Role of Gut Microbiota in Modulating Oxidative Stress Induced by Environmental Factors
bDepartment of Medical Biology and Biochemistry, Division of Ecology and Environmental Protection, Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Toruń, M. Skłodowska-Curie St. 9, 85-094 Bydgoszcz, Poland,
cDepartment of Biotechnology, Institute of Biological Sciences, Faculty of Biological Sciences, University of Zielona Góra, Prof. Z. Szafran St. 1, 65-516 Zielona Góra, Poland
Keywords
Abstract
The widespread presence of environmental pollutants, including toxic metals, microplastics, and antibiotics, has significantly altered gut microbiota composition and functionality, leading to dysbiosis and oxidative stress. These changes contribute to various adverse physiological effects, including systemic inflammation, mitochondrial dysfunction, and intestinal barrier dysfunction. This review provides a comprehensive analysis of the molecular mechanisms by which these environmental factors induce oxidative damage, emphasising the importance of redox imbalance, the overproduction of reactive oxygen species, and inflammatory signalling pathways. Key pathways involved include NF-κB, Nrf2/Keap1, PI3K/AKT, p38-MAPK, JAK/STAT and TLR4/MyD88. These pathways collectively contribute to the progression of chronic inflammatory conditions. Furthermore, this article synthesises findings from 354 studies published between 2016 and 2024, integrating human and animal research evidence. Existing literature suggests that gut dysbiosis exacerbates oxidative stress through impaired short-chain fatty acid production, downregulation of peroxisome proliferator-activated receptor gamma, and disruption of antioxidant enzyme activity. This review explores these mechanisms in more detail. Additionally, the review evaluates studies investigating microbiota-targeted therapeutic interventions to mitigate oxidative stress. These interventions include probiotics, prebiotics, polyphenols, and postbiotics, focusing on their reported modulation of Nrf2 and AMPK signalling pathways. The potential of faecal microbiota transplantation as an innovative approach to restoring a healthy gut ecosystem and counteracting pollutant-induced oxidative damage is also discussed. In light of the growing global exposure to environmental pollutants and their associated long-term health implications, it is imperative to gain a deeper understanding of their impact on gut microbiota and oxidative stress. This topic remains at the forefront of biomedical research due to its implications for public health, disease prevention, and developing novel therapeutic strategies.Introduction
Environmental pollutants include a wide range of substances that have adverse effects on both ecosystems and human health. Among the most important are toxic metals, including lead (Pb), mercury (Hg), cadmium (Cd), and arsenic (As), which accumulate in soil and water mainly as a result of industrial activities, mining, and improper waste disposal. These metals are characterised by long-term persistence in the environment, contributing to widespread contamination [1, 2]. In addition to toxic metals, persistent organic pollutants (POPs), such as polychlorinated biphenyls (PCBs) and dioxins, pose a significant environmental threat [3, 4]. These compounds, mainly by-products of industrial processes, have high potential for bioaccumulation in the food chain [3]. Another emerging environmental concern is the presence of microplastics, i.e. tiny plastic particles resulting from the degradation of larger plastic materials, which have invaded both marine and terrestrial ecosystems, affecting wildlife [5, 6].
It is crucial to understand how toxic metals alter gut microbial diversity and function, as these disruptions contribute to inflammation and oxidative stress, which are key factors in the development of various chronic diseases. Exposure to toxic metals has been shown to disrupt gut microbial diversity and metabolic functions, leading to the proliferation of pathogenic species and the resulting inflammation and oxidative stress [7–9]. Distinct microbial shifts are associated with such exposure, including an increased prevalence of Firmicutes and Proteobacteria alongside a reduction in beneficial Bacteroidetes populations, thereby exacerbating gut dysbiosis [8]. Identifying these shifts can inform targeted interventions aimed at restoring gut homeostasis and mitigating adverse health outcomes.
In addition to traditional pollutants, such as metals and plastics, the widespread use of chemical compounds, including antibiotics, pesticides, and herbicides, poses a significant threat to today's ecosystems [10, 11]. The excessive and often indiscriminate use of these substances in agriculture and medicine has led to their widespread presence in water sources and contributed to the emergence of antibiotic-resistant bacteria [12]. The presence of various environmental pollutants has been shown to have significant effects on the composition of the gut microbiota, often leading to dysbiosis and oxidative stress [13, 14]. The gut microbiota, a crucial regulator of human health, plays a fundamental role in metabolism, immune function, and oxidative homeostasis [15].
Redox imbalance resulting from reduced cellular antioxidant capacity leads to the excessive accumulation of ROS, which in turn cause oxidative damage to cell membranes, DNA, and proteins [16]. Furthermore, prolonged exposure to elevated ROS levels has been shown to activate inflammation-associated signalling pathways such as NF-κB, thereby amplifying oxidative stress and perpetuating inflammatory states [17]. These processes contribute to the progressive deterioration of the gut microbiota composition and the onset of chronic inflammation, creating a self-perpetuating cycle that is detrimental to overall health [18].
Changes in the gut microbiota associated with exposure to environmental pollutants have been implicated in the pathogenesis of metabolic disorders, including obesity [19, 20], insulin resistance [21], and type 2 diabetes [22, 23]. The gut microbiota plays a critical role in the regulation of glucose metabolism, lipid storage, and cellular energy homeostasis. Disruption of this microbial ecosystem can lead to hormonal imbalances and impaired nutrient processing [24]. Furthermore, dysbiosis has been linked to an increased risk of cardiovascular disease, as microbial dysbiosis contributes to chronic inflammation and dysregulation of lipid metabolism – both of which are key risk factors for atherosclerosis and hypertension [25, 26]. Consequently, prolonged exposure to environmental pollutants can significantly increase susceptibility to a wide range of serious health conditions [27].
In order to develop effective strategies to mitigate the adverse health effects of environmental pollutants, it is essential to elucidate their underlying mechanisms. Research on the gut microbiota should prioritise the identification of pollutant sources and their molecular effects on human physiology [13]. In particular, studying the interactions between microbial communities and oxidative processes may offer new therapeutic avenues, including dietary interventions, probiotic supplementation, and antioxidant-based strategies. Furthermore, reduction of environmental exposures to toxic metals, microplastics, and excessive antibiotics may be an important approach to preserve gut microbial homeostasis and prevent related diseases [14].
This study investigates the impact of environmental pollutants, including toxic metals, microplastics and antibiotics, on the human gut microbiota, and the mechanisms leading to dysbiosis and oxidative stress. The focus is on how exposure to these pollutants may contribute to chronic diseases such as metabolic disorders, inflammatory bowel disease, autoimmune conditions and cardiovascular pathologies. The main objective is to elucidate the molecular mechanisms involved, particularly redox imbalance, excessive reactive oxygen species (ROS) production, and activation of inflammatory pathways, which drive physiological changes in affected individuals. The study also evaluates the long-term health effects of exposure to pollutants and assesses the associated public health risks. To achieve this, a thorough review of the scientific literature will be conducted to identify the key pollutants that impact the gut microbiota and to determine their prevalence and distribution across different ecosystems.
Environmental pollutants disrupt the balance and composition of the gut microbiota, triggering an increase in oxidative stress by enhancing the production of reactive oxygen species. This oxidative stress damages gut tissues and microbial communities, thereby exacerbating dysbiosis. Consequently, a self-perpetuating cycle is established where oxidative stress and dysbiosis continuously influence and amplify each other [15]. The specific aim of the study is to elucidate the molecular mechanisms underlying these phenomena, including redox imbalance, excessive ROS production, and activation of inflammatory pathways. These mechanisms are thought to be responsible for the observed changes in human organisms. In addition, the study was designed to evaluate the long-term effects of exposure to environmental pollutants and to assess the potential risks they pose to public health. The study has been designed to analyse the scientific literature and available research to identify the most important pollutants affecting the gut microbiota and to assess their prevalence and presence in different ecosystems. An important component of the study will also be to explore potential health protection methods, such as dietary modification, probiotics, or the use of antioxidants, which could mitigate the negative effects of dysbiosis and oxidative stress caused by pollutants.
Another key component of the study is to explore potential health-protective strategies, e.g. dietary modification, probiotics, and the use of antioxidants, that may mitigate the adverse effects of dysbiosis and oxidative stress induced by environmental pollutants. This research is inherently interdisciplinary, integrating concepts from microbiology, toxicology, physiology, and molecular biology to provide a holistic perspective on the multifaceted effects of pollutants on the gut microbiota and human health. The novelty of the study lies in the in-depth analysis of key molecular pathways, such as NF-κB, Nrf2/Keap1, PI3K/AKT, p38-MAPK, JAK/STAT, and TLR4/MyD88, which establish mechanistic links between dysbiosis, inflammatory processes, and oxidative stress. In addition, the focus on antibiotic resistance genes as a critical aspect of contemporary health challenges opens new avenues for research into targeted therapeutic interventions.
Materials and Methods
The bibliographic databases used for this study included PubMed, Scopus, and Google Scholar. An initial literature search yielded 501 studies. Following rigorous screening of titles, abstracts and full texts, 147 studies were excluded due to duplication, irrelevance to the research focus, poor methodological quality or failure to meet the established inclusion criteria. Thus, a total of 354 studies were retained for the final analysis. The literature search covered the period from 2016 to 2024 to ensure inclusion of the most recent and relevant studies. Search criteria included articles, reviews, and clinical trials investigating the effects of environmental contaminants, e.g. toxic metals, microplastics, and antibiotics, on gut microbiota, oxidative stress, and dysbiosis. A comprehensive search strategy was implemented using a combination of the following keywords: 'pollutants', 'environmental contaminants', 'gut microbiota', 'dysbiosis', 'oxidative stress', 'heavy metals', 'microplastics', and 'antibiotics' to maximise the retrieval of relevant publications.
