Kynurenic Acid Levels and Kynurenine Aminotransferase I, II and III Activities in Ganglia, Heart and Liver of Snail Helix Pomatia
bFaculty for Biology, University of Würzburg, Germany,
cDepartment of Biomedical Sciences, University of Veterinary Medicine Vienna, Austria,
cformer Institution,
+person is deceased
Keywords
Abstract
Background/Aims:
Kynurenic acid (KYNA), a tryptophan metabolite along the kynurenine pathway, is an endogenous antagonist of glutamate ionotropic excitatory amino acid (EAA) receptors and the α7 nicotinic acetylcholine receptor (nAChR). The involvement of KYNA in various pathological conditions and during the aging process is significant. KYNA synthesis from L-kynurenine (L-KYN), through the action of several kynurenine aminotransferases (KATs), is present in the central nervous system (CNS) and periphery of mammals. We were interested in investigating the ability of the brain and peripheral organs of Helix pomatia snails to synthesize KYNA, in an in vitro study. In comparative studies between rat and snail, we looked for the synthesis of KYNA in the liver. We then looked for an effect of age on KYNA synthesis.Materials:
Ten shell parameters of the Helix pomatia snail were used to establish an Age Rating Scale (ARS), i.e. body weight, shell weight, shell length, width and height, shell opening length and width, lip width, number of shell turns and external shell growth rings. An age of the snails was determined according to the ARS and the snails were divided into three groups, i.e. young, middle and old age. Homogenates of dissected regions, i.e. cerebral ganglia (CG), subpharyngeal ganglia (SG) consisting of pedal, visceral and pleural ganglia, heart and liver, were examined. KYNA was measured by high performance liquid chromatography (HPLC) and KAT activities were measured by an enzymatic method.Results:
With respect to ARS, an evaluation of the age of the snails between young (1-2 years), middle (5-7 years) and old (9-13 years) showed significant differences (p<0.001). Analysis of KYNA levels in different snail tissues, i.e. CG, SG, heart and liver, showed an occurrence in the low femtomolar range. Marked and significant increases of KYNA were found in the liver of middle and old age groups. In the SG, KYNA decreased significantly with age. There were no differences in KYNA levels between groups in CG and heart. The lowest KAT activity was found in CG and SG (5 pmol/mg/h), while in heart and liver the values were visibly higher (between 8 and 80 pmol/mg/h). Only in the liver, and exceptionally only for KAT I, the activity increased significantly with age, i.e. up to 14 years. No age-related changes in KAT I, II and III activities were found in CG and SG. Snail liver shows a different pattern of KAT activities compared to the rat liver.Conclusion:
Regions of the CNS and periphery of the snail Helix pomatia are able to synthesize KYNA due to KAT activities. In the snail liver, KAT I activity increased with age. Notably, there was no age-related increase in KAT activities in the heart and especially in the CNS of Helix pomatia, indicating significant differences from mammals. A moderate KYNA metabolism in the Helix pomatia snail in the periods studied, up to 14 years, could be a physiological phenomenon that protects organs from possible functional insufficiency due to high KYNA levels, as has been suggested. It is reasonable to search for the factor(s) that could regulate the concentration of KYNA in the body of the snail.Introduction
The kynurenine pathway of tryptophan metabolism is the main pathway of tryptophan catabolism [1]. The first step in tryptophan catabolism along this pathway is cleavage of the indole ring (Fig. 1). Two different enzymes, tryptophan 2, 3-dioxygenase or indolamine 2, 3-dioxygenase, catalyze this reaction, resulting in the formation of N-formyl-kynurenine, which is further metabolized by formamidase to L-kynurenine (L-KYN). L-KYN can be degraded by kynurenine aminotransferase (KAT) to kynurenic acid (KYNA) or by kynurenine monooxygenase or kynureninase to 3-hydroxykynurenine or anthranilic acid, respectively.
KYNA is significantly synthesized in the mammalian periphery and also in the CNS, but to a lesser extent [2-6]. Three KATs (KAT I, KAT II, KAT III) have been identified in rat and human brain tissue [7-13]. All three KATs have specific enzymatic properties and their different physiological roles have been suggested. KAT I, with a pH optimum of about 9.6, has been suggested to be particularly important under pathological conditions in an alkaline cellular milieu [9, 11]. KAT II, with a pH optimum of about 7.4, may be the primary enzyme for KYNA synthesis under physiological conditions [3, 4, 7]. KAT III, with a pH optimum of about 8.0, may be active under both physiological and pathological conditions [4, 6, 13]. Experimental studies in rats have shown that approximately 80 % of synthesized KYNA is released from the cell into the medium in vitro [14], and importantly, both the synthesis and release can be significantly influenced by various pharmacological approaches [14, 15].
KYNA has neuroprotective and anticonvulsant activities [5, 16] and exerts antagonistic effects on the ionotropic glutamate receptors α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) [17], kainate [18], N -methyl-d-aspartate (NMDA) [5, 17, 18] and on α7 nicotinic acetylcholine receptors (nAChR) [19]. It is also a ligand for the metabotropic glutamate receptor G protein-coupled receptor 35 (GPR35) [20].
Changes in KYNA levels and of KAT activities over the lifespan have been reported in several mammalian species. In the rat and sheep CNS, KYNA increased during the embryonic stage until the day of birth and then decreased dramatically [21, 22]. In the rat, there was also a low level of KAT activity during the first week after birth, which slowly and progressively increased during ontogenesis and maturation [23]. Increased KYNA levels and KAT activity were found in the brain of old rats, suggesting an age-dependent increase in KYNA metabolism [23, 24]. Furthermore, a brain region-specific increase in KAT activity and an increase in KYNA have been reported throughout the life [23-25]. In particular, a mild increase in KAT activity was found in the cerebellum and substantia nigra compared to other brain regions [23, 24]. No significant changes in KAT activity were found in rat liver during the aging process [24-26]. Interestingly, a positive correlation between KYNA levels and age was also found in human cerebro spinal fluid (CSF), but not in serum [27].
Significant alterations in KYNA metabolism have been found under pathological conditions. Increased KYNA metabolism has been observed in the CNS and/or periphery of patients with Down syndrome [28], Alzheimer’s disease [29], schizophrenia patients [30], HIV-1 infected patients [31], encephalomyocarditis [32], hydrocephalus patients [33], and during the aging process [27]. In patients with Parkinson’s disease, KYNA levels were elevated in those with dementia but not in those without dementia symptoms [34]. In fact, experimental studies have also shown a correlation between working memory deficits and increasing KYNA levels [35-37]. In addition, several other studies have reported significant observations that elevated brain KYNA is associated with cognitive deficits [29, 38, 39] and that reducing brain KYNA could improve cognitive functions and memory [40-47].
In the early seventies, scientists were interested in tryptophan metabolism and serotonin synthesis in neurons of Helix pomatia snails [48]. Recently, Benatti C et al. published an interesting paper dealing with the identification and characterization of the kynurenine pathway in the pond snail Lymnaea stagnalis using genome and transcriptome [49]. The authors identified putative transcripts encoding several kynurenine pathway enzymes, including KAT, in the CNS, gut, stomach, muscle, penis, and the hemocytes of six-month-old Lymnaea stagnalis snails [49].
Although there is no universal aging technique for all species, individual techniques have varying degrees of success for different species groups and economic interests. The use of shell characteristics to age juvenile and adult Roman snails Helix pomatia was described by Pollard et al. in 1977 [50]. The authors stated that the raised lip of Helix pomatia made the aging method applicable to this species in the field. In addition, shell characteristics have been used to age snails of determinate growth. Shell increments have also been successfully used to demonstrate growth rates and aging in marine bivalves [51, 52]. Raboud, 1985 presented various methods of age determination, the pulmonate snail Arianta arbustorum from the Swiss Alps was aged using thin sections of the shell margins cut from marked individuals [53]. Shell layers at the opening lip and growth breaks in the juvenile can provide a reliable estimate of age.
In the review by Bökenhans et al. 2016, the authors described a number of techniques that can be used to estimate age and growth rates for many marine gastropod species [54]. Hollyman et al. 2018, presented an overview of the available techniques for age and growth rate determination in gastropods [55]. The authors described many different options, depending on the type of gastropod species and the potential age registration structures available.
We were interested to study the formation of KYNA in the CNS and periphery in the snail Helix pomatia , and also to study an effect of KYNA metabolism on age, up to 14 years.
Since snail growth is highly dependent on both environmental and laboratory conditions [56, 57], it was reasonable for us to use an approach that included measurements of parameters that characterize the snail body. We used body weight, shell weight, shell length, width and height, shell opening length and shell opening width, lip width, number of shell turns, and internal/external growth rings. All parameters of each snail were evaluated and combined, and an age rating scale (ARS) of the snail was established. Subsequently, three different age groups of snails were constructed: young, middle and old. The KYNA concentrations and the KAT I, II and III activities were measured in the upper and lower parts of the esophageal nerve ring and in the heart and liver of the edible snails in three different age groups. An alteration of the biochemical data with age progression was of interest. In comparative studies, between snail and rat, we also studied the synthesis of KYNA in the liver. We investigated the influence of snail mucus on KYNA synthesis in rat liver homogenate. Preliminary data have been presented in an abstract form [58].
Materials and Methods
Materials
Chemicals. L-kynurenine (L-KYN), kynurenic acid (KYNA), pyruvate (PYR), α-ketoglutarate (α-KGL), α-ketoisocaproate (α-KIS), pyridoxal 5’-phosphate (P5P), and 2-amino-2-methyl-1-propanol (AMPOL) were purchased from Sigma-Aldrich Handel’s GmbH, Vienna, Austria. All other chemicals used were of the highest commercial purity.
Animals.
Snails, Helix pomatia , were obtained from Gugumuck, Vienna Snail Manufacturer, Austria. Snails were housed in a large group in an indoor enclosure with free access to food (vegetables such as lettuce or carrots) under a 12-hour light/12-hour dark cycle and watered once daily to maintain optimal humidity.
Male, 3-month-old, Sprague-Dawley rats (Him:OFA/SPF) were obtained from the breeding facility of the University of Veterinary Medicine Vienna, Austria and housed under standard laboratory conditions with a 12-hour light/12-hour dark cycle and free access to standard animal chow and water.
Methods
Establishment of the snail Age Rating Scale (ARS). To establish the ARS, 10 different parameters of snails were used and evaluated, i.e. body weight, shell weight, shell length, shell width and shell height, length and width of the shell opening, width of the lip, number of shell turns and external growth rings of the shell (Table 1). A value of selected parameters of each snail was combined and the obtained data formed the basis of a scale, which was evaluated by using significance analysis. Subsequently, the values of ARS were used to create three age groups of snails, followed by statistical analysis.
