoda Arachchige AS: Human NK cells: From development to effector functions. Innate Immun 2021;27:212-229. https://doi.org/10.1177/17534259211001512 |
||||
| 152 Chan CJ, Smyth MJ, Martinet L: Molecular mechanisms of natural killer cell activation in response to cellular stress. Cell Death Differ 2014;21:5-14. https://doi.org/10.1038/cdd.2013.26 |
||||
| 153 Vidal SM, Khakoo SI, Biron CA: Natural killer cell responses during viral infections: flexibility and conditioning of innate immunity by experience. Curr Opin Virol 2011;1:497-512. https://doi.org/10.1016/j.coviro.2011.10.017 |
||||
| 154 Diab M, Schmiedel D, Seidel E, Bacharach E, Mandelboim O: Human Metapneumovirus Escapes NK Cell Recognition through the Downregulation of Stress-Induced Ligands for NKG2D. Viruses 2020;12:781. https://doi.org/10.3390/v12070781 |
||||
| 155 Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, Xu Y, Tian Z: Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 2020;17:533-535. https://doi.org/10.1038/s41423-020-0402-2 |
||||
| 156 Poznanski SM, Ashkar AA: What Defines NK Cell Functional Fate: Phenotype or Metabolism? Front Immunol 2019;10:1414. https://doi.org/10.3389/fimmu.2019.01414 |
||||
| 157 Zhou G, Juang SW, Kane KP: NK cells exacerbate the pathology of influenza virus infection in mice. Eur J Immunol 2013;43:929-938. https://doi.org/10.1002/eji.201242620 |
||||
| 158 Li F, Zhu H, Sun R, Wei H, Tian Z: Natural killer cells are involved in acute lung immune injury caused by respiratory syncytial virus infection. J Virol 2012;86:2251-2258. https://doi.org/10.1128/JVI.06209-11 |
||||
| 159 Harker JA, Godlee A, Wahlsten JL, Lee DC, Thorne LG, Sawant D, Tregoning JS, Caspi RR, Bukreyev A, Collins PL, Openshaw PJ: Interleukin 18 coexpression during respiratory syncytial virus infection results in enhanced disease mediated by natural killer cells. J Virol 2010;84:4073-4082. https://doi.org/10.1128/JVI.02014-09 |
||||
| 160 Li A, He M, Wang H, Qiao B, Chen P, Gu H, Zhang M, He S: All-trans retinoic acid negatively regulates cytotoxic activities of nature killer cell line 92. Biochem Biophys Res Commun 2007;352:42-47. https://doi.org/10.1016/j.bbrc.2006.10.132 |
||||
| 161 Dawson HD, Li NQ, DeCicco KL, Nibert JA, Ross AC: Chronic marginal vitamin A status reduces natural killer cell number and function in aging Lewis rats. J Nutr 1999;129:1510-1517. https://doi.org/10.1093/jn/129.8.1510 |
||||
| 162 Bowman TA, Goonewardene IM, Pasatiempo AM, Ross AC, Taylor CE: Vitamin A deficiency decreases natural killer cell activity and interferon production in rats. J Nutr 1990;120:1264-1273. https://doi.org/10.1093/jn/120.10.1264 |
||||
| 163 Vassiliou AG, Jahaj E, Pratikaki M, Keskinidou C, Detsika M, Grigoriou E, Psarra K, Orfanos SE, Tsirogianni A, Dimopoulou I, Kotanidou A: Vitamin D deficiency correlates with a reduced number of natural killer cells in intensive care unit (ICU) and non-ICU patients with COVID-19 pneumonia. Hellenic J Cardiol 2021;62:381-383. https://doi.org/10.1016/j.hjc.2020.11.011 |
||||
| 164 Lee KN, Kang HS, Jeon JH, Kim EM, Yoon SR, Song H, Lyu CY, Piao ZH, Kim SU, Han YH, Song SS, Lee YH, Song KS, Kim YM, Yu DY, Choi I: VDUP1 is required for the development of natural killer cells. Immunity 2005;22:195-208. https://doi.org/10.1016/j.immuni.2004.12.012 |
||||
| 165 Al-Jaderi Z, Maghazachi AA: Effects of vitamin D3, calcipotriol and FTY720 on the expression of surface molecules and cytolytic activities of human natural killer cells and dendritic cells. Toxins (Basel) 2013;5:1932-1947. https://doi.org/10.3390/toxins5111932 |
||||
| 166 Lee GY, Park CY, Cha KS, Lee SE, Pae M, Han SN: Differential effect of dietary vitamin D supplementation on natural killer cell activity in lean and obese mice. J Nutr Biochem 2018;55:178-184. https://doi.org/10.1016/j.jnutbio.2018.01.004 |
||||
| 167 Park CY, Han SN: The Role of Vitamin D in Adipose Tissue Biology: Adipocyte Differentiation, Energy Metabolism, and Inflammation. J Lipid Atheroscler 2021;10:130-144. https://doi.org/10.12997/jla.2021.10.2.130 |
||||
| 168 Huijskens MJ, Walczak M, Sarkar S, Atrafi F, Senden-Gijsbers BL, Tilanus MG, Bos GM, Wieten L, Germeraad WT: Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy 2015;17:613-620. https://doi.org/10.1016/j.jcyt.2015.01.004 |
||||
| 169 Wu CY, Zhang B, Kim H, Anderson SK, Miller JS, Cichocki F: Ascorbic Acid Promotes KIR Demethylation during Early NK Cell Differentiation. J Immunol 2020;205:1513-1523. https://doi.org/10.4049/jimmunol.2000212 |
||||
| 170 Muzzioli M, Stecconi R, Moresi R, Provinciali M: Zinc improves the development of human CD34+ cell progenitors towards NK cells and increases the expression of GATA-3 transcription factor in young and old ages. Biogerontology 2009;10:593-604. https://doi.org/10.1007/s10522-008-9201-3 |
||||
| 171 van Helden MJ, Goossens S, Daussy C, Mathieu AL, Faure F, Marcais A, Vandamme N, Farla N, Mayol K, Viel S, Degouve S, Debien E, Seuntjens E, Conidi A, Chaix J, Mangeot P, de Bernard S, Buffat L, Haigh JJ, Huylebroeck D, et al.: Terminal NK cell maturation is controlled by concerted actions of T-bet and Zeb2 and is essential for melanoma rejection. J Exp Med 2015;212:2015-2025. https://doi.org/10.1084/jem.20150809 |
||||
| 172 Samson SI, Richard O, Tavian M, Ranson T, Vosshenrich CA, Colucci F, Buer J, Grosveld F, Godin I, Di Santo JP: GATA-3 promotes maturation, IFN-gamma production, and liver-specific homing of NK cells. Immunity 2003;19:701-711. https://doi.org/10.1016/S1074-7613(03)00294-2 |
||||
| 173 Kumar S, Rajagopalan S, Sarkar P, Dorward DW, Peterson ME, Liao HS, Guillermier C, Steinhauser ML, Vogel SS, Long EO: Zinc-Induced Polymerization of Killer-Cell Ig-like Receptor into Filaments Promotes Its Inhibitory Function at Cytotoxic Immunological Synapses. Mol Cell 2016;62:21-33. https://doi.org/10.1016/j.molcel.2016.03.009 |
||||
| 174 Chaigne-Delalande B, Li FY, O'Connor GM, Lukacs MJ, Jiang P, Zheng L, Shatzer A, Biancalana M, Pittaluga S, Matthews HF, Jancel TJ, Bleesing JJ, Marsh RA, Kuijpers TW, Nichols KE, Lucas CL, Nagpal S, Mehmet H, Su HC, Cohen JI, et al.: Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science 2013;341:186-191. https://doi.org/10.1126/science.1240094 |
||||
| 175 Medzhitov R: The spectrum of inflammatory responses. Science 2021;374:1070-1075. https://doi.org/10.1126/science.abi5200 |
||||
| 176 Abdulkhaleq LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee MNM: The crucial roles of inflammatory mediators in inflammation: A review. Vet World 2018;11:627-635. https://doi.org/10.14202/vetworld.2018.627-635 |
||||
| 177 Pober JS, Sessa WC: Inflammation and the blood microvascular system. Cold Spring Harb Perspect Biol 2014;7:a016345. https://doi.org/10.1101/cshperspect.a016345 |
||||