The inclusion criteria were carefully defined to find studies published in peer-reviewed journals, research conducted in human or animal models, and articles addressing molecular mechanisms by which contaminants affect the gut microbiota. The exclusion criteria included studies not related to the environmental contaminants of interest, articles lacking sufficient data on microbiota changes, and research focusing on non-ecological or non-biological aspects of contaminants. In addition, non-English language articles were excluded unless an English abstract was available. The review prioritised high quality peer-reviewed sources to ensure the reliability and validity of the findings.
Gut microbiota and its role in metabolic, immune, and neurological homeostasis
Understanding the microbiota-gut-brain axis and neuronal homeostasis is crucial for grasping the systemic effects of oxidative stress induced by environmental factors. The role of specific bacterial taxa in modulating oxidative stress is clearer when considered alongside their broader influence on the interconnected metabolic, immune and neurological systems - a relationship that is mediated by the human gut microbiota. This complex ecosystem comprises approximately 1, 000 bacterial species and plays a pivotal role in maintaining these physiological systems. Representatives of the Firmicutes phylum, including the genera Clostridium, Lactobacillus and Ruminococcus, are particularly important in this context as they ferment dietary fibre into short-chain fatty acids (SCFAs), such as butyrate, acetate and propionate [30, 31]. These metabolites support intestinal barrier integrity, modulate immune responses and provide an energy source for colonocytes [32]. Dysbiosis, or disturbances in the microbial composition, has been associated with a variety of health conditions, including metabolic disorders, inflammatory diseases, and mental disorders [30-34] (Fig. 1).

Fig. 1: Changes in the composition and diversity of the gut microbiota have been identified as potential non-invasive biomarkers for the early detection of disease. Specific microbial profiles have been associated with conditions such as inflammatory bowel disease, diabetes and mental disorders, facilitating early diagnosis and personalised therapeutic approaches. Therapeutic interventions include the administration of probiotics and prebiotics to restore microbial balance and enhance SCFA production, the use of faecal microbiota transplantation to treat severe dysbiosis, including recurrent Clostridioides difficile infections, and advanced therapeutic approaches targeting the microbiota through innovative pharmacological agents and dietary interventions to improve health outcomes.
The existing literature on the gut-brain-microbiota axis highlights the complex interplay between gut health, the gut microbiome, and the central nervous system. Bonaz et al. highlight the pivotal role of the vagus nerve as a primary communication pathway within this axis, demonstrating its essential function in mediating interactions between the gut microbiota and the brain. Their review suggests that vagus nerve stimulation may influence the gut microbiota composition while exerting anti-inflammatory effects, offering potential therapeutic applications for gastrointestinal disorders and systemic inflammation [33]. In a previous study, Bonaz et al. further explored the involvement of the vagus nerve in the neuro-immune axis, highlighting its role in modulating immune responses and intestinal permeability. Their findings suggest that vagal signalling has important implications for the pathophysiology of gastrointestinal disorders, particularly inflammatory bowel disease, thereby linking gut dysfunction to systemic inflammation [34].
Milani et al. and Turroni et al. explore the importance of the gut microbiota in early life and its long-term impact on health [35, 36]. Milani et al. describe the infant gut microbiota as a critical “microbial organ” that plays a fundamental role in shaping the development of the immune system and metabolic processes. The composition of this microbiota is influenced by various factors, such as the mode of birth and early nutrition, and its disruption through antibiotic use or dietary factors has been linked to increased susceptibility to infection, allergy, and autoimmune disease later in life [35]. Turroni et al. further expand on the composition of microbial communities in infants and highlight their role in establishing immune homeostasis [36], illustrating the complex interplay between gut microbiota and host health from an early age [35, 37]. In addition, studies have highlighted the impact of early life microbial exposures, including breastfeeding and exposure to diverse environments, on the resilience of the immune system in adulthood [38, 39].
Recent scientific investigations have increasingly focused on elucidating the mechanisms by which the gut microbiota regulate intestinal permeability, with the aim of elucidating their role in the pathogenesis of various gastrointestinal disorders [40]. The human gut microbiome is known to contain several beneficial bacterial taxa that play an essential role in maintaining physiological homeostasis. For example, Lactobacillus spp. facilitate digestion, enhance immune function, and produce lactic acid [41], while Bifidobacterium spp. contribute to the integrity of the intestinal barrier, inhibit the proliferation of pathogenic bacteria, and produce SCFAs [4]. In addition, Akkermansia muciniphila has been implicated in strengthening the mucosal barrier and promoting metabolic health [43], while Faecalibacterium prausnitzii synthesises anti-inflammatory butyrate, which supports colonic homeostasis [44].
Eubacterium spp. also play an important role in fibre fermentation and SCFA production [45]. However, adverse conditions, such as dysbiosis induced by poor diet, psychological stress, or excessive use of antibiotics, can disrupt the microbial balance and favour the proliferation of opportunistic and potentially pathogenic bacteria [46, 47]. These include Clostridium difficile, which is associated with colitis and diarrhoea [48], pathogenic strains of Escherichia coli, which contribute to intestinal inflammation and infection [49], Klebsiella spp., which are associated with inflammatory bowel disease [50], Salmonella spp., a common cause of foodborne illness [51], and members of the Proteobacteria phylum, e.g. Enterobacter spp., which have been implicated in chronic intestinal inflammation and dysbiosis-related disorders [52, 53]. In addition to traditional risk factors, there is growing evidence that environmental factors, such as pollution, climate change, and overuse of antibiotics can exacerbate the imbalance of the gut microbiota, leading to the onset of chronic disease and a decline in immune system function [13, 54].
Collectively, these studies provide valuable insights into the multifaceted role of the gut microbiota in maintaining overall health, particularly in relation to gut-brain interactions and immune regulation [33, 35]. The importance of gut barrier integrity and the vagus nerve in maintaining physiological balance has been consistently emphasised in multiple studies [33-35, 37], highlighting their relevance in the pathophysiology of microbiota-related diseases.
Environmental pollutants and gut microbiota dysbiosis
Toxic metals. Existing research on the effects of toxic metals on the gut microbiota highlights the complex interactions between environmental contaminants, microbial communities, and host physiology [7-9]. Several studies have explored these relationships and elucidated the key molecular mechanisms underlying the dysregulation of the gut microbiota by metal exposure. Assefa and Köhler highlight that toxic metal exposure alters microbial diversity and promotes the proliferation of pathogenic species while inducing inflammation and oxidative stress [9]. Richardson et al. further demonstrate that such exposure leads to specific microbial shifts characterised by an increased abundance of Firmicutes and Proteobacteria and a concomitant decrease in beneficial Bacteroidetes populations [8]. Zhang et al. highlight that prolonged exposure to toxic metals not only reduces gut microbial diversity but also disrupts microbial metabolic functions, exacerbating oxidative stress [7].
The existing literature on the effects of toxic metal exposure on the gut microbiota consistently shows that contaminants, such as lead, mercury, and cadmium, significantly alter the microbial composition, resulting in dysbiosis [55, 56]. In addition, the potential role of probiotics in mitigating the adverse effects of toxic metal exposure on the gut microbiota has received attention in the literature [57]. Duan et al. highlight that probiotic supplementation, particularly with Lactobacillus and Bifidobacterium strains, may help to restore the microbial balance disrupted by contaminants [58]. Probiotics have shown promise in animal models by reducing the intestinal absorption of toxic metals and promoting the proliferation of beneficial bacteria [59, 60]. This has led to the proposal that probiotics could serve as a means of preventive or therapeutic intervention to reduce the harmful effects of environmental pollutants on gut health.
Arun et al. further discuss the therapeutic potential of probiotics, suggesting that these microorganisms could alleviate oxidative stress, inflammation, and microbial imbalances induced by toxic metal exposure [61]. This protective effect is attributed to the ability of probiotics to modulate the intestinal immune response, reduce ROS production, and support the restoration of intestinal barrier integrity [58]. The ability of probiotics to protect the gut microbiota highlights their potential as an innovative therapeutic strategy to counteract the harmful effects of environmental pollutants [61]. In addition, the metabolite profiles produced by the gut microbiota are crucial for a full understanding of the toxicological effects of metal exposure [58]. Santiago et al. propose that metabolites produced by microbial fermentation, particularly SCFAs, are essential for maintaining gut integrity and modulating immune responses [62]. However, dysbiosis resulting from toxic metal exposure may disrupt the production of these metabolites, thereby exacerbating inflammation and compromising gut barrier function [63].
In addition, Bist and Choudhary found that toxic metals, particularly cadmium and lead, promote the overgrowth of pathogenic bacteria, such as Enterococcus, Clostridium, and Escherichia coli [64]. While these bacteria are normally present in low concentrations in a healthy gut, their proliferation in the presence of toxic metals contributes to an inflammatory environment and disrupts the delicate microbial balance [41, 65]. Overgrowth of these pathogenic bacteria is associated with the production of harmful metabolites, including endotoxins, which exacerbate gut inflammation and compromise the immune system [66]. In addition, a shift towards a more pathogenic microbiota can facilitate the translocation of harmful microbes across the gut barrier, leading to infection and systemic inflammation [47].
In a related study, Richardson et al. examined the gut microbiota of rats following exposure to cadmium and arsenic and found that the metal exposure induced distinct microbial responses, resulting in shifts in the gut microbiota composition [8]. The study highlighted an increase in Firmicutes and Proteobacteria coupled with a decrease in Bacteroidetes. These changes in the microbial composition were associated with impaired gut function and increased systemic inflammation. Notably, the study also identified changes in microbial metabolites, including SCFAs, which are essential for maintaining gut integrity and immune responses, contributing to the development of chronic diseases, such as liver and kidney damage [8].