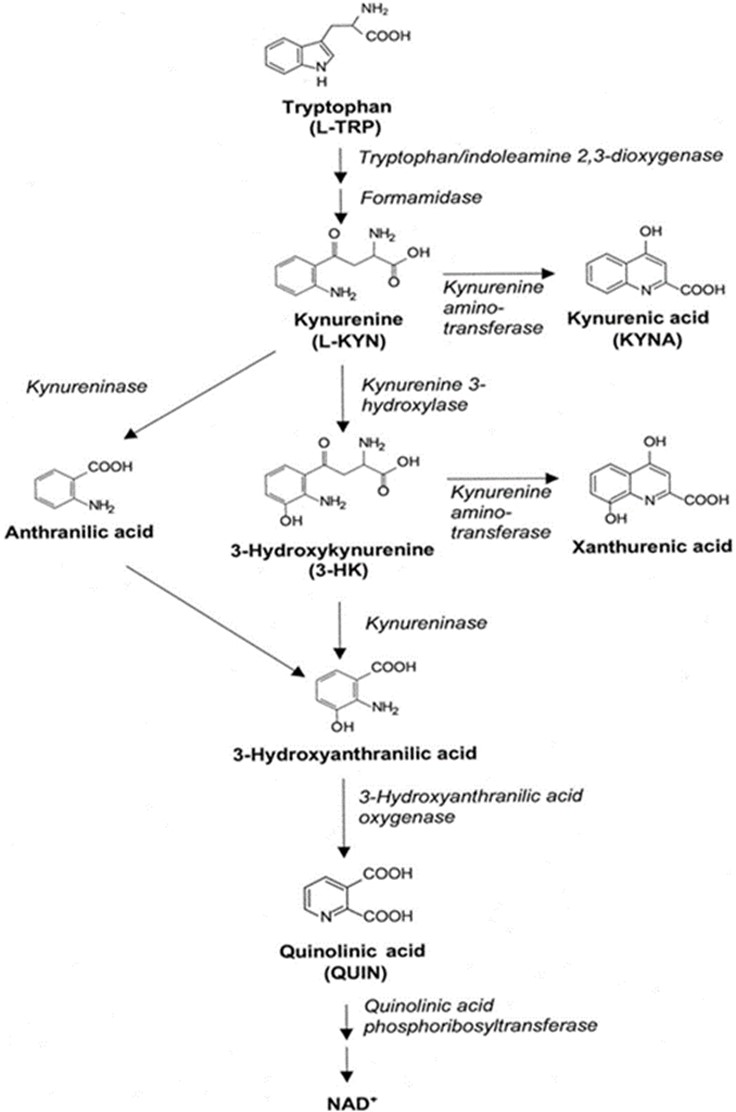
Fig. 1:
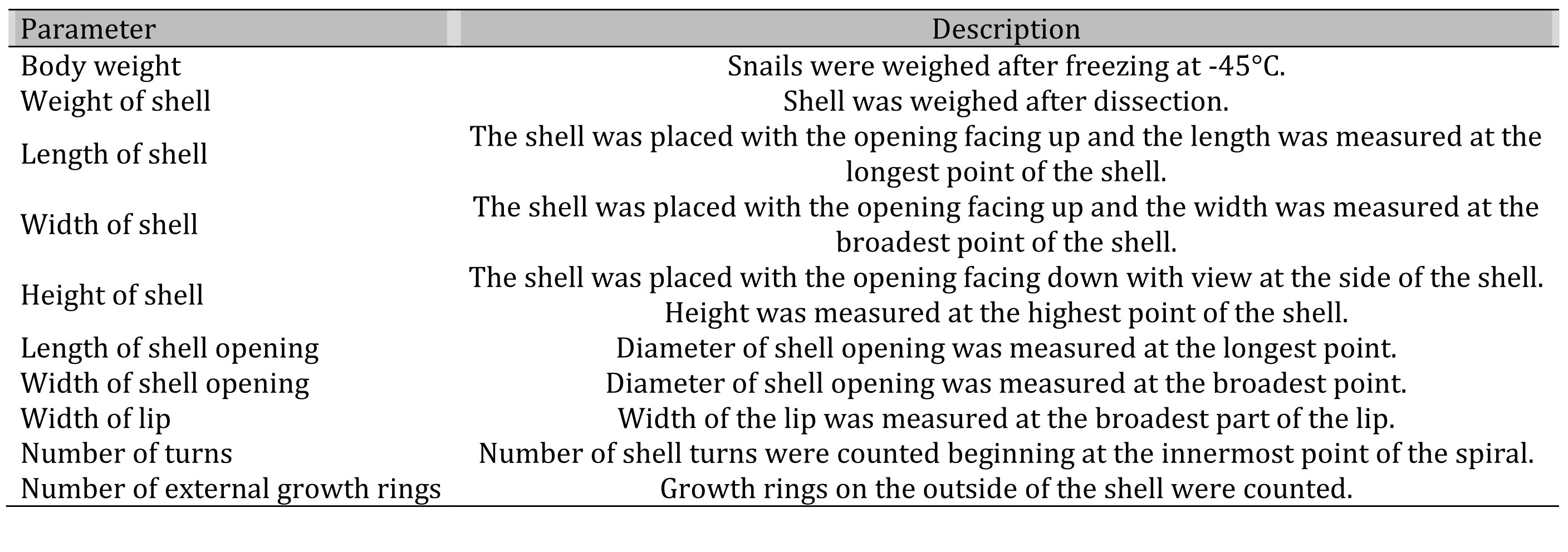
Table 1: List of parameters used to determine the Age Rating Scale (ARS) of snail Helix pomatia
Internal growth rings. In a separate experiment, internal growth rings were estimated in 37 snail shells, i.e. 12 shells from the young and middle group and 13 shells from the old group. To determine the number of internal growth rings, a 0.5 x 0.5 cm piece was cut out of the shell at the lip using a Dremel [59]. The sides of the piece were sanded smooth and then treated with 10% HCl. The number of internal growth rings was then counted under a microscope.
Age Rating Scale (ARS) and snail age. The number of external and internal growth rings was determined. One external or one internal ring corresponds to 1 year [59]. Correlation analyses were performed between ARS values and the number of growth rings (external and internal) and age.
Extraction of snail mucus.
Snails produce two types of mucus. One constantly covers the snail’s skin and is used for locomotion and protection against dehydration. It has a yellowish color. The second type, produced by the snail in stressful situations, is white to translucent in color.
The yellow slime was obtained by gently scraping the snail’s foot with a spatula. The white slime was obtained by gently touching the snail, causing it to retreat into its shell and start producing slime as a defensive reaction. The newly produced mucus was collected with a pipette.
Dissection. Snails were killed at -45oC and stored at -45oC. The snails were then thawed and the nerve ring, heart and liver were rapidly dissected. The upper part (cerebral ganglia, CG) and the lower part (subpharyngeal ganglia, SG) consisting of pedal, visceral and pleural ganglia of the esophageal nerve ring were prepared. For a single data point of CG or SG samples (i.e. n=1), equal amounts of tissue from CG or SG were pooled. Ten snails were pooled for the young group, seven for the middle group and five for the old group. For heart samples, equal amounts of heart tissue were pooled, five snails for young group, three for middle group and one for old group. The dissection was performed according to the procedure [60] using microscope (x10) and cooled glass plate, and the tissue was stored at -45oC, until use. The study was performed at the Karl Landsteiner Research Institute Mauer according to the Austrian Ethical Code. Helix pomatia snails are not subject to the Animal Experiments Act, version 2012 dated of 08.11.2021, Austria.
Rats. Animals (N=5) were killed by decapitation. Liver dissection was performed at the University of Veterinary Medicine Vienna according to Austrian ethical guidelines. The excised liver was immediately frozen at -45oC and stored until analysis.
Preparation of homogenates.
Snail tissues from dissected regions, i.e. CG, SG, heart and liver, were homogenized after the addition of 10 volumes (wt/vol) of distilled water according to Baran et al. [6]. The resulting homogenate of each region was divided, one part for KYNA and the other for KAT determination. For KAT activity measurements, snail homogenates were diluted 1:2 with homogenization buffer (5 mM tris-acetate buffer pH 8.0 containing 50 mM P5P and 10 mM mercaptoethanol).
Rat liver homogenates were prepared by adding 100 volumes (wt/vol) of homogenization buffer to the liver probe and homogenized. All steps were performed on ice.
Kynurenic acid (KYNA) determination.
For KYNA determination, snail homogenates were further diluted 1:2 with distilled water and 64 µl of homogenate (1:20) was mixed with 4 µl 50% trichloroacetic acid, 14 µl 0.8 M perchloric acid and 318 µl distilled water. Denatured proteins were removed by centrifugation (11, 700 rpm, 20 min, 7oC) and KYNA levels were determined using a high performance liquid chromatography (HPLC) system. KYNA identification involved separation of compounds on DOWEX and identification on the HPLC [14].
The measurement of KYNA was performed according to the method described by Swartz et al. 1990 [61] with minor modifications. The HPLC system consisted of a Merck Hitachi Elite LaChrom L-2130 pump, an L-2200 autosampler, an L-2485 fluorescence detector, and a computer. The mobile phase consisted of 50 mM sodium acetate, 250 mM zinc acetate and 5% acetonitrile, pH 6.15, and was pumped through a Chromolith Performance RP-18e column at a flow rate of 0.7 ml/min. The fluorescence detector was set at an excitation wavelength of 340 nm and an emission wavelength of 398 nm. The injection volume was 50 µl. The detection limit for KYNA was five fmol per injection.
Determination of kynurenine aminotransferases (KATs) activities. The activities of KAT I, KAT II and KAT III were measured by an enzymatic assay as described by Baran et al. [6]. The reaction mixture contained 10 µl of snail homogenate 1:20 (CG, SG, heart or liver), 100 µM L-KYN, 70 µM P5P, 1 mM PYR and buffer for KATs in a total volume of 100 µl. 150 mM AMPOL buffer with pH 9.6 was used for KAT I, 150 mM tris-acetate buffer with pH 7.4 was used for KAT II, and 150 mM tris-acetate buffer with pH 8.0 was used for KAT III. After incubation at 37°C for 2 hours, the reaction was stopped by adding 7 µl of 50% trichloroacetic acid and 500 µl of 0.1 M HCl. Blanks were prepared by adding 7 µl of 50% trichloroacetic acid prior to incubation. Denatured proteins were removed by centrifugation (11, 700 rpm, 20 min, 7°C) and synthesized KYNA was measured by HPLC as described.
Tissue and time dependence.
Using standard assay conditions for KAT activity measurements, snail tissue homogenates, i.e. 5, 10, 15 and 20 µl from CG, SG, heart or liver, were prepared as described in Materials and Methods, and synthesized KYNA levels due to KAT I, KAT II and KAT III were measured in a dose-dependent manner in an in vitro study (data not shown).
Using different incubation times i.e. 1, 2 and 4 h, snail tissue homogenates i.e. from CG, SG, heart or liver (10 µl) were prepared and synthesized KYNA was measured in a time-dependent manner up to 4 h, in an in vitro study (data not shown).
Experiments
Co-substrate specificity for KAT I, II and III. For co-substrate specificity, three 2-oxo acids, i.e., PYR, α-KGL and α-KIS, each at 1 mM final concentration, were used for the KAT assay, as described above.
Kinetic Analysis. Under standard assay conditions, using different concentrations of L-KYN (0.5, 1, 2, 3, 4, 5 mM), P5P (50, 100, 200, 400, 600, 800 µM) and PYR (0.5, 1, 2, 3, 4, 5 mM), Michaelis-Menten saturation curves and Lineweaver-Burk plots were generated for KAT I, KAT II and KAT III in snail liver homogenates. Km and Vmax values were calculated.
Kynurenine aminotransferase (KAT), kynurenic acid (KYNA) and aging. KAT activity and KYNA concentration were analyzed in snail liver, heart, CG and SG. Correlation analysis between biochemical parameters and aging groups was performed.
KAT I, KAT II and KAT III activities in rat liver homogenates. Rat and snail liver homogenates were used to compare KAT activity between species.
Effect of snail mucus on KAT I, KAT II and KAT III in rat liver homogenate. KAT activity was measured as previously described [6]. A volume of 50 µl of snail mucus was added to the reaction mixtures prior to incubation at 37°C for 2 hours.
Statistical Analysis. Results are expressed as mean ± standard error of the mean (SEM). One-way ANOVA analysis and Student’s t-test were used. Asterisks indicate a significant difference: *p<0.05 ; **p<0.01 ; ***p<0.001
Results
Snail Age Rating Scale (ARS)
Evaluation of shell parameters. Using linear regression analysis, we found a significant correlation between the number of external growth rings and the shell parameters used (Fig. 2). The following significant correlations were obtained: body weight (y=4.3468x-1.8878, R2=0.78187, p<0.0001 , Fig. 2A), shell weight (y=0.589x+1.0981, R2=0.66709, p<0001 , Fig. 2B), shell length (y=0.2207x+2.7669, R2=0.76881, p<0.0001 , Fig. 2C), shell width (y=0.2596x+1.8963, R2=0.76299, p<0.0001 , Fig. 2D), shell height (y=0.2209x+1.5976, R2=0.77643, p<0.0001 , Fig. 2E), shell opening length (y=0.1238x+1.296, R2=0.67857, p<0.0001 , Fig. 2F), shell opening width (y=0.1526x+1.5546, R2=0.76266, p<0.0001 , Fig. 2G), lip thickness of (y=0.5873x+2.9229, R2=0.59338, p<0.0001 , Fig. 2H) and number of shell turns (y=0.1308x+3.4986, R2=0.54561, p<0.0001 , Fig. 2I).
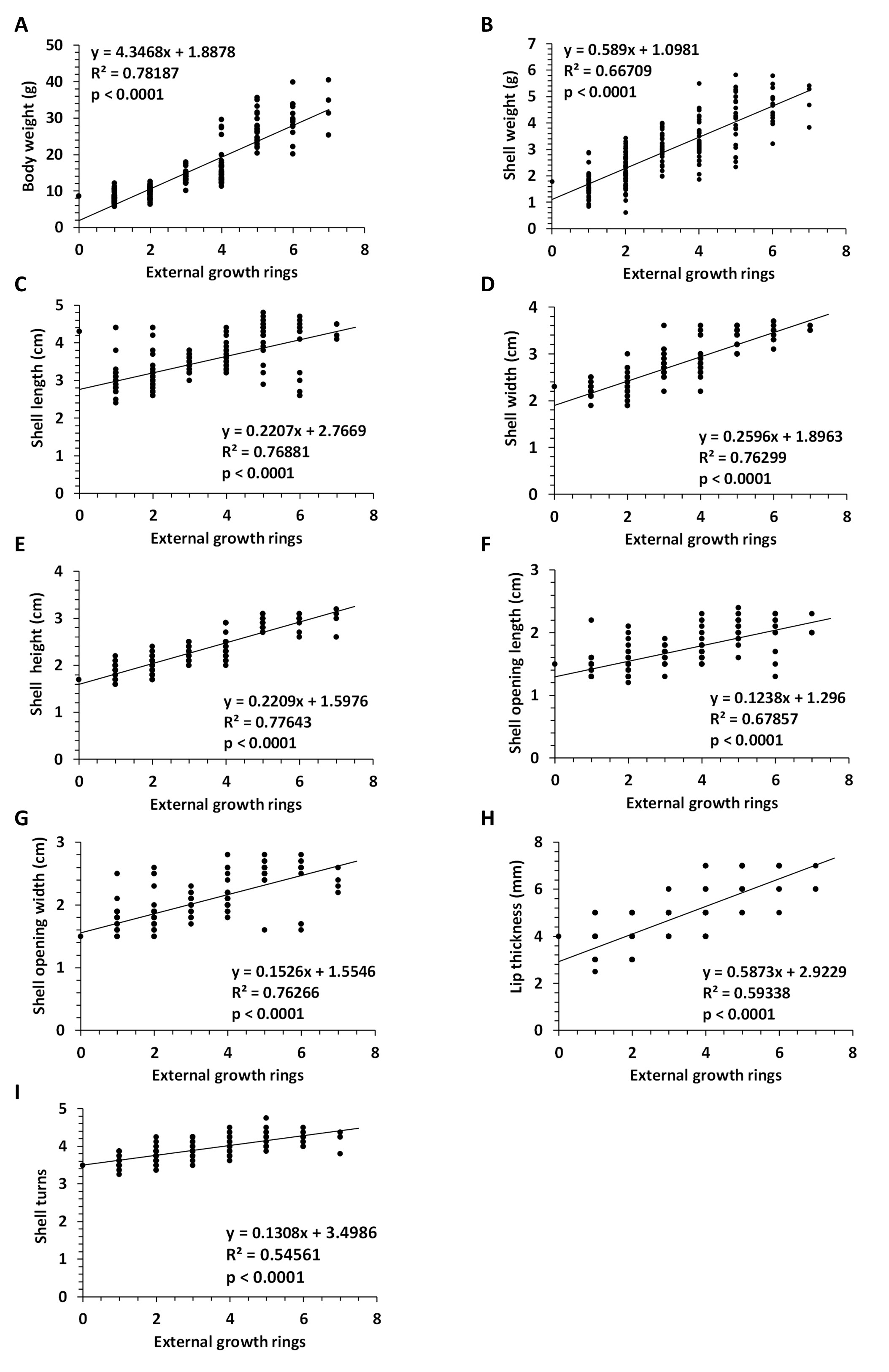
Fig. 2: Linear regression between number of external growth rings and parameters. Regression analysis between number of external growth rings and body weight (A), shell weight (B), shell length(C), shell width (D), shell height (E), shell opening length (F), shell opening width (G), lip thickness (H), and number of shell turns (I). Abbreviations: N (number). N=205 snails, Helix pomatia.