| 178 Miteva KT, Pedicini L, Wilson LA, Jayasinghe I, Slip RG, Marszalek K, Gaunt HJ, Bartoli F, Deivasigamani S, Sobradillo D, Beech DJ, McKeown L: Rab46 integrates Ca(2+) and histamine signaling to regulate selective cargo release from Weibel-Palade bodies. J Cell Biol 2019;218:2232-2246. https://doi.org/10.1083/jcb.201810118 |
||||
| 179 Holthenrich A, Drexler HCA, Chehab T, Nass J, Gerke V: Proximity proteomics of endothelial Weibel-Palade bodies identifies novel regulator of von Willebrand factor secretion. Blood 2019;134:979-982. https://doi.org/10.1182/blood.2019000786 |
||||
| 180 Farooque SP, Arm JP, Lee TH: Lipid Mediators: Leukotrienes, Prostanoids, Lipoxins, and Platelet-Activating Factor; in: Allergy and Allergic Diseases, Blackwell Scientific, 2008, pp 566-633. https://doi.org/10.1002/9781444300918.ch26 |
||||
| 181 Usatyuk PV, Kotha SR, Parinandi NL, Natarajan V: Phospholipase D signaling mediates reactive oxygen species-induced lung endothelial barrier dysfunction. Pulm Circ 2013;3:108-115. https://doi.org/10.4103/2045-8932.109925 |
||||
| 182 Patel RB, Kotha SR, Sherwani SI, Sliman SM, Gurney TO, Loar B, Butler SO, Morris AJ, Marsh CB, Parinandi NL: Pulmonary fibrosis inducer, bleomycin, causes redox-sensitive activation of phospholipase D and cytotoxicity through formation of bioactive lipid signal mediator, phosphatidic acid, in lung microvascular endothelial cells. Int J Toxicol 2011;30:69-90. https://doi.org/10.1177/1091581810388850 |
||||
| 183 Han M, Pendem S, Teh SL, Sukumaran DK, Wu F, Wilson JX: Ascorbate protects endothelial barrier function during septic insult: Role of protein phosphatase type 2A. Free Radic Biol Med 2010;48:128-135. https://doi.org/10.1016/j.freeradbiomed.2009.10.034 |
||||
| 184 Roy J, Galano JM, Durand T, Le Guennec JY, Lee JC: Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J 2017;31:3729-3745. https://doi.org/10.1096/fj.201700170R |
||||
| 185 Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W: ROS and ROS-Mediated Cellular Signaling. Oxid Med Cell Longev 2016;2016:4350965. https://doi.org/10.1155/2016/4350965 |
||||
| 186 Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK: Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ Res 2018;122:877-902. https://doi.org/10.1161/CIRCRESAHA.117.311401 |
||||
| 187 Corcoran SE, O'Neill LA: HIF1alpha and metabolic reprogramming in inflammation. J Clin Invest 2016;126:3699-3707. https://doi.org/10.1172/JCI84431 |
||||
| 188 Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Gorlach A: Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol 2007;27:755-761. https://doi.org/10.1161/01.ATV.0000258979.92828.bc |
||||
| 189 Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB: Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem 2003;278:24233-24241. https://doi.org/10.1074/jbc.M212389200 |
||||
| 190 Schoonbroodt S, Ferreira V, Best-Belpomme M, Boelaert JR, Legrand-Poels S, Korner M, Piette J: Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of I kappa B alpha in NF-kappa B activation by an oxidative stress. J Immunol 2000;164:4292-4300. https://doi.org/10.4049/jimmunol.164.8.4292 |
||||
| 191 Wu X, Xu F, Liu J, Wang G: Comparative study of dendritic cells matured by using IL-1beta, IL-6, TNF-alpha and prostaglandins E2 for different time span. Exp Ther Med 2017;14:1389-1394. https://doi.org/10.3892/etm.2017.4649 |
||||
| 192 Vega-Ramos J, Roquilly A, Zhan Y, Young LJ, Mintern JD, Villadangos JA: Inflammation conditions mature dendritic cells to retain the capacity to present new antigens but with altered cytokine secretion function. J Immunol 2014;193:3851-3859. https://doi.org/10.4049/jimmunol.1303215 |
||||
| 193 Turner MD, Nedjai B, Hurst T, Pennington DJ: Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta 2014;1843:2563-2582. https://doi.org/10.1016/j.bbamcr.2014.05.014 |
||||
| 194 Fullerton JN, Gilroy DW: Resolution of inflammation: a new therapeutic frontier. Nature reviews Drug discovery 2016;15:551-567. https://doi.org/10.1038/nrd.2016.39 |
||||
| 195 Shu X, Keller TCt, Begandt D, Butcher JT, Biwer L, Keller AS, Columbus L, Isakson BE: Endothelial nitric oxide synthase in the microcirculation. Cell Mol Life Sci 2015;72:4561-4575. https://doi.org/10.1007/s00018-015-2021-0 |
||||
| 196 Serhan CN, Chiang N, Dalli J, Levy BD: Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol 2014;7:a016311. https://doi.org/10.1101/cshperspect.a016311 |
||||
| 197 Spite M, Serhan CN: Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res 2010;107:1170-1184. https://doi.org/10.1161/CIRCRESAHA.110.223883 |
||||
| 198 Yoshimura A, Ito M, Chikuma S, Akanuma T, Nakatsukasa H: Negative Regulation of Cytokine Signaling in Immunity. Cold Spring Harb Perspect Biol 2018;10:a028571. https://doi.org/10.1101/cshperspect.a028571 |
||||
| 199 Sun SC: A20 restricts inflammation via ubiquitin binding. Nat Immunol 2020;21:362-364. https://doi.org/10.1038/s41590-020-0632-6 |
||||
| 200 Proto JD, Doran AC, Gusarova G, Yurdagul A, Jr., Sozen E, Subramanian M, Islam MN, Rymond CC, Du J, Hook J, Kuriakose G, Bhattacharya J, Tabas I: Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity 2018;49:666-677 e666. https://doi.org/10.1016/j.immuni.2018.07.015 |
||||
| 201 Croasdell Lucchini A, Gachanja NN, Rossi AG, Dorward DA, Lucas CD: Epithelial Cells and Inflammation in Pulmonary Wound Repair. Cells 2021;10:339. https://doi.org/10.3390/cells10020339 |
||||
| 202 Raghavan S, Leo MD: Histamine Potentiates SARS-CoV-2 Spike Protein Entry Into Endothelial Cells. Front Pharmacol 2022;13:872736. https://doi.org/10.3389/fphar.2022.872736 |
||||
| 203 Veenith T, Martin H, Le Breuilly M, Whitehouse T, Gao-Smith F, Duggal N, Lord JM, Mian R, Sarphie D, Moss P: High generation of reactive oxygen species from neutrophils in patients with severe COVID-19. Sci Rep 2022;12:10484. https://doi.org/10.1038/s41598-022-13825-7 |
||||
| 204 Tian M, Liu W, Li X, Zhao P, Shereen MA, Zhu C, Huang S, Liu S, Yu X, Yue M, Pan P, Wang W, Li Y, Chen X, Wu K, Luo Z, Zhang Q, Wu J: HIF-1alpha promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19. Signal Transduct Target Ther 2021;6:308. https://doi.org/10.1038/s41392-021-00726-w |
||||
| 205 Morris DR, Qu Y, Agrawal A, Garofalo RP, Casola A: HIF-1alpha Modulates Core Metabolism and Virus Replication in Primary Airway Epithelial Cells Infected with Respiratory Syncytial Virus. Viruses 2020;12:1088. https://doi.org/10.3390/v12101088 |
||||
| 206 Ling L, Chen Z, Lui G, Wong CK, Wong WT, Ng RWY, Tso EYK, Fung KSC, Chan V, Yeung ACM, Hui DSC, Chan PKS: Longitudinal Cytokine Profile in Patients With Mild to Critical COVID-19. Front Immunol 2021;12:763292. https://doi.org/10.3389/fimmu.2021.763292 |
||||
| 207 Gu Y, Zuo X, Zhang S, Ouyang Z, Jiang S, Wang F, Wang G: The Mechanism behind Influenza Virus Cytokine Storm. Viruses 2021;13:1362. https://doi.org/10.3390/v13071362 |
||||
