Exosome-Mediated Mechanisms of Drug Resistance in Lung Cancer: Molecular Mechanisms and Therapeutic Strategies
bDepartment of Histology and Embryology, Poznan University of Medical Sciences, 60-781 Poznan, Poland,
cDepartment of Morphological and Physiological Sciences, Faculty of Medicine, Collegium Medicum, Mazovian Academy in Płock, 09-402 Płock, Poland
Keywords
Abstract
Lung cancer, one of the leading causes of cancer-related deaths globally, is notorious for its poor prognosis and limited response to conventional therapies. Despite advancements in chemotherapy, targeted therapies, and immunotherapy, the efficacy of these treatments is often undermined by the development of resistance, particularly multidrug resistance (MDR). MDR in lung cancer is primarily driven by various mechanisms, including the overexpression of ATP-binding cassette (ABC) transporters like P-glycoprotein (ABCB1), which actively pump chemotherapeutic drugs out of cancer cells, reducing their intracellular concentration and effectiveness. Additionally, genetic mutations, enhanced DNA repair mechanisms, and alterations in drug targets contribute to this phenomenon. The complexity of MDR not only complicates treatment regimens but also contributes to the high mortality rate associated with lung cancer. Understanding the underlying mechanisms of MDR and developing strategies to overcome this resistance are critical for improving patient outcomes. The objective of this review is to present a comprehensive summary of the current knowledge on conventional and emerging mechanisms of drug resistance, with a particular focus on the involvement of exosomes and exosome-mediated factors that mediate drug resistance in lung cancer. Exosomes, tiny vesicles secreted by cells, play a critical role in drug resistance, especially in lung cancer. They carry genetic material and proteins that can alter the behavior of recipient cells, promoting resistance. In lung cancer, exosomes transfer miRNAs and other molecules that enhance survival pathways and inhibit cell death, contributing to chemoresistance. Recent research highlights the potential of targeting exosomal pathways to develop new therapeutic strategies.
Introduction
Lung cancer is one of the leading cancer deaths throughout the world. It is the second most frequently diagnosed cancer in men, after prostate cancer, and the second most common cancer in women, after breast cancer [1]. Lung cancer remains a significant global health challenge, with statistics in 2024 reflecting its widespread impact. In the United States alone, it is estimated that there will be around 238, 340 new cases of lung cancer diagnosed and approximately 127, 070 deaths from the disease in 2024, World Health Organization (WHO). Around 80% of lung cancer deaths are caused by smoking. Other risk factors include exposure to radon and asbestos, prolonged and cumulative exposure to air pollution (especially emissions of polycyclic aromatic hydrocarbons, or PAHs), and a personal or family history of lung cancer. Histologically, lung cancer has been broadly categorized into two groups: small cell lung carcinoma (SCLC) that encompasses 15% of all lung cancers, and non-small cell lung carcinoma (NSCLC) that is categorized into lung adenocarcinoma (LUAD), squamous cell carcinoma (SqCC) and large cell carcinoma (LCC) [2].
The prognosis of non-small cell lung cancer (NSCLC) is challenging due to the unavailability of a platform for early-stage diagnosis and the late appearance of symptoms in disease development, which may limit treatment choices and survival [3]. The 5-year survival rate for patients with limited-stage SCLC who receive treatment (typically a combination of chemotherapy and radiation) is approximately 15-30%. Without treatment, the prognosis is poor, with survival typically only a few months [4]. It is encouraging to note that lung cancer survival has only marginally improved over the last several decades. However, there is still much to be done to improve outcomes for patients. The availability of screening and early detection by low-dose computer tomography and advances in targeted treatments and immunotherapy will likely decrease mortality rates and improve patient survival outcomes in the near future.
As a major contributor of global mortality caused by cancers, lung cancer is currently mainly treated by surgical resections, including the dissection of mediastinal lymph nodes by lobectomy operations, especially for patients suffering from non-metastatic NSCLC [5]. Nevertheless, in many patients suffering from non-metastatic NSCLC, surgical operations are out of consideration because these patients have poor pulmonary functions and a high risk of cardiovascular diseases and other comorbidities. For these patients, chemotherapy and adjuvant therapies are used as the first-line treatments [6]. In the treatment of lung cancer, drug resistance is one of the most urgent problems. Some of the cancers show primary resistance to the drugs used, while others, initially sensitive, acquire the trait of drug resistance during chemotherapy. The tumor can trigger various, often complex, mechanisms of defense against cytostatic activity of the drugs used, such as detoxification processes or active removal of the drug from the cell [7]. Mechanisms that allow the development of multidrug resistance (MDR), as well as ways to prevent or eliminate it, are still the subject of intensive research aimed at discovering new, effective MDR inhibitors. A promising direction of research may be the development of diagnostic methods that could allow monitoring of the full state of tumor sensitivity in individual patients in order to select effective chemotherapy.
Cancer resistance can be divided into two broad categories: primary resistance, which involves early tumor progression without prior tumor response, and secondary (acquired) resistance, which occurs after initial tumor responses [8]. Recently, several studies have shown that drug combinations can selectively kill resistant cells while protecting normal cells. For example, chemotherapeutic drugs that induce apoptosis in both normal and cancer cells by activating caspases can be used in combination with caspase inhibitors that block chemotherapy-induced apoptosis. As a result, sensitive cells may be protected while drug-resistant cells undergo apoptosis. This may be due to increased expression of drug pumps in drug-resistant cells that export caspase inhibitors from MDR cells [9]. However, these protective and selective effects may not be achieved if the normal cells overexpress drug pumps, or if the drug resistant cells are deficient in these drug efflux proteins, thereby limiting clinical application [10]. The majority of research to date has focused solely on reversing MDR rather than preventing it. Understanding the molecular mechanisms underlying the development of MDR during chemotherapy may help in the development of novel strategies to overcome MDR. In recent years, studies have focused on new strategies to prevent the emergence of MDR in cancer [9]. The Fig. 1. shows the treatment options for lung cancer.
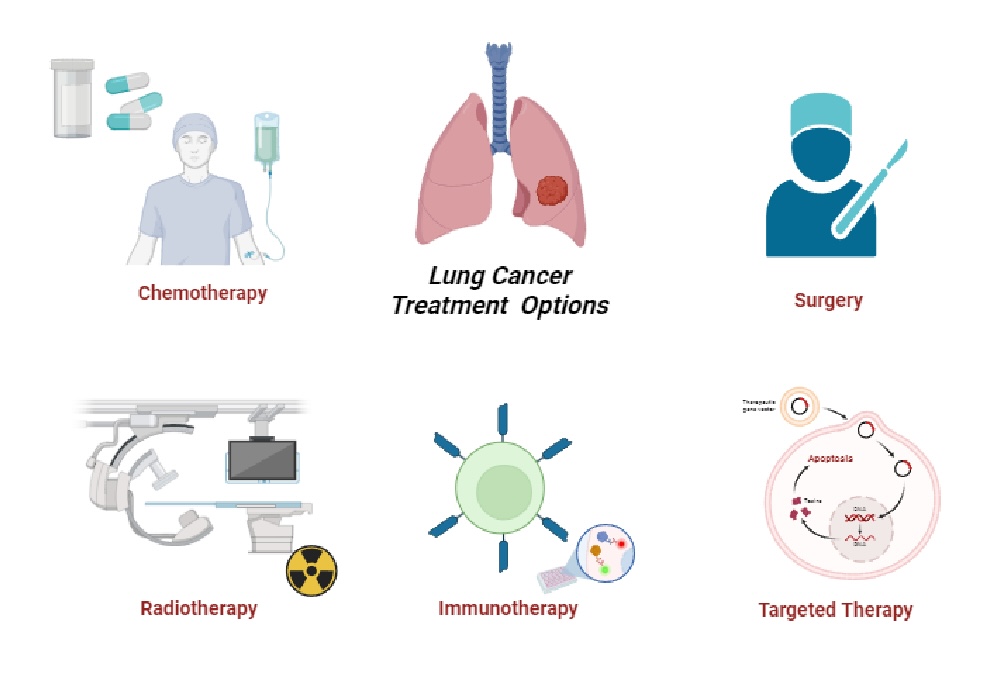
Fig. 1: The treatment options for lung cancer (created with BioRender.com, modified) [11].
The treatment of NSCLC may entail surgical, chemotherapeutic, radiotherapeutic, targeted or a combination of these modalities. In contrast, the treatment of small cell lung cancer typically comprises radiation therapy and chemotherapy.
Mechanisms of drug resistance in lung cancer
Tumor cells are capable of developing resistance to chemotherapeutic drugs not only through intrinsic cellular mechanisms but also through interactions with their surrounding microenvironment. Microenvironment-mediated drug resistance (EM-DR) can generally be divided into two main categories: intrinsic soluble factor-mediated drug resistance (SM-DR) and cell adhesion-mediated drug resistance (CAM-DR) [12]. SM-DR refers to the resistance mechanisms that involve soluble factors within the tumor microenvironment that can influence the survival and proliferation of tumor cells. Various cytokines and growth factors secreted by tumor cells or stromal cells within the tumor microenvironment can promote drug resistance. CAM-DR refers to the resistance to chemotherapy that cancer cells develop when they adhere to components of the extracellular matrix (ECM) or to other cells in their microenvironment [13, 14]. Both CAM-DR and SM-DR contribute to the development of multidrug resistance in lung cancer through mechanisms that involve the upregulation and enhanced function of ABC transporters [15]. By promoting the efflux of chemotherapy drugs from cancer cells, these transporters reduce drug efficacy and contribute to the survival and proliferation of resistant cancer cell populations [16]. The development of cross-resistance involves various cellular mechanisms, such as alterations in drug entry and transport, changes in enzyme affinity for cytostatics, activation or inactivation of pharmacological compounds, disruption of apoptosis regulation, changes in DNA repair processes, and active removal of cytostatics by membrane transport proteins. Increased expression of transport proteins is a frequently reported factor associated with resistance to cancer treatment with cytostatics [7]. MDR is defined as the acquisition by tumor cells of simultaneous insensitivity to administered therapeutic agents that are structurally dissimilar and have diverse molecular points of entry. MDR is a unique type of resistance in which cancer cells develop cross-resistance to a broad spectrum of anti-cancer agents [17]. The mechanisms underlying MDR include ATP-binding cassette (ABC) transporter overexpression, autophagy, DNA damage repair, cancer stem cells, genetic mutations and DNA methylation [18]. The Fig. 2. shows the different mechanisms of multidrug resistance in cancer. The MDR phenomenon is one of the causes of ineffective pharmacotherapy.