Age Rating Scale (ARS).
The snails were divided into three age groups, according to the ARS values: ARS from 23.1 to 35.0 was young age, ARS from 36.3 to 48.4 was middle age and ARS from 50.1 to 79.9 was old age (Table 2). Evaluation of ARS values between these three age groups, i.e. young, middle and old, revealed statistically significant differences with p <0.001, (Table 2, Fig. 3). One-way ANOVA analysis of ARS between these three age groups showed statistically significant differences p=2.3704E-101, F=952.181.
To evaluate the efficiency of shell parameters for ARS use, we calculated the degree of increase of each parameter among three age groups, i.e., young, middle and old (Fig. 3). As shown in Fig. 3, using the young group as 100%, we found a visible and significant increase in parameter values among these three groups. The highest effect was observed in the number of external growth rings, followed by body weight and shell weight.
In a separate experiment, total growth rings, i.e. external and internal growth rings, were calculated. The number of internal growth rings on the shell lip was determined for each age group (Fig. 4). No internal growth rings were observed in the young age group, while two to four and four to six internal growth rings were observed in the middle age and old age groups (Fig 4). Using linear regression analyses, we found a significant correlation between the total number of rings, i.e. the number of external and internal growth rings, and the parameters used (Fig. 5A-I), including ARS, p = 7.59E-23, (Fig. 5J).
Furthermore, using linear regression analyses, we found a significant correlation between the number of internal growth rings and ARS, p=2.2E-25, (Fig. 6A) or the number of external growth rings counted on the outside of the shell, p=0.012, (Fig. 6B). Snail age as the sum of external and internal growth rings (total rings) correlated significantly with ARS, p= 7.59E-23, (Fig. 6C).
Evaluation of the correlation between ARS and total rings showed that the young group of snails was approximately 1-2 years old, the middle group was 5-7 years old, and the old group was 9-14 years old (Fig. 4 and Fig. 6C).
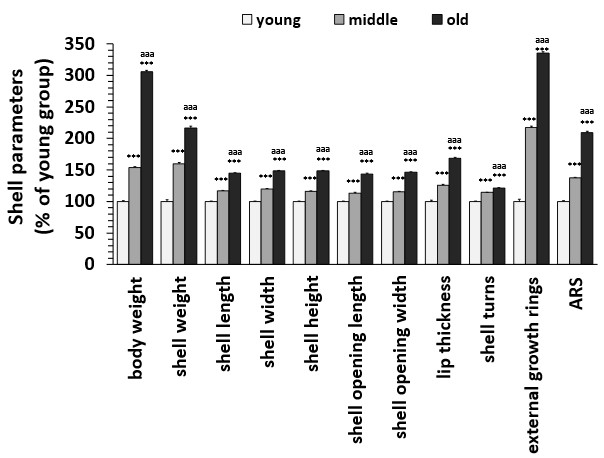
Fig. 3: Differences in shell parameters between young, middle and old age groups of Helix pomatia. Data are mean ± SEM. ANOVA analysis of variance revealed significant differences between age groups for each parameter (body weight P = 1.03 E-96, F = 794.16, shell weight P = 2.67 E-47, F = 191.05, shell length P = 1.57 E-88, F = 642.44, shell width P = 2.43 E-83, F = 559.79, shell height P = 1.16 E-91, F = 697.20, shell opening length P = 2.48 E-75, F = 449.93, shell opening width P = 1.51 E-88, F = 642.71, lip thickness P = 3.42 E-52, F = 224.73, number of shell turns P = 1.58 E-38, F = 137.77, number of external rings P = 2.30, F = 623.00). Student's t-test revealed significant differences between young and middle-aged or old groups, as indicated by asterisks: ***p < 0.001. Student's t-test revealed significant differences between middle-aged and old group as indicated by letters: aaa p < 0.001. Abbreviations: N (number). Young snails: N=95, middle snails: N=63, old snails: N=47.
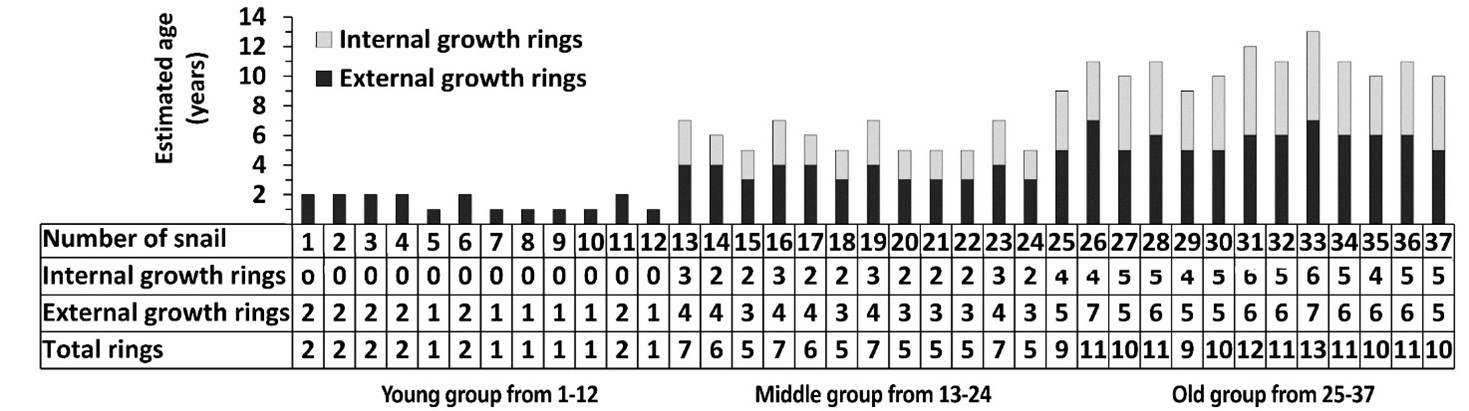
Fig. 4: Estimation of the total number of growth rings in the Helix pomatia snail. Number of internal and external growth rings in 37 snails of 3 different age groups, i.e. young, middle-aged and old. The estimated age of the snails was obtained by summing the external and internal growth rings.
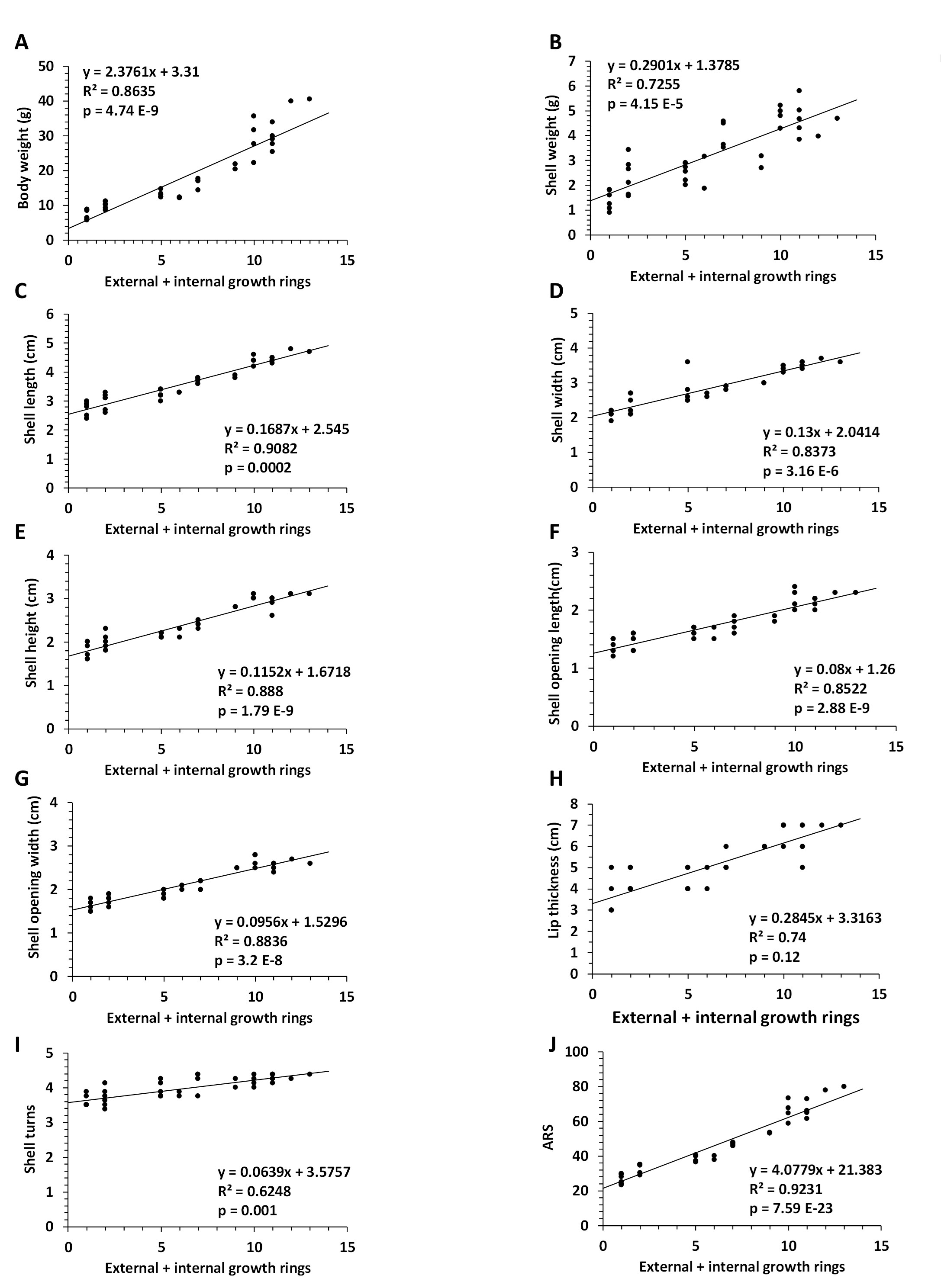
Fig. 5: Correlation between number of external and internal growth rings and parameters of the Helix pomatia snail. Linear regression between number of external and internal growth rings and body weight (A), shell weight (B), shell length (C), shell width (D), shell height (E), shell opening length (F), shell opening width (G), lip thickness (H), number of shell turns (I), and ARS (J). Abbreviations: ARS (Age Rating Scale); N (number); N=37 snails.