| 208 Lage SL, Amaral EP, Hilligan KL, Laidlaw E, Rupert A, Namasivayan S, Rocco J, Galindo F, Kellogg A, Kumar P, Poon R, Wortmann GW, Shannon JP, Hickman HD, Lisco A, Manion M, Sher A, Sereti I: Persistent Oxidative Stress and Inflammasome Activation in CD14(high)CD16(-) Monocytes From COVID-19 Patients. Front Immunol 2021;12:799558. https://doi.org/10.3389/fimmu.2021.799558 |
||||
| 209 Lim JY, Oh E, Kim Y, Jung WW, Kim HS, Lee J, Sul D: Enhanced oxidative damage to DNA, lipids, and proteins and levels of some antioxidant enzymes, cytokines, and heat shock proteins in patients infected with influenza H1N1 virus. Acta Virol 2014;58:253-260. https://doi.org/10.4149/av_2014_03_253 |
||||
| 210 Nin N, Sanchez-Rodriguez C, Ver LS, Cardinal P, Ferruelo A, Soto L, Deicas A, Campos N, Rocha O, Ceraso DH, El-Assar M, Ortin J, Fernandez-Segoviano P, Esteban A, Lorente JA: Lung histopathological findings in fatal pandemic influenza A (H1N1). Med Intensiva 2012;36:24-31. https://doi.org/10.1016/j.medin.2011.10.005 |
||||
| 211 Lee JS, Park S, Jeong HW, Ahn JY, Choi SJ, Lee H, Choi B, Nam SK, Sa M, Kwon JS, Jeong SJ, Lee HK, Park SH, Park SH, Choi JY, Kim SH, Jung I, Shin EC: Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol 2020;5:eabd1554. https://doi.org/10.1126/sciimmunol.abd1554 |
||||
| 212 Kim H, Jang M, Kim Y, Choi J, Jeon J, Kim J, Hwang YI, Kang JS, Lee WJ: Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. J Pharm Pharmacol 2016;68:406-420. https://doi.org/10.1111/jphp.12529 |
||||
| 213 Fisher BJ, Seropian IM, Kraskauskas D, Thakkar JN, Voelkel NF, Fowler AA, 3rd, Natarajan R: Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit Care Med 2011;39:1454-1460. https://doi.org/10.1097/CCM.0b013e3182120cb8 |
||||
| 214 Huang A, Vita JA, Venema RC, Keaney JF, Jr.: Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem 2000;275:17399-17406. https://doi.org/10.1074/jbc.M002248200 |
||||
| 215 Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER: L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem 2001;276:40-47. https://doi.org/10.1074/jbc.M004392200 |
||||
| 216 Varadharaj S, Steinhour E, Hunter MG, Watkins T, Baran CP, Magalang U, Kuppusamy P, Zweier JL, Marsh CB, Natarajan V, Parinandi NL: Vitamin C-induced activation of phospholipase D in lung microvascular endothelial cells: regulation by MAP kinases. Cell Signal 2006;18:1396-1407. https://doi.org/10.1016/j.cellsig.2005.10.019 |
||||
| 217 Granger M, Eck P: Dietary Vitamin C in Human Health. Adv Food Nutr Res 2018;83:281-310. https://doi.org/10.1016/bs.afnr.2017.11.006 |
||||
| 218 Carcamo JM, Pedraza A, Borquez-Ojeda O, Zhang B, Sanchez R, Golde DW: Vitamin C is a kinase inhibitor: dehydroascorbic acid inhibits IkappaBalpha kinase beta. Mol Cell Biol 2004;24:6645-6652. https://doi.org/10.1128/MCB.24.15.6645-6652.2004 |
||||
| 219 Osipyants AI, Poloznikov AA, Smirnova NA, Hushpulian DM, Khristichenko AY, Chubar TA, Zakhariants AA, Ahuja M, Gaisina IN, Thomas B, Brown AM, Gazaryan IG, Tishkov VI: L-ascorbic acid: A true substrate for HIF prolyl hydroxylase? Biochimie 2018;147:46-54. https://doi.org/10.1016/j.biochi.2017.12.011 |
||||
| 220 Trinh TA, Hoang TX, Kim JY: All-trans retinoic acid increases NF-kappaB activity in PMA-stimulated THP-1 cells upon unmethylated CpG challenge by enhancing cell surface TLR9 expression. Mol Cell Biochem 2020;473:167-177. https://doi.org/10.1007/s11010-020-03817-4 |
||||
| 221 Alatshan A, Kovacs GE, Aladdin A, Czimmerer Z, Tar K, Benko S: All-Trans Retinoic Acid Enhances both the Signaling for Priming and the Glycolysis for Activation of NLRP3 Inflammasome in Human Macrophage. Cells 2020;9:1591. https://doi.org/10.3390/cells9071591 |
||||
| 222 Wang X, Allen C, Ballow M: Retinoic acid enhances the production of IL-10 while reducing the synthesis of IL-12 and TNF-alpha from LPS-stimulated monocytes/macrophages. J Clin Immunol 2007;27:193-200. https://doi.org/10.1007/s10875-006-9068-5 |
||||
| 223 Austenaa LM, Carlsen H, Hollung K, Blomhoff HK, Blomhoff R: Retinoic acid dampens LPS-induced NF-kappaB activity: results from human monoblasts and in vivo imaging of NF-kappaB reporter mice. J Nutr Biochem 2009;20:726-734. https://doi.org/10.1016/j.jnutbio.2008.07.002 |
||||
| 224 Nurrahmah QI, Madhyastha R, Madhyastha H, Purbasari B, Maruyama M, Nakajima Y: Retinoic acid abrogates LPS-induced inflammatory response via negative regulation of NF-kappa B/miR-21 signaling. Immunopharmacol Immunotoxicol 2021;43:299-308. https://doi.org/10.1080/08923973.2021.1902348 |
||||
| 225 Moreno-Vinasco L, Verbout NG, Fryer AD, Jacoby DB: Retinoic acid prevents virus-induced airway hyperreactivity and M2 receptor dysfunction via anti-inflammatory and antiviral effects. Am J Physiol Lung Cell Mol Physiol 2009;297:L340-346. https://doi.org/10.1152/ajplung.90267.2008 |
||||
| 226 Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, Slominski AT: 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One 2009;4:e5988. https://doi.org/10.1371/journal.pone.0005988 |
||||
| 227 Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E: Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 2012;188:2127-2135. https://doi.org/10.4049/jimmunol.1102412 |
||||
| 228 Dauletbaev N, Herscovitch K, Das M, Chen H, Bernier J, Matouk E, Berube J, Rousseau S, Lands LC: Down-regulation of IL-8 by high-dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up-regulation of DUSP1. Br J Pharmacol 2015;172:4757-4771. https://doi.org/10.1111/bph.13249 |
||||
| 229 Wang Q, He Y, Shen Y, Zhang Q, Chen D, Zuo C, Qin J, Wang H, Wang J, Yu Y: Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J Biol Chem 2014;289:11681-11694. https://doi.org/10.1074/jbc.M113.517581 |
||||
| 230 Liang S, Cai J, Li Y, Yang R: 1, 25DihydroxyVitamin D3 induces macrophage polarization to M2 by upregulating Tcell Igmucin3 expression. Mol Med Rep 2019;19:3707-3713. https://doi.org/10.3892/mmr.2019.10047 |
||||
| 231 Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC: Vitamin D receptor inhibits nuclear factor kappaB activation by interacting with IkappaB kinase beta protein. J Biol Chem 2013;288:19450-19458. https://doi.org/10.1074/jbc.M113.467670 |
||||
| 232 Sun J, Kong J, Duan Y, Szeto FL, Liao A, Madara JL, Li YC: Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab 2006;291:E315-322. https://doi.org/10.1152/ajpendo.00590.2005 |
||||
| 233 Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, Sheikh A, Griffiths CJ: Vitamin D for the management of asthma. Cochrane Database Syst Rev 2016;9:CD011511. https://doi.org/10.1002/14651858.CD011511 |
||||
| 234 Li X, He J, Yu M, Sun J: The efficacy of vitamin D therapy for patients with COPD: a meta-analysis of randomized controlled trials. Ann Palliat Med 2020;9:286-297. https://doi.org/10.21037/apm.2020.02.26 |
||||
| 235 Barrea L, Grant WB, Frias-Toral E, Vetrani C, Verde L, de Alteriis G, Docimo A, Savastano S, Colao A, Muscogiuri G: Dietary Recommendations for Post-COVID-19 Syndrome. Nutrients 2022;14:1305. https://doi.org/10.3390/nu14061305 |