A study conducted by Liu et al. investigated the toxic effects of lead (Pb) exposure on Carassius auratus, focusing on intestinal damage, oxidative stress, immune response, and microbiota dysbiosis [67]. The results of the study showed that the Pb exposure led to significant morphological changes in the intestine, including increased wall thickness and goblet cell number as well as reduced crypt depth. Their gene expression analysis showed increased oxidative stress and inflammatory markers, while 16S rRNA sequencing revealed a decrease in microbial diversity and an increase in pathogenic bacteria. Further analysis of gene expression changes showed a decrease in the expression of claudin-7 and villin-1 and a significant increase in oxidative stress and immune-related markers, such as glutathione transferase (GST), glutathione (GSH), catalase (CAT), interleukins IL-8, IL-10, and IL-1, and tumour necrosis factor α (TNF-α). In addition, 16S rRNA sequencing revealed a decrease in microbial diversity with an increase in pathogenic bacteria, including Erysipelotrichaceae, Weeksellaceae, and Vibrionaceae, in response to the Pb exposure. This dysbiosis was associated with functional changes in the gut microbiota, including activation of the PPAR signalling pathway and immune dysfunction, suggesting that the gut microbiota may serve as a biomarker for assessing heavy metal toxicity and contamination [67]. In a related study, Liu et al. observed similar results, showing that Pb exposure in silver carp (Hypophthalmichthys molitrix) caused significant intestinal structural damage, digestive stress, altered immune response, and microbiota dysbiosis [68]. These findings further highlight the critical role of the gut microbiota in mediating lead toxicity and its overall impact on gut health.
As shown in Fig. 2, the effects of heavy metals on the gut microbiota are complex and can be explored through several key mechanisms. Toxic metals, such as lead, cadmium, and mercury, have been shown to disrupt the gut microbiota by inducing oxidative stress, altering microbial diversity, damaging the gut barrier, impairing metabolite production, inducing inflammation, promoting antibiotic resistance, and affecting gut-brain axis communication [56]. These effects may have long-lasting and transgenerational health consequences, highlighting the need for further research in this area (Fig. 2).

Fig. 2: Key mechanisms of toxic metal effects on gut microbiota and their implications. The impact of toxic metals such as lead, cadmium and mercury on the gut microbiota is complex and can be broadly categorised into several key mechanisms. These include induction of oxidative stress, alteration of microbial diversity, damage to the gut barrier, impairment of metabolite production, induction of inflammation, promotion of antibiotic resistance, and effects on gut-brain axis communication. These mechanisms have the potential to result in long-term and transgenerational health effects.
Zhang et al. investigated the long-term effects of toxic metal pollution on the gastrointestinal microbiota of Bufo raddei and found that prolonged mercury exposure led to significant changes in the diversity and abundance of gut bacteria [7]. Over time, a decrease in microbial diversity was observed, which contributed to increased oxidative stress and inflammatory responses. Similarly, Liu et al. investigated the role of the gut microbiota in mediating cadmium-induced liver injury and demonstrated that the cadmium exposure disrupted the gut microbiota and interfered with bile acid metabolism – a pathway critical for liver function. Specifically, the cadmium-induced changes in the microbial composition led to an imbalance in bile acid homeostasis, which in turn impaired the farnesoid X receptor (FXR) signalling axis, a key regulator of liver function and metabolism. This dysregulation exacerbated liver injury, highlighting the central role of the gut microbiota in mediating the toxic effects of environmental pollutants [69]. Furthermore, Liu et al. extended these findings by investigating the protective potential of melatonin in attenuating cadmium-induced liver fibrosis. Their results suggest that melatonin administration improves liver function by modulating gut microbiota and bile acid metabolism, thereby counteracting the deleterious effects of toxic metals on the gut-liver axis. These findings suggest that modulation of the gut microbiota may be a promising therapeutic strategy to prevent or treat metal-induced liver injury [70].
In summary, the body of research collectively highlights the critical role of the gut microbiota in mediating the effects of toxic metal exposure. Studies reported by Porru et al [55]., Bist and Choudhary [64], and Duan et al [58]. demonstrate that the restoration of microbial balance through probiotic supplementation and other microbiome-based strategies has significant potential to mitigate the adverse health effects of environmental contaminants. Furthermore, understanding the changes in microbial metabolites resulting from pollutant exposure opens up new opportunities for targeted therapeutic interventions. The molecular mechanisms identified in these studies, such as oxidative stress, inflammatory pathways, and altered microbial metabolism, provide valuable insights into the effects of pollutants on gut health and highlight the importance of developing effective strategies to protect the gut microbiota and prevent pollutant-induced diseases.
Microplastics. Plastic pollution is a major environmental challenge that originates from both terrestrial and aquatic sources and contributes to widespread ecological and human health concerns [71]. The major contributors include plastic packaging, consumer goods, textiles, and industrial products. Single-use plastics, such as bottles, bags, and food packaging, are of particular concern due to their widespread use and significant environmental impacts. In addition, synthetic textiles, including polyester and nylon, have been identified as sources of microplastic contamination, as these materials release plastic particles during washing [72]. Recent studies estimate that washing synthetic textiles can release up to 700, 000 microplastic fibres per load, which subsequently enter wastewater systems and accumulate in aquatic environments [73]. The increasing reliance on plastic materials in various industries, including agriculture and medicine, further exacerbates the problem [74, 75].
Another important source of plastic pollution is the degradation of larger plastic debris into microplastics and nanoplastics through environmental processes, such as exposure to ultraviolet (UV) radiation, mechanical abrasion, and weathering [76, 77]. These plastic particles enter biological organisms through a variety of pathways. In aquatic ecosystems, fish and other marine species ingest microplastics and nanoplastics either directly or indirectly through contaminated food sources. After ingestion, these particles can accumulate in the digestive tract, enter the circulatory system, or even penetrate internal organs [5, 78].
The health effects of microplastics are an emerging area of concern, particularly in relation to the gut-liver axis [79]. Recently, Zhang et al. have investigated the effects of polystyrene (PS) microplastics on liver injury and demonstrated that PS-induced changes in the gut microbiota significantly contribute to liver damage [80]. Their findings highlight that perturbations in the gut-liver axis occur via inflammatory and oxidative stress pathways. Specifically, increased intestinal permeability facilitates the translocation of harmful microbial metabolites into the bloodstream, thereby promoting hepatic inflammation and oxidative stress. This process involves the activation of pro-inflammatory cytokines, including tumour necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which subsequently trigger NF-κB signalling, further exacerbating liver injury. These findings highlight the critical role of gut microbiota dysbiosis in mediating the systemic effects of microplastic exposure, particularly with regard to liver health [80]. As shown in Fig. 3, microplastics exert a profound influence on the composition of the gut microbiota, with far-reaching physiological consequences.
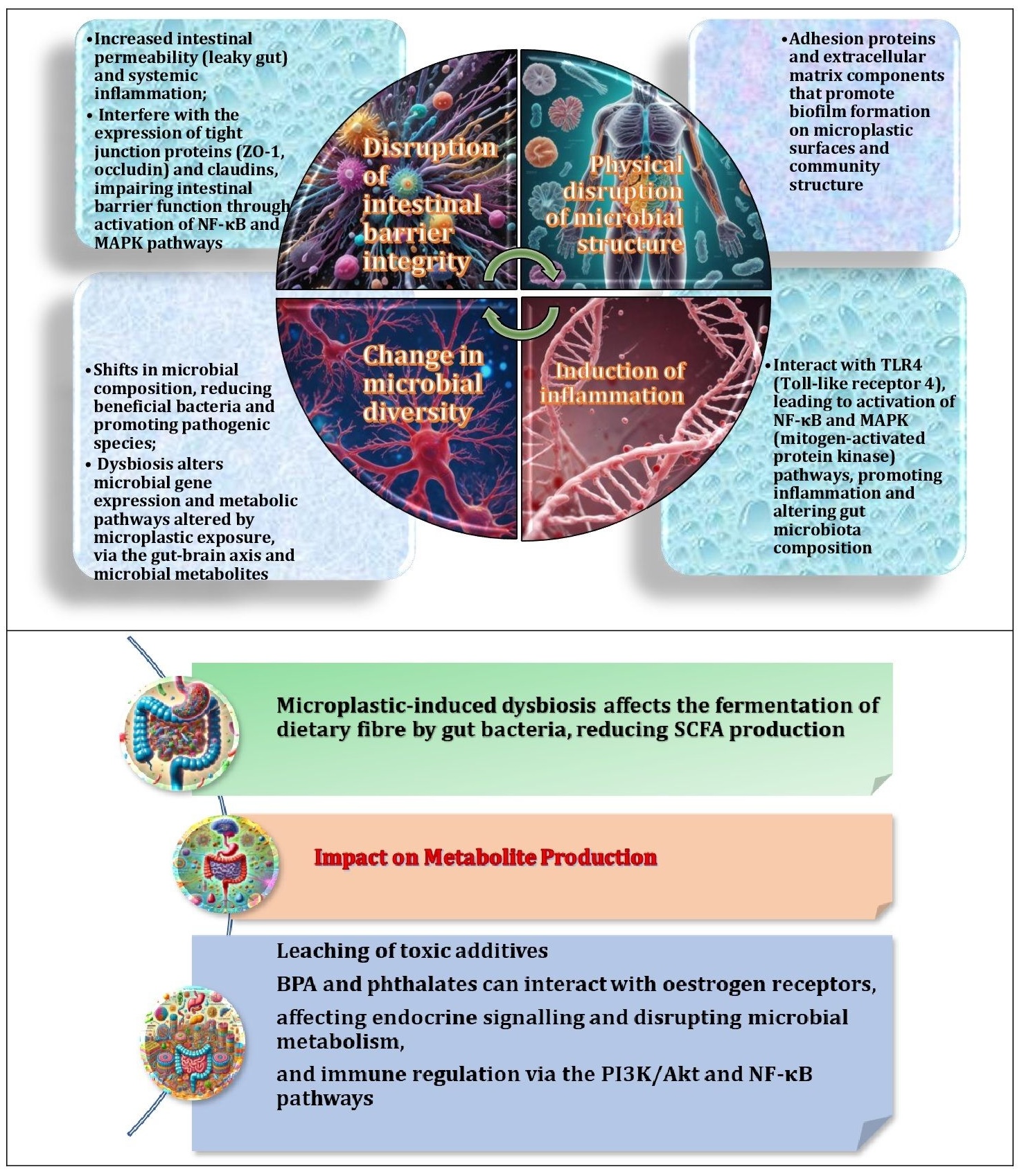
Fig. 3: Key mechanisms of microplastic effects on gut microbiota and their implications. Microplastics affect the gut microbiota by promoting biofilm formation, inducing inflammation through TLR4 and NF-κB pathways, causing oxidative stress through ROS and JNK/p38 MAPK activation, altering microbial diversity, disrupting gut barrier integrity through tight junction impairment, leaching toxic additives such as BPA and phthalates that affect endocrine signalling, and reducing short-chain fatty acid production by interfering with microbial fermentation, all of which contribute to systemic health effects.