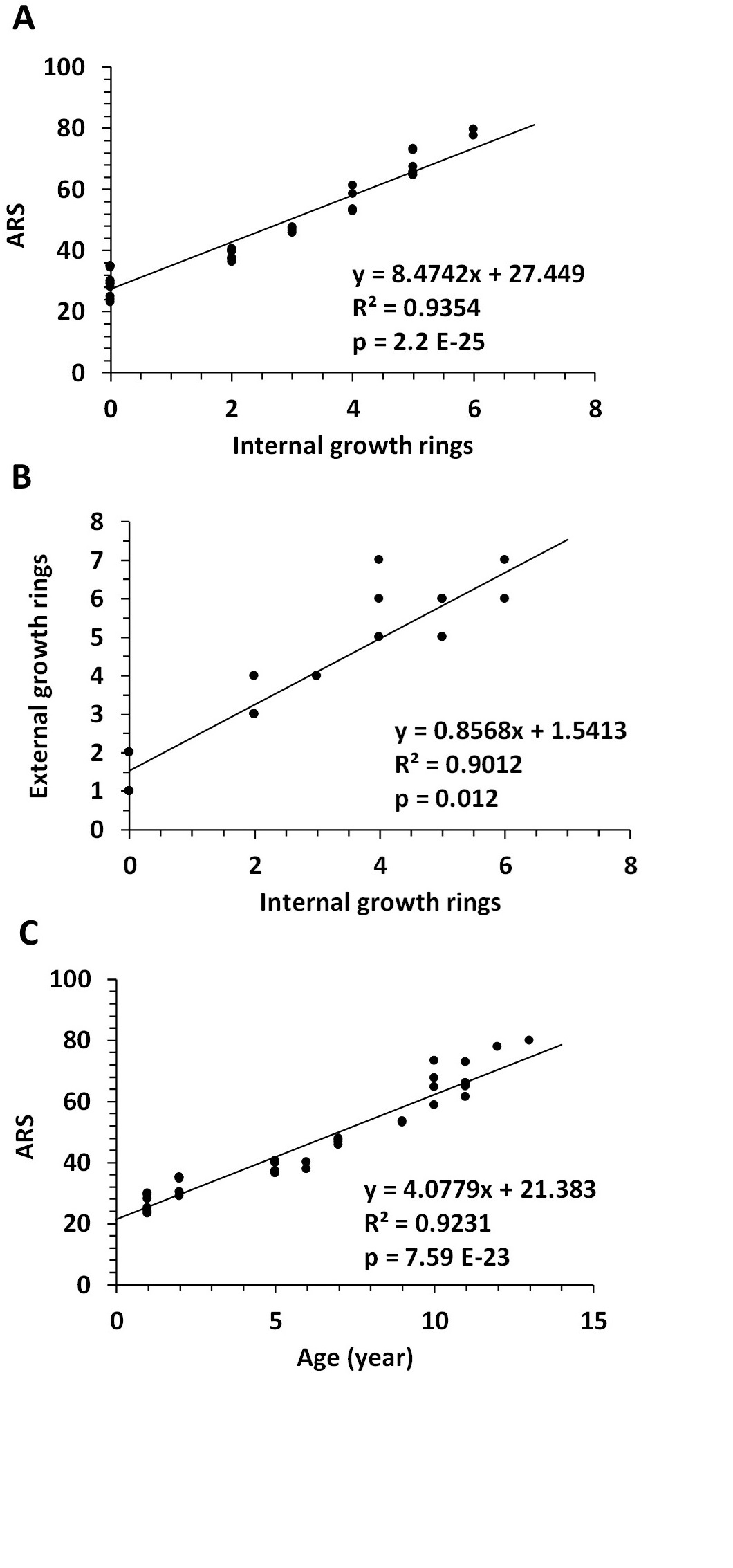
Fig. 6: Correlation between internal and external growth rings and age rating scale (ARS) of the Helix pomatia snail. Linear regression between internal growth rings and ARS (A), external growth rings (B), and between ARS and total rings, respectively age (C). Abbreviations: N (number). N=37 snails.
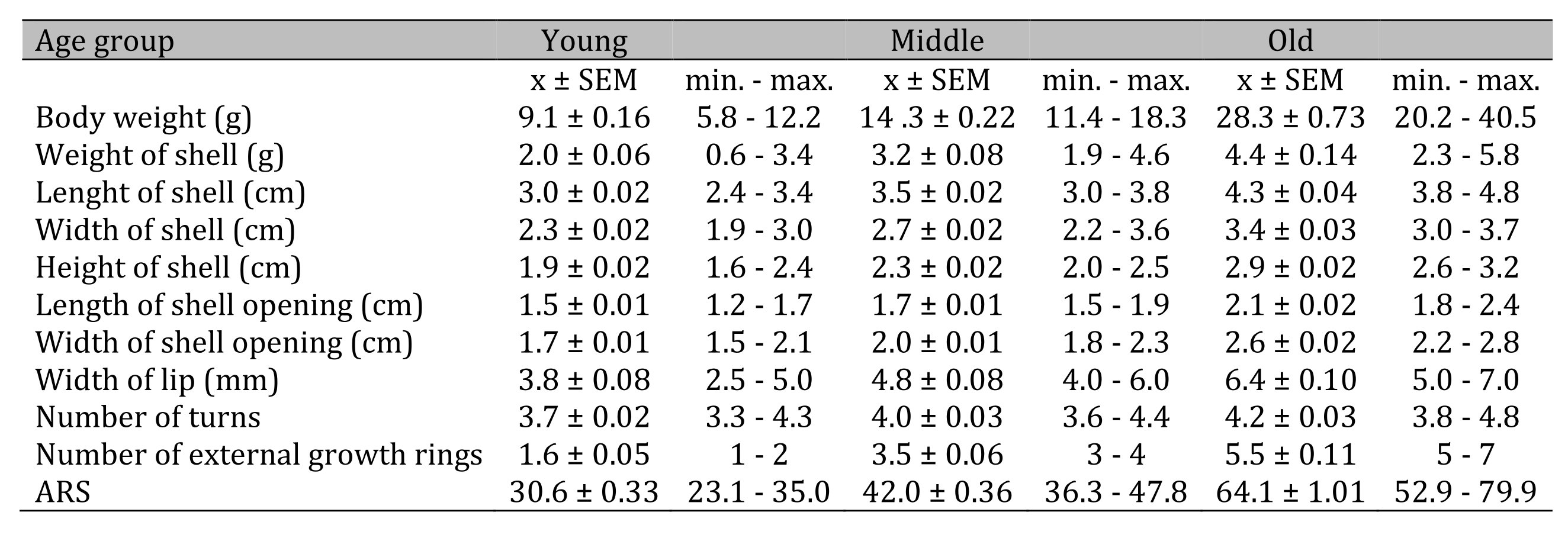
Table 2: Values of the parameters of the snail Helix pomatia in different age groups, i.e. young, middle and old. Abbreviations: N (number); max (maximum); min (minimum); number of snails was: N=94 for young; N=62 for middle and N=46 for old group
Biochemical Investigation
Characterization of Kynurenine Aminotransferases in Snail Tissue
Co-substrate specificity. The co-substrate specificity of KAT I, KAT II and KAT III in snail liver homogenate was investigated by comparing the efficacy among three 2-oxo acids, i.e. PYR, α-KGL and α-KIS (Fig. 7). Within the three 2-oxo acids investigated, no significant differences in KYNA formation were observed between the co-substrates. In the presence of α-KIS, KYNA formation was slightly weaker for each KAT.
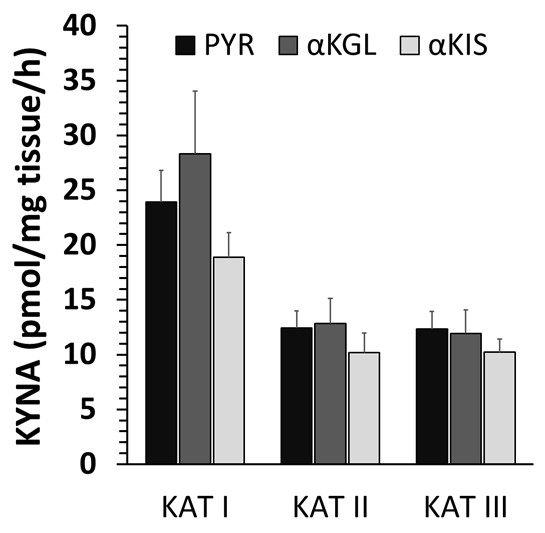
Fig. 7: Influence of co-substrate on liver kynurenine aminotransferase (KAT) I, II and III activities of the snail Helix pomatia. Co-substrate specificity for KAT I, II and III using pyruvate (PYR), α-ketoglutarate (α-KGL) or α-ketoisocaproate (α-KIS). Data are the mean of 4 animals ± SEM.
Kinetic Analyses - Vmax and Km for KAT I, KAT II and KAT III. Using different concentrations of L-KYN, PYR or P5P, Vmax and the Michaelis-Menten constant Km were determined for KAT I (Fig. 8), KAT II (Fig. 9) and KAT III (Fig. 10) in crude snail liver homogenate. The Km values for the KATs are listed in Table 3.
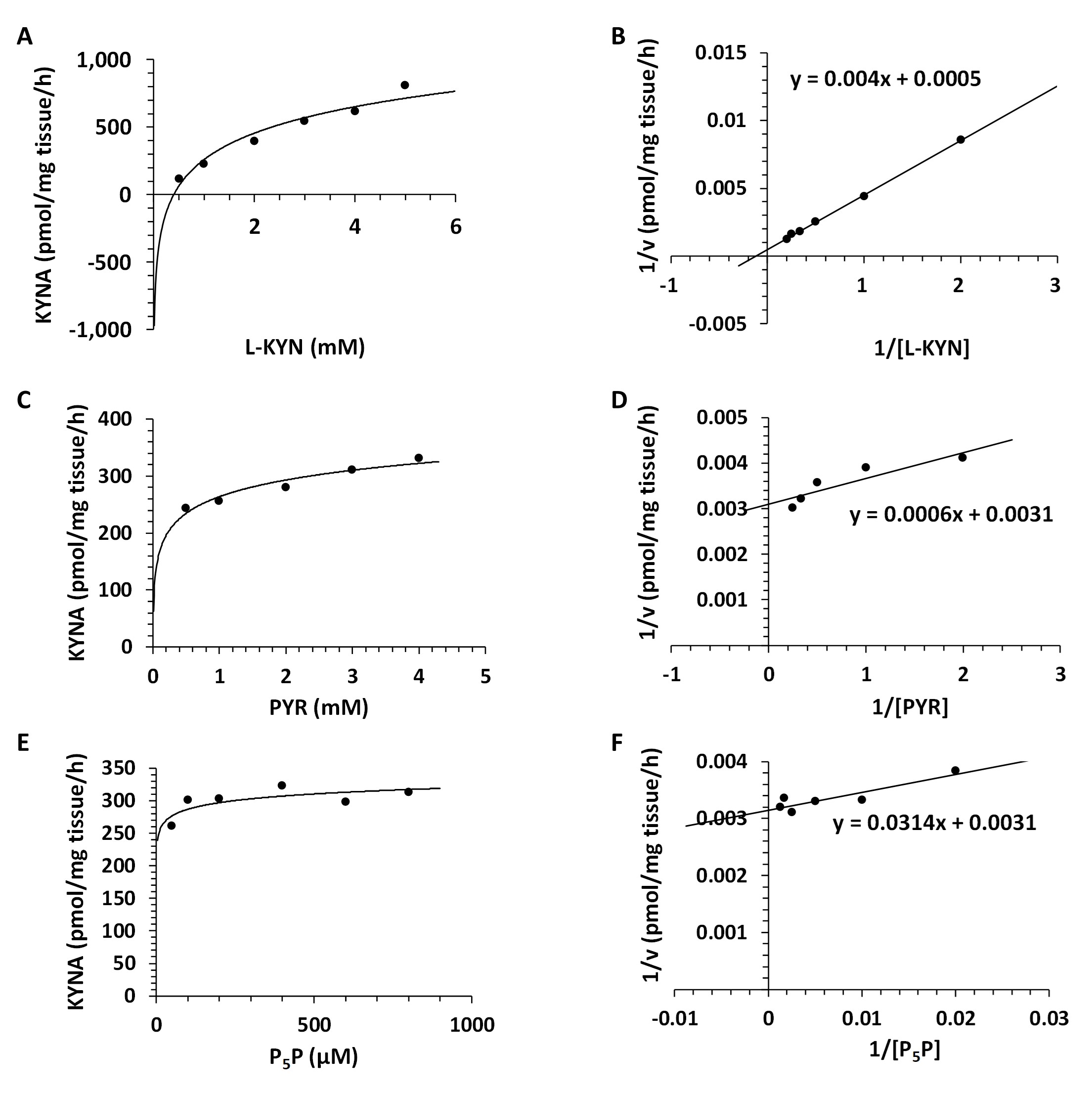
Fig. 8: Enzyme kinetics of kynurenine aminotransferase I (KAT I) in the Helix pomatia liver. A: Michaelis-Menten saturation curve for L-kynurenine (L-KYN), Vmax. B: Lineweaver-Burk plot for L-kynurenine (L-KYN), Km. C: Michaelis-Menten saturation curve for pyruvate (PYR), Vmax. D: Lineweaver-Burk plot for pyruvate (PYR), Km. E: Michaelis-Menten saturation curve for pyridoxal 5'-phosphate (P5P), Vmax. F: Lineweaver-Burk plot for pyridoxal 5'-phosphate (P5P), Km.
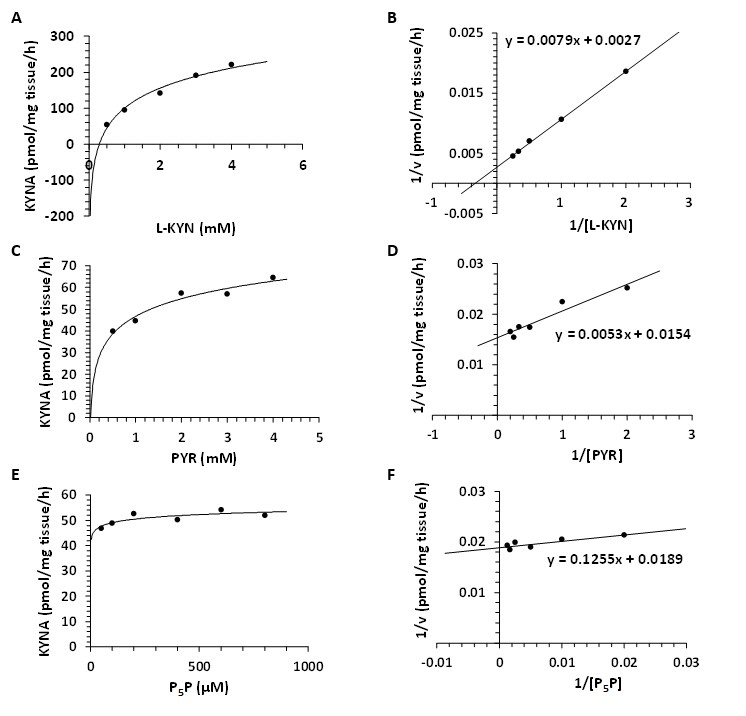
Fig. 9: Enzyme kinetics of kynurenine aminotransferase II (KAT II) in Helix pomatia liver. A: Michaelis-Menten saturation curve for L-kynurenine (L-KYN), Vmax. B: Lineweaver-Burk plot for L-kynurenine (L-KYN), Km. C: Michaelis-Menten saturation curve for pyruvate (PYR), Vmax. D: Lineweaver-Burk plot for pyruvate (PYR), Km. E: Michaelis-Menten saturation curve for pyridoxal 5'-phosphate (P5P), Vmax. F: Lineweaver-Burk plot for pyridoxal 5'-phosphate (P5P), Km.