||||
| 236 Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, et al.: Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583. https://doi.org/10.1136/bmj.i6583 |
||||
| 237 Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP: Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020;12:988. https://doi.org/10.3390/nu12040988 |
||||
| 238 Rocksen D, Ekstrand-Hammarstrom B, Johansson L, Bucht A: Vitamin E reduces transendothelial migration of neutrophils and prevents lung injury in endotoxin-induced airway inflammation. Am J Respir Cell Mol Biol 2003;28:199-207. https://doi.org/10.1165/rcmb.4899 |
||||
| 239 Wang Y, Jiang Q: gamma-Tocotrienol inhibits lipopolysaccharide-induced interlukin-6 and granulocyte colony-stimulating factor by suppressing C/EBPbeta and NF-kappaB in macrophages. J Nutr Biochem 2013;24:1146-1152. https://doi.org/10.1016/j.jnutbio.2012.08.015 |
||||
| 240 Wang Y, Park NY, Jang Y, Ma A, Jiang Q: Vitamin E gamma-Tocotrienol Inhibits Cytokine-Stimulated NF-kappaB Activation by Induction of Anti-Inflammatory A20 via Stress Adaptive Response Due to Modulation of Sphingolipids. J Immunol 2015;195:126-133. https://doi.org/10.4049/jimmunol.1403149 |
||||
| 241 Yang C, Jiang Q: Vitamin E delta-tocotrienol inhibits TNF-alpha-stimulated NF-kappaB activation by up-regulation of anti-inflammatory A20 via modulation of sphingolipid including elevation of intracellular dihydroceramides. J Nutr Biochem 2019;64:101-109. https://doi.org/10.1016/j.jnutbio.2018.10.013 |
||||
| 242 Das T, Chen Z, Hendriks RW, Kool M: A20/Tumor Necrosis Factor alpha-Induced Protein 3 in Immune Cells Controls Development of Autoinflammation and Autoimmunity: Lessons from Mouse Models. Front Immunol 2018;9:104. https://doi.org/10.3389/fimmu.2018.00104 |
||||
| 243 Kim MH, Jeong HJ: Zinc Oxide Nanoparticles Suppress LPS-Induced NF-kappaB Activation by Inducing A20, a Negative Regulator of NF-kappaB, in RAW 264.7 Macrophages. J Nanosci Nanotechnol 2015;15:6509-6515. https://doi.org/10.1166/jnn.2015.10319 |
||||
| 244 von Bulow V, Rink L, Haase H: Zinc-mediated inhibition of cyclic nucleotide phosphodiesterase activity and expression suppresses TNF-alpha and IL-1 beta production in monocytes by elevation of guanosine 3',5'-cyclic monophosphate. J Immunol 2005;175:4697-4705. https://doi.org/10.4049/jimmunol.175.7.4697 |
||||
| 245 von Bulow V, Dubben S, Engelhardt G, Hebel S, Plumakers B, Heine H, Rink L, Haase H: Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B. J Immunol 2007;179:4180-4186. https://doi.org/10.4049/jimmunol.179.6.4180 |
||||
| 246 Takahashi N, Tetsuka T, Uranishi H, Okamoto T: Inhibition of the NF-kappaB transcriptional activity by protein kinase A. Eur J Biochem 2002;269:4559-4565. https://doi.org/10.1046/j.1432-1033.2002.03157.x |
||||
| 247 Constantino L, Goncalves RC, Giombelli VR, Tomasi CD, Vuolo F, Kist LW, de Oliveira GM, Pasquali MA, Bogo MR, Mauad T, Horn A, Jr., Melo KV, Fernandes C, Moreira JC, Ritter C, Dal-Pizzol F: Regulation of lung oxidative damage by endogenous superoxide dismutase in sepsis. Intensive Care Med Exp 2014;2:17. https://doi.org/10.1186/2197-425X-2-17 |
||||
| 248 Malpuech-Brugere C, Nowacki W, Rock E, Gueux E, Mazur A, Rayssiguier Y: Enhanced tumor necrosis factor-alpha production following endotoxin challenge in rats is an early event during magnesium deficiency. Biochim Biophys Acta 1999;1453:35-40. https://doi.org/10.1016/S0925-4439(98)00081-7 |
||||
| 249 Zhang L, Yang L, Xie X, Zheng H, Zheng H, Zhang L, Liu C, Piao JG, Li F: Baicalin Magnesium Salt Attenuates Lipopolysaccharide-Induced Acute Lung Injury via Inhibiting of TLR4/NF-kappaB Signaling Pathway. J Immunol Res 2021;2021:6629531. https://doi.org/10.1155/2021/6629531 |
||||
| 250 Libako P, Nowacki W, Castiglioni S, Mazur A, Maier JA: Extracellular magnesium and calcium blockers modulate macrophage activity. Magnes Res 2016;29:11-21. https://doi.org/10.1684/mrh.2016.0398 |
||||
| 251 Bussiere FI, Gueux E, Rock E, Mazur A, Rayssiguier Y: Protective effect of calcium deficiency on the inflammatory response in magnesium-deficient rats. Eur J Nutr 2002;41:197-202. https://doi.org/10.1007/s00394-002-0376-0 |
||||
| 252 Reina-Campos M, Scharping NE, Goldrath AW: CD8(+) T cell metabolism in infection and cancer. Nat Rev Immunol 2021;21:718-738. https://doi.org/10.1038/s41577-021-00537-8 |
||||
| 253 Bhat P, Leggatt G, Waterhouse N, Frazer IH: Interferon-gamma derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis 2017;8:e2836. https://doi.org/10.1038/cddis.2017.67 |
||||
| 254 McMaster SR, Wilson JJ, Wang H, Kohlmeier JE: Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production. J Immunol 2015;195:203-209. https://doi.org/10.4049/jimmunol.1402975 |
||||
| 255 Schmidt ME, Varga SM: The CD8 T Cell Response to Respiratory Virus Infections. Front Immunol 2018;9:678. https://doi.org/10.3389/fimmu.2018.00678 |
||||
| 256 McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ: CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc Natl Acad Sci U S A 2011;108:7529-7534. https://doi.org/10.1073/pnas.1103782108 |
||||
| 257 Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y, Kobayashi Y, Flavell RA, Kleinstein SH, Craft J, Kaech SM: Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat Immunol 2015;16:871-879. https://doi.org/10.1038/ni.3224 |
||||
| 258 de Goer de Herve MG, Jaafoura S, Vallee M, Taoufik Y: FoxP3(+) regulatory CD4 T cells control the generation of functional CD8 memory. Nat Commun 2012;3:986. https://doi.org/10.1038/ncomms1992 |
||||
| 259 Erickson JJ, Lu P, Wen S, Hastings AK, Gilchuk P, Joyce S, Shyr Y, Williams JV: Acute Viral Respiratory Infection Rapidly Induces a CD8+ T Cell Exhaustion-like Phenotype. J Immunol 2015;195:4319-4330. https://doi.org/10.4049/jimmunol.1403004 |
||||
| 260 Rutigliano JA, Sharma S, Morris MY, Oguin TH, 3rd, McClaren JL, Doherty PC, Thomas PG: Highly pathological influenza A virus infection is associated with augmented expression of PD-1 by functionally compromised virus-specific CD8+ T cells. J Virol 2014;88:1636-1651. https://doi.org/10.1128/JVI.02851-13 |
||||
| 261 Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Liu Y, Wang G, Yuan Z, Feng Z, Zhang Y, Wu Y, Chen Y: Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol 2020;11:827. https://doi.org/10.3389/fimmu.2020.00827 |
||||
| 262 Dzhagalov I, Chambon P, He YW: Regulation of CD8+ T lymphocyte effector function and macrophage inflammatory cytokine production by retinoic acid receptor gamma. J Immunol 2007;178:2113-2121. https://doi.org/10.4049/jimmunol.178.4.2113 |
||||
| 263 Guo Y, Lee YC, Brown C, Zhang W, Usherwood E, Noelle RJ: Dissecting the role of retinoic acid receptor isoforms in the CD8 response to infection. J Immunol 2014;192:3336-3344. https://doi.org/10.4049/jimmunol.1301949 |
||||