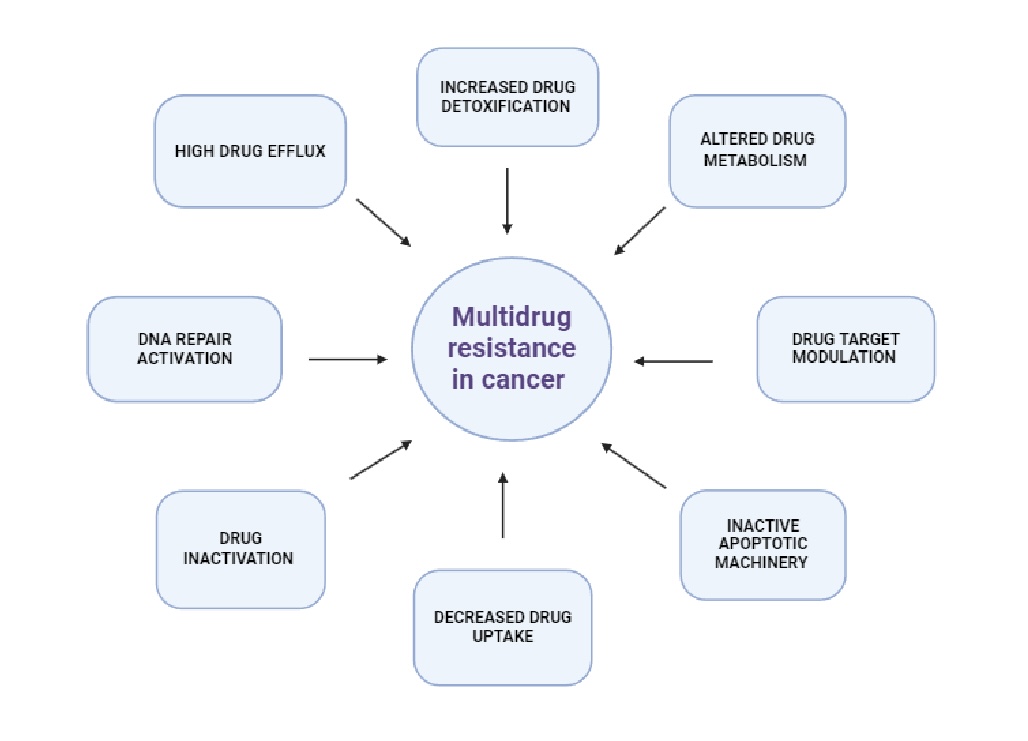
Fig. 2: Mechanisms of multidrug resistance in cancer (created with BioRender.com, modified) [20]. The mechanisms of multidrug resistance (MDR) in tumors are complex. The primary mechanism of MDR is the overexpression of ATP-binding cassette (ABC) transporters, which increases drug efflux and consequently reduces intracellular drug concentration. Other mechanisms of MDR include the reduction of drug uptake by influx transporters, the boosting of drug metabolism, the blocking of apoptotic signaling pathways, the elevation of adaptability through epigenetic and microRNA regulation, the mutation of drug targets or the feedback activation of other targets and signaling pathways, and the alteration of the tumor microenvironment.
The membrane proteins of the ATP-binding cassette (ABC) family are mainly responsible for the MDR phenomenon. The ABC family comprises 49 proteins, which are classified into seven subfamilies (A to G). The proteins of each subfamily have a transmembrane spanning domain (MSD) and an ATP-binding domain (NBD) [19].
The diversity of ABC proteins reflects their crucial roles in cellular physiology and their involvement in MDR of various diseases, including cancer. Among all ABCB1, ABCC1, ABCC3 and ABCG2 have been extensively studied, and they are associated with MDR in cancer cells [21]. Fig. 3. shows the ABC protein family.
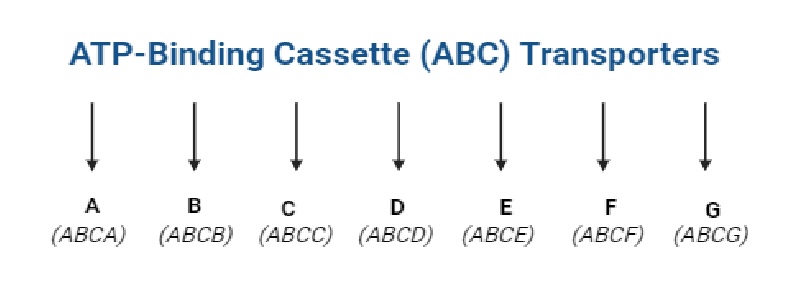
Fig. 3: The family ABC (created with BioRender.com). ATP-binding cassette (ABC) proteins are a large family of transmembrane proteins that utilize the energy derived from ATP hydrolysis to transport various molecules across cellular membranes. They transport a wide variety of substrates across extra- and intracellular membranes, including metabolic products, lipids and sterols, and drugs. ABC transporters play a role in tumor resistance, cystic fibrosis and a range of other inherited human diseases, as well as the development of resistance to multiple drugs.
ABCB1, also commonly known as P-glycoprotein (P-gp), is a transmembrane glycoprotein present in cells of various organs, including the adrenal cortex, biliary canaliculi, endothelium of the blood-brain and blood-testicle barriers, placenta, gastrointestinal epithelium, proximal renal tubuli, and some bone marrow stem cells [19]. P-gp acts as a detoxifying agent in many of organs by pumping toxins or xenobiotics, including anticancer agents, out of cells. ABCB1 is known to mediate MDR to numerous anticancer drugs such as vincristine, paclitaxel, doxorubicin, colchicine, vinblastine, and etoposide by pumping them out of drug-resistant cancer cells [22]. Tumors expressing P-gp can be divided into two groups. The first group includes cancers originating from tissues that primarily express P-gp (including the liver, kidneys, pancreas, intestines and adrenal cortex), which are considered primary resistant. The second group includes cancers originating from tissues that initially have low P-gp concentrations and develop resistance during chemotherapy, which persists after its completion. This group includes, among others: breast cancer, small cell lung cancer, acute and chronic myeloid leukemia, chronic lymphocytic leukemia [7]. High P-gp expression was found in small cell (80%) and non-small cell (100%) lung cancer. The development of P-gp inhibitors that are able to reestablish drug sensitivity of resistant cells when co-administered with anticancer drugs has been considered a promising approach. P-gp is expressed in more than 50% of cancers exhibiting MDR and is associated with the induction of chemotherapy [23]. Another study demonstrated that P-gp is capable of transporting approximately 20 distinct cytotoxic drugs, including paclitaxel and doxorubicin. Several P-gp inhibitors have reached clinical trials, but no inhibitors are clinically available. To solve this issue, many researchers are currently focused on natural products as a source of effective new MDR reversers [24, 25]. There are studies demonstrating that MDR could be potentially overcome by circumventing P-gp-mediated drug efflux with nano-drug delivery system (NDDS), such as nanotube, micelle, liposome, and nanometal material. The NDDS utilizes smart and special mechanisms to achieve drug release in designated circumstances (such as pH, hypoxia, and reducibility) or drug accumulation in tumors via passive targeting (EPR effect) or active targeting (ligand-receptor binding) [8].
MRP1 (ABCC1) is an ATP-binding cassette (ABC) transporter that mediates the efflux of various substrates, including anticancer drugs, thereby contributing to MDR in cancers [26]. The role of MRP1 in drug resistance is more variable but still relevant in certain subtypes, such as adenocarcinoma [27]. Its expression can correlate with poorer prognosis and treatment outcomes. The expression of MRP1 and P-gp in the lung has been the subject of particular study in the context of SCLC and NSCLC [28]. The prominent expression of P-gp and MRP1 in the human lung suggests that these transporters may be pivotal in the protection against endogenous or exogenous toxic compounds entering the lung.
ABCG2, also known as breast cancer resistance protein (BCRP), is highly expressed in many tissues, including the mammary glands, prostate, small intestine, brain, colon, liver, and kidney. It acts as an essential component of the cell defense system and is also associated with cell stemness [29]. ABCG2 is known to mediate MDR to numerous anticancer drugs including topotecan, mitoxantrone, irinotecan, and SN-38 [30]. This protein has the ability to transport both hydrophobic molecules and hydrophilic inorganic conjugates containing sulphate residues. By this ability, it is functionally similar to the Multidrug resistance-associated protein 1 (MRP1/ABCC1). However, this transporter is capable of shedding molecules of many chemotherapeutics outside the cell, such as doxorubicin, daunorubicin, methotrexate, topotecan, irinotecan, the tyrosine kinase inhibitors imatinib and gefitinib [31]. In the human body, BCRP functions as a protective pump, removing toxic substances of exo- and endogenous origin from cells, which is why its high concentration is observed in tissues of organs responsible for detoxification processes. Overexpression of BCRP has been reported in acute lympho- and myeloblastic leukaemia cells, multiple myeloma, prostate cancer and breast, lung, kidney and endometrial cancer [32].
ABCC3 is a member of the multidrug resistance-associated protein (MRP) subfamily which is involved in MDR [33]. Structurally, ABCC3 consists of two transmembrane domains (TMDs), each containing multiple membrane-spanning α-helices, and two nucleotide-binding domains (NBDs) responsible for ATP binding and hydrolysis. The TMDs form the translocation pathway through which substrates are transported across the membrane, while the NBDs drive the transport process by alternating between ATP-bound and ADP-bound states [34, 35]. ABCC3 is expressed in various tissues, including the liver, intestines, and lungs. ABCC3 expression has been shown to be higher in NSCLC than that in SCLC and ABCC3 expression correlated with decreased sensitivity of lung cancer cells to anticancer drugs (vincristine, etoposide and cisplatine and especially to methotrexate and doxorubicin in NSCLC [36]. Hypoxic conditions, which are prevalent in tumor microenvironments, have been observed to upregulate ABCC3 expression via the activation of hypoxia-inducible factors (HIFs). Furthermore, microRNAs (miRNAs) have been identified as post-transcriptional regulators of ABCC3, influencing its expression levels and, consequently, its function in drug resistance [37, 38].