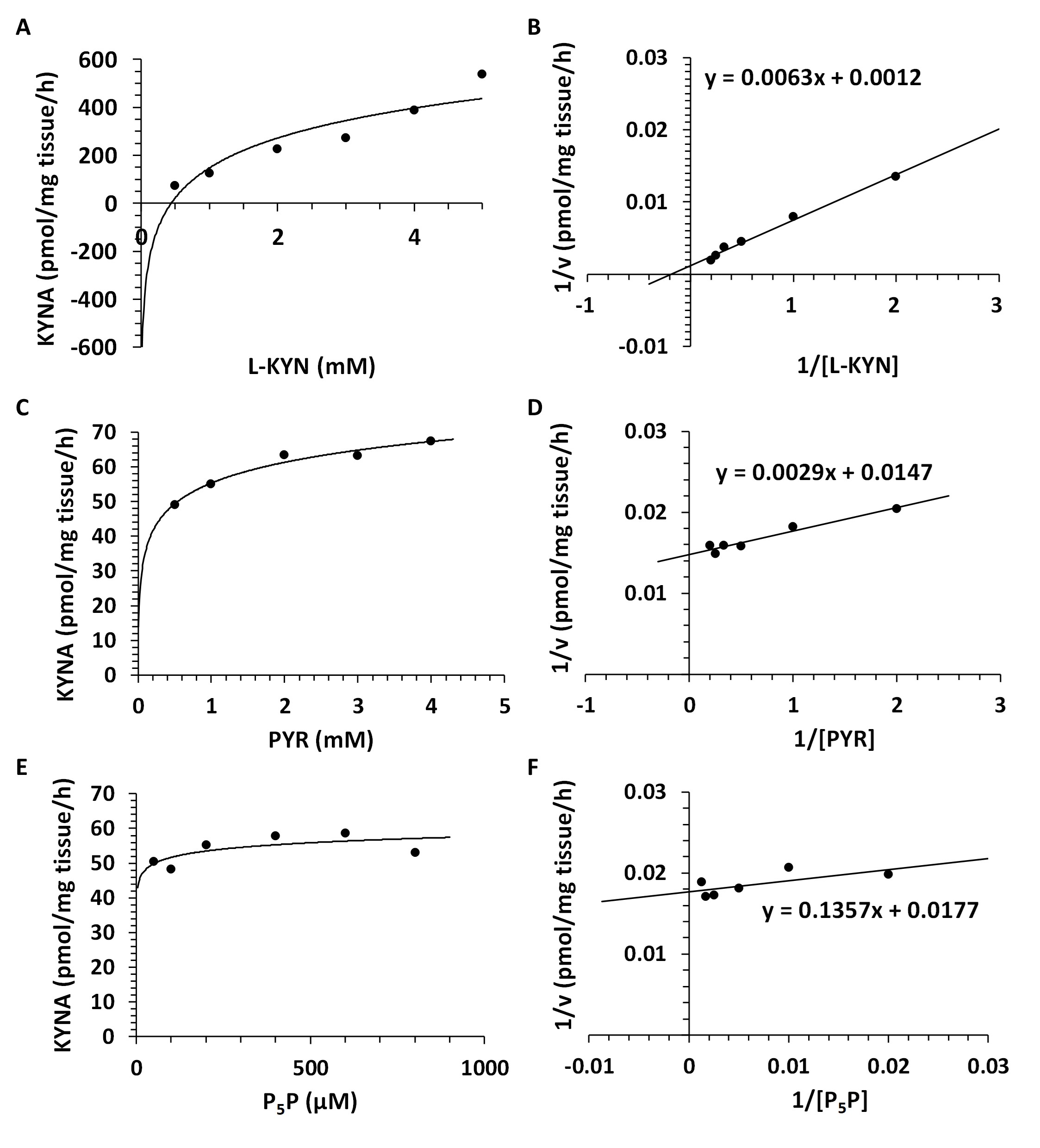
Fig. 10: Enzyme kinetics of kynurenine aminotransferase III (KAT III) in Helix pomatia liver. A: Michaelis-Menten saturation curve for L-kynurenine (L-KYN), Vmax. B: Lineweaver-Burk plot for L-kynurenine (L-KYN), Km. C: Michaelis-Menten saturation curve for pyruvate (PYR), Vmax. D: Lineweaver-Burk plot for pyruvate (PYR), Km. E: Michaelis-Menten saturation curve for pyridoxal 5'-phosphate (P5P), Vmax. F: Lineweaver-Burk plot for pyridoxal 5'-phosphate (P5P), Km.
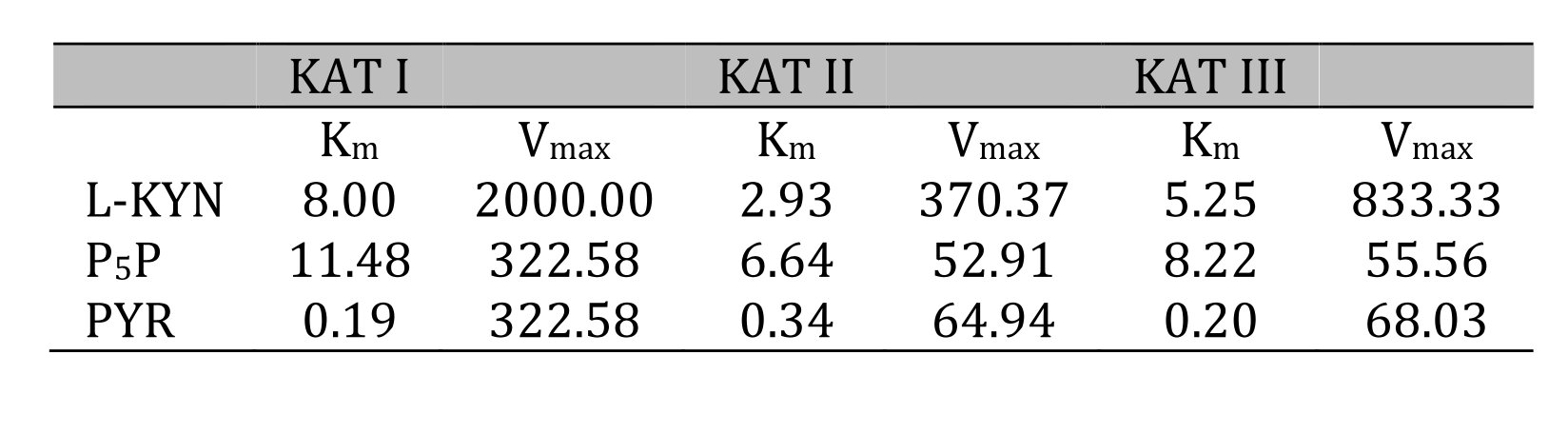
Table 3: Kinetic analysis for kynurenine aminotransferase (KAT) I, II and III in Helix pomatia liver. Michaelis-Menten constant (Km) and maximum velocity (Vmax) of KAT I, KAT II and KAT III in crude liver homogenate for L-kynurenine (L-KYN), pyruvate (PYR) and pyridoxal 5'-phosphate (P5P). Km values for KAT I, II and III as a function of L-KYN, PYR and P5P. Km for L-KYN and PYR in mM; Km for P5P in µM; Vmax is expressed in pmol/mg tissue/h synthesized kynurenic acid (KYNA)
Regional distribution of KAT activities in snail tissues and aging.
KAT I, KAT II and KAT III activities were present in all regions examined. The lowest activities were measured in CG and SG (Fig. 11A, 11B), while the highest activities were found in the heart and liver (Fig. 11C, 11D).
In CG, KATs activities were ranged from 4 to 6 pmol/mg tissue/h and no significant differences between KATs within age groups were observed (Fig. 11A). In SG, we found that KATs activities ranged from 3 to 5 pmol/mg tissue/h (Fig. 11B). KATs I and II activities were significantly reduced (p<0.05 ) in the middle but not in the old group, compared to the young group (Fig. 11B).
No changes in KATs activities were observed in CG and SG due to the aging process (Fig. 11A and 11B).
In the heart, KAT activity ranged from 7 to 15 pmol/mg tissue/h (Fig. 11C). Regarding the aging process, there was no correlation between the studied groups for KAT I, KAT II or KAT III in the heart. Higher levels of KAT I, II and III activities were observed in the middle group, which significantly decreased in the old group (p<0.05 ) (Fig. 11C).
In the liver, KAT I expressed the highest activity values between 50 and 90, followed by KAT III between 15 and 30 and KAT II between 10 and 15 pmol/mg tissue/h, respectively. Regarding the aging process, we found a significant increase of KAT I but not of KAT II and III in the liver with age progression. KAT II and III activities increased only in the old group (Fig. 11D).
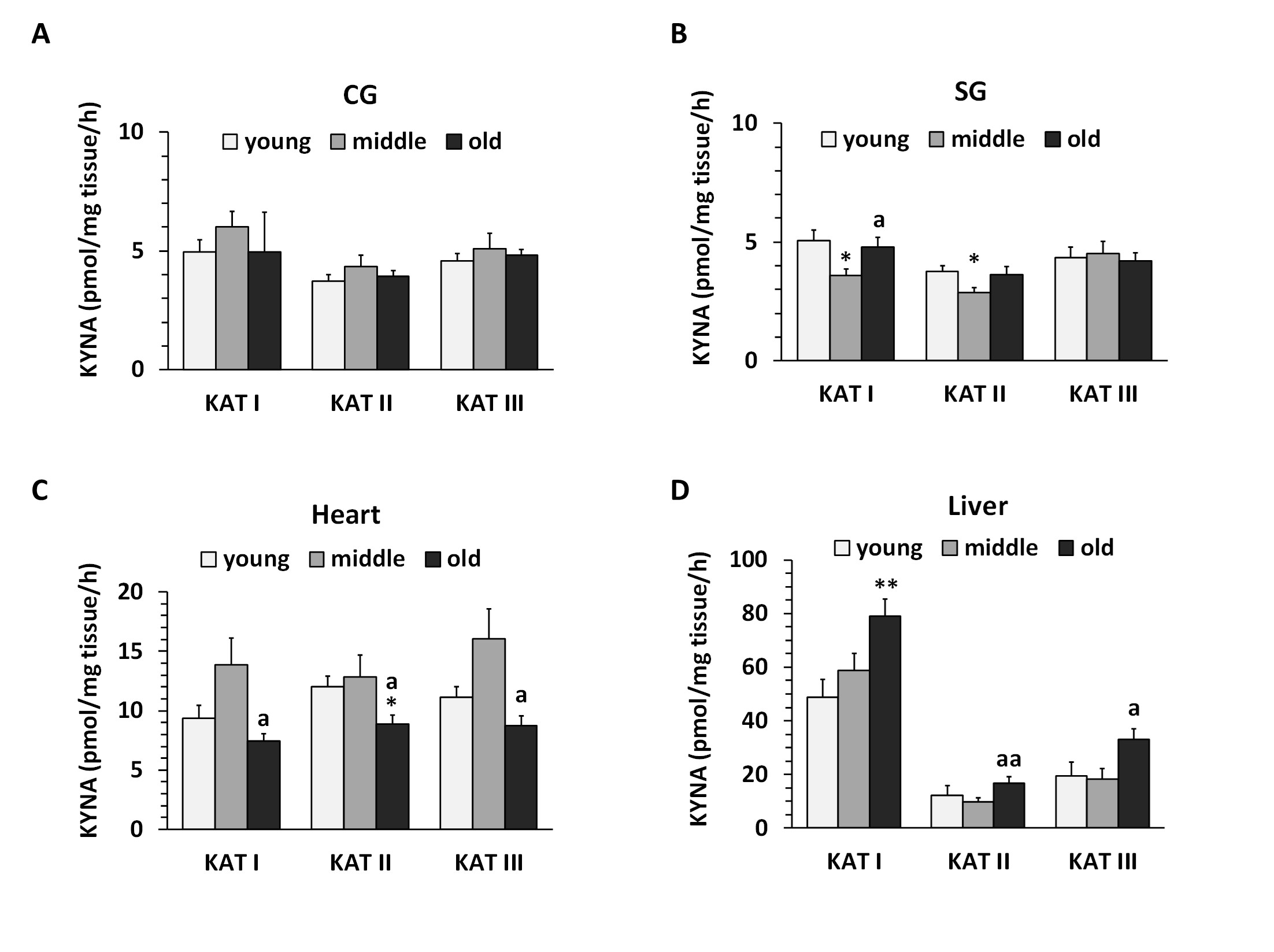
Fig. 11: Kynurenine aminotransferase (KAT) I, II and III activities in cerebral ganglia (CG), subpharyngeal ganglia (SG), heart and liver of Helix pomatia. Data are the mean of 7-10 animals ± SEM. Asterisks indicate significant differences compared to the young group: Student's t-test: *p< 0.05; **p< 0.01. Letters indicate significant differences compared to the middle-aged group: ap< 0.05; aap< 0.01.