The impact of microplastics on the gut microbiota is complex and involves multiple biological mechanisms. These include promotion of biofilm formation, activation of inflammatory pathways through Toll-like receptor 4 (TLR4) and nuclear factor kappa B (NF-κB) signalling, and induction of oxidative stress via ROS generation and c-Jun N-terminal kinase (JNK)/p38 mitogen-activated protein kinase (MAPK) activation [81]. In addition, microplastics alter microbial diversity, compromise gut barrier integrity by impairing tight junctions, and facilitate the release of toxic additives, such as bisphenol A (BPA) and phthalates, which disrupt endocrine signalling [82]. Studies have shown that exposure to microplastics can significantly reduce beneficial bacteria, such as Lactobacillus and Bifidobacterium, while promoting the growth of opportunistic pathogens [79]. They also reduce the production of short-chain fatty acids by interfering with microbial fermentation processes [31]. Together, these mechanisms contribute to systemic health effects.
Ke et al. (2023) conducted a pilot study to investigate the presence of microplastics in preschool children and their effects on the gut microbiota composition [83]. Their results indicate that children exposed to microplastics had altered gut microbiota profiles, potentially increasing their susceptibility to diseases associated with microbial dysbiosis. The study also suggests that microplastic exposure contributes to gut epithelial damage, leading to systemic inflammation. An increased abundance of pathogenic bacteria producing lipopolysaccharide (LPS), which exacerbates gut inflammation and triggers immune activation, was also found. These findings suggest that exposure to microplastics early in life, when the immune system is still developing, may have long-term health consequences [83]. Exposure to microplastics has also been associated with developmental and neurobehavioural effects in young children, raising concerns about long-term cognitive and immune system effects [84, 85].
Jing et al. (2022) investigated the haematopoietic effects of polystyrene (PS) microplastics, focusing on the interplay between gut microbiota, microbial metabolites, and cytokine-mediated immune responses [86]. Their study showed that exposure to PS microplastics resulted in haematopoietic dysfunction, primarily through changes in the composition of the gut microbiota. The underlying molecular mechanisms included perturbations in microbiota-derived metabolites, e.g. SCFAs, which play a critical role in immune regulation. In addition, PS microplastics stimulated the release of pro-inflammatory cytokines, including IL-6 and TNF-alpha, which contributed to haematopoietic suppression and immune dysregulation through activation of the Janus kinase/signal transducer and activator of transcription (JAK-STAT) pathway. These findings highlight the complex interactions between gut microbiota, the immune system, and microplastic-induced inflammatory responses [86].
Similarly, Jin et al. demonstrated that exposure to PS microplastics significantly compromised gut barrier integrity in a mouse model, leading to increased gut permeability, inflammation, and microbial dysbiosis [87]. Their study showed that the exposure to PS microplastics resulted in a shift in the composition of the gut microbiota, characterised by a decrease in beneficial bacterial populations and an overrepresentation of potentially pathogenic species. These microbial changes had downstream effects on metabolic functions, particularly the production of SCFAs, which are essential for maintaining gut homeostasis. Consistent with these findings, Jing et al. further confirmed that PS microplastics induced haematopoietic damage by modulating the gut microbiota composition and increasing the levels of inflammatory cytokines, such as IL-6 and TNF-α. These immune and microbiota perturbations were implicated in impaired blood cell production and systemic inflammation, highlighting the broad physiological consequences of microplastic exposure [87].
These findings highlight the systemic effects of microplastics on health, particularly through the gut-brain axis, as demonstrated in animal models. The effects of environmental contaminants on the gut microbiota composition and the mechanisms underlying these changes are summarised in Table 1.
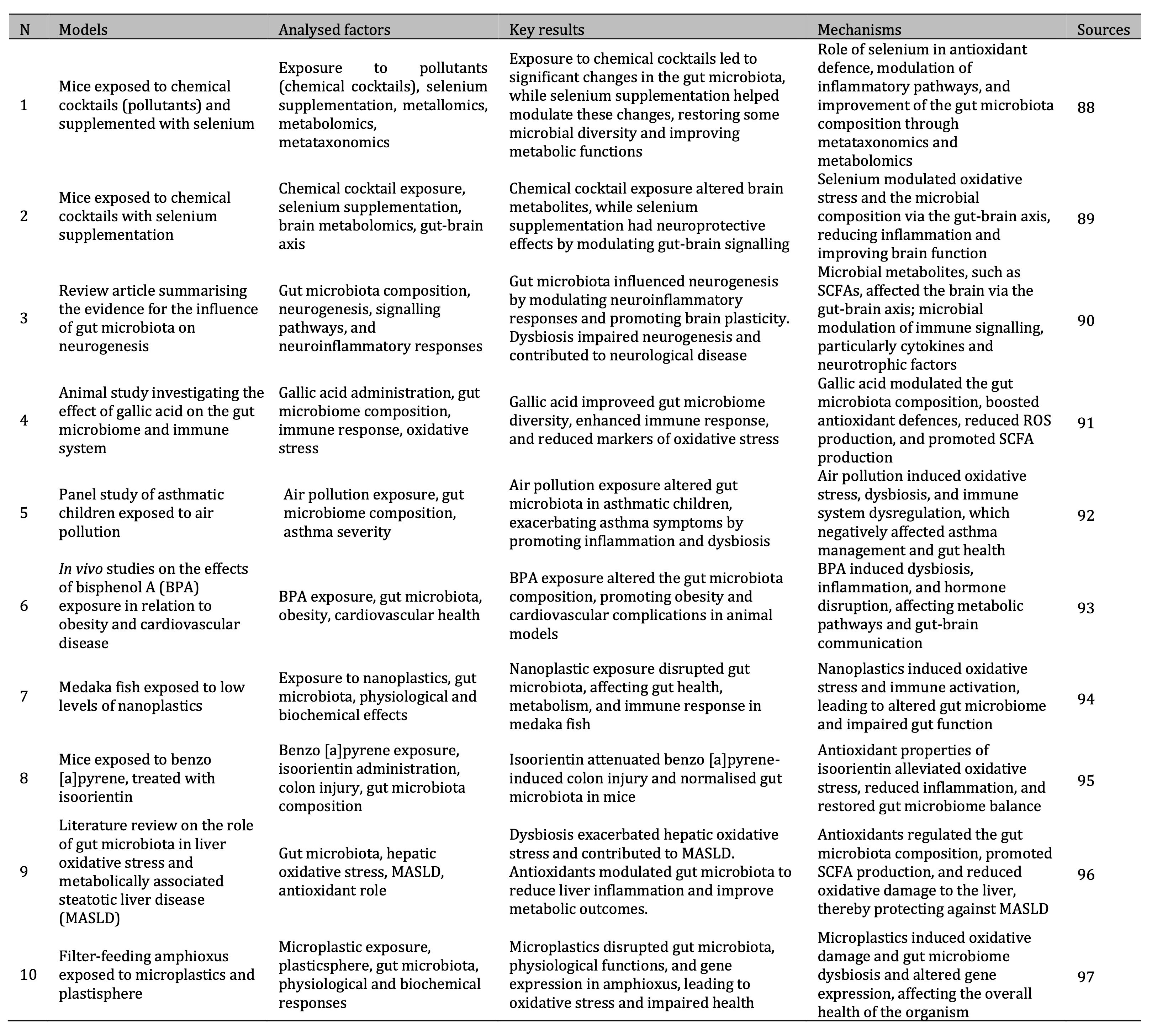
Table 1: Summary of research studies on the effects of environmental pollutants on gut microbiota and health mechanisms
Collectively, these studies illustrate the interactions of microplastics with the gut microbiota, initiating a cascade of molecular mechanisms that contribute to various adverse health outcomes. Extensive research has elucidated the key pathways involved in these processes, in particular inflammation, oxidative stress, and alterations in intestinal permeability [80, 86, 98]. These molecular perturbations play a central role in mediating the systemic consequences of microplastic exposure, particularly in relation to liver function, immune regulation, and haematopoiesis [83, 99]. Future research should focus on identifying potential therapeutic interventions, such as probiotics or dietary changes, to mitigate the adverse effects of microplastic-induced gut dysbiosis.
Pesticide-induced toxicity. Extensive research has shown that pesticides have a profound effect on the gut microbiota, leading to disruptions in microbial diversity and changes in the balance between commensal and pathogenic species [100]. The effects of various classes of pesticides, including organophosphates and pyrethroids, on the composition and function of the gut microbiota have been well documented, with results indicating a decrease in microbial diversity accompanied by an overgrowth of pathogenic strains [101-103]. Such perturbations have been shown to affect the production of SCFAs, which play a critical role in maintaining the integrity of the gut barrier and modulating immune responses [102]. Furthermore, Meng et al. (2020) highlight that these microbial alterations can have systemic effects, including inflammation and metabolic dysregulation, highlighting the sensitivity of the gut microbiota to pesticide exposure and its role as an unintended target of toxic effects [104].
The gut microbiota is increasingly being recognised as a central component of the gut-brain axis, influencing a range of systemic physiological processes, including neurological health [105]. Pesticide exposure has been implicated in exacerbating gut dysbiosis, a phenomenon implicated in the pathogenesis of neurodegenerative diseases, e.g. Parkinson's disease [106]. The disruption of microbial metabolite profiles induced by pesticide exposure has been shown to promote inflammation and oxidative stress, both of which contribute to neurodegenerative pathways [106]. Recent studies have also identified changes in neurotransmitter synthesis, i.e. decreased levels of gamma-aminobutyric acid (GABA) and serotonin, further linking pesticide-induced gut dysbiosis to neurological dysfunction [107]. In addition, non-target effects of pesticides on the gut microbiota have been found to increase health risks, highlighting the need to understand these interactions in order to mitigate their long-term consequences [105].