In lung cancer, understanding the regulation of these transporters, their interaction with cancer therapies, and their potential role in chemoresistance could lead to novel strategies to improve treatment outcomes. Modulating the activity of these transporters or finding ways to overcome their drug efflux function remains an area of intense research, particularly in improving the efficacy of chemotherapy in lung cancer patients.
The role of the tumor microenvironment in drug resistance
For many years, cancer research concentrated on the tumor cells themselves. However, as scientific knowledge advanced in this field, the crucial role of the tumor microenvironment (TME) and its communication pathways was revealed. The exchange of information between tumor cells and their environment can affect tumor growth either positively or negatively. It has been shown that the microenvironment of unadvanced tumors, often inhibits their growth, and only as the disease progresses do tumor cells reprogram the microenvironment to support their growth [39]. Communication involves both cell secretion products, which are cytokines, chemokines and metabolites, and various processes occurring in the tumor microenvironment, including hypoxia, angiogenesis, intercellular matrix remodelling. All these signals and processes have a significant impact on the development of the disease and its heterogeneity, making the tumor microenvironment an important source for potential targets for new anti-cancer therapies. TME is composed of a variety of cellular elements, including cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), T cells, natural killer (NK) cells, B cells, endothelial cells, pericytes, and other cell types. These elements engage in a dynamic interplay with tumor cells, influencing their behavior and response to therapeutic intervention [40]. Recent research has elucidated the multifaceted roles of the tumor microenvironment (TME) in mediating drug resistance, thereby providing new insights into potential therapeutic targets [41]. Cancer-associated fibroblasts (CAFs) represent a dominant component of the TME, exerting profound effects on tumor biology. These fibroblasts secrete various cytokines, growth factors, and ECM (extracellular matrix) components that facilitate tumor growth and metastasis [42]. CAFs contribute to drug resistance by remodeling the ECM, which can physically impede drug penetration, and by secreting factors that activate survival pathways in cancer cells [43]. Recent studies have highlighted the role of CAF-derived exosomes in transferring resistance-related molecules, such as microRNAs and proteins, to tumor cells, thereby enhancing their survival and drug resistance. Understanding the intricate interactions within the TME has led to the development of novel therapeutic strategies aimed at overcoming drug resistance [44]. Targeting CAFs and their secreted factors, modulating immune cell function, and altering ECM dynamics are promising approaches [40]. For instance, inhibitors of CAF-related signaling pathways and ECM-modifying enzymes are being explored to enhance drug delivery and efficacy. Immunotherapies targeting TAMs and other immunosuppressive cells within the TME are also under investigation, aiming to reprogram the immune microenvironment to support anti-tumor activity [45]. In NSCLC, CAFs secrete interleukin-6 (IL-6), which activates the JAK2-STAT3 signaling pathway in cancer cells, promoting metastasis and contributing to a drug-resistant phenotype [46, 47]. Targeting the IL-6/JAK2/STAT3 axis has emerged as a promising strategy to overcome CAF-induced resistance in NSCLC. Several therapeutic approaches are being investigated, for example: monoclonal antibodies targeting IL-6 (e.g., siltuximab) or its receptor IL-6R (e.g., tocilizumab), along with small-molecule inhibitors of JAK2 such as ruxolitinib, can inhibit downstream signaling pathways and have shown potential in preclinical studies to sensitize NSCLC cells to chemotherapy and immunotherapy, potentially reversing drug resistance [48, 49]. Small-molecule inhibitors of JAK2, such as ruxolitinib, have shown potential in preclinical studies to sensitize NSCLC cells to chemotherapy and immunotherapy. SCLC is characterized by a paucity of actionable driver mutations, which limits targeted therapeutic options. Unlike NSCLC, where specific mutations can be targeted with precision therapies, SCLC typically lacks such identifiable mutations, making treatment more challenging. Additionally, the tumor microenvironment in SCLC is notably dense and rich in stromal components. This environment, combined with elevated levels of interleukin-6 (IL-6), fosters the survival of chemoresistant cancer cell subpopulations [50, 51].
Hypoxia, a common feature of the TME, profoundly affects the efficacy of cancer therapies, including chemotherapy and immunotherapy. Tumor hypoxia results from the rapid proliferation of cancer cells outpacing the development of new blood vessels, leading to regions with low oxygen levels [52]. Recent research has revealed the mechanisms by which hypoxia contributes to therapeutic resistance and explored strategies to mitigate its effects. Hypoxia leads to the stabilization and activation of hypoxia-inducible factors (HIFs), particularly HIF-1α, which drive the transcription of genes associated with survival, angiogenesis, and metabolic adaptation [53, 54]. In SCLC, hypoxic regions within the tumor microenvironment play a significant role in promoting chemoresistance. For instance, a study on the effects of HIF-1α on gene expression profiles of NCI-H446 cells, a human SCLC cell line, demonstrated that HIF-1α influences the expression of multiple genes involved in tumor progression and resistance mechanisms [55, 56]. Additionally, research has indicated that IL-6 can increase the expression of HIF-1α through STAT3, promoting glycolysis and contributing to a chemoresistant phenotype [57]. These findings underscore the importance of the HIF-1α and IL-6/STAT3 signaling axis in the development of chemoresistance in SCLC, suggesting that therapeutic interventions targeting these pathways may enhance treatment efficacy [54, 58]. HIF-1α upregulates the expression of genes involved in drug resistance, such as MDR1, which encodes P-glycoprotein, a drug efflux pump that reduces intracellular drug accumulation, thereby diminishing the efficacy of chemotherapeutic agents [58]. Recent studies have shown that hypoxia can also alter the cellular response to chemotherapy by inducing a quiescent state in cancer cells, making them less susceptible to drugs targeting rapidly dividing cells [59]. Additionally, hypoxia-induced autophagy can promote cell survival during chemotherapy, further contributing to drug resistance [60]. Hypoxia impacts immunotherapy by creating an immunosuppressive TME. HIF-1α enhances the expression of immune checkpoint molecules, such as PD-L1, on tumor cells, promoting immune evasion. Studies have demonstrated that hypoxia-induced PD-L1 expression can be a significant barrier to effective immune checkpoint blockade therapy [61]. Furthermore, hypoxia promotes the recruitment and activity of immunosuppressive cells, including regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), which inhibit the anti-tumor immune response. Hypoxia-induced adenosine production, through the enzymatic activity of CD39 and CD73, also plays a role in suppressing T cell function and promoting tumor progression [62]. Targeting tumor hypoxia is likely to become a key strategy in anticancer therapy. This may be accomplished by either reducing hypoxia with hypoxia-reducing agents or by directly targeting these oxygen-deprived regions. The use of nanocarriers designed to target hypoxic regions represents a promising strategy for the delivery of anticancer drugs in a targeted manner. Developing a polymeric nanocarrier, or polymersome, loaded with two distinct anticancer agents: an immunostimulant targeting the stimulator of interferon genes (STING pathway) and a chemotherapeutic [63]. These agents will be selectively released in hypoxic tumor regions following systemic administration. Hypoxia-activated prodrugs (HAPs) have been demonstrated to exhibit selective cytotoxicity within hypoxic tumor regions, while modulating hypoxia-inducible factor (HIF) signaling and overcoming immunosuppressive mechanisms within the hypoxic microenvironment. These properties have shown promise in enhancing the efficacy of immunotherapy [64]. Oxygenation strategies, such as hyperbaric oxygen therapy (HBO) and oxygen-carrying agents, have the potential to enhance tumor oxygenation and render tumors more susceptible to a range of treatment modalities, including radiation therapy and photodynamic therapy (PDT) [65]. Furthermore, combining immunotherapy with hypoxia-targeted approaches or developing hypoxia-specific immunotherapies may result in enhanced efficacy and improved treatment outcomes.
The role of exosomes in drug resistance
Exosomes as mediators of intercellular communication
The
development of MDR resistance represents a significant challenge in
the management of lung cancer. Despite
the development of novel molecularly targeted drugs to promote
therapeutic efficacy, the
5-year survival rate remains low [66].
Recent molecular studies have identified various genes and signaling
pathways that contribute to chemoresistance, enhancing understanding
of tumor biology and resistance mechanisms. A significant emerging
mechanism is the transfer of extracellular vesicles (EVs),
like exosomes, between cancer cells and their non-cancerous
surroundings, which facilitates resistance to chemotherapy. These
small vesicles are defined as small lipid nanovesicles with a
diameter of 30–150 nm which are secreted by most cell types,
influence numerous physiological processes and play an important role
in diseases, including cancer [67].
Exosomes are key mediators of intercellular communication,
facilitating the transfer of various biomolecules like proteins,
lipids, and RNA between cells. By transferring molecules, exosomes
help regulate immune responses, modulate the tumor microenvironment,
and contribute to drug resistance, metastasis, and tumor progression
[68].
Their role in cellular communication makes them essential for
understanding disease mechanisms and developing therapeutic
strategies.
Exosomes
play a dynamic role within the tumor microenvironment (TME) by
actively interacting with surrounding stromal and immune cells,
thereby modulating various TME components. Under conditions such as
hypoxia, nutrient deprivation, and acidosis, tumor cells increase the
release of exosomes, which in turn alter the behavior of adjacent
cells to create a supportive niche for tumor growth and survival [69,
70].
Furthermore, exosomes can convert normal stromal cells into
cancer-associated fibroblasts, amplifying the pro-tumorigenic signals
within the TME [71].
They also facilitate the establishment of an immunosuppressive
microenvironment by delivering immune checkpoint molecules and
cytokines that inhibit anti-tumor immune responses [72].
The effect of exosomes on resistance
One
of the principal mechanisms by which exosomes contribute to the
development of drug resistance is through the transfer of microRNAs
(miRNAs). These small, non-coding RNAs are capable of regulating gene
expression post-transcriptionally. Tumor-derived exosomes frequently
contain miRNAs that target genes involved in cell survival, apoptosis
and drug metabolism [73,
74].