Kynurenic acid (KYNA) in snail tissues. KYNA was found in the low femtomolar range in all regions examined. The lowest levels were found in the CG and SG, followed by the heart and liver (Fig. 12A-D). In CG and heart, KYNA levels were around 50 fmol/mg tissue in all age groups (Fig. 12A, 12C). In SG, KYNA levels decreased significantly with age, and in old snails, the KYNA levels were reduced by 50% (p<0.01 ) compared to the young group (Fig. 12B). In the liver, KYNA was 70 fmol/mg tissue in the young group and increased significantly to 120 fmol/mg tissue in the middle and old age groups p<0.05 (Fig. 12D).
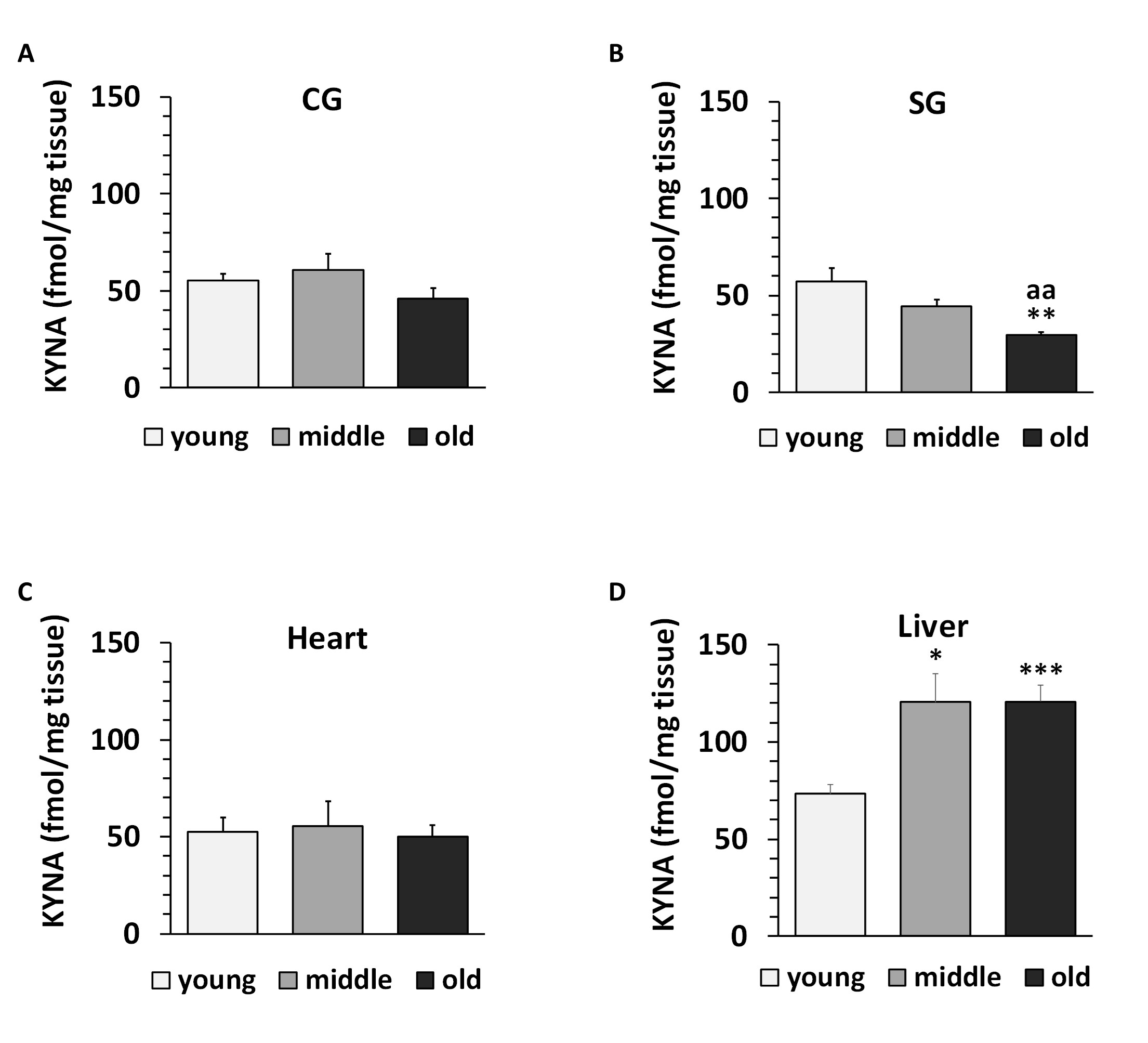
Fig. 12: Kynurenic acid (KYNA) concentration in cerebral ganglia (CG), subpharyngeal ganglia (SG), heart and liver of Helix pomatia. Data are mean ± SEM of 94 animals for the young group, 62 for the middle group and 46 for the old group. Asterisks indicate significant differences compared to the young group: Student's t-test: *p< 0.05, ***p< 0.001. Letters indicate significant differences compared to the middle group: aap< 0.01.
KAT activities in rat liver. The activities of KAT I, KAT II and KAT III in rat liver are shown in Fig. 13. The highest KAT activity was observed for KAT II, followed by KAT III and KAT I. KAT I was significantly different from KAT II and KAT III (p=0.0006 for KAT II, p=0.0100 for KAT III).
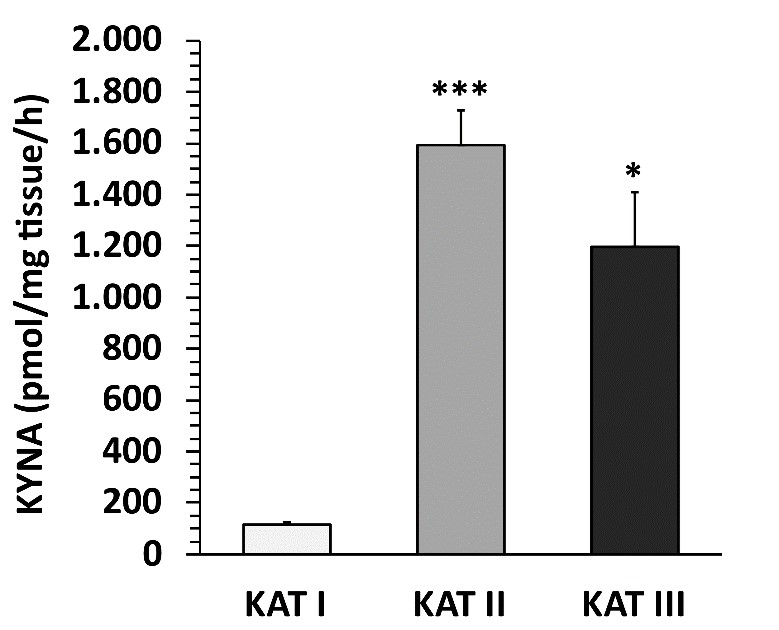
Fig. 13: Kynurenine aminotransferase (KAT) I, II and III activities in rat liver. Data are mean ± SEM of 5 animals. Student's t-test revealed significant differences from KAT I to KAT II and KAT III as indicated by asterisks: *p< 0.05; ***p< 0.001.
Influence of snail mucus on KAT activity in rat liver. Two different snail fluids exerted different effects on KYNA formation in rat liver homogenates (Fig. 14). While yellow and white snail mucus tended to increase KAT I activity, yellow snail mucus tended to decrease KAT II and III activity, whereas white snail mucus tended to increase KAT II and III activity.
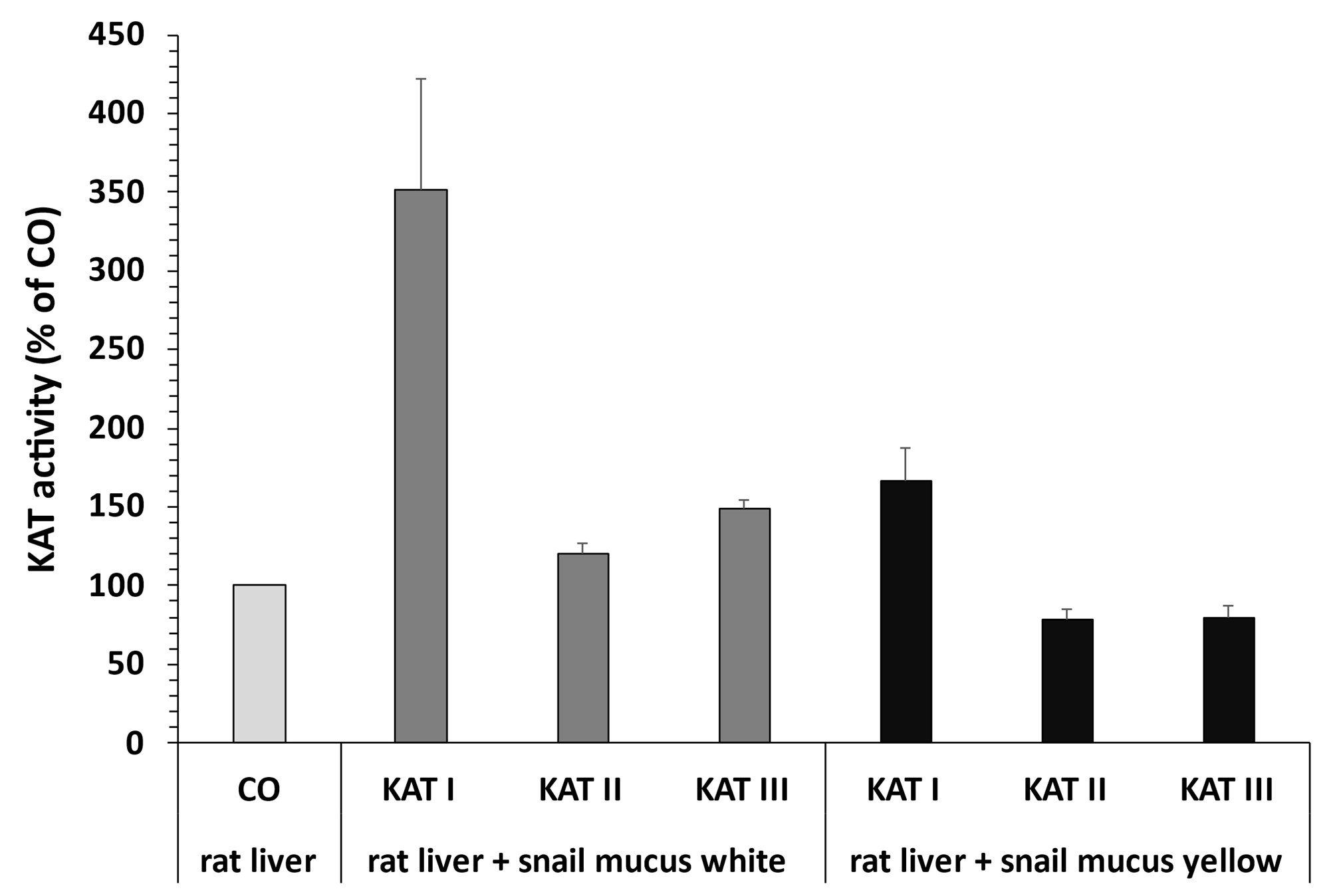
Fig. 14: Influence of rat liver kynurenine aminotransferase (KAT) I, II and III activities by Helix pomatia mucus. Data are mean ± SEM of 4 determinations. Student's t-test showed no significant differences.
Discussion
Using the values of ten selected shell parameters, we developed an ARS to determine the age of the Helix pomatia snail. There are data indicating that growth rings are significantly associated with the age of the snail [59]. In fact, correlation analyses between the number of growth rings and different shell parameters showed a significant dependence and indicated the relevance of using these parameters for age estimation of Helix pomatia snails. Indeed, a significant correlation was obtained between the number of growth rings and body weight, shell length, shell width, shell height, shell opening length, and shell opening width, respectively, and each correlation was statistically significant. Three age groups of Helix pomatia snails, young (1-2 years), middle (5-7 years) and old (9-13 years) were identified, and used for neurochemical evaluation.
Among the examined parameters, four of them, such as rings, body weight, shell weight and shell thickness increased dominantly with age, showing a proportional progression of about 50% for each group, i.e. middle and old group, respectively, while other parameters also increased significantly, but only by 25% for each group, i.e. middle and old group.
Furthermore, we found that the summation of all parameters of each snail resulted in a precise value on the ARS scale, and the obtained ARS values corresponded significantly with the total number of rings and age, respectively. We are convinced that the developed ARS has a significant advantage for studies with the snail Helix pomatia , since the individual parameters can be determined very easily, especially in field studies where the age of individual animals is unknown.
The advantage of the method presented in our study compared to previously published data is the significant increase of the ARS with increasing number of internal or external rings, up to 14 years.
In particular, using the ARS method, we can clearly see the differences between each parameter during the progression of age, without damaging of the structure of the skull. All the parameters together form a pattern of characteristics that could be characteristic for each species of clam.
For the first time, our data showed that snail tissues are able to synthesize KYNA in peripheral organs and CNS regions. Interestingly, snail liver showed a significantly higher KAT I level compared to KAT II or KAT III, and KAT I activity increased significantly with aging; surprisingly, KAT II and III were significantly increased only in the old group. There was no such difference between KAT I and KAT II or KAT III in snail heart, CG or SG. In fact, the value of KAT I, KAT II and KAT III activities in the snail CG and SG were in a similar range during the studied life span.