The public health implications of pesticide-induced disruption of the gut microbiota have been widely discussed [103, 106], with studies highlighting the need for a comprehensive assessment of pesticide safety, particularly with regard to unintended effects on microbial ecosystems [101]. These authors further argue that the gut microbiota serves as a critical mediator in the translation of environmental exposures into systemic health effects, including immune dysregulation and chronic disease progression. For example, long-term exposure to pesticides has been associated with increased susceptibility to inflammatory bowel disease and metabolic disorders, such as obesity and type 2 diabetes, due to microbiota-induced immune dysfunction [101, 103]. In addition, Abou Diwan et al. provide evidence that maternal exposure to pesticides induces microbiota perturbations in both the mother and the offspring, with potential transgenerational effects [108]. Given the broad implications of gut microbiota perturbations for metabolic, immune and neurological health, addressing these effects is imperative for public health.
Environmental pollutants, gut microbiota dysbiosis, and antibiotic resistance
Perturbations in microbial homeostasis, caused by environmental pollutants such as toxic metals and microplastics, have been shown to significantly alter the composition and diversity of the gut microbiota, leading to dysbiosis. This condition is characterised by a reduction in beneficial microbial populations, such as Lactobacillus and Bifidobacterium, and a concurrent increase in opportunistic pathogens like Escherichia and Enterococcus [56, 109, 110]. For instance, exposure to heavy metals, including cadmium, lead, and mercury, has been demonstrated to disrupt microbial communities [79, 111, 112]. Furthermore, microplastics have been shown to exacerbate dysbiosis by adsorbing environmental toxins and acting as vectors for pathogenic bacteria [112]. These imbalances have been shown to activate molecular pathways, including immune dysregulation and increased intestinal permeability, which in turn facilitate oxidative stress.
It has been established that chronic exposure to pollutants, such as bisphenol A (BPA), is associated with elevated ROS production. This, in turn, has been shown to result in oxidative damage to intestinal epithelial cells and compromise barrier integrity [13, 14, 113]. This cascade has been demonstrated to promote chronic inflammation, immune dysfunction and systemic diseases [79]. Furthermore, microbial dysbiosis has been demonstrated to impact pivotal metabolic processes, including nutrient absorption and bile acid metabolism, thereby exacerbating gastrointestinal disorders and predisposing individuals to metabolic and liver diseases, such as metabolic syndrome, non-alcoholic fatty liver disease (NAFLD), and insulin resistance [21, 114–118].
The long-term ramifications of dysbiosis are further compounded by the emergence of antibiotic resistance (ABR), a phenomenon that is exacerbated by the persistent utilisation of antibiotics and environmental contamination. The presence of pollutants such as antibiotics and heavy metals in the environment has been demonstrated to facilitate the horizontal transfer of antibiotic resistance genes (ARGs), thereby creating reservoirs of resistant bacterial strains [119–122]. It has been demonstrated that heavy metals, including arsenic and copper, have the capacity to co-select for antibiotic resistance. This phenomenon occurs through the induction of stress responses that promote genetic adaptations, thereby enhancing bacterial survival under conditions that are detrimental to their growth [123, 124]. Consequently, multidrug-resistant bacteria proliferate in the gastrointestinal tract, limiting therapeutic options and enabling the persistence of pathogenic microorganisms [125, 126].
It is also important to note that microbial alterations in the gut microbiota can promote the carriage of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, further complicating antimicrobial treatments [127]. This dynamic interplay between dysbiosis and the dissemination of ABR genes signifies a pivotal public health concern, bearing substantial ramifications for both individual health and global health security.
Environmental spread of antibiotic resistance genes
Recent research highlights the escalating and multifaceted challenge of antibiotic resistance (AR) in the environment, with profound implications for both public health and ecosystem stability. The ubiquitous presence of antibiotic resistance genes (ARGs) in aquatic environments, as demonstrated by Liu et al. [128] and Shao et al. [129], highlights the contamination of natural water bodies and their potential role as reservoirs for the transmission of resistance to humans and animals. This contamination is further exacerbated by the accumulation of heavy metals, microplastics, and other contaminants, which have been shown to co-select for ARGs, thereby enhancing their persistence and mobility within microbial communities. These contaminants are mainly derived from agricultural runoff, wastewater, and improper disposal of pharmaceuticals, facilitating the widespread distribution of ARGs in different ecological systems [130, 131]. In addition, anthropogenic activities, including industrial waste discharges and aquaculture practices, have been identified as significant contributors to the spread of ARGs, further exacerbating the environmental burden of antibiotic resistance [130, 132].
The scientific literature on ARGs has extensively documented the complex mechanisms that govern their persistence and spread in environmental ecosystems, raising significant global public health concerns. Jian et al. provide a comprehensive review of the occurrence, transmission, and mitigation strategies associated with ARGs, highlighting their widespread presence in various environmental matrices, including wastewater, agricultural soils, and natural ecosystems [133]. The authors highlight the critical role of mobile genetic elements (MGEs) in promoting horizontal gene transfer between bacterial populations, thereby accelerating the spread of resistance. In particular, integrons, transposons, and plasmids play a pivotal role in this genetic exchange, enabling bacteria to acquire and disseminate resistance determinants even in the absence of direct antibiotic selection pressure [134]. They also identify key factors driving the persistence of ARGs, such as the overuse and misuse of antibiotics in clinical and agricultural settings as well as environmental contamination through wastewater discharge [133]. Recent metagenomic studies have further demonstrated that ARGs can persist in environmental reservoirs for extended periods, with some resistant bacteria exhibiting enhanced survival strategies, including biofilm formation and efflux pump activation, which confer additional resilience to environmental stressors [135]. Given the potential risks posed by the unchecked spread of ARGs, the authors advocate stringent monitoring and control measures to mitigate the impact of antibiotic resistance on public and environmental health. Emerging bioremediation strategies, such as bacteriophage therapy, advanced oxidation processes, and constructed wetlands, have been proposed as innovative approaches to reduce the prevalence of ARGs in contaminated environments and offer potential ways to mitigate the global threat of antibiotic resistance [130].
Environmental drivers of antibiotic resistance
As highlighted in the relevant literature, significant environmental factors contributing to antibiotic resistance include the presence of microorganisms carrying ARGs in different environmental compartments and the impact of human activities on their spread [10]. For example, Jian et al. highlight that bacteria carrying ARGs are found in various environments, including soil, water, and industrial waste. In particular, improper disposal of antibiotic-contaminated waste, especially through wastewater contamination, has been identified as a major factor in the spread of antibiotic resistance [133]. In addition, traces of antibiotics and ARGs have been detected in water used for agricultural and livestock purposes, contributing to the spread of these genes and facilitating their further spread among microorganisms in the natural environment [136]. Increased agricultural intensification, with overuse of antibiotics, has further exacerbated the problem by providing a continuous source of resistance genes in the environment [12].
In addition, industrial waste pollution and the use of antibiotics in aquaculture have been identified as major sources of ARGs in natural ecosystems [137]. Yuan et al. showed that the routine use of antibiotics in fish farming contributes to an increase in antibiotic-resistant bacteria, thereby facilitating the spread of resistance [138]. Aquaculture practices, especially in large-scale operations, have been shown to contribute significantly to environmental contamination with ARGs due to the widespread and often indiscriminate use of antibiotics. Nguyen et al. conducted a comprehensive analysis of current strategies for monitoring ARGs in wastewater treatment and highlighted the challenges associated with detecting and removing these genes from wastewater effluents [136]. Their findings suggest that wastewater treatment plants often fail to effectively eliminate ARGs, resulting in the spread of resistant bacteria to the surrounding environment. Technological advances in the detection and removal of ARGs are needed to mitigate their environmental impact and prevent the further evolution of resistance in bacterial populations.
Yuan et al. review the role of aquaculture in the spread of ARGs, emphasising the contamination of aquatic environments due to the extensive use of antibiotics in fish farming [138]. Their review highlights that the overuse of antibiotics in aquaculture has led to a significant increase in the prevalence of antibiotic-resistant bacteria, which are capable of transferring ARGs to other microbial populations in the water. This process, known as the 'resistance cycle', not only exacerbates environmental contamination but also poses a potential risk to human health through the consumption of contaminated seafood [138].
Research focused on the prevalence, distribution, and transfer of ARGs in different environmental settings, highlighting the complex interactions within the One Health framework. Liu et al. investigated the presence of ARGs in the surface water of a subtropical drinking water river-reservoir system, revealing significant contamination in aquatic environments with serious implications for both environmental and human health [128]. Kim et al. investigated the dynamics of ARG gain and loss in multidrug-resistant bacteria, with particular emphasis on the implications of these processes for human and environmental health. Their findings suggest that the environment acts as both a reservoir and a mediator for the persistence and spread of resistance genes [139].
Ajayi et al. provide a comprehensive review of ARGs across different ecosystems, reinforcing the interconnectedness of animal, human, and environmental health and highlighting the need for a holistic approach to managing antimicrobial resistance [140]. Shao et al. reviewed the movement, transformation, and distribution of antibiotics and ARGs in aquatic systems and identified key environmental factors that facilitate the spread of resistance. Their work highlights the importance of the aquatic environment as a conduit for the spread of ARGs, which can exacerbate resistance in both the environment and human populations [129].
Thus, these studies underscore the ubiquitous presence of ARGs in various environments and the need for integrated strategies to combat antimicrobial resistance. The growing body of research highlights the urgent need for comprehensive monitoring, reduction, and remediation of ARGs in the environment. Interdisciplinary approaches involving environmental management, technological innovation in waste treatment, and regulation of antibiotic use in all sectors are essential to effectively protect public health and mitigate the environmental drivers of antibiotic resistance.