For instance, exosomes derived from cancerous cells have been found
to contain a greater number of miRNAs, which are closely associated
with cancer invasion, and drug resistance, than exosomes derived from
normal cells [75].
For example, miR-21 (MicroRNA-21)
is commonly found in exosomes from various cancers and has been shown
to downregulate the expression of phosphatase
and tensin homolog (PTEN),
a tumor suppressor gene, thereby promoting cell survival and
resistance to chemotherapy [76].
This
miRNA transfer alters the recipient cell’s phenotype, making it
more adept at evading the effects of anticancer drugs. Exosomes
also transport proteins that contribute directly to drug resistance.
These include MDR proteins such as P-gp and MRPs. Furthermore, other
efflux transporters, including ABCB2, ABCA3, and MRP1, have been
demonstrated to regulate drug resistance via exosome transport [77].
These findings provide a novel molecular mechanism for how
chemotherapeutic drugs help sensitive cancer cells acquire drug
resistance. Recent reports underline that exosomes affect the
distribution of membrane transport proteins between drug-resistant
and sensitive cells, thereby inducing the production of
drug-resistant proteins [11,
76].
Exosomes
play a crucial role in resistance signaling by transferring growth
factors and signaling molecules between tumor cells, thus promoting
the development of drug resistance. Tumor-derived exosomes are often
enriched with growth factors like vascular endothelial growth factor
(VEGF), transforming growth factor-beta (TGF-β),
and epidermal growth factor (EGF) [78]. These growth factors, upon
transfer to neighboring tumor or stromal cells, activate signaling
pathways that promote cellular proliferation, angiogenesis, and
survival. For example, VEGF in exosomes can stimulate endothelial
cell proliferation, fostering the development of a vascular network
that supports tumor growth and offers a protective barrier against
therapeutic agents. A research study on glioblastoma demonstrated the
crucial role of vesicles in the exchange of information between
malignant and vascular cells, the release of growth factors and
cytokines by endothelial cells, the activation of the PI3K/AKT
signaling pathway, and the movement of pericytes [79]. It was
therefore demonstrated that these tumour-related extracellular
vesicles are capable of autonomously stimulating the proliferation
and migration of tumor cells. Furthermore, it was found that they are
able to modify the tumor microenvironment in a manner that supports
the growth and spread of tumors. This is achieved through the
disruption of the ECM and the release of growth factors that
facilitate tumor cell migration. Additionally, the vesicles were
found to contain cytokines that triggered immune and inflammatory
responses, along with VEGF, which contributed to pro-angiogenic
functions [78]. Targeting exosome-mediated angiogenesis presents a
novel approach in cancer therapy. Strategies to inhibit exosome
production, release, or uptake could potentially reduce angiogenesis
and, consequently, tumor growth and metastasis. Additionally,
exosomes themselves could be engineered to deliver anti-angiogenic
agents directly to the tumor site, providing a targeted therapeutic
approach [80].
Exosomes
are important in modulating the immune response, thereby contributing
to the immunosuppressive environment that is often found in tumors.
This immunosuppression is a key mechanism by which tumors evade the
immune system and continue to grow unchecked. By delivering
immunosuppressive molecules, exosomes can alter the behavior and
function of immune cells within the tumor microenvironment [81]. This
modification supports the establishment of an environment that is
conducive to tumor growth and metastasis while hindering the body's
natural immune response. For example, exosomal PD-L1(Programmed
Cell Death Ligand 1)
can bind to PD-1 on T cells, inhibiting their cytotoxic activity and
allowing tumor cells to evade immune destruction. Additionally,
exosomes can deliver miRNAs and proteins that suppress the function
of dendritic cells, macrophages, and natural killer cells, further
contributing to an immunosuppressive tumor microenvironment [82].
Understanding the role of exosomes in immunosuppression opens new
avenues for cancer therapy. Targeting exosomal pathways could help in
reversing the immunosuppressive tumor microenvironment. Strategies
might include blocking the release or uptake of exosomes, inhibiting
the exosomal PD-L1 interaction with PD-1, or altering the cargo of
exosomes to boost immune responses against tumors. Tumor-associated
exosomes play a key role in mediating alterations within the TME
through endocrinal effects, including hypoxia, starvation and
acidosis [83]. In response to these stressful environments, the
release of tumor-associated exosomes is further increased, forming a
feedback loop that ultimately promotes tumor drug resistance [84].
Exosomes
have been demonstrated to facilitate intercellular communication by
transporting a range of bio-molecules between tumor cells and their
microenvironment. Moreover, exosomes are capable of transporting
signalling molecules that promote cancer cell survival and
proliferation, thereby providing resistance to chemotherapy.
Furthermore, exosomes can modulate the tumor microenvironment by
inducing phenotypic changes that impede drug response. It is
therefore imperative to gain a deeper understanding of the role of
exosomes in mediating drug resistance in lung cancer in order to
develop new therapeutic strategies and biomarkers to overcome
treatment limitations [85].
In
the context of lung cancer, exosomes have garnered significant
interest due to their involvement in the pathogenesis of both SCLC
and NSCLC. For instance, a 2024 study highlighted the potential of
exosome-based therapies in NSCLC, demonstrating that exosome-based
treatments could decrease tumor growth, metastasis, and drug
resistance in preclinical models [86].
Exosomes also play a significant role in modulating the response of
cancer cells to treatments like chemotherapy and radiation. In NSCLC,
radioresistance is a major challenge in treatment, and recent studies
have examined the influence of exosomes in this process [87,
88].
A 2021 study explored how exosomes derived from NSCLC cells can
affect radiosensitivity [89].
The researchers found that tumor-derived exosomes influence the
response of NSCLC cells to radiation therapy by transferring
molecules that either enhance or diminish the cells' sensitivity to
radiation. Exosomal cargo such as microRNAs, proteins, and long
non-coding RNAs (lncRNAs) can alter DNA repair pathways, apoptosis,
and cell cycle progression, leading to either increased resistance or
susceptibility to radiation [90].
This ability to modulate the tumor's response to therapy makes
exosomes an important target for improving the efficacy of
radiotherapy in NSCLC patients. Recent studies have underscored the
potential of exosome-based therapies in combating tumor growth,
metastasis, and drug resistance [91,
92].
Furthermore, exosomes show promise as biomarkers for early detection,
diagnosis, and prognosis in NSCLC patients. As research progresses,
targeting exosomes or harnessing their therapeutic potential could
offer new strategies for improving treatment outcomes in lung cancer
[93,
94].
It is important to note that the therapeutic strategies discussed in
these studies are predominantly focused on non‐small
cell lung cancer (NSCLC), which accounts for approximately 85% of all
lung cancer cases due to its distinct molecular profile and tumor
microenvironment compared to small cell lung cancer (SCLC).
Beyond
lung cancer, exosomes play a crucial role in drug resistance across
various cancer types. In breast cancer, exosomes carry microRNAs such
as miR-222 and miR-223, which modulate the expression of
resistance-associated proteins like PTEN and FOXO3a, leading to
increased chemoresistance [95–98].
Similarly, in ovarian cancer, exosomal miR-21 contributes to
cisplatin resistance by downregulating PTEN and activating the
PI3K/AKT pathway [99–101].
In prostate cancer, exosomes transport proteins and RNA molecules
associated with resistance to hormonal therapy, promoting tumor
progression [102,
103].
Melanoma-derived exosomes, enriched with miR-155 and miR-210, enhance
resistance to BRAF inhibitors by modifying the tumor microenvironment
and supporting angiogenesis [101,
104].
These findings highlight the universal role of exosomes in
facilitating drug resistance across different malignancies,
underscoring the need for targeted strategies to counteract their
effects in NSCLC and beyond. Given
their crucial role in shaping therapeutic responses, exosomes
represent both a challenge and an opportunity in modern oncology.
Understanding their mechanisms of action opens the door to innovative
therapeutic strategies aimed at overcoming drug resistance and
improving patient outcomes.
Modern therapeutic approaches and strategies to overcome resistance
The
advent of modern therapeutics has greatly improved cancer treatment.
However, drug resistance remains a huge challenge. In
addition to exosomes, other biomarkers within the TME provide
valuable insights into cancer resistance. These include circulating
tumor DNA (ctDNA), TAMs and cytokines, all of which contribute to the
complex interplay of factors that drive resistance [105].
ctDNA fragments, shed by tumor cells into the bloodstream, offer a
non-invasive means of monitoring genetic changes associated with
resistance. The analysis of ctDNA enables the identification of
mutations and alterations in real-time, thereby providing a dynamic
view of tumor evolution. TAMs represent a pivotal element of the TME,
exerting a profound influence on tumor growth and therapeutic
responsiveness. The presence and activity of these cells may be
indicative of resistance mechanisms, such as immune evasion and the
suppression of anti-tumor responses.
Cytokines,
released by both tumor and stromal cells, regulate various aspects of
the immune response and can contribute to drug resistance by
promoting a microenvironment conducive to the growth and spread of
cancer cells [106].
The monitoring of cytokine levels can provide insights into the
inflammatory state of the TME and its impact on therapy resistance.
The integration of exosomes and other TME-derived biomarkers into
clinical practice offers a powerful approach for real-time monitoring
of cancer resistance [107,
108].
Liquid
biopsy enables the non-invasive monitoring of resistance, allowing
for timely adjustments to treatment regimens. It also facilitates
early detection of relapse, potentially improving patient outcomes
through prompt intervention [109].
The integration of exosomes and other TME-derived biomarkers into
clinical practice offers a powerful approach for real-time monitoring
of cancer resistance. Techniques such as liquid biopsies enable the
non-invasive collection and analysis of these biomarkers,
facilitating the timely adaptation of therapeutic strategies [110].
Liquid biopsies involve the analysis of biomarkers in bodily fluids,
such as blood or urine, providing a less invasive alternative to
traditional tissue biopsies [111].
This approach allows for the continuous monitoring of tumor dynamics
and the detection of emerging resistance. Understanding the role of
exosomes and other biomarkers in resistance can inform the
development of novel therapeutic strategies. Targeting exosomal
pathways, for instance, could enhance the sensitivity of tumors to
existing treatments and overcome resistance. Due
to rapidly developing nanotechnologies, exosomes could be used for
therapeutic applications.