In particular, our study showed for the first time that there was no increase in KAT I, KAT II or KAT III activity in the CG and SG regions of Helix pomatia, among three age groups, indicating that KAT activity in the CNS does not increase with the age.
Surprisingly, KAT activity in the heart was significantly decreased in old snails, suggesting a novel biochemical pattern of KAT alteration with age. In a previous study, we demonstrated that high KYNA levels significantly affect mitochondrial respiratory parameters in rat heart, in an in vitro study [62] and the involvement of KYNA in cardiovascular diseases such as stroke has been reported [63]. Lowering of serum KYNA levels by exercise using stochastic resonance therapy has been reported [64] and this therapeutic approach was also successful in stroke patients, suggesting that lowering of KYNA in the periphery is a beneficial event for stroke patients [64].
The highest KAT activities were found in the liver of Helix pomatia , which corresponded well with the highest levels of endogenous KYNA found in the liver, followed by the heart, CG and SG, too. Unfortunately, the linear regression analysis between endogenous KYNA levels and KAT I, KAT II and KAT III activities showed no significant correlation, suggesting the presence of events regulating the synthesis or content of KYNA. Remarkably, the similarly high hepatic KYNA levels in the middle and old groups of Helix pomatia actually suggest the presence of events that could regulate KYNA levels during the maturation and aging process. Interestingly, Benatti and co-authors found the highest expression of KAT-like in the gut, and the lowest in the CNS in the six month old pond snail Lymnaea stagnalis [49]. They also found that other kynurenine pathway enzymes are also expressed in the gut [49]. In rodents, KYAT-I and KYAT-III have similar expression patterns: their mRNA levels are much higher in the liver and neuroendocrine tissues than in the CNS [65]. This trend of low KAT activity in the CNS was observed in Helix pomatia [58, and present study], as well as, in Lymnaea stagnalis for Lym KYAT-like [49]. In addition, the young Helix pomatia snails in the line have a body length of 24 mm, which is comparable to the body of the six-month-old pond snail Lymnaea stagnalis with a shell length of 20-25 mm [49].
The marked capacity of KYNA synthesis, found in the picomolar range per mg of tissue per hour, and the low endogenous KYNA found in the femtomolar range lead to the assumption, that there are endogenous factors responsible for the decrease of endogenous KYNA and thus the absence of a possible functional insufficiency [35, 37-40, 62]. The level of KYNA in snail was in femtomolar concentration and was low compared to the amount of KYNA observed in rat or human [2, 5, 14]. A low level of KYNA metabolism has also been reported in piglets [42, 66]. Interestingly, we found that piglet homogenate blocks rat KAT activity [42] and furthermore, several herbs also block KAT activities, as demonstrated in an in vitro study [46, 47]. Importantly, Baran et al. 2010 demonstrated that human body fluids, such as serum or CSF also have the ability to block KYNA formation [44]. The authors postulated the presence of a glia-depressing factor (GDF), and its involvement in pathological conditions such as multiple sclerosis has been suggested [44]. Our data also suggest the presence of such factor(s) in the snail Helix pomatia , since snail mucus could also influence KAT I, II and III activities or KYNA formation, in rat liver homogenate, in an in vitro study. It is still questionable whether these factor(s) could have similar effects on KYNA synthesis in the CNS and periphery of the snail Helix pomatia and during the aging process. Nevertheless, there are still unclear mechanisms of KYNA that need to be considered in the near future [67].
The pharmacological approach in an in vitro and in vivo study showed that synthesized KYNA was immediately released into the medium [14] and transported by probenecid-dependent transport [68]. Uwai et al. 2012 and 2013 demonstrated different transport efficiencies of KYNA by human organic anion transporters hOAT1 (SLC22A6) and hOAT3 (SLC22A8) [68, 69] and the authors showed species differences between rat and human [69]. Probenecid inhibited KYNA transport by hOAT1 and hOAT3 [68, 69]. The authors suggested that these transporters are involved in the disposition of KYNA [68, 69]. Unfortunately, no data on KYNA transport are available for the snail Helix pomatia .
Storage of synthesized KYNA has also been suggested [70], and degradation of KYNA in the periphery has also been documented [71], but these events have not yet been investigated for kynurenine metabolites in Helix pomatia .
Furthermore, the biochemical studies and evaluation of enzyme properties for KAT I, KAT II or KAT III in Helix pomatia suggest some similarities with KATs found in other species including, humans [3, 4, 9, 6-15], but there are also differences, as our data showed. Importantly, we found no significant effect of 2-oxo acids on the efficiency of KYNA formation in Helix pomatia , in contrast to humans or rats, where 2-oxo acids such as PYR, α-KGL or α-KIS play a notable role [3, 4, 9].
In Helix pomatia liver, pH plays an important role in the efficiency of KYNA formation. Vmax is visibly increased with higher pH, i.e. Vmax of KAT II (pH=7.4) < Vmax of KAT III (pH=8.0) < Vmax of KAT I (pH=9.6). Whereas in rat liver, KAT activities increase with decreasing pH value, i.e. KAT I (pH=9.6) < KAT III (pH=8.0) < KAT II (pH=7.4). The accumulated data indicate that, in contrast to the rat, the snail Helix pomatia showed the opposite pattern of KAT I, II and III activities. Therefore, fluctuations in pH could affect KAT activity and KYNA formation. For a healthy environment of the gut microbiome, the different KAT activities within different species may be beneficial. Despite their different properties, snail KAT I shares some characteristics with rat kidney and brain KATs [3, 4, 9]. For example, both enzymes have Km values for L-KYN in the millimolar range.
It is also noteworthy to speculate on the presence of a KAT protein in snail liver responsible for KYNA formation.
The different pattern of KAT I, KAT II and KAT III distribution in the liver between different species such as the snail Helix pomatia and rats, may be important and may have an impact on different physiological roles between species. It is an interesting observation that the increase of KAT II activity during the aging process was observed in the rat CNS but not in the liver [23-26], whereas in the snail KAT I activity significantly increased with age in the liver but not in the CNS.
These data, which indicate significant species-dependent differences in the biochemistry of KYNA and probably in its function, suggest a remarkable phenomenon in the aging biology of the snail Helix pomatia .
Conclusion
The application of the rating scale for age determination in the snail Helix pomatia revealed an interesting pattern of parameter changes during the whole life span. We found that KAT I is the dominant enzyme for KYNA synthesis in the liver of Helix pomatia, and interestingly, an increase in KAT I activity with age was observed. Whereas, a moderate increase in KAT II and III activities could be detected in the liver of the oldest group.
Furthermore, we found that KAT I, II and III activities did not show significant changes between different age groups in the CNS, suggesting a physiological phenomenon that is species dependent. Notably, we found the SG as a specific brain region in the CNS of Helix pomatia during the aging process due to decreased KYNA content.
In terms of KYNA formation, our data indicate a significant species-dependent difference in KYNA metabolism between Helix pomatia and mammals throughout the life span. New pharmacological approaches to regulate KYNA synthesis and its physiological significance for therapeutic application to enhance the learning process are in the research pipeline.
Abbreviations
AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, α-KGL: α-ketoglutarate, α-KIS: α-ketoisocaproate, AMPOL: 2-amino-2-methyl-1-propanol, ARS: age rating scale, EAA: excitatory amino acid, nAChR: nicotinic -acetylcholine receptor, CG: cerebral ganglia, CNS: central nervous system, CSF: cerebro spinal fluid, GDF: glia depressing factor, GPR35: G protein-coupled receptor 35, HPLC: high performance liquid chromatography, KAT: kynurenine aminotransferase, Km: Michaelis-Menten constant, KYNA: kynurenic acid, L-KYN: L-kynurenine, N: number, NMDA: N-methyl-D-aspartate, P5P: pyridoxal 5’-phosphate, PYR: pyruvate, SG: subpharyngeal ganglia, TRP: tryptophan, Vmax: maximal velocity.
Acknowledgements
Study was supported in part by Science and Research of the Lower Austria, St. Pölten (Project K3-F- 995/001-2021 to H. Baran) and by the Austrian National Bank (Jubiläumsfond, Grant 12316 to H. Baran).
H. Baran designed the study. C. Kronsteiner performed the analysis, and B. Kepplinger and H. Baran contributed to the analysis. C. Kronsteiner calculated and assessed the data and wrote the first draft of manuscript. All authors contributed to the final version of the manuscript, which was approved by H. Baran and C. Kronsteiner.
Disclosure Statement
The authors declare no competing interests.
References
| 1 | Hayaishi O: Newer Aspects of tryptophan metabolism; in: Hayaishi O, Ishimura Y, Kido R (eds): Biochemical and Medical Aspects of Tryptophan Metabolism. Developments in Biochemistry. Elsevier/North-Holland Biomedical Press Amsterdam, New York, Oxford,1980, vol 16, pp 16-30.
|
| 2 | Moroni F, Russi P, Lombardi G, Beni M, Carlà V: Presence of Kynurenic Acid in the Mammalian Brain. J Neurochem 1988;51:177-180.
https://doi.org/10.1111/j.1471-4159.1988.tb04852.x |
| 3 | Ishikawa T, Okuno E, Tsujimoto M, Nakamura M, Kido R: Kynurenine-pyruvate aminotransferase in rat kidney and brain; in: Schwarcz R, Young SN, Brown RR (eds): Kynurenine and Serotonin Pathways. Adv Exp Med Biol. New York, Plenum Press, 1991;294:567-573.
https://doi.org/10.1007/978-1-4684-5952-4_68 |
| 4 | Kido R: Kynurenate forming enzymes in liver, kidney and brain, in: Schwarcz R, Young SN, Brown RR (eds): Kynurenine and Serotonin Pathways. Adv Exp Med Biol. New York, Plenum Press, 1991, vol 294, pp 201-205.
https://doi.org/10.1007/978-1-4684-5952-4_18 |
| 5 | Stone TW: Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev 1993;45:309-379.
|
| 6 | Baran H, Amann G, Lubec B, Lubec G: Kynurenic acid and kynurenine aminotransferase in heart. Pediatr Res 1997;41:404-410.
https://doi.org/10.1203/00006450-199703000-00017 |
| 7 | Okuno E, Nakamura M, Schwarcz R: Two kynurenine aminotransferases in human brain. Brain Res 1991;534:307-312.
https://doi.org/10.1016/0006-8993(91)91583-M |
| 8 | Okuno E, Du F, Ishikawa T, Tsujimoto M, Nakamura M, Schwarcz R, Kido R: Purification and characterization of kynurenine-pyruvate aminotransferase from rat kidney and brain. Brain Res 1990;542:307-312.
https://doi.org/10.1016/0006-8993(91)91583-M |
| 9 | Baran H, Okuno E, Kido R, Schwarcz R: Purification and characterization of kynurenine aminotransferase I from human brain. J Neurochem 1994;62:730-738.
https://doi.org/10.1046/j.1471-4159.1994.62020730.x |
| 10 | Han Q, Li J: pH dependence, substrate specificity and inhibition of human kynurenine aminotransferase I. J Mol Cell Biol 2004;271:4804-4814.
https://doi.org/10.1111/j.1432-1033.2004.04446.x |
| 11 | Baran H, Hainfellner JA, Kepplinger B: Kynurenic acid metabolism in various types of brain pathology in HIV-1 infected patients. Int J Tryptophan Res 2012;5:49-64.
https://doi.org/10.4137/IJTR.S10627 |
| 12 | Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R: Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem 2007;102:103-111.
https://doi.org/10.1111/j.1471-4159.2007.04556.x |
| 13 | Yu P, Li Z, Zhang L, Tagle DA, Cai T: Characterization of kynurenine aminotransferase III, a novel member of a phylogenetically conserved KAT family. Gene 2006;365:111-118.
https://doi.org/10.1016/j.gene.2005.09.034 |
| 14 | Turski WA, Gramsbergen JBP, Traitler H, Schwarcz R: Rat brain slices produce and liberate kynurenic acid upon expose to L-kynurenine. J Neurochem 1989;52:1629-1636.
https://doi.org/10.1111/j.1471-4159.1989.tb09218.x |
| 15 | Schwarcz R, Baran H, Wu HQ, Du F, McMaster O: The Neurochemistry of quinolinic acid and kynurenate, in: Meldrum BS, Moroni F, Simon RP, Woods JH (eds): Current Concepts. Excitatory Amino Acids. New York, Raven Press, 1991, pp 365-375.
|
| 16 | Foster AC, Vezzani A, French ED, Schwarcz R: Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett 1984;48:273-278.
https://doi.org/10.1016/0304-3940(84)90050-8 |
| 17 | Weber M, Dietrich D, Gräsel I, Reuter G, Seifert G, Steinhäuser C: 6-Hydroxykynurenic acid and kynurenic acid differently antagonize AMPA and NMDA receptors in hippocampal neurons. J Neurochem 2001;77:1108-1115.
https://doi.org/10.1046/j.1471-4159.2001.00340.x |
| 18 | Bertolino M, Vicini S, Costa E: Kynurenic acid inhibits the activation of kainic and N-methyl-d-aspartic acid-sensitive ionotropic receptors by a different mechanism. Neuropharmacology 1989;28:453-457.
https://doi.org/10.1016/0028-3908(89)90078-6 |
| 19 | Hilmas C, Pereira EFR, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX: The brain metabolite kynurenic acid inhibits 7 nicotinic receptor activity and increases non-7 nicotinic receptor expression: Physiopathological implications. J Neurosci 2001;21:7463-7473.