| 264 Jansa P, Forejt J: A novel type of retinoic acid response element in the second intron of the mouse H2Kb gene is activated by the RAR/RXR heterodimer. Nucleic Acids Res 1996;24:694-701. https://doi.org/10.1093/nar/24.4.694 |
||||
| 265 Tan X, Sande JL, Pufnock JS, Blattman JN, Greenberg PD: Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. J Virol 2011;85:8316-8327. https://doi.org/10.1128/JVI.00781-11 |
||||
| 266 Yuzefpolskiy Y, Baumann FM, Penny LA, Studzinski GP, Kalia V, Sarkar S: Vitamin D receptor signals regulate effector and memory CD8 T cell responses to infections in mice. J Nutr 2014;144:2073-2082. https://doi.org/10.3945/jn.114.202895 |
||||
| 267 Li P, Zhu X, Cao G, Wu R, Li K, Yuan W, Chen B, Sun G, Xia X, Zhang H, Wang X, Yin Z, Lu L, Gao Y: 1alpha,25(OH)2D3 reverses exhaustion and enhances antitumor immunity of human cytotoxic T cells. J Immunother Cancer 2022;10:e003477. https://doi.org/10.1136/jitc-2021-003477 |
||||
| 268 Zhu Y, van Essen D, Saccani S: Cell-type-specific control of enhancer activity by H3K9 trimethylation. Mol Cell 2012;46:408-423. https://doi.org/10.1016/j.molcel.2012.05.011 |
||||
| 269 Kroening PR, Barnes TW, Pease L, Limper A, Kita H, Vassallo R: Cigarette smoke-induced oxidative stress suppresses generation of dendritic cell IL-12 and IL-23 through ERK-dependent pathways. J Immunol 2008;181:1536-1547. https://doi.org/10.4049/jimmunol.181.2.1536 |
||||
| 270 Jeong YJ, Hong SW, Kim JH, Jin DH, Kang JS, Lee WJ, Hwang YI: Vitamin C-treated murine bone marrow-derived dendritic cells preferentially drive naive T cells into Th1 cells by increased IL-12 secretions. Cell Immunol 2011;266:192-199. https://doi.org/10.1016/j.cellimm.2010.10.005 |
||||
| 271 Jeong YJ, Kim JH, Hong JM, Kang JS, Kim HR, Lee WJ, Hwang YI: Vitamin C treatment of mouse bone marrow-derived dendritic cells enhanced CD8(+) memory T cell production capacity of these cells in vivo . Immunobiology 2014;219:554-564. https://doi.org/10.1016/j.imbio.2014.03.006 |
||||
| 272 Luchtel RA, Bhagat T, Pradhan K, Jacobs WR, Jr., Levine M, Verma A, Shenoy N: High-dose ascorbic acid synergizes with anti-PD1 in a lymphoma mouse model. Proc Natl Acad Sci U S A 2020;117:1666-1677. https://doi.org/10.1073/pnas.1908158117 |
||||
| 273 Pilipow K, Scamardella E, Puccio S, Gautam S, De Paoli F, Mazza EM, De Simone G, Polletti S, Buccilli M, Zanon V, Di Lucia P, Iannacone M, Gattinoni L, Lugli E: Antioxidant metabolism regulates CD8+ T memory stem cell formation and antitumor immunity. JCI Insight 2018;3:e122299. https://doi.org/10.1172/jci.insight.122299 |
||||
| 274 Kanellopoulou C, George AB, Masutani E, Cannons JL, Ravell JC, Yamamoto TN, Smelkinson MG, Jiang PD, Matsuda-Lennikov M, Reilley J, Handon R, Lee PH, Miller JR, Restifo NP, Zheng L, Schwartzberg PL, Young M, Lenardo MJ: Mg(2+) regulation of kinase signaling and immune function. J Exp Med 2019;216:1828-1842. https://doi.org/10.1084/jem.20181970 |
||||
| 275 Shipkova M, Wieland E: Surface markers of lymphocyte activation and markers of cell proliferation. Clin Chim Acta 2012;413:1338-1349. https://doi.org/10.1016/j.cca.2011.11.006 |
||||
| 276 Lotscher J, Marti ILAA, Kirchhammer N, Cribioli E, Giordano Attianese GMP, Trefny MP, Lenz M, Rothschild SI, Strati P, Kunzli M, Lotter C, Schenk SH, Dehio P, Loliger J, Litzler L, Schreiner D, Koch V, Page N, Lee D, Grahlert J, et al.: Magnesium sensing via LFA-1 regulates CD8(+) T cell effector function. Cell 2022;185:585-602 e529. https://doi.org/10.1016/j.cell.2021.12.039 |
||||
| 277 Harker N, Naito T, Cortes M, Hostert A, Hirschberg S, Tolaini M, Roderick K, Georgopoulos K, Kioussis D: The CD8alpha gene locus is regulated by the Ikaros family of proteins. Mol Cell 2002;10:1403-1415. https://doi.org/10.1016/S1097-2765(02)00711-6 |
||||
| 278 Bilic I, Koesters C, Unger B, Sekimata M, Hertweck A, Maschek R, Wilson CB, Ellmeier W: Negative regulation of CD8 expression via Cd8 enhancer-mediated recruitment of the zinc finger protein MAZR. Nat Immunol 2006;7:392-400. https://doi.org/10.1038/ni1311 |
||||
| 279 Omilusik KD, Best JA, Yu B, Goossens S, Weidemann A, Nguyen JV, Seuntjens E, Stryjewska A, Zweier C, Roychoudhuri R, Gattinoni L, Bird LM, Higashi Y, Kondoh H, Huylebroeck D, Haigh J, Goldrath AW: Transcriptional repressor ZEB2 promotes terminal differentiation of CD8+ effector and memory T cell populations during infection. J Exp Med 2015;212:2027-2039. https://doi.org/10.1084/jem.20150194 |
||||
| 280 Clambey ET, Collins B, Young MH, Eberlein J, David A, Kappler JW, Marrack P: The Ikaros transcription factor regulates responsiveness to IL-12 and expression of IL-2 receptor alpha in mature, activated CD8 T cells. PLoS One 2013;8:e57435. https://doi.org/10.1371/journal.pone.0057435 |
||||
| 281 Luckheeram RV, Zhou R, Verma AD, Xia B: CD4(+)T cells: differentiation and functions. Clin Dev Immunol 2012;2012:925135. https://doi.org/10.1155/2012/925135 |
||||
| 282 Olatunde AC, Hale JS, Lamb TJ: Cytokine-skewed Tfh cells: functional consequences for B cell help. Trends Immunol 2021;42:536-550. https://doi.org/10.1016/j.it.2021.04.006 |
||||
| 283 Zhu J, Yamane H, Paul WE: Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 2010;28:445-489. https://doi.org/10.1146/annurev-immunol-030409-101212 |
||||
| 284 Gil-Etayo FJ, Garcinuno S, Utrero-Rico A, Cabrera-Marante O, Arroyo-Sanchez D, Mancebo E, Pleguezuelo DE, Rodriguez-Frias E, Allende LM, Morales-Perez P, Castro-Panete MJ, Lalueza A, Lumbreras C, Paz-Artal E, Serrano A: An Early Th1 Response Is a Key Factor for a Favorable COVID-19 Evolution. Biomedicines 2022;10:296. https://doi.org/10.3390/biomedicines10020296 |
||||
| 285 Brown DM, Lee S, Garcia-Hernandez Mde L, Swain SL: Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J Virol 2012;86:6792-6803. https://doi.org/10.1128/JVI.07172-11 |
||||
| 286 Zhang S, Zhang H, Zhao J: The role of CD4 T cell help for CD8 CTL activation. Biochem Biophys Res Commun 2009;384:405-408. https://doi.org/10.1016/j.bbrc.2009.04.134 |
||||
| 287 Huang S, He Q, Zhou L: T cell responses in respiratory viral infections and chronic obstructive pulmonary disease. Chin Med J (Engl) 2021;134:1522-1534. https://doi.org/10.1097/CM9.0000000000001388 |
||||
| 288 Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY: A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 2015;162:1078-1089. https://doi.org/10.1016/j.cell.2015.08.021 |
||||
| 289 Muramatsu M, Yoshida R, Yokoyama A, Miyamoto H, Kajihara M, Maruyama J, Nao N, Manzoor R, Takada A: Comparison of antiviral activity between IgA and IgG specific to influenza virus hemagglutinin: increased potential of IgA for heterosubtypic immunity. PLoS One 2014;9:e85582. https://doi.org/10.1371/journal.pone.0085582 |
||||