These
nanobioconjugates may actively target tumors by accurately locating
on the surface of tumor cells, blocking signaling, and enhancing
macrophage phagocytosis [112]
[113,
114].
Further research is being conducted on the potential use of synthetic
exosomes as therapeutic agents or active drug delivery systems. By
using the exosomal organotropic properties, it may be possible to
specifically target a recipient cell for gene therapy using exosomes
that deliver therapeutic substances [115].
In
NSCLC, exosomes derived from bone marrow mesenchymal stem cells
(MSCs) have been demonstrated to enhance chemosensitivity to
cisplatin by delivering microRNA-193a, which targets leucine-rich
repeat-containing protein 1 (LRRC1) and reduces drug resistance
[116].
Conversely, lung cancer cell-derived exosomes (LCCDEs) have been
shown to play a role in the development of drug resistance through
the transfer of resistance-associated proteins and RNA. This offers a
promising new avenue for the development of more effective
therapeutic interventions [87].
To
further address drug resistance in NSCLC, emerging combination
therapies are under investigation that simultaneously target exosome
biogenesis and conventional treatment pathways [117–119].
For example, the use of GW4869 – an inhibitor of the ceramide
pathway—in combination with targeted agents such as osimertinib has
shown potential in sensitizing NSCLC cells to therapy [120].
Additionally, novel approaches are exploring the engineering of
exosomes as delivery vehicles for small interfering RNAs (siRNAs) or
chemotherapeutic agents that directly modulate resistance pathways
(e.g., PI3K/AKT or TGF-β)
[121].
Furthermore, immunotherapeutic strategies aimed at neutralizing
exosome-mediated immune suppression—such as the use of antibodies
to block exosomal PD-L1—are being investigated to enhance the
efficacy of immune checkpoint inhibitors in NSCLC [122].
Integrating liquid biopsy monitoring of exosomal biomarkers into
clinical practice allows for real-time adjustments to treatment
regimens, enabling early detection of emerging resistance mechanisms.
Additionally, small extracellular vesicles (sEVs) isolated from
biological fluids have shown promise as valuable biomarkers for
chemotherapy resistance, offering potential for predicting treatment
non-responsiveness.
Exosomal microRNAs (miRs), proteins, and other biomolecules have been
demonstrated to be correlated with drug resistance and high
recurrence rates in a range of cancers. In this context, a reduced
serum concentration of sEV miR-146a-5p was associated with an
increased risk of NSCLC recurrence and cisplatin resistance, whereas
elevated levels of sEV miR-222-3p were predictive of gemcitabine
sensitivity [123,
124].
Conclusion
Drug resistance remains a significant challenge in treating lung cancer, diminishing the effectiveness of both conventional chemotherapy and novel targeted therapies. Exosomes, small extracellular vesicles secreted by tumor cells, play a critical role in this resistance by transferring molecules such as microRNAs, proteins, and growth factors between cells. These molecules alter cellular phenotypes, promote angiogenesis, and suppress immune responses, creating an environment that supports tumor survival and proliferation. Targeting exosomal pathways holds potential for novel cancer therapies. Strategies may include inhibiting exosome release or uptake, blocking interactions like PD-L1/PD-1, or engineering exosomes to deliver therapeutic agents, thus enhancing immune responses and reducing tumor progression.
Acknowledgements
Author Contributions
Vita
Havryliuk and Agnieszka Żuryń focused on developing chapters:
Introduction, “Mechanisms
of drug resistance in lung cancer” and “The
role of the tumor microenvironment in drug resistance”.
Karolina
Wojtowicz has drafted a chapter about “The
role of exosomes in drug resistance”.
Maciej
Gagat has described the chapter about “Modern
therapeutic approaches and strategies to overcome resistance”.
Funding Sources
No funding.
Disclosure of AI Assistance
In
the process of writing, we utilized artificial intelligence tools,
specifically language models, to assist with text development,
formulating precise statements, and improving grammar and style. AI
was used exclusively for the editorial process and did not influence
the substantive aspects of the research, data analysis, or
interpretation of results.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
| 1 | Parkin D, Bray F, Ferlay J, Pisani P: Global Cancer Statistics, 2002. CA Cancer J Clin. 2005 Mar;55(2):74-108.
https://doi.org/10.3322/canjclin.55.2.74 |
| 2 | Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A: Epidemiology of lung cancer. Contemp Oncol Poznan Pol. 2021;25(1):45-52.
https://doi.org/10.5114/wo.2021.103829 |
| 3 | Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, Mukherjee A, Manash P: Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023 Feb;22(1):40.
https://doi.org/10.1186/s12943-023-01740-y |
| 4 | Blackhall F, Girard N, Livartowski A, McDonald L, Roset M, Lara N, García A: Treatment patterns and outcomes among patients with small-cell lung cancer (SCLC) in Europe: a retrospective cohort study. BMJ Open. 2023 Feb;13(2):e052556.
https://doi.org/10.1136/bmjopen-2021-052556 |
| 5 | Manfredini B, Zirafa CC, Filosso PL, Stefani A, Romano G, Davini F, Melfi F: The Role of Lymphadenectomy in Early-Stage NSCLC. Cancers. 2023 Jul;15(14):3735.
https://doi.org/10.3390/cancers15143735 |
| 6 | Wei Z, Chen J, Zuo F, Guo J, Sun X, Liu D, Liu C: Traditional Chinese Medicine has great potential as candidate drugs for lung cancer: A review. J Ethnopharmacol. 2023 Jan;300:115748.
https://doi.org/10.1016/j.jep.2022.115748 |
| 7 | Katarzyna Lenart, Anna Szyda, Marek Kiełbasiński, Danuta Duś, Maria Podolak-Dawidziak : Clinical effects of multidrug resistance in neoplasms. Via Medica ISSN 1734-3542.2005
|
| 8 | Sun X, Zhao P, Lin J, Chen K, Shen J : Recent advances in access to overcome cancer drug resistance by nanocarrier drug delivery system. Cancer Drug Resist. 2023;6(2):390-415.
https://doi.org/10.20517/cdr.2023.16 |
| 9 | Wang J, Seebacher N, Shi H, Kan Q, Duan Z: Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget. 2017 Oct;8(48):84559-71.
https://doi.org/10.18632/oncotarget.19187 |
| 10 | Engle K, Kumar G: Cancer multidrug-resistance reversal by ABCB1 inhibition: A recent update. Eur J Med Chem. 2022 Sep;239:114542.
https://doi.org/10.1016/j.ejmech.2022.114542 |
| 11 | Ashrafi A, Akter Z, Modareszadeh P, Modareszadeh P, Berisha E, Alemi PS, Chacon Castro MDC, Deese AR , Zhang L : Current Landscape of Therapeutic Resistance in Lung Cancer and Promising Strategies to Overcome Resistance. Cancers. 2022 Sep;14(19):4562.
https://doi.org/10.3390/cancers14194562 |
| 12 | Huang Y, Wang Y, Tang J, Qin S, Shen X, He S, Ju S: CAM-DR: Mechanisms, Roles and Clinical Application in Tumors. Front Cell Dev Biol. 2021 Jul;9:698047.
https://doi.org/10.3389/fcell.2021.698047 |
| 13 | Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B: The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv Pharm Bull. 2017 Sep;7(3):339-48.
https://doi.org/10.15171/apb.2017.041 |
| 14 | Wu P, Gao W, Su M, Nice EC, Zhang W, Lin J, Xie N: Adaptive Mechanisms of Tumor Therapy Resistance Driven by Tumor Microenvironment. Front Cell Dev Biol. 2021 Mar;9:641469.
https://doi.org/10.3389/fcell.2021.641469 |
| 15 | Zhang L, Ye B, Chen Z, Chen Z-S: Progress in the studies on the molecular mechanisms associated with multidrug resistance in cancers. Acta Pharm Sin B. 2023 Mar;13(3):982-97.
https://doi.org/10.1016/j.apsb.2022.10.002 |
| 16 | Popęda M, Płuciennik E, Bednarek AK: Proteins in cancer multidrug resistance. Postępy Hig Med Dośw. 2014 May;68:616-32.
https://doi.org/10.5604/17322693.1103268 |
| 17 | Sawicka E, Wolniak M, Piwowar A : Mechanisms of cancer multidrug resistance with particular reference to breast cancer. Farm Pol. 2018 Aug;74(8):500-4.
https://doi.org/10.32383/farmpol/118703 |
| 18 | Tian Y, Lei Y, Wang Y, Lai J, Wang J, Xia F: Mechanism of multidrug resistance to chemotherapy mediated by P‑glycoprotein (Review). Int J Oncol. 2023 Aug;63(5):119.
https://doi.org/10.3892/ijo.2023.5567 |
| 19 | Pilotto Heming C, Muriithi W, Wanjiku Macharia L, Niemeyer Filho P, Moura-Neto V, Aran V: P-glycoprotein and cancer: what do we currently know? Heliyon. 2022 Oct;8(10):e11171.
https://doi.org/10.1016/j.heliyon.2022.e11171 |
| 20 | Costea T, Vlad OC, Miclea L-C, Ganea C, Szöllősi J, Mocanu M-M: Alleviation of Multidrug Resistance by Flavonoid and Non-Flavonoid Compounds in Breast, Lung, Colorectal and Prostate Cancer. Int J Mol Sci. 2020 Jan;21(2):401.
https://doi.org/10.3390/ijms21020401 |
| 21 | Xiao H, Zheng Y, Ma L, Tian L, Sun Q: Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front Pharmacol. 2021 Apr;12:648407.
https://doi.org/10.3389/fphar.2021.648407 |
| 22 | Skinner KT, Palkar AM, Hong AL: Genetics of ABCB1 in Cancer. Cancers. 2023 Aug;15(17):4236.
https://doi.org/10.3390/cancers15174236 |
| 23 | Steinbichler TB, Dudás J, Skvortsov S, Ganswindt U, Riechelmann H, Skvortsova I-I: Therapy resistance mediated by exosomes. Mol Cancer. 2019 Dec;18(1):58.