Environmental pollutants, oxidative stress, and gut microbiome dysbiosis
Excessive production of ROS is a critical mechanism by which environmental pollution affects the human gut microbiome and overall health. Pollutants, such as heavy metals, pesticides, and particulate matter, generate ROS in biological systems, leading to oxidative stress [16]. In the gut, oxidative stress disrupts the balance of microbial communities, leading to dysbiosis, i.e. an imbalance between beneficial and harmful microbes [141]. Environmental pollutants interfere with antioxidant enzyme activity through a variety of mechanisms, as shown in Fig. 4. They inhibit the expression of antioxidant enzymes, deplete essential cofactors, and generate excessive ROS, overwhelming the body's antioxidant defences [142, 143]. In addition, pollutants can alter the activity of key enzymes involved in antioxidant defence, aggravating oxidative stress and exacerbating the negative impact on microbial health [144].
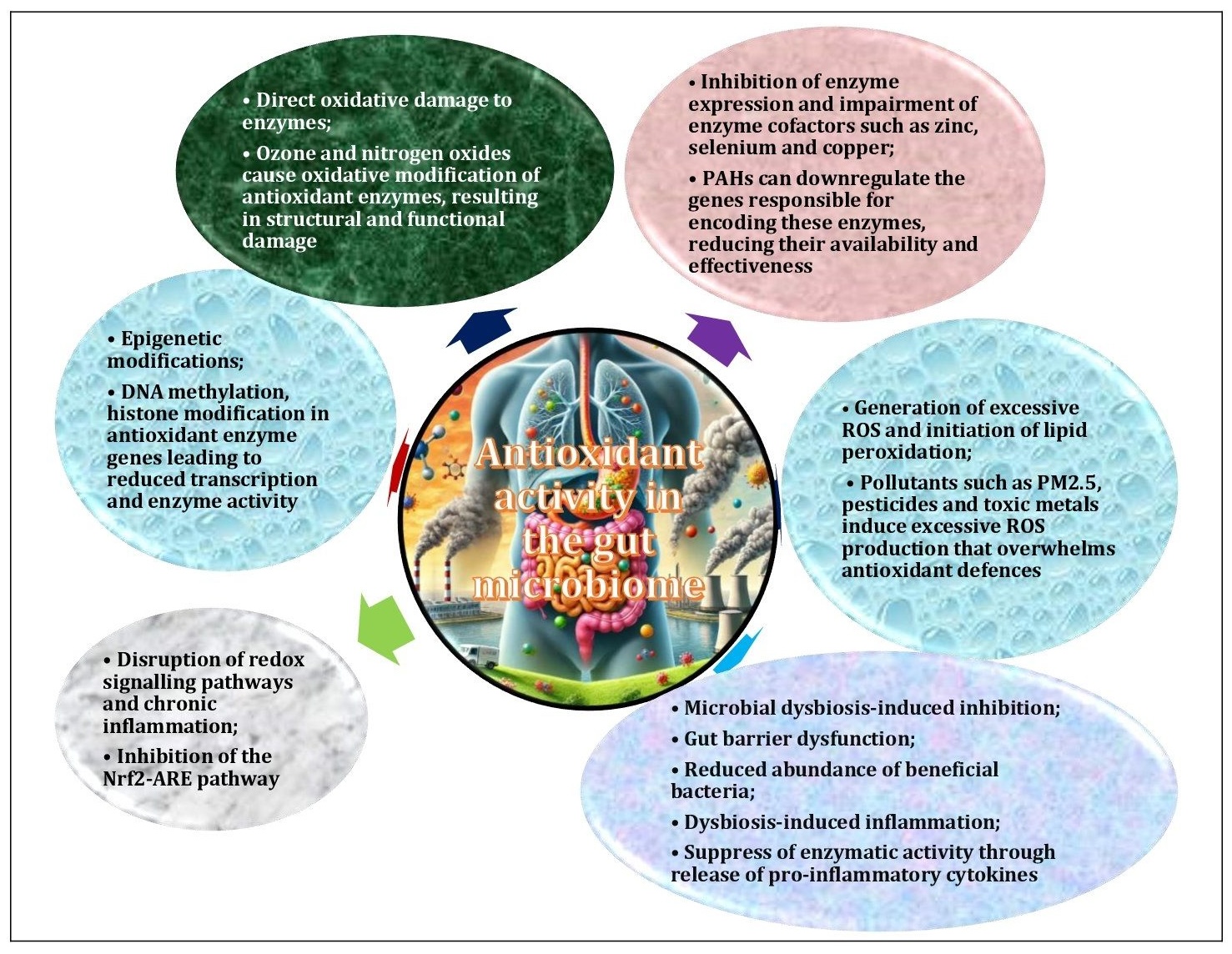
Fig. 4: Mechanisms of pollutant-induced disruption of antioxidant function in the gut microbiome and in the organism. Environmental pollutants interfere with antioxidant enzyme activity by a variety of mechanisms. They inhibit enzyme expression by binding directly to active sites or by downregulating encoding genes, generate excessive reactive oxygen species (ROS) that overwhelm defences, and impair essential cofactors such as zinc and selenium. In addition, toxins induce gut microbiome dysbiosis, oxidative damage to enzymes and epigenetic modifications, while also disrupting redox signalling pathways and gut barrier integrity. Chronic inflammation and lipid peroxidation products further exacerbate oxidative stress and exacerbate the suppression of antioxidant enzyme activity.
In addition, these pollutants induce gut microbiome dysbiosis, cause oxidative damage to enzymes, and induce chronic inflammation, all of which further suppress enzyme activity [14]. These combined effects contribute to systemic oxidative stress and impaired redox balance, as shown in Fig. 4. This systemic oxidative stress is associated with a number of metabolic disorders that compromise immune function and increase susceptibility to various diseases [145]. In turn, oxidative stress promotes the production of ROS through microbial metabolism, creating a vicious cycle of oxidative damage. This imbalance can compromise the integrity of the intestinal barrier, exacerbate inflammation, and affect overall systemic health [141].
A wide range of diseases have been linked to prolonged exposure to environmental pollutants and the mechanisms outlined above, including irritable bowel syndrome [146], Crohn's disease [147], ulcerative colitis [148], type 2 diabetes [149], obesity [150], insulin resistance [151], rheumatoid arthritis [152], systemic lupus erythematosus [153], non-alcoholic fatty liver disease [154], liver cirrhosis [155], atherosclerosis [156], hypertension [157], myocardial infarction [158], Alzheimer's disease [159], Parkinson's disease [160], amyotrophic lateral sclerosis [161], colorectal cancer [162], liver cancer [163], chronic obstructive pulmonary disease [164], and asthma [150]. These diseases share common pathogenic mechanisms driven by environmental pollutants, gut dysbiosis, and oxidative stress, highlighting the need for integrated strategies to address these interrelated factors [146-164].
Paun and Danska [165] emphasised the pivotal role of the gut microbiome in influencing the risk of type 1 and type 2 diabetes, highlighting its bidirectional relationship with systemic metabolic health. Similarly, Zhao et al. established a link between exposure to PM2.5 particulate matter and insulin resistance via microbiome-mediated mechanisms [166]. Together, these findings emphasise the complex interplay between environmental pollutants, microbial dysbiosis and host physiology, and highlight the importance of addressing environmental exposures to mitigate their adverse health effects.
Environmental pollution significantly disrupts antioxidant enzyme activity in both the gut microbiome and the organism through a variety of mechanisms. Pollutants, such as heavy metals, particulate matter, and pesticides, have been shown to inhibit the expression or activity of critical enzymes, including superoxide dismutase (SOD) and glutathione peroxidase (GPx). These pollutants act either by binding to the active sites of these enzymes or by down-regulating genes responsible for their synthesis [167]. In addition, pollutants, such as pesticides and heavy metals, can act as direct enzyme inhibitors, interfering with their function at the molecular level [168, 169].
Pollutants also induce dysbiosis, which has been shown to reduce populations of beneficial bacteria that contribute to antioxidant activity [14]. This microbial imbalance further compromises the host's ability to mitigate oxidative damage. Inflammation, often triggered by environmental pollutants, has been shown to suppress antioxidant enzyme synthesis through the action of pro-inflammatory cytokines [79]. This inflammatory response can lead to a cascade of adverse events, ultimately compromising the body's ability to effectively manage oxidative stress. Pollutants can also cause direct oxidative modifications to enzymes, damaging their structure and function [145]. In addition, epigenetic changes, e.g. DNA methylation, have been shown to inhibit the transcription of antioxidant genes, compounding the effects of pollution on enzyme expression [170, 171].
The results of the present study are consistent with those reported by Omar et al., who demonstrated that rifaximin treatment in rats conferred protection against malathion-induced testicular toxicity [172]. This protective effect was attributed to modulation of the gut microbiome and mitigation of oxidative stress through mitophagy. These observations highlight a recurring theme across multiple studies: pollution-induced oxidative stress significantly influences the composition of the gut microbiome, which in turn contributes to a range of systemic health dysfunctions [172]. In support of these conclusions, research focusing on prenatal environmental risk factors, for instance a study conducted by Love et al. (2024), further reinforces the growing recognition of the impact of environmental exposures on microbiome health [173]. This highlights the importance of understanding the mechanism of environmental contaminants in the disruption of microbial homeostasis from an early stage of development.