https://doi.org/10.1523/JNEUROSCI.21-19-07463.2001 |
| 20 | Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L: Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem 2006;281:22021-22028.
https://doi.org/10.1074/jbc.M603503200 |
| 21 | Beal MF, Swartz KJ, Isacson O: Developmental changes in brain kynurenic acid concentrations. Dev Brain Res 1992;68:136-139.
https://doi.org/10.1016/0165-3806(92)90256-V |
| 22 | Walker DW, Curtis B, Lacey B, Nitsos I: Kynurenic acid in brain and cerebrospinal fluid of fetal, newborn, and adult sheep and effects of placental embolization. Pediatr Res 1999;45:820-826.
https://doi.org/10.1203/00006450-199906000-00007 |
| 23 | Baran H, Schwarcz R: Regional differences in the ontogenetic pattern of kynurenine aminotransferase in the rat brain. Dev Brain Res 1993;74:283-286.
https://doi.org/10.1016/0165-3806(93)90014-2 |
| 24 | Gramsbergen JBP, Schmidt W, Turski WA, Schwarcz R: Age-related changes in kynurenic acid production in rat brain. Brain Res 1992;588:1-5.
https://doi.org/10.1016/0006-8993(92)91337-E |
| 25 | Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Grant R: Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J 2011;278:4425-4434.
https://doi.org/10.1111/j.1742-4658.2011.08366.x |
| 26 | Comai S, Costa CVL, Ragazzi E, Bertazzo A, Allegri G: The effect of age on the enzyme activities of tryptophan metabolism along the kynurenine pathway in rats. Clin Chim Acta 2005;360:67-80.
https://doi.org/10.1016/j.cccn.2005.04.013 |
| 27 | Kepplinger B, Baran H, Kainz A, Ferraz-Leite H, Newcombe J, Kalina P: Age-related increase of kynurenic acid in human cerebrospinal fluid - IgG and β2-microglobulin changes. Neurosignals 2005;14:126-135.
https://doi.org/10.1159/000086295 |
| 28 | Baran H, Cairns N, Lubec B, Lubec G: Increased kynurenic acid levels and decreased brain kynurenine aminotransferase I in patients with Down syndrome. Life Sci 1996;58:1891-1899.
https://doi.org/10.1016/0024-3205(96)00173-7 |
| 29 | Baran H, Jellinger K, Deecke L: Kynurenine metabolism in Alzheimer's disease. J Neural Transm 1999;106:165-181.
https://doi.org/10.1007/s007020050149 |
| 30 | Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Robert RC: Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 2001;50:521-530.
https://doi.org/10.1016/S0006-3223(01)01078-2 |
| 31 | Baran H, Hainfellner JA, Kepplinger B, Mazal PR, Schmid H, Budka H: Kynurenic acid metabolism in the brain of HIV-1 infected patients. J Neural Transm 2000;107:1127-1138.
https://doi.org/10.1007/s007020070026 |
| 32 | Baran H, Draxler M, Kronsteiner C, Kepplinger B: Increase of kynurenic acid after encephalomyocarditis virus infection and its significances. Neurosignals 2021;29:24-34.
https://doi.org/10.33594/000000434 |
| 33 | Kepplinger B, Baran H, Kronsteiner C, Reuss J: Increased levels of kynurenic acid in the cerebrospinal fluid in patients with hydrocephalus. Neurosignals 2019;27(1):1-11.
https://doi.org/10.33594/000000095 |
| 34 | Ogawa T, Matson WR, Beal MF, Myers RH, Bird ED, Milbury P, Saso S: Kynurenine pathway abnormalities in Parkinson's disease. Neurology 1992;42:1702-1706.
https://doi.org/10.1212/WNL.42.9.1702 |
| 35 | Chess AC, Simoni MK, Alling TE, Bucci DJ: Elevations of kynurenic acid produce working memory deficits. Schizophr Bull 2007;33:797-804.
https://doi.org/10.1093/schbul/sbl033 |
| 36 | Steele RJ, Stewart MG: 7-Chlorokynurenate, an antagonist of the glycine binding site on the NMDA receptor, inhibits memory formation in day-old chicks (Gallus domesticus). Behav Neural Biol 1993;60:89-92.
https://doi.org/10.1016/0163-1047(93)90145-8 |
| 37 | Vohra M, Lemieux GA, Lin L, Ashrafi K: Kynurenic acid accumulation underlies learning and memory impairment associated with aging. Genes Dev 2018;32:14-19.
https://doi.org/10.1101/gad.307918.117 |
| 38 | Kozak R, Campbell BM, Strick CA, Horner W, Hoffmann WE, Kiss T, Chapin DS, McGinnis D, Abbott AL, Roberts BM, Fonseca K, Guanowsky V, Young DA, Seymour PA, Dounay A, Hajos M, Williams GV, Castner SA: Reduction of brain kynurenic acid improves cognitive function. J Neurosci 2014;34:10592-10602.
https://doi.org/10.1523/JNEUROSCI.1107-14.2014 |
| 39 | Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R: Fluctuations in Endogenous Kynurenic Acid Control Hippocampal Glutamate and Memory. Neuropsychopharmacol 2011;36:2357-2367.
https://doi.org/10.1038/npp.2011.127 |
| 40 | Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu HQ, Schwarcz R: Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity and cognitive behavior. Neuropsychopharmacol 2010;35:1734-1742.
https://doi.org/10.1038/npp.2010.39 |
| 41 | Vohra M, Lemieux GA, Lin L, Ashrafi K. The beneficial effects of dietary restriction on learning are distinct from its effects on longevity and mediated by depletion of a neuroinhibitory metabolite. PLoS Biol DOI: 10.1371/journal.pbio.2002032.
https://doi.org/10.1371/journal.pbio.2002032 |
| 42 | Baran H, Kepplinger B: Porcine tissues influence kynurenic acid formation. Parkinsonism Relat Disord 2006;12:36-36.
https://doi.org/10.1016/S1353-8020(07)70122-0 |
| 43 | Baran H, Kepplinger B: Cerebrolysin lowers kynurenic acid formation - An in vitro study. Eur Neuropsychopharmacol 2009;19:161-168.
https://doi.org/10.1016/j.euroneuro.2008.09.003 |
| 44 | Baran H, Kepplinger B, Draxler M: Endogenous Kynurenine aminotransferases inhibitor is proposed to act as "Glia Depressing Factor" (GDF). Int J Tryptophan Res 2010;3:13-22.
https://doi.org/10.4137/IJTR.S3682 |
| 45 | Baran H, Kepplinger B: D-Cycloserine lowers kynurenic acid formation - new mechanism of action. Eur Neuropsychopharmacol 2013;24:639-644.
https://doi.org/10.1016/j.euroneuro.2013.10.006 |
| 46 | Baran H, Pietryja MJ, Kronsteiner C, Kepplinger B: Jerusalem balsam lowers kynurenic acid formation: an in vitro study. J Tradit Med Clin Naturopat 2017;3:1-5.
https://doi.org/10.4172/2573-4555.1000224 |
| 47 | Kepplinger B, Baran H, Kronsteiner C, Więcek M: Hawthorn berry extract lowers kynurenic acid and anthranilic acid formation. Clin Pharmacol Biopharm 2017;6:57.
|
| 48 | Osborne NN: Tryptophan metabolism in characterized neurones of Helix. Br J Pharmacol 1973;48:546-549.
https://doi.org/10.1111/j.1476-5381.1973.tb08361.x |
| 49 | Benatti C, Rivi V, Alboni S, Grilli A, Castellano S, Pani L, Brunello N, Blom JMC, Bicciato S, Tascedda F: Identification and characterization of the kynurenine pathway in the pond snail Lymnaea stagnalis Sci Rep. 2022;12:15617.
|
| 50 | Pollard E, Cooke AS, Welch JM: The use of shell features in age determination of juvenile and adult Roman snails Helix pomatia. J Zool 1977;183:269-279.
https://doi.org/10.1111/j.1469-7998.1977.tb04186.x |
| 51 | Pannella G, Macclintock C: Biological and environmental rhythms reflected in molluscan shell growth. J Paleontol 1968;42:64-80.
https://doi.org/10.1017/S0022336000061655 |
| 52 | Rhoads DC, Lutz RA: Skeletal growth of aquatic organisms: Biological records of environmental changes; in Rhoads DC, Lutz RA (eds): Topics in Geobiology. New York, Springer, 2013, vol 1, pp 763.
|
| 53 | Raboud C: Age determination of Arianta Arbustorum (L.) (Pulmonata) based on growth and inner Layers. J Moll Stud 1986;52:243-247.
https://doi.org/10.1093/mollus/52.3.243 |
| 54 | Bökenhans V, Bigatti G, Averbuj A: Age estimation methods in the marine gastropod Buccinanops globulosus comparing shell marks and opercula growth rings. Mar Biol Res 2016;12:1-7.
https://doi.org/10.1080/17451000.2016.1209526 |
| 55 | Hollyman PR, Laptikhovsky VV, Christopher A, Richardson CA: Techniques for Estimating the Age and Growth of Molluscs: Gastropoda. J Shellfish Res 2018;37:773-782.
https://doi.org/10.2983/035.037.0408 |
| 56 | García A, Perea JM, Mayoral A, Acero R, Martos J, Gómez G and F: Laboratory rearing conditions for improved growth of juvenile Helix aspersa Müller snails. Lab Anim 2006;40:309-316.
https://doi.org/10.1258/002367706777611505 |
| 57 | Sampelayo RS, Fonolla J, Extremera FG: Land snails as experimental animals: a study of the variability and distribution of individual weight in Helix asparsa snails born from the same clutch. Lab Anim 1990;24:1-4.
https://doi.org/10.1258/002367790780890275 |
| 58 | Kronsteiner C, Kepplinger B, Baran H: Kynurenic acid and kynurenine aminotransferase activity in ganglia and liver of Helix pomatia. 15th Meeting of the Austrian Neuroscience Association (ANA), 24th-26th September 2017, Institute of Science and Technology (IST) Austria, Abstracts book, p. 64.
|
| 59 | Pollard E: Growth classes in the adult roman snail (Helix pomatia L.). Oecologia 1973;12:209-212.
https://doi.org/10.1007/BF00345518 |
| 60 | Löw P, Molnár K, Kriska G: Dissection of a Snail (Helix pomatia), in Löw P, Molnár K, Kriska G (eds): Atlas of Animal Anatomy and Histology. Springer Cham, 2016, vol 1, pp 49-77.
https://doi.org/10.1007/978-3-319-25172-1_5 |
| 61 | Swartz KJ, Matson WR, MacGarvey U, Ryan EA, Beal MF: Measurement of kynurenic acid in mammalian brain extracts and cerebrospinal fluid by high-performance liquid chromatography with fluorometric and coulometric electrode array detection. Anal Biochem 1990;185:363-376.
https://doi.org/10.1016/0003-2697(90)90309-W |
| 62 | Baran H, Staniek K, Bertignol-Spörr M, Attam M, Kronsteiner C, Kepplinger B: Effects of various kynurenine metabolites on respiratory parameters of rat brain, liver and heart mitochondria. Int J Tryptophan Res 2016;17:17-29.
https://doi.org/10.4137/IJTR.S37973 |
| 63 | Kepplinger B, Sedlnitzky-Semler B, Eigner S, Kalina P, Berger P, Baran H: Stroke Patients after repetitive Transcranial Magnetic Stimulation (rTMS) - Alteration of tryptophan metabolites in the serum. Int J Neurorehabil 2014;1:128.
https://doi.org/10.4172/2376-0281.1000128 |
| 64 | Kepplinger B, Baran H, Sedlnitzky-Semler B, Badawi NR, Erhart H: Stochastic Resonance activity influences serum tryptophan Metabolism in healthy human subjects. Int J Tryptophan Res 2011;4:49-60.
https://doi.org/10.4137/IJTR.S7986 |
| 65 | Han Q, Cai T, Tagle DA, Li J: Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell Mol Life Sci 2010;67:353-368.
https://doi.org/10.1007/s00018-009-0166-4 |
| 66 | Baran H, Kepplinger B, Draxler M, Ferraz-Leite H: Kynurenic acid metabolism in rat, piglet and human tissues, in: Battistin L (ed): 7th Congress of the European Society for Clinical Neuropharmacology. J Neural Transm 2004; 111:227-231.
https://doi.org/10.1007/s00702-004-0132-0 |
| 67 | Stone TW: Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J Neurochem 2020;152:627-649.
https://doi.org/10.1111/jnc.14907 |
| 68 | Uwai Y, Honjo H, Iwamoto K: Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol Res 2012;65:254-60.
https://doi.org/10.1016/j.phrs.2011.11.003 |
| 69 | Uwai Y, Kawasaki T, Nabekura T: Caffeic acid inhibits organic anion transporters OAT1 and OAT3 in rat kidney. Drug Metabol Drug Interact 2013;28:247-250.
https://doi.org/10.1515/dmdi-2013-0050 |
| 70 | Wu HQ, Baran H, Ungerstedt U, Schwarcz R: Kynurenic Acid in the Quinolinate-lesioned Rat Hippocampus: Studies In vitro and In vivo. Eur J Neurosci 1992;4:1264-1270.
https://doi.org/10.1111/j.1460-9568.1992.tb00152.x |
| 71 | Kaihara M, Price JM, Takahashi H: The conversion of kynurenic acid to quinaldic acid by humans and rats. J Biol Chem 1956;223:705-708.
https://doi.org/10.1016/S0021-9258(18)65070-7 |