https://doi.org/10.1186/s12943-019-0970-x |
| 24 | U. Ferreira M-J: Natural Product-Derived Compounds for Targeting Multidrug Resistance in Cancer and Microorganisms. Int J Mol Sci. 2023 Sep;24(18):14321.
https://doi.org/10.3390/ijms241814321 |
| 25 | Xue X, Liang X-J: Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin J Cancer. 2012 Feb;31(2):100-9.
https://doi.org/10.5732/cjc.011.10326 |
| 26 | Cole SPC: Multidrug Resistance Protein 1 (MRP1, ABCC1), a "Multitasking" ATP-binding Cassette (ABC) Transporter. J Biol Chem. 2014 Nov;289(45):30880-8.
https://doi.org/10.1074/jbc.R114.609248 |
| 27 | Lu JF, Pokharel D, Bebawy M: MRP1 and its role in anticancer drug resistance. Drug Metab Rev. 2015 Oct;47(4):406-19.
https://doi.org/10.3109/03602532.2015.1105253 |
| 28 | Berger W, Setinek U, Hollaus P, Zidek T, Steiner E, Elbling L, Cantonati H, Attems J, Gsur A, Micksche M: Multidrug resistance markers P-glycoprotein, multidrug resistance protein 1, and lung resistance protein in non-small cell lung cancer: prognostic implications. J Cancer Res Clin Oncol. 2005 Jun;131(6):355-63.
https://doi.org/10.1007/s00432-004-0653-9 |
| 29 | Noguchi K, Katayama K, Sugimoto Y: Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics. Pharmacogenomics Pers Med. 2014 Feb;53.
https://doi.org/10.2147/PGPM.S38295 |
| 30 | Fan Y, Tao T, Guo Z, Wah To KK, Chen D, Wu S, Yang C, Li J, Luo M, Wang F,Fu L: Lazertinib improves the efficacy of chemotherapeutic drugs in ABCB1 or ABCG2 overexpression cancer cells in vitro, in vivo, and ex vivo. Mol Ther - Oncolytics. 2022 Mar;24:636-49.
https://doi.org/10.1016/j.omto.2022.02.006 |
| 31 | Hegedüs C, Truta-Feles K, Antalffy G, Várady G, Német K, Özvegy-Laczka C, Kéri G, Orfi L, Szakács G, Settleman J, Váradi A, Sarkadi B: Interaction of the EGFR inhibitors gefitinib, vandetanib, pelitinib and neratinib with the ABCG2 multidrug transporter: Implications for the emergence and reversal of cancer drug resistance. Biochem Pharmacol. 2012 Aug;84(3):260-7.
https://doi.org/10.1016/j.bcp.2012.04.010 |
| 32 | Ni Z, Bikadi Z, F. Rosenberg M, Mao Q: Structure and Function of the Human Breast Cancer Resistance Protein (BCRP/ABCG2). Curr Drug Metab. 2010 Sep;11(7):603-17.
https://doi.org/10.2174/138920010792927325 |
| 33 | Choi Y, Yu A-M: ABC Transporters in Multidrug Resistance and Pharmacokinetics, and Strategies for Drug Development. Curr Pharm Des. 2014 Feb;20(5):793-807.
https://doi.org/10.2174/138161282005140214165212 |
| 34 | Badiee SA, Isu UH, Khodadadi E, Moradi M: The Alternating Access Mechanism in Mammalian Multidrug Resistance Transporters and Their Bacterial Homologs. Membranes. 2023 May;13(6):568.
https://doi.org/10.3390/membranes13060568 |
| 35 | Fan W, Shao K, Luo M: Structural View of Cryo-Electron Microscopy-Determined ATP-Binding Cassette Transporters in Human Multidrug Resistance. Biomolecules. 2024 Feb;14(2):231.
https://doi.org/10.3390/biom14020231 |
| 36 | Zhao Y, Lu H, Yan A, Yang Y, Meng Q, Sun L, Pang H, Li C, Dong X, Cai L: ABCC3 as a marker for multidrug resistance in non-small cell lung cancer. Sci Rep. 2013 Nov;3(1):3120.
https://doi.org/10.1038/srep03120 |
| 37 | Roy S, Kumaravel S, Sharma A, Duran CL, Bayless KJ, Chakraborty S: Hypoxic tumor microenvironment: Implications for cancer therapy. Exp Biol Med. 2020 Jul;245(13):1073-86.
https://doi.org/10.1177/1535370220934038 |
| 38 | Bae T, Hallis SP, Kwak M-K: Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer. Exp Mol Med. 2024 Mar;56(3):501-14.
https://doi.org/10.1038/s12276-024-01180-8 |
| 39 | Mayer S, Milo T, Isaacson A, Halperin C, Miyara S, Stein Y, Lior C, Pevsner-Fischer M, Tzahor E , Mayo A, Alon U, Scherz-Shouval R : The tumor microenvironment shows a hierarchy of cell-cell interactions dominated by fibroblasts. Nat Commun. 2023 Sep;14(1):5810.
https://doi.org/10.1038/s41467-023-41518-w |
| 40 | Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017 Mar;214(3):579-96.
https://doi.org/10.1084/jem.20162024 |
| 41 | Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, Hynes RO, Janowitz T: A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020 Mar;20(3):174-86.
https://doi.org/10.1038/s41568-019-0238-1 |
| 42 | Zhao Z, Li T, Sun L, Yuan Y, Zhu Y: Potential mechanisms of cancer-associated fibroblasts in therapeutic resistance. Biomed Pharmacother. 2023 Oct;166:115425.
https://doi.org/10.1016/j.biopha.2023.115425 |
| 43 | Zheng J, Hao H: The importance of cancer-associated fibroblasts in targeted therapies and drug resistance in breast cancer. Front Oncol. 2024 Jan;13:1333839.
https://doi.org/10.3389/fonc.2023.1333839 |
| 44 | Peng Z, Tong Z, Ren Z, Ye M, Hu K: Cancer-associated fibroblasts and its derived exosomes: a new perspective for reshaping the tumor microenvironment. Mol Med. 2023 May;29(1):66.
https://doi.org/10.1186/s10020-023-00665-y |
| 45 | Balkwill FR, Capasso M, Hagemann T: The tumor microenvironment at a glance. J Cell Sci. 2012 Dec;125(23):5591-6.
https://doi.org/10.1242/jcs.116392 |
| 46 | Huang S, Chung JY-F, Li C, Wu Y, Qiao G, To K-F, Tang PM-K: Corrigendum to "Cellular dynamics of tumor microenvironment driving immunotherapy resistance in non-small-cell lung carcinoma" [Cancer Lett. 604 (2024) 217272]. Cancer Lett. 2024 Dec;217425.
https://doi.org/10.1016/j.canlet.2024.217425 |
| 47 | Shien K, Papadimitrakopoulou VA, Ruder D, Behrens C, Shen L, Kalhor N, Lee J, Wang J, Tang X, Girard L, Kurie JM, Herbst RS, Minna JD: JAK1/STAT3 Activation through a Proinflammatory Cytokine Pathway Leads to Resistance to Molecularly Targeted Therapy in Non-Small Cell Lung Cancer. Mol Cancer Ther. 2017 Oct;16(10):2234-45.
https://doi.org/10.1158/1535-7163.MCT-17-0148 |
| 48 | Wang L, Cao L, Wang H, Liu B, Zhang Q, Meng Z, Wu X, Zhou Q, Xu Ke: Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget. 2017 Sep;8(44):76116-28.
https://doi.org/10.18632/oncotarget.18814 |
| 49 | Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Wu Y-L, Paz-Ares L: Lung cancer: current therapies and new targeted treatments. The Lancet. 2017 Jan;389(10066):299-311.
https://doi.org/10.1016/S0140-6736(16)30958-8 |
| 50 | Denninghoff V, Russo A, De Miguel-Pérez D, Malapelle U, Benyounes A, Gittens A, Cardona AF, Rolfo C: Small Cell Lung Cancer: State of the Art of the Molecular and Genetic Landscape and Novel Perspective. Cancers. 2021 Apr;13(7):1723.
https://doi.org/10.3390/cancers13071723 |
| 51 | Shintani Y, Fujiwara A, Kimura T, Kawamura T, Funaki S, Minami M, Okumura M: IL-6 Secreted from Cancer-Associated Fibroblasts Mediates Chemoresistance in NSCLC by Increasing Epithelial-Mesenchymal Transition Signaling. J Thorac Oncol. 2016 Sep;11(9):1482-92.
https://doi.org/10.1016/j.jtho.2016.05.025 |
| 52 | Emami Nejad A, Najafgholian S, Rostami A, Sistani A, Shojaeifar S, Esparvarinha M, Nedaeinia R, Haghjooy Javanmard S, Taherian S, Ahmadlou M, Salehi R, Sadeghi B, Manian M: The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int. 2021 Jan;21(1):62.
https://doi.org/10.1186/s12935-020-01719-5 |
| 53 | Wu H-M, Jiang Z-F, Ding P-S, Shao L-J, Liu R-Y: Hypoxia-induced autophagy mediates cisplatin resistance in lung cancer cells. Sci Rep. 2015 Jul;5:12291.
https://doi.org/10.1038/srep12291 |
| 54 | Shi Y, Lin X, Wang J, Zhou Z, Chen S, Chen G: Advances of HIF‑1α/glycolysis axis in non‑small cell lung cancer (Review). Oncol Rep. 2024 Feb;51(4):55.
https://doi.org/10.3892/or.2024.8714 |
| 55 | Karetsi E, Ioannou MG, Kerenidi T, Minas M, Molyvdas PA, Gourgoulianis KI, Paraskeva E: Differential expression of hypoxia-inducible factor 1α in non-small cell lung cancer and small cell lung cancer. Clinics. 2012;67(12):1373-8.
https://doi.org/10.6061/clinics/2012(12)05 |
| 56 | Wan J, Ma J, Mei J, Shan G: The effects of HIF-1alpha on gene expression profiles of NCI-H446 human small cell lung cancer cells. J Exp Clin Cancer Res. 2009 Dec;28(1):150.
https://doi.org/10.1186/1756-9966-28-150 |
| 57 | Afsar CU, Uysal P: HIF-1α Levels in patients receiving chemoradiotherapy for locally advanced non-small cell lung carcinoma. Rev Assoc Médica Bras. 2019 Oct;65(10):1295-9.