As demonstrated by Klimkaite et al., exposure to pollutants such as air pollution has been shown to induce changes in the airway microbiome, leading to molecular perturbations [174]. This finding is consistent with the research conducted by Gao et al., who demonstrated a direct correlation between oxidative stress and intestinal health. Their study revealed that Bacillus coagulans, a probiotic bacterium, can alleviate copper-induced oxidative stress by regulating oxidative pathways and modulating the composition of the gut microbiome [175]. These studies emphasise the complex interconnection between environmental pollutants, microbiome health, and the adaptive or protective functions of microbial communities against external stressors. Further evidence indicates that environmental pollution plays a pivotal role in the initiation of oxidative stress, the disruption of the gut microbiome, and the influence on systemic health. Pollutants such as microplastics, toxic metals, particulate matter, polycyclic aromatic hydrocarbons, ozone, nitrogen oxides, second hand smoke, pesticides, ultrafine soot, and general air pollution have been demonstrated to cause immediate oxidative damage [176–179]. These pollutants have also been shown to induce long-term shifts in microbial populations in the gut and other organs. These persistent microbial disturbances contribute to a wide range of diseases, including cognitive disorders, metabolic dysfunctions, and reproductive toxicity [180], emphasising the chronic and multifaceted impacts of environmental pollution on health and the challenges it poses for disease prevention and management.
Consequently, the growing body of evidence underscores the urgent need for strategies aimed at reducing environmental exposures to pollutants and protecting the gut microbiome to prevent or mitigate the development of chronic diseases. A more comprehensive understanding of the interactions between environmental stressors, oxidative stress, and microbiome health is therefore essential for formulating more effective public health interventions and treatments [13]. This integrated approach to the study of environmental health could lead to improved strategies to mitigate the effects of pollution on human health and well-being.
Molecular pathways linking environmental pollutants to gut microbiota dysregulation
The molecular pathways involved in the regulation of the gut microbiota in response to environmental pollutants encompass several critical mechanisms, including inflammatory signalling, immune modulation, and gut barrier integrity pathways [181]. In particular, the nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways, which are essential for cellular stress responses and inflammatory regulation, have been identified as particularly vulnerable to pollutant exposure. Their dysregulation leads to intestinal inflammation and microbial dysbiosis [182, 183]. Toll-like receptors (TLRs), particularly TLR4 and TLR2, have been observed to be the key mediators of pollutant-induced immune activation, initiating inflammatory cascades that profoundly affect the composition of the gut microbiota [184, 185]. In addition, pollutants have been shown to impair the function of regulatory T cells (Tregs), which are essential for maintaining immune tolerance and suppressing excessive inflammation [186].
Perturbations in cytokine homeostasis further exacerbate gut microbiome dysbiosis [187]. Increased levels of pro-inflammatory cytokines, such as tumour necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β), and decreased levels of anti-inflammatory cytokines, particularly interleukin-10 (IL-10), contribute to immune dysregulation [188]. Toxins also compromise the integrity of the intestinal barrier by damaging tight junction proteins, including zonula occludens-1 (ZO-1), occludin, and claudin-1, thereby increasing intestinal permeability and facilitating the translocation of harmful microbial products into the systemic circulation [189, 190]. In addition, epithelial growth factors (EGFs) and trefoil factors (TFFs), which are critical for intestinal repair and epithelial cell homeostasis, have been shown to be impaired by contaminants, preventing proper mucosal regeneration and exacerbating barrier dysfunction [191].
In addition to immune modulation and gut barrier integrity, contaminants have been shown to disrupt microbiota-host interactions by altering the production of SCFAs, including butyrate, acetate, and propionate, which are essential for immune regulation, gut epithelial maintenance, and barrier function [63]. Furthermore, changes in microbial metabolites, such as secondary bile acids and tryptophan-derived indole compounds, have been observed to disrupt both gut microbiota diversity and host metabolic homeostasis [192].
Oxidative stress pathways, particularly through the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, are also affected by pollutants, including heavy metals and air pollution, leading to oxidative damage in the gut and dysbiosis. ROS generated by pollutants further increase inflammation and epithelial injury [193]. In addition, cytochrome P450 (CYP) enzymes, which play a central role in xenobiotic metabolism and detoxification, can modulate microbial exposure to environmental toxins, thereby exerting a significant influence on the composition of the gut microbiota [194]. Also, through cannabinoid receptors CB1 and CB2, the endocannabinoid system (ECS) is highly sensitive to contaminant-induced alterations, affecting gastrointestinal motility, intestinal permeability, and microbial diversity, which collectively contribute to gut dysfunction and systemic metabolic disorders [195, 196].
The molecular pathways highlight the intricate interplay between environmental pollutants and gut health, underscoring their widespread impact on microbial balance, immune homeostasis, and overall gut functionality, as illustrated in Fig. 5. Importantly, these pathways are not isolated but rather form an interconnected regulatory network in which pollutants can disrupt multiple mechanisms simultaneously. It is hypothesised that such perturbations contribute to gut dysbiosis, increased inflammation, compromised barrier function, and impaired immune responses, ultimately increasing susceptibility to metabolic, autoimmune, and inflammatory disorders. Understanding these mechanisms is essential for developing targeted interventions to mitigate the adverse effects of environmental pollution on gut and systemic health.

Fig. 5: Key molecular pathways involved in the regulation of gut microbiota and contaminants. Key molecular pathways regulating gut microbiota and pollutants include inflammation via NF-κB and MAPK pathways, immune modulation via TLRs and Tregs, gut barrier integrity via tight junction proteins and epithelial growth factors, microbial interaction via SCFAs and metabolites, and oxidative stress. The influence of contaminants on these processes and their potential consequences, such as microbiota dysbiosis, gut inflammation and compromised gut health, are the subject of ongoing research.
NF-κB as a mediator of environmental pollutant-induced gut microbiota dysbiosis and inflammation. The transcription factor NF-κB serves as a central regulator of inflammation within the gut microbiome, particularly in response to environmental pollutants [197]. A growing body of research has elucidated the mechanisms by which pollutants disrupt gut microbiota homeostasis, contributing to systemic inflammation and disease pathogenesis. For example, polyethylene microplastics (PE-MPs) have been shown to induce gut microbiota dysbiosis in mice, leading to liver injury through activation of the Toll-like receptor 2 (TLR2)/NF-κB/NOD-like receptor family pyrin domain containing 3 (NLRP3) pathway [198]. This finding highlights the inflammatory potential of microplastics, with gut microbial imbalances triggering liver injury via inflammation-related mechanisms. In a related study, Lin et al. demonstrated that nano-sized polystyrene particles exhibit greater toxicity than micro-sized polystyrene particles, primarily due to their more pronounced effects on the gut microbiota composition. This highlights the size-dependent toxicity of microplastics and reinforces their role in exacerbating systemic inflammation and toxicity [199].
Further supporting this line of research, Liu et al. investigated the effects of exposure to oil mist particulate matter and found that it promotes hyperlipidaemia-related inflammation via the microbiota/SCFAs/G-protein-coupled receptor 43 (GPR43) axis and TLR4/NF-κB activation. These findings reveal a complex interplay between environmental pollution, gut microbiome alterations, and inflammation in the regulation of lipid metabolism [200]. Collectively, these studies suggest that various pollutants, e.g. microplastics, airborne particulate matter, and industrial chemicals such as benzimidazole fungicides, disrupt the gut microbiota balance and activate inflammatory pathways, positioning the TLR/NF-κB axis as a central mediator in these processes.
Consistent with these findings, Lu et al. (2025) reported that carbendazim, a widely used benzimidazole fungicide in agriculture, induced intestinal inflammation in grass carp (Ctenopharyngodon idella) via the TLR5/NF-κB pathway [201]. This observation highlights the potential environmental and ecological consequences of agricultural pollutants on gut health. In a parallel study, Duan et al. highlighted the therapeutic potential of ginsenoside Rg3 in alleviating acute radiation proctitis through modulation of the TLR4/MyD88/NF-κB pathway, highlighting the critical role of TLR/NF-κB signalling in both gut inflammation and microbial homeostasis [202]. Furthermore, Liu et al. demonstrated that exposure to antimicrobials, such as benzalkonium chloride and triclosan, leads to sex-specific changes in the gut microbiota composition and overall health in zebrafish [203]. This suggests that chemical pollutants may have sex-specific effects on the microbiome structure and host health outcomes and warrants further investigation into sex-specific responses to environmental pollutants.
NF-κB is a key transcription factor activated by several inflammatory stimuli, including environmental pollutants, such as particulate matter (PM2.5), heavy metals, and microplastics. These pollutants have been shown to disrupt the microbial balance in the gut, leading to inflammatory responses and systemic health complications [204-207]. In addition, extensive research has highlighted a significant impact of environmental pollutants, particularly microplastics, airborne particulate matter, and agricultural pesticides, on the gut microbiota composition and the initiation of systemic inflammation via inflammatory pathways such as the Toll-like receptor (TLR)/NF-κB axis [206, 208, 209]. These pollutants have been shown to induce gut dysbiosis, which subsequently leads to serious health problems, including liver damage, hyperlipidaemia, and gastrointestinal inflammation [76, 101, 102]. Emerging evidence suggests that pollutants also affect tight junction proteins, such as ZO-1, occludin, and claudin-1, thereby increasing intestinal permeability and the risk of inflammatory diseases [210].
These studies highlight the central role of the TLR/NF-κB pathway in mediating pollutant-induced gut microbiota dysbiosis and inflammation [198]. These findings have important implications for understanding the interplay between environmental pollution, gut health, and systemic disease development and highlight the need for targeted strategies to mitigate pollutant exposure and its adverse health consequences.
Nrf2/Keap1 pathway as a regulator of oxidative stress and gut microbiome homeostasis. The Nrf2/Keap1 signalling pathway plays a critical role in regulating antioxidant responses, protecting cells from oxidative stress and maintaining cellular homeostasis [211]. In normal physiological conditions, nuclear factor erythroid 2-related factor 2 (Nrf2) remains bound to Kelch-like ECH-associated protein 1 (Keap1), facilitating its ubiquitination and subsequent degradation, thereby maintaining low basal levels of Nrf2 activity [212]. However, upon exposure to oxidative stress or environmental pollutants, e.g. airborne particulate matter (PM2.5), heavy metals, and microplastics, Nrf2 is released from Keap1 and translocates to the nucleus, where it binds to the antioxidant response element (ARE) and upregulates the expression of antioxidant genes and detoxifying enzymes, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and heme oxygenase-1 (HO-1) [213, 214]. This activation plays a protective role by neutralising ROS and preserving cellular structures, particularly within the intestinal epithelium. In the context of gut health, Nrf2 activation is essential for maintaining microbial homeostasis, regulating inflammatory responses, and preventing gut dysbiosis caused by oxidative stress. In particular, prolonged exposure to environmental pollutants can impair Nrf2 activation, leading to an imbalance in antioxidant defences, increased intestinal permeability and systemic inflammation [215].