| 290 Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claer L, Quentric P, Fadlallah J, Devilliers H, Ghillani P, Gunn C, Hockett R, Mudumba S, Guihot A, Luyt CE, Mayaux J, Beurton A, Fourati S, Bruel T, Schwartz O, et al.: IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 2021;13:eabd2223. https://doi.org/10.1126/scitranslmed.abd2223 |
||||
| 291 Walsh EE, Falsey AR: Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis 2004;190:373-378. https://doi.org/10.1086/421524 |
||||
| 292 van Erp EA, Luytjes W, Ferwerda G, van Kasteren PB: Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front Immunol 2019;10:548. https://doi.org/10.3389/fimmu.2019.00548 |
||||
| 293 Shen L, Li S, Zhu Y, Zhao J, Tang X, Li H, Xing H, Lu M, Frederick C, Huang C, Wong G, Wang C, Lan J: Clinical and laboratory-derived parameters of 119 hospitalized patients with coronavirus disease 2019 in Xiangyang, Hubei Province, China. J Infect 2020;81:147-178. https://doi.org/10.1016/j.jinf.2020.03.038 |
||||
| 294 Lalueza A, Folgueira D, Diaz-Pedroche C, Hernandez-Jimenez P, Ayuso B, Castillo C, Laureiro J, Trujillo H, Torres M, Lumbreras C: Severe lymphopenia in hospitalized patients with influenza virus infection as a marker of a poor outcome. Infect Dis (Lond) 2019;51:543-546. https://doi.org/10.1080/23744235.2019.1598572 |
||||
| 295 Gul A, Khan S, Arshad M, Anjum SI, Attaullah S, Ali I, Rauf A, Arshad A, Alghanem SM, Khan SN: Peripheral blood T cells response in human parainfluenza virus-associated lower respiratory tract infection in children. Saudi J Biol Sci 2020;27:2847-2852. https://doi.org/10.1016/j.sjbs.2020.07.005 |
||||
| 296 Ramos-Fernandez JM, Moreno-Perez D, Antunez-Fernandez C, Milano-Manso G, Cordon-Martinez AM, Urda-Cardona A: [Lower lymphocyte response in severe cases of acute bronchiolitis due to respiratory syncytial virus]. An Pediatr (Engl Ed) 2018;88:315-321. https://doi.org/10.1016/j.anpede.2017.07.005 |
||||
| 297 Liu S, Huang Z, Fan R, Jia J, Deng X, Zou X, Li H, Cao B: Cycling and activated CD8(+) T lymphocytes and their association with disease severity in influenza patients. BMC Immunol 2022;23:40. https://doi.org/10.1186/s12865-022-00516-1 |
||||
| 298 Roman M, Calhoun WJ, Hinton KL, Avendano LF, Simon V, Escobar AM, Gaggero A, Diaz PV: Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Respir Crit Care Med 1997;156:190-195. https://doi.org/10.1164/ajrccm.156.1.9611050 |
||||
| 299 Pinto RA, Arredondo SM, Bono MR, Gaggero AA, Diaz PV: T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics 2006;117:e878-886. https://doi.org/10.1542/peds.2005-2119 |
||||
| 300 Guo-Parke H, Linden D, Mousnier A, Scott IC, Killick H, Borthwick LA, Fisher AJ, Weldon S, Taggart CC, Kidney JC: Altered Differentiation and Inflammation Profiles Contribute to Enhanced Innate Responses in Severe COPD Epithelium to Rhinovirus Infection. Front Med (Lausanne) 2022;9:741989. https://doi.org/10.3389/fmed.2022.741989 |
||||
| 301 Liu Y, Qin T, Zhao X, Dong S, Zhu J, Peng D, Zhong J, Li T, Chen X: Skewed balance of regulatory T cell and inflammatory T cell in IL-17 defect with human metapneumovirus infection. Cell Immunol 2018;331:161-167. https://doi.org/10.1016/j.cellimm.2018.06.007 |
||||
| 302 Choreno-Parra JA, Jimenez-Alvarez LA, Cruz-Lagunas A, Rodriguez-Reyna TS, Ramirez-Martinez G, Sandoval-Vega M, Hernandez-Garcia DL, Choreno-Parra EM, Balderas-Martinez YI, Martinez-Sanchez ME, Marquez-Garcia E, Sciutto E, Moreno-Rodriguez J, Barreto-Rodriguez JO, Vazquez-Rojas H, Centeno-Saenz GI, Alvarado-Pena N, Salinas-Lara C, Sanchez-Garibay C, Galeana-Cadena D, et al.: Clinical and Immunological Factors That Distinguish COVID-19 From Pandemic Influenza A(H1N1). Front Immunol 2021;12:593595. https://doi.org/10.3389/fimmu.2021.593595 |
||||
| 303 Habibi MS, Jozwik A, Makris S, Dunning J, Paras A, DeVincenzo JP, de Haan CA, Wrammert J, Openshaw PJ, Chiu C, Mechanisms of Severe Acute Influenza Consortium I: Impaired Antibody-mediated Protection and Defective IgA B-Cell Memory in Experimental Infection of Adults with Respiratory Syncytial Virus. Am J Respir Crit Care Med 2015;191:1040-1049. https://doi.org/10.1164/rccm.201412-2256OC |
||||
| 304 Oh JE, Song E, Moriyama M, Wong P, Zhang S, Jiang R, Strohmeier S, Kleinstein SH, Krammer F, Iwasaki A: Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Sci Immunol 2021;6:eabj5129. https://doi.org/10.1126/sciimmunol.abj5129 |
||||
| 305 Kim MH, Kim HJ, Chang J: Superior immune responses induced by intranasal immunization with recombinant adenovirus-based vaccine expressing full-length Spike protein of Middle East respiratory syndrome coronavirus. PLoS One 2019;14:e0220196. https://doi.org/10.1371/journal.pone.0220196 |
||||
| 306 Chan RWY, Chan KCC, Lui GCY, Tsun JGS, Chan KYY, Yip JSK, Liu S, Yu MWL, Ng RWY, Chong KKL, Wang MH, Chan PKS, Li AM, Lam HS: Mucosal Antibody Response to SARS-CoV-2 in Paediatric and Adult Patients: A Longitudinal Study. Pathogens 2022;11:397. https://doi.org/10.3390/pathogens11040397 |
||||
| 307 Smith-Norowitz TA, Mandal M, Joks R, Norowitz LT, Weaver D, Durkin HG, Bluth MH, Kohlhoff S: IgE anti-respiratory syncytial virus antibodies detected in serum of pediatric patients with asthma. Hum Immunol 2015;76:519-524. https://doi.org/10.1016/j.humimm.2015.06.002 |
||||
| 308 Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F: A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 2007;204:1757-1764. https://doi.org/10.1084/jem.20070590 |
||||
| 309 Agrawal S, Ganguly S, Tran A, Sundaram P, Agrawal A: Retinoic acid treated human dendritic cells induce T regulatory cells via the expression of CD141 and GARP which is impaired with age. Aging (Albany NY) 2016;8:1223-1235. https://doi.org/10.18632/aging.100973 |
||||
| 310 Zai K, Yuzuriha K, Kishimura A, Mori T, Katayama Y: Preparation of Complexes between Ovalbumin Nanoparticles and Retinoic Acid for Efficient Induction of Tolerogenic Dendritic Cells. Anal Sci 2018;34:1243-1248. https://doi.org/10.2116/analsci.18P252 |
||||
| 311 Geissmann F, Revy P, Brousse N, Lepelletier Y, Folli C, Durandy A, Chambon P, Dy M: Retinoids regulate survival and antigen presentation by immature dendritic cells. J Exp Med 2003;198:623-634. https://doi.org/10.1084/jem.20030390 |
||||
| 312 Brown CC, Esterhazy D, Sarde A, London M, Pullabhatla V, Osma-Garcia I, Al-Bader R, Ortiz C, Elgueta R, Arno M, de Rinaldis E, Mucida D, Lord GM, Noelle RJ: Retinoic acid is essential for Th1 cell lineage stability and prevents transition to a Th17 cell program. Immunity 2015;42:499-511. https://doi.org/10.1016/j.immuni.2015.02.003 |
||||
| 313 Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK: Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol 2008;181:2277-2284. https://doi.org/10.4049/jimmunol.181.4.2277 |
||||
| 314 Stephensen CB, Rasooly R, Jiang X, Ceddia MA, Weaver CT, Chandraratna RA, Bucy RP: Vitamin A enhances in vitro Th2 development via retinoid X receptor pathway. J Immunol 2002;168:4495-4503. https://doi.org/10.4049/jimmunol.168.9.4495 |
||||