https://doi.org/10.1590/1806-9282.65.10.1295 |
| 58 | Chen Z, Han F, Du Y, Shi H, Zhou W: Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023 Feb;8(1):70.
https://doi.org/10.1038/s41392-023-01332-8 |
| 59 | Harris AL: Hypoxia - a key regulatory factor in tumour growth. Nat Rev Cancer. 2002 Jan;2(1):38-47.
https://doi.org/10.1038/nrc704 |
| 60 | Mowers EE, Sharifi MN, Macleod KF: Autophagy in cancer metastasis. Oncogene. 2017 Mar;36(12):1619-30.
https://doi.org/10.1038/onc.2016.333 |
| 61 | Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S: PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014 May;211(5):781-90.
https://doi.org/10.1084/jem.20131916 |
| 62 | Allard D, Allard B, Stagg J: On the mechanism of anti-CD39 immune checkpoint therapy. J Immunother Cancer. 2020 Feb;8(1):e000186.
https://doi.org/10.1136/jitc-2019-000186 |
| 63 | Pan J, Fei C-J, Hu Y, Wu X-Y, Nie L, Chen J: Current understanding of the cGAS-STING signaling pathway: Structure, regulatory mechanisms, and related diseases. Zool Res. 2023;44(1):183-218.
https://doi.org/10.24272/j.issn.2095-8137.2022.464 |
| 64 | Ciepła J, Smolarczyk R: Tumor hypoxia unveiled: insights into microenvironment, detection tools and emerging therapies. Clin Exp Med. 2024 Oct;24(1):235.
https://doi.org/10.1007/s10238-024-01501-1 |
| 65 | Wang P, Wang X-Y, Man C-F, Gong D-D, Fan Y: Advances in hyperbaric oxygen to promote immunotherapy through modulation of the tumor microenvironment. Front Oncol. 2023 Sep;13:1200619.
https://doi.org/10.3389/fonc.2023.1200619 |
| 66 | Sandúa A, Alegre E, González Á: Exosomes in Lung Cancer: Actors and Heralds of Tumor Development. Cancers. 2021 Aug;13(17):4330.
https://doi.org/10.3390/cancers13174330 |
| 67 | Duréndez-Sáez E, Torres-Martinez S, Calabuig-Fariñas S, Meri-Abad M, Ferrero-Gimeno M, Camps C: Exosomal microRNAs in non-small cell lung cancer. Transl Cancer Res. 2021 Jun;10(6):3128-39.
https://doi.org/10.21037/tcr-20-2815 |
| 68 | Taghvimi S, Vakili O, Soltani Fard E, Khatami SH, Karami N, Taheri‐Anganeh M, Salehi M, Negahdari, Ghasemi H, Movahedpour A : Exosomal microRNAs and long noncoding RNAs: Novel mediators of drug resistance in lung cancer. J Cell Physiol. 2022 Apr;237(4):2095-106.
https://doi.org/10.1002/jcp.30697 |
| 69 | Mito I, Takahashi H, Kawabata-Iwakawa R, Horikawa M, Ida S, Tada H, Matsuyama T, Misawa K, Takeda S, Chikamatsu K : Tumor-derived exosomes elicit cancer-associated fibroblasts shaping inflammatory tumor microenvironment in head and neck squamous cell carcinoma. Oral Oncol. 2023 Jan;136:106270.
https://doi.org/10.1016/j.oraloncology.2022.106270 |
| 70 | He G, Peng X, Wei S, Yang S, Li X, Huang M, Tang S, Jin H, Liu J, Zhang S, Zheng H, Fan Q : Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications. Mol Cancer. 2022 Dec;21(1):19.
https://doi.org/10.1186/s12943-021-01440-5 |
| 71 | Yang X, Li Y, Zou L, Zhu Z : Role of Exosomes in Crosstalk Between Cancer-Associated Fibroblasts and Cancer Cells. Front Oncol. 2019;9:356.
https://doi.org/10.3389/fonc.2019.00356 |
| 72 | Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Guan L, Li T, Liu X, Liu S, Yang R, Lu Y, Dong L, Gou W, Lu Y : Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018 Aug;560(7718):382-6.
https://doi.org/10.1038/s41586-018-0392-8 |
| 73 | Zelli V, Compagnoni C, Capelli R, Corrente A, Di Vito Nolfi M, Zazzeroni F, Alesse E, Tessitore A: Role of exosomal microRNAs in cancer therapy and drug resistance mechanisms: focus on hepatocellular carcinoma. Front Oncol. 2022 Jul;12:940056.
https://doi.org/10.3389/fonc.2022.940056 |
| 74 | Wang M, Yu F, Ding H, Wang Y, Li P, Wang K : Emerging Function and Clinical Values of Exosomal MicroRNAs in Cancer. Mol Ther - Nucleic Acids. 2019 Jun;16:791-804.
https://doi.org/10.1016/j.omtn.2019.04.027 |
| 75 | Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM: Exosome mediated communication within the tumor microenvironment. J Controlled Release. 2015 Dec;219:278-94.
https://doi.org/10.1016/j.jconrel.2015.06.029 |
| 76 | Xue D, Han J, Liang Z, Jia L, Liu Y, Tuo H, Peng Y : Current Perspectives on the Unique Roles of Exosomes in Drug Resistance of Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2022 Feb;Volume 9:99-112.
https://doi.org/10.2147/JHC.S351038 |
| 77 | Xu Y, Qiu A, Peng F, Tan X, Wang J, Gong X : Exosomal transfer of circular RNA FBXW7 ameliorates the chemoresistance to oxaliplatin in colorectal cancer by sponging miR-18b-5p. Neoplasma. 2021;68(01):108-18.
https://doi.org/10.4149/neo_2020_200417N414 |
| 78 | Gao J, Zhang X, Jiang L, Li Y, Zheng Q : Tumor endothelial cell-derived extracellular vesicles contribute to tumor microenvironment remodeling. Cell Commun Signal. 2022 Dec;20(1):97.
https://doi.org/10.1186/s12964-022-00904-5 |
| 79 | Ateeq M, Broadwin M, Sellke FW, Abid MR : Extracellular Vesicles' Role in Angiogenesis and Altering Angiogenic Signaling. Med Sci. 2024 Jan;12(1):4.
https://doi.org/10.3390/medsci12010004 |
| 80 | Ahmadi M, Rezaie J : Tumor cells derived-exosomes as angiogenenic agents: possible therapeutic implications. J Transl Med. 2020 Dec;18(1):249.
https://doi.org/10.1186/s12967-020-02426-5 |
| 81 | Liu S-L, Sun P, Li Y, Liu S-S, Lu Y : Exosomes as critical mediators of cell-to-cell communication in cancer pathogenesis and their potential clinical application. Transl Cancer Res. 2019 Feb;8(1):298-311.
https://doi.org/10.21037/tcr.2019.01.03 |
| 82 | Poggio M, Hu T, Pai C-C, Chu B, Belair CD, Chang A, Montabana E, Lang UE, Fu Q, Fong L, Blelloch R : Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell. 2019 Apr;177(2):414-427.e13.
https://doi.org/10.1016/j.cell.2019.02.016 |
| 83 | Da Costa VR, Araldi RP, Vigerelli H, D'Ámelio F, Mendes TB, Gonzaga V, Policíquio B, Colozza-Gama GA, Kerkis I, Valverde CV: Exosomes in the Tumor Microenvironment: From Biology to Clinical Applications. Cells. 2021 Oct;10(10):2617.
https://doi.org/10.3390/cells10102617 |
| 84 | Ren B, Li X, Zhang Z, Tai S, Yu S: Exosomes: a significant medium for regulating drug resistance through cargo delivery. Front Mol Biosci. 2024 Jul;11:1379822.
https://doi.org/10.3389/fmolb.2024.1379822 |
| 85 | Shimada Y, Minna JD: Exosome mediated phenotypic changes in lung cancer pathophysiology. Transl Cancer Res. 2017 Aug;6(S6):S1040-2.
https://doi.org/10.21037/tcr.2017.07.09 |
| 86 | Orooji N, Fadaee M, Kazemi T, Yousefi B: Exosome therapeutics for non-small cell lung cancer tumorigenesis. Cancer Cell Int. 2024 Oct;24(1):360.
https://doi.org/10.1186/s12935-024-03544-6 |
| 87 | Li M-Y, Liu L-Z, Dong M: Progress on pivotal role and application of exosome in lung cancer carcinogenesis, diagnosis, therapy and prognosis. Mol Cancer. 2021 Jan;20(1):22.
https://doi.org/10.1186/s12943-021-01312-y |
| 88 | Xu K, Zhang C, Du T, Gabriel ANA, Wang X, Li X, Sun Li, Wang N, Jiang X, Zhang Yi: Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed Pharmacother. 2021 Feb;134:111111.
https://doi.org/10.1016/j.biopha.2020.111111 |
| 89 | Fang J, Rao X, Wang C, Wang Y, Wu C, Zhou R: Role of exosomes in modulating non-small cell lung cancer radiosensitivity. Front Pharmacol. 2024 Dec;15:1471476.
https://doi.org/10.3389/fphar.2024.1471476 |
| 90 | Sun H, Sun R, Song X, Gu W, Shao Y: Mechanism and clinical value of exosomes and exosomal contents in regulating solid tumor radiosensitivity. J Transl Med. 2022 Dec;20(1):189.
https://doi.org/10.1186/s12967-022-03392-w |
| 91 | Baran K, Waśko J, Kryczka J, Boncela J, Jabłoński S, Kolesińska B, Brzeziańska-Lasota B, Kordiak J: The Comparison of Serum Exosome Protein Profile in Diagnosis of NSCLC Patients. Int J Mol Sci. 2023 Sep;24(18):13669.
https://doi.org/10.3390/ijms241813669 |
| 92 | Jakobsen KR, Paulsen BS, Bæk R, Varming K, Sorensen BS, Jørgensen MM: Exosomal proteins as potential diagnostic markers in advanced non‐small cell lung carcinoma. J Extracell Vesicles. 2015 Jan;4(1):26659.