| 315 Iwata M, Eshima Y, Kagechika H: Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol 2003;15:1017-1025. https://doi.org/10.1093/intimm/dxg101 |
||||
| 316 Koning JJ, Rajaraman A, Reijmers RM, Konijn T, Pan J, Ware CF, Butcher EC, Mebius RE: Development of follicular dendritic cells in lymph nodes depends on retinoic acid-mediated signaling. Development 2021;148:dev199713. https://doi.org/10.1242/dev.199713 |
||||
| 317 Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, Fagarasan S: The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity 2010;33:71-83. https://doi.org/10.1016/j.immuni.2010.07.003 |
||||
| 318 Watanabe K, Sugai M, Nambu Y, Osato M, Hayashi T, Kawaguchi M, Komori T, Ito Y, Shimizu A: Requirement for Runx proteins in IgA class switching acting downstream of TGF-beta 1 and retinoic acid signaling. J Immunol 2010;184:2785-2792. https://doi.org/10.4049/jimmunol.0901823 |
||||
| 319 Chen X, Welner RS, Kincade PW: A possible contribution of retinoids to regulation of fetal B lymphopoiesis. Eur J Immunol 2009;39:2515-2524. https://doi.org/10.1002/eji.200939374 |
||||
| 320 Chen X, Esplin BL, Garrett KP, Welner RS, Webb CF, Kincade PW: Retinoids accelerate B lineage lymphoid differentiation. J Immunol 2008;180:138-145. https://doi.org/10.4049/jimmunol.180.1.138 |
||||
| 321 Chen Q, Ross AC: Vitamin A and immune function: retinoic acid modulates population dynamics in antigen receptor and CD38-stimulated splenic B cells. Proc Natl Acad Sci U S A 2005;102:14142-14149. https://doi.org/10.1073/pnas.0505018102 |
||||
| 322 Ertesvag A, Aasheim HC, Naderi S, Blomhoff HK: Vitamin A potentiates CpG-mediated memory B-cell proliferation and differentiation: involvement of early activation of p38MAPK. Blood 2007;109:3865-3872. https://doi.org/10.1182/blood-2006-09-046748 |
||||
| 323 Lee BO, Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Makris M, Sprague F, Lund FE, Randall TD: CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J Immunol 2005;175:5827-5838. https://doi.org/10.4049/jimmunol.175.9.5827 |
||||
| 324 Marks E, Ortiz C, Pantazi E, Bailey CS, Lord GM, Waldschmidt TJ, Noelle RJ, Elgueta R: Retinoic Acid Signaling in B Cells Is Required for the Generation of an Effective T-Independent Immune Response. Front Immunol 2016;7:643. https://doi.org/10.3389/fimmu.2016.00643 |
||||
| 325 Ferreira GB, Gysemans CA, Demengeot J, da Cunha JP, Vanherwegen AS, Overbergh L, Van Belle TL, Pauwels F, Verstuyf A, Korf H, Mathieu C: 1, 25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J Immunol 2014;192:4210-4220. https://doi.org/10.4049/jimmunol.1302350 |
||||
| 326 Ferreira GB, van Etten E, Verstuyf A, Waer M, Overbergh L, Gysemans C, Mathieu C: 1, 25-Dihydroxyvitamin D3 alters murine dendritic cell behaviour in vitro and in vivo . Diabetes Metab Res Rev 2011;27:933-941. https://doi.org/10.1002/dmrr.1275 |
||||
| 327 Sheikh V, Kasapoglu P, Zamani A, Basiri Z, Tahamoli-Roudsari A, Alahgholi-Hajibehzad M: Vitamin D3 inhibits the proliferation of T helper cells, downregulate CD4(+) T cell cytokines and upregulate inhibitory markers. Hum Immunol 2018;79:439-445. https://doi.org/10.1016/j.humimm.2018.03.001 |
||||
| 328 Sloka S, Silva C, Wang J, Yong VW: Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. J Neuroinflammation 2011;8:56. https://doi.org/10.1186/1742-2094-8-56 |
||||
| 329 Zhou Q, Qin S, Zhang J, Zhon L, Pen Z, Xing T: 1, 25(OH)2D3 induces regulatory T cell differentiation by influencing the VDR/PLC-gamma1/TGF-beta1/pathway. Mol Immunol 2017;91:156-164. https://doi.org/10.1016/j.molimm.2017.09.006 |
||||
| 330 Wang Z, Wang Y, Xu B, Liu J, Ren Y, Dai Z, Cui D, Su X, Si S, Song SJ: Vitamin D improves immune function in immunosuppressant mice induced by glucocorticoid. Biomed Rep 2017;6:120-124. https://doi.org/10.3892/br.2016.817 |
||||
| 331 Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE: Modulatory effects of 1, 25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 2007;179:1634-1647. https://doi.org/10.4049/jimmunol.179.3.1634 |
||||
| 332 Danner OK, Matthews LR, Francis S, Rao VN, Harvey CP, Tobin RP, Wilson KL, Alema-Mensah E, Newell Rogers MK, Childs EW: Vitamin D3 Suppresses Class II Invariant Chain Peptide Expression on Activated B-Lymphocytes: A Plausible Mechanism for Downregulation of Acute Inflammatory Conditions. J Nutr Metab 2016;2016:4280876. https://doi.org/10.1155/2016/4280876 |
||||
| 333 Treptow S, Grun J, Scholz J, Radbruch A, Heine G, Worm M: 9-cis Retinoic acid and 1.25-dihydroxyvitamin D3 drive differentiation into IgA(+) secreting plasmablasts in human naive B cells. Eur J Immunol 2021;51:125-137. https://doi.org/10.1002/eji.202048557 |
||||
| 334 Kim HW, Cho SI, Bae S, Kim H, Kim Y, Hwang YI, Kang JS, Lee WJ: Vitamin C Up-regulates Expression of CD80, CD86 and MHC Class II on Dendritic Cell Line, DC-1 Via the Activation of p38 MAPK. Immune Netw 2012;12:277-283. https://doi.org/10.4110/in.2012.12.6.277 |
||||
| 335 Sasidharan Nair V, Song MH, Oh KI: Vitamin C Facilitates Demethylation of the Foxp3 Enhancer in a Tet-Dependent Manner. J Immunol 2016;196:2119-2131. https://doi.org/10.4049/jimmunol.1502352 |
||||
| 336 Liu Z, Cao W, Xu L, Chen X, Zhan Y, Yang Q, Liu S, Chen P, Jiang Y, Sun X, Tao Y, Hu Y, Li C, Wang Q, Wang Y, Chen CD, Shi Y, Zhang X: The histone H3 lysine-27 demethylase Jmjd3 plays a critical role in specific regulation of Th17 cell differentiation. J Mol Cell Biol 2015;7:505-516. https://doi.org/10.1093/jmcb/mjv022 |
||||
| 337 Song MH, Nair VS, Oh KI: Vitamin C enhances the expression of IL17 in a Jmjd2-dependent manner. BMB Rep 2017;50:49-54. https://doi.org/10.5483/BMBRep.2017.50.1.193 |
||||
| 338 Ichiyama K, Mitsuzumi H, Zhong M, Tai A, Tsuchioka A, Kawai S, Yamamoto I, Gohda E: Promotion of IL-4- and IL-5-dependent differentiation of anti-mu-primed B cells by ascorbic acid 2-glucoside. Immunol Lett 2009;122:219-226. https://doi.org/10.1016/j.imlet.2009.01.007 |
||||
| 339 Qi T, Sun M, Zhang C, Chen P, Xiao C, Chang X: Ascorbic Acid Promotes Plasma Cell Differentiation through Enhancing TET2/3-Mediated DNA Demethylation. Cell Rep 2020;33:108452. https://doi.org/10.1016/j.celrep.2020.108452 |
||||
| 340 Woo A, Kim JH, Jeong YJ, Maeng HG, Lee YT, Kang JS, Lee WJ, Hwang YI: Vitamin C acts indirectly to modulate isotype switching in mouse B cells. Anat Cell Biol 2010;43:25-35. https://doi.org/10.5115/acb.2010.43.1.25 |
||||
| 341 Quek AML, Ooi DSQ, Teng O, Chan CY, Ng GJL, Ng MY, Yee S, Cheong EW, Weng R, Cook AR, Hartman M, Angeli V, Tambyah PA, Seet RCS: Zinc and vitamin C intake increases spike and neutralising antibody production following SARS-CoV-2 infection. Clin Transl Med 2022;12:e731. https://doi.org/10.1002/ctm2.731 |
||||
| 342 George MM, Subramanian Vignesh K, Landero Figueroa JA, Caruso JA, Deepe GS, Jr.: Zinc Induces Dendritic Cell Tolerogenic Phenotype and Skews Regulatory T Cell-Th17 Balance. J Immunol 2016;197:1864-1876. https://doi.org/10.4049/jimmunol.1600410 |
||||
| 343 Song XT, Evel-Kabler K, Shen L, Rollins L, Huang XF, Chen SY: A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat Med 2008;14:258-265. https://doi.org/10.1038/nm1721 |
||||
| 344 Maywald M, Wang F, Rink L: The Intracellular Free Zinc Level Is Vital for Treg Function and a Feasible Tool to Discriminate between Treg and Activated Th Cells. Int J Mol Sci 2018;19:3575. https://doi.org/10.3390/ijms19113575 |
||||
| 345 Powell MD, Read KA, Sreekumar BK, Oestreich KJ: Ikaros Zinc Finger Transcription Factors: Regulators of Cytokine Signaling Pathways and CD4(+) T Helper Cell Differentiation. Front Immunol 2019;10:1299. https://doi.org/10.3389/fimmu.2019.01299 |
||||
| 346 Anzilotti C, Swan DJ, Boisson B, Deobagkar-Lele M, Oliveira C, Chabosseau P, Engelhardt KR, Xu X, Chen R, Alvarez L, Berlinguer-Palmini R, Bull KR, Cawthorne E, Cribbs AP, Crockford TL, Dang TS, Fearn A, Fenech EJ, de Jong SJ, Lagerholm BC, et al.: An essential role for the Zn(2+) transporter ZIP7 in B cell development. Nat Immunol 2019;20:350-361. https://doi.org/10.1038/s41590-018-0295-8 |
||||
| 347 Miyai T, Hojyo S, Ikawa T, Kawamura M, Irie T, Ogura H, Hijikata A, Bin BH, Yasuda T, Kitamura H, Nakayama M, Ohara O, Yoshida H, Koseki H, Mishima K, Fukada T: Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc Natl Acad Sci U S A 2014;111:11780-11785. https://doi.org/10.1073/pnas.1323549111 |
||||
| 348 Fields S, Ternyak K, Gao H, Ostraat R, Akerlund J, Hagman J: The 'zinc knuckle' motif of Early B cell Factor is required for transcriptional activation of B cell-specific genes. Mol Immunol 2008;45:3786-3796. https://doi.org/10.1016/j.molimm.2008.05.018 |
||||
| 349 Jurado S, Gleeson K, O'Donnell K, Izon DJ, Walkley CR, Strasser A, Tarlinton DM, Heierhorst J: The Zinc-finger protein ASCIZ regulates B cell development via DYNLL1 and Bim. J Exp Med 2012;209:1629-1639. https://doi.org/10.1084/jem.20120785 |
||||
| 350 Arenzana TL, Smith-Raska MR, Reizis B: Transcription factor Zfx controls BCR-induced proliferation and survival of B lymphocytes. Blood 2009;113:5857-5867. https://doi.org/10.1182/blood-2008-11-188888 |
||||
| 351 Kosan C, Saba I, Godmann M, Herold S, Herkert B, Eilers M, Moroy T: Transcription factor miz-1 is required to regulate interleukin-7 receptor signaling at early commitment stages of B cell differentiation. Immunity 2010;33:917-928. https://doi.org/10.1016/j.immuni.2010.11.028 |
||||
| 352 Mega T, Lupia M, Amodio N, Horton SJ, Mesuraca M, Pelaggi D, Agosti V, Grieco M, Chiarella E, Spina R, Moore MA, Schuringa JJ, Bond HM, Morrone G: Zinc finger protein 521 antagonizes early B-cell factor 1 and modulates the B-lymphoid differentiation of primary hematopoietic progenitors. Cell Cycle 2011;10:2129-2139. https://doi.org/10.4161/cc.10.13.16045 |
||||
| 353 Taniguchi M, Fukunaka A, Hagihara M, Watanabe K, Kamino S, Kambe T, Enomoto S, Hiromura M: Essential role of the zinc transporter ZIP9/SLC39A9 in regulating the activations of Akt and Erk in B-cell receptor signaling pathway in DT40 cells. PLoS One 2013;8:e58022. https://doi.org/10.1371/journal.pone.0058022 |
||||
| 354 Hojyo S, Miyai T, Fujishiro H, Kawamura M, Yasuda T, Hijikata A, Bin BH, Irie T, Tanaka J, Atsumi T, Murakami M, Nakayama M, Ohara O, Himeno S, Yoshida H, Koseki H, Ikawa T, Mishima K, Fukada T: Zinc transporter SLC39A10/ZIP10 controls humoral immunity by modulating B-cell receptor signal strength. Proc Natl Acad Sci U S A 2014;111:11786-11791. https://doi.org/10.1073/pnas.1323557111 |
||||
| 355 Tepaamorndech S, Oort P, Kirschke CP, Cai Y, Huang L: ZNT7 binds to CD40 and influences CD154-triggered p38 MAPK activity in B lymphocytes-a possible regulatory mechanism for zinc in immune function. FEBS Open Bio 2017;7:675-690. https://doi.org/10.1002/2211-5463.12211 |
||||
| 356 Klein U, Tu Y, Stolovitzky GA, Keller JL, Haddad J, Jr., Miljkovic V, Cattoretti G, Califano A, Dalla-Favera R: Transcriptional analysis of the B cell germinal center reaction. Proc Natl Acad Sci U S A 2003;100:2639-2644. https://doi.org/10.1073/pnas.0437996100 |
||||
| 357 Rolles B, Maywald M, Rink L: Intracellular zinc during cell activation and zinc deficiency. J Trace Elem Med Biol 2021;68:126864. https://doi.org/10.1016/j.jtemb.2021.126864 |
||||
| 358 Sakurai N, Maeda M, Lee SU, Ishikawa Y, Li M, Williams JC, Wang L, Su L, Suzuki M, Saito TI, Chiba S, Casola S, Yagita H, Teruya-Feldstein J, Tsuzuki S, Bhatia R, Maeda T: The LRF transcription factor regulates mature B cell development and the germinal center response in mice. J Clin Invest 2011;121:2583-2598. https://doi.org/10.1172/JCI45682 |
||||
| 359 Chevrier S, Emslie D, Shi W, Kratina T, Wellard C, Karnowski A, Erikci E, Smyth GK, Chowdhury K, Tarlinton D, Corcoran LM: The BTB-ZF transcription factor Zbtb20 is driven by Irf4 to promote plasma cell differentiation and longevity. J Exp Med 2014;211:827-840. https://doi.org/10.1084/jem.20131831 |
||||
| 360 Tellier J, Shi W, Minnich M, Liao Y, Crawford S, Smyth GK, Kallies A, Busslinger M, Nutt SL: Blimp-1 controls plasma cell function through the regulation of immunoglobulin secretion and the unfolded protein response. Nat Immunol 2016;17:323-330. https://doi.org/10.1038/ni.3348 |
||||
| 361 Xie B, Khoyratty TE, Abu-Shah E, P FC, MacLean AJ, Pirgova G, Hu Z, Ahmed AA, Dustin ML, Udalova IA, Arnon TI: The Zinc Finger Protein Zbtb18 Represses Expression of Class I Phosphatidylinositol 3-Kinase Subunits and Inhibits Plasma Cell Differentiation. J Immunol 2021;206:1515-1527. https://doi.org/10.4049/jimmunol.2000367 |
||||
| 362 Qu B, Al-Ansary D, Kummerow C, Hoth M, Schwarz EC: ORAI-mediated calcium influx in T cell proliferation, apoptosis and tolerance. Cell Calcium 2011;50:261-269. https://doi.org/10.1016/j.ceca.2011.05.015 |
||||
| 363 Krishnamoorthy M, Buhari FHM, Zhao T, Brauer PM, Burrows K, Cao EY, Moxley-Paquette V, Mortha A, Zuniga-Pflucker JC, Treanor B: The ion channel TRPM7 is required for B cell lymphopoiesis. Sci Signal 2018;11:eaan2693. https://doi.org/10.1126/scisignal.aan2693 |
||||
| 364 Gotru SK, Gil-Pulido J, Beyersdorf N, Diefenbach A, Becker IC, Vogtle T, Remer K, Chubanov V, Gudermann T, Hermanns HM, Nieswandt B, Kerkau T, Zernecke A, Braun A: Cutting Edge: Imbalanced Cation Homeostasis in MAGT1-Deficient B Cells Dysregulates B Cell Development and Signaling in Mice. J Immunol 2018;200:2529-2534. https://doi.org/10.4049/jimmunol.1701467 |
||||
| 365 Mwanza-Lisulo M, Kelly P: Potential for use of retinoic acid as an oral vaccine adjuvant. Philos Trans R Soc Lond B Biol Sci 2015;370:20140145. https://doi.org/10.1098/rstb.2014.0145 |
||||
| 366 Dhawan M, Priyanka, Choudhary OP: Immunomodulatory and therapeutic implications of vitamin D in the management of COVID-19. Hum Vaccin Immunother 2022;18:2025734. https://doi.org/10.1080/21645515.2022.2025734 |
||||