https://doi.org/10.3402/jev.v4.26659 |
| 93 | Sandfeld-Paulsen B, Aggerholm-Pedersen N, Bæk R, Jakobsen KR, Meldgaard P, Folkersen BH, Rasmussen TR, Varming K, Jørgensen MM, Sorensen BS : Exosomal proteins as prognostic biomarkers in non‐small cell lung cancer. Mol Oncol. 2016 Dec;10(10):1595-602.
https://doi.org/10.1016/j.molonc.2016.10.003 |
| 94 | Hsu M-T, Wang Y-K, Tseng YJ: Exosomal Proteins and Lipids as Potential Biomarkers for Lung Cancer Diagnosis, Prognosis, and Treatment. Cancers. 2022 Jan;14(3):732.
https://doi.org/10.3390/cancers14030732 |
| 95 | Piombino C, Mastrolia I, Omarini C, Candini O, Dominici M, Piacentini F, Toss A : The Role of Exosomes in Breast Cancer Diagnosis. Biomedicines. 2021 Mar;9(3):312.
https://doi.org/10.3390/biomedicines9030312 |
| 96 | Liu T, Hooda J, Atkinson JM, Whiteside TL, Oesterreich S, Lee AV: Exosomes in Breast Cancer - Mechanisms of Action and Clinical Potential. Mol Cancer Res. 2021 Jun;19(6):935-45.
https://doi.org/10.1158/1541-7786.MCR-20-0952 |
| 97 | Liu Q, Peng F, Chen J: The Role of Exosomal MicroRNAs in the Tumor Microenvironment of Breast Cancer. Int J Mol Sci. 2019 Aug;20(16):3884.
https://doi.org/10.3390/ijms20163884 |
| 98 | Yu D, Wu Y, Zhang X, Lv M, Chen W, Chen X, Yang SY, Shen H, Zhong S-L, Tang J-H, Zhao J-H : Exosomes from adriamycin-resistant breast cancer cells transmit drug resistance partly by delivering miR-222. Tumor Biol. 2016 Mar;37(3):3227-35.
https://doi.org/10.1007/s13277-015-4161-0 |
| 99 | Chen B, Qiu X, Li Y: Exosomes in ovarian cancer: impact on drug resistance and advances in SERS detection techniques. J Pharm Anal. 2024 Dec;101170.
https://doi.org/10.1016/j.jpha.2024.101170 |
| 100 | Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q, Feng F, Liu Y, Xu W, Li Y: Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. 2019 Dec;38(1):81.
https://doi.org/10.1186/s13046-019-1095-1 |
| 101 | Ralser DJ, Condic M, Egger E, Koensgen D, Mustea A, Stope MB: Evaluation of the Diagnostic Potential of Circulating MicroRNAs miR-1 and miR-21 in Patients With Ovarian Cancer. Anticancer Res. 2022 Dec;42(12):5839-45.
https://doi.org/10.21873/anticanres.16092 |
| 102 | Feng S, Lou K, Zou X, Zou J, Zhang G: The Potential Role of Exosomal Proteins in Prostate Cancer. Front Oncol. 2022 Jun;12:873296.
https://doi.org/10.3389/fonc.2022.873296 |
| 103 | Lorenc T, Klimczyk K, Michalczewska I, Słomka M, Kubiak-Tomaszewska G, Olejarz W: Exosomes in Prostate Cancer Diagnosis, Prognosis and Therapy. Int J Mol Sci. 2020 Mar;21(6):2118.
https://doi.org/10.3390/ijms21062118 |
| 104 | Tan Y, Tang F, Li J, Yu H, Wu M, Wu Y, Zeng H, Hou K, Zhang Q: Tumor-derived exosomes: the emerging orchestrators in melanoma. Biomed Pharmacother. 2022 May;149:112832.
https://doi.org/10.1016/j.biopha.2022.112832 |
| 105 | Turabi K, Klute K, Radhakrishnan P: Decoding the Dynamics of Circulating Tumor DNA in Liquid Biopsies. Cancers. 2024 Jul;16(13):2432.
https://doi.org/10.3390/cancers16132432 |
| 106 | Kartikasari AER, Huertas CS, Mitchell A, Plebanski M: Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front Oncol. 2021 Jul;11:692142.
https://doi.org/10.3389/fonc.2021.692142 |
| 107 | Mosquera-Heredia MI, Morales LC, Vidal OM, Barceló E, Silvera-Redondo C, Vélez JI, Garavito-Galofre P: Exosomes: Potential Disease Biomarkers and New Therapeutic Targets. Biomedicines. 2021 Aug;9(8):1061.
https://doi.org/10.3390/biomedicines9081061 |
| 108 | Hanjani NA, Esmaelizad N, Zanganeh S, Gharavi AT, Heidarizadeh P, Radfar M, Omidi F, MacLoughlin R, Doroudian M: Emerging role of exosomes as biomarkers in cancer treatment and diagnosis. Crit Rev Oncol Hematol. 2022 Jan;169:103565.
https://doi.org/10.1016/j.critrevonc.2021.103565 |
| 109 | Hussen BM, Faraj GSH, Rasul MF, Hidayat HJ, Salihi A, Baniahmad A, Taheri M, Ghafouri-Frad S : Strategies to overcome the main challenges of the use of exosomes as drug carrier for cancer therapy. Cancer Cell Int. 2022 Oct;22(1):323.
https://doi.org/10.1186/s12935-022-02743-3 |
| 110 | Garg P, Malhotra J, Kulkarni P, Horne D, Salgia R, Singhal SS: Emerging Therapeutic Strategies to Overcome Drug Resistance in Cancer Cells. Cancers. 2024 Jul;16(13):2478.
https://doi.org/10.3390/cancers16132478 |
| 111 | Maqsood Q, Sumrin A, Saleem Y, Wajid A, Mahnoor M: Exosomes in Cancer: Diagnostic and Therapeutic Applications. Clin Med Insights Oncol. 2024 Jan;18:11795549231215966.
https://doi.org/10.1177/11795549231215966 |
| 112 | Ming‐Kun C, Zi‐Xian C, Mao‐Ping C, Hong C, Zhuang‐Fei C, Shan‐Chao Z: Engineered extracellular vesicles: A new approach for targeted therapy of tumors and overcoming drug resistance. Cancer Commun. 2024 Feb;44(2):205-25.
https://doi.org/10.1002/cac2.12518 |
| 113 | Shao Y, Wang Y, Su R, Pu W, Chen S, Fu L, Yu H, Qiu Y: Dual identity of tumor-associated macrophage in regulated cell death and oncotherapy. Heliyon. 2023 Jul;9(7):e17582.
https://doi.org/10.1016/j.heliyon.2023.e17582 |
| 114 | Zhang W, Taheri-Ledari R, Ganjali F, Afruzi FH, Hajizadeh Z, Saeidirad M, Qazi F, Kashtiaray A, Sehat SS, Hamblin MR, Maleki A: Nanoscale bioconjugates: A review of the structural attributes of drug-loaded nanocarrier conjugates for selective cancer therapy. Heliyon. 2022 Jun;8(6):e09577.
https://doi.org/10.1016/j.heliyon.2022.e09577 |
| 115 | Bahadorani M, Nasiri M, Dellinger K, Aravamudhan S, Zadegan R: Engineering Exosomes for Therapeutic Applications: Decoding Biogenesis, Content Modification, and Cargo Loading Strategies. Int J Nanomedicine. 2024 Jul;Volume 19:7137-64.
https://doi.org/10.2147/IJN.S464249 |
| 116 | Wu H, Mu X, Liu L, Wu H, Hu X, Chen L, Liu J, Mu Y, Yuan F, Liu W, Zhao Y: . Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 2020 Sep;11(9):801.
https://doi.org/10.1038/s41419-020-02962-4 |
| 117 | Han Q-F, Li W-J, Hu K-S, Gao J, Zhai W-L, Yang J-H, Zhang S-J: Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer. 2022 Nov;21(1):207.
https://doi.org/10.1186/s12943-022-01671-0 |
| 118 | Wan X, Fang Y, Du J, Cai S, Dong H: GW4869 Can Inhibit Epithelial-Mesenchymal Transition and Extracellular HSP90α in Gefitinib-Sensitive NSCLC Cells. OncoTargets Ther. 2023 Nov;Volume 16:913-22.
https://doi.org/10.2147/OTT.S428707 |
| 119 | Wandrey M, Jablonska J, Stauber RH, Gül D: Exosomes in Cancer Progression and Therapy Resistance: Molecular Insights and Therapeutic Opportunities. Life. 2023 Oct;13(10):2033.
https://doi.org/10.3390/life13102033 |
| 120 | Lin C-C, Wu C-Y, Tseng JT-C, Hung C-H, Wu S-Y, Huang Y-T, Chang W-Y,Su P-L, Su W-C: Extracellular Vesicle miR-200c Enhances Gefitinib Sensitivity in Heterogeneous EGFR-Mutant NSCLC. Biomedicines. 2021 Feb;9(3):243.
https://doi.org/10.3390/biomedicines9030243 |
| 121 | Wu Y, Fu H, Hao J, Yang Z, Qiao X, Li Y, Zhao R, Lin T, Wang Y, Wang M: Tumor‐derived exosomal PD-L1: a new perspective in PD-1/PD-L1 therapy for lung cancer. Front Immunol. 2024 Mar;15:1342728.
https://doi.org/10.3389/fimmu.2024.1342728 |
| 122 | Wu B, Huang X, Shi X, Jiang M, Liu H, Zhao L: LAMTOR1 decreased exosomal PD-L1 to enhance immunotherapy efficacy in non-small cell lung cancer. Mol Cancer. 2024 Sep;23(1):184.
https://doi.org/10.1186/s12943-024-02099-4 |
| 123 | Shams SG: Decoding the secrets of small extracellular vesicle communications: exploring the inhibition of vesicle-associated pathways and interception strategies for cancer treatment. Am J Cancer Res. 2024;14(5):1957-80.
https://doi.org/10.62347/JWMX3035 |
| 124 | Wei F, Ma C, Zhou T, Dong X, Luo Q, Geng L, Ding L, Zhang Y, Zhang L, Li N, Li Y, Liu Y: Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol Cancer. 2017 Dec;16(1):132.
https://doi.org/10.1186/s12943-017-0694-8 |