From a molecular biological and physiological perspective, Nrf2 activation represents a fundamental cellular defence mechanism against oxidative damage, particularly in the intestinal tract and liver [215]. Saeedi et al. demonstrated that gut-dwelling lactobacilli stimulate hepatic Nrf2 activation, thereby enhancing the liver's resilience to oxidative stress. The study highlighted that lactobacilli modulate the expression of hepatic antioxidant enzymes through Nrf2 signalling, thereby mitigating ROS accumulation resulting from environmental pollutants and dietary imbalances. This highlights the intricate gut-liver axis in which microbiota-derived metabolites influence systemic oxidative responses [216]. Similarly, Wang et al. provided evidence that exposure to real ambient PM2.5 particles induces oxidative stress in both lung and gut, leading to Nrf2 activation as a compensatory mechanism to counteract pollutant-induced damage. These findings suggest that the gut microbiota – particularly beneficial bacteria such as lactobacilli – play a critical role in modulating oxidative stress in distant organs via the Nrf2 pathway, thereby enhancing the gut's contribution to systemic protection against environmental pollutants [217].
These studies highlight the Nrf2/Keap1 axis as a key molecular pathway mediating cellular defence against oxidative stress and pollution-induced damage. Given its central role in modulating antioxidant defences, reducing inflammation, and maintaining microbiome homeostasis, targeting Nrf2 offers significant therapeutic potential for the prevention and treatment of pollution-induced diseases. Future research should further explore pharmacological and dietary strategies aimed at enhancing Nrf2 activation to counteract oxidative damage and inflammation associated with environmental exposures.
PI3K/AKT and MAPK pathways as key regulators of cellular stress response to environmental pollutants. The PI3K/AKT and p38-MAPK pathways are essential cellular stress response mechanisms that are vital for maintaining homeostasis in the presence of environmental stressors, including exposure to various pollutants [218]. These signalling cascades enable cells to detect and respond to external stressors, such as oxidative stress, inflammation, and DNA damage, which are often induced by environmental pollutants, including air pollution, heavy metals, and particulate matter. The PI3K/AKT/mTOR signalling pathway is known to play a pivotal role in regulating fundamental cellular processes, such as cell survival, autophagy, and apoptosis, in response to pollutants and toxins [219, 220]. Studies reported by Guo et al. [221] and Yin et al. [222] provide compelling evidence that metals, such as arsenic and nickel, can activate the PI3K/AKT/mTOR pathway in a variety of organisms, including common carp and mouse models. Specifically, the activation of PI3K/AKT/mTOR enhances cellular survival under oxidative stress and toxin exposure, thereby reducing apoptosis and mitigating pollutant-induced damage [221, 222].
In particular, Guo et al. (2021) demonstrated that zinc can counteract arsenic-induced toxicity in fish by modulating the PI3K/AKT/mTOR signalling cascade, thereby protecting the intestine from arsenic-induced damage. This finding highlights the adaptive function of this signalling pathway in mitigating the toxic effects of environmental pollutants, particularly heavy metals [221]. Furthermore, the role of PI3K/AKT/mTOR in promoting autophagy was emphasised by Yin et al. (2021), who demonstrated that nickel exposure disrupts cellular homeostasis by impairing autophagic mechanisms [222]. In parallel, this pathway closely interacts with MAPK signalling and autophagic processes to regulate inflammation, metabolism, and immune responses. Studies conducted by Zhang et al. [223] and Lu et al. [224] highlight the importance of the PI3K/AKT/mTOR axis in controlling apoptosis and autophagy in cells exposed to air pollutants, including PM2.5 and microplastics. Zhang et al. identified the role of the PI3K/AKT/mTOR pathway in modulating autophagy in alveolar epithelial cells, where chronic exposure to PM2.5 promotes apoptosis and disrupts cellular homeostasis. Dysregulation of these pathways due to prolonged pollution exposure has been linked to chronic inflammation, gut dysbiosis, and impaired barrier function, highlighting their relevance in environmental health research [223, 224].
Furthermore, Sun et al. [225] and Dong et al. [226] have shown that environmental pollutants such as bisphenol A activate the PI3K/AKT/mTOR pathway, leading to abnormal autophagy and immune dysfunction. This dysregulation has been implicated in the pathogenesis of autoimmune diseases, highlighting the broader health implications of environmental toxin exposure [225, 226]. Bisphenol A, a widely recognised environmental contaminant commonly found in plastics, exerts profound effects on cellular stress response mechanisms by disrupting PI3K/AKT/mTOR signalling, thereby altering immune homeostasis and increasing susceptibility to disease [226].
In addition, exposure to bisphenol A has been shown to cause a wide range of adverse health effects, particularly when individuals are concurrently exposed to other environmental contaminants. BPA acts as an endocrine-disrupting chemical, interfering with hormonal signalling and leading to developmental disruption, neurotoxicity, and reproductive health disturbances [227]. Early life exposure to BPA has been linked to childhood obesity, neurodevelopmental disorders, and increased susceptibility to metabolic diseases later in life [228]. BPA exposure has also been associated with liver toxicity and may result in transgenerational effects, as seen in a study reporting liver defects in medaka fish after multiple generations of BPA exposure [229]. Furthermore, the combination of BPA with other environmental chemicals, such as phenols, pesticides, and phthalates, has been shown to exacerbate obesity and increase health risks [230]. The complexity of BPA mechanisms highlights the importance of understanding its interaction with other contaminants in order to assess its long-term effects on human health [231].
The results of studies further highlight the significant effects of environmental toxicants, including microplastics, cadmium, arsenic, and nanoparticles, on various biological systems, in particular through inflammatory responses and cellular damage via MAPK signalling [232]. Trophic transfer of nanoplastics leads to intestinal inflammation, dysbiosis, and activation of inflammatory pathways in zebrafish, highlighting the environmental risks of microplastic pollution. Xie et al. [233] and Li et al. [234] showed that polystyrene microplastics induce reproductive toxicity and oxidative stress via MAPK pathways, exerting deleterious effects on multiple organs, including the heart.
Furthermore, Cao et al. observed that cadmium exposure induced apoptosis and mitochondrial damage in BEAS-2B cells through MAPK signalling [235]. Furthermore, Liu et al. [236] and Ijomone et al. (2021) [237] observed that heavy metals such as arsenic induce depression and anxiety-like behaviours in mice through ROS/p38 MAPK/NLRP3 inflammasome activation, linking environmental stressors to neurobehavioural health. In addition, Han et al. investigated the effects of nanoparticle exposure on the lungs, demonstrating how viral reactivation can exacerbate lung damage and contribute to emphysema [238]. Taken together, these studies highlight the widespread activation of MAPK signalling pathways, which are central to the toxicological mechanisms behind the deleterious effects of various environmental pollutants on cellular integrity, inflammation, and general health.
Thus, the PI3K/AKT/mTOR pathway is a critical player in cellular stress responses to environmental pollutants. This pathway influences cell survival, inflammation, and immune responses and is essential for protecting cells from oxidative stress and regulating apoptosis and autophagy in response to pollutants. The interaction of this pathway with MAPK signalling and its ability to modulate immune responses emphasises its importance in environmental health and disease prevention. Thus, further research into the modulation of these signalling mechanisms offers significant potential for therapeutic intervention in the prevention of pollution-related diseases.
Role of JAK/STAT signalling in the effects of environmental pollutants, gut microbiome dysbiosis, and immune dysregulation. The gut microbiome plays a critical role in modulating immune responses, and its composition can be significantly altered by various environmental factors, including pollution [13, 14]. Emerging evidence suggests that exposure to pollutants, such as heavy metals, polycyclic aromatic hydrocarbons (PAHs), and persistent organic pollutants (POPs), is associated with changes in microbial diversity that impair host immunity and metabolic functions [63, 109]. In particular, pollutants, especially fine particulate matter (PM2.5) and chemical compounds, have been shown to affect the structure of the microbiome, leading to dysbiosis (an imbalance in microbial communities), which subsequently affects immune function and homeostasis [14].
A fundamental mechanism by which environmental pollution modulates immune responses is the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathway. This pathway is essential for mediating inflammatory and immune responses and plays a critical role in cellular proliferation, differentiation, and apoptosis [239, 240]. Dysregulation of this pathway, induced by microbial shifts resulting from contaminant exposure, can lead to chronic inflammation, immune suppression and the onset of autoimmune and inflammatory diseases [241]. In addition, oxidative stress and changes in gut permeability driven by pollutant-induced microbiome perturbations may further contribute to immune dysfunction, exacerbating inflammatory bowel disease and metabolic syndrome [14, 18].
Several biomarkers can be used to monitor the impact of pollution on both the gut microbiome and the immune system, highlighting the need to maintain microbiome balance in response to environmental stressors. In particular, various biomarkers, such as pro-inflammatory cytokines (e.g. interleukin-6, tumour necrosis factor-α), acute-phase proteins (C-reactive protein), faecal calprotectin, and SCFAs, serve as key indicators of microbial and immune dysregulation [242, 243]. Biomarkers associated with the gut microbiome and immune dysregulation, particularly those related to the JAK/STAT pathway in response to contaminant exposure, are shown in Fig. 6.
Fig. 6: Biomarkers associated with the gut microbiome and immune dysregulation, particularly through the JAK/STAT pathway in response to environmental pollution.
Furthermore, Jin et al.) showed that beryllium sulphate induces epithelial-mesenchymal transition (EMT) via JAK/STAT modulation, illustrating the involvement of this pathway in cellular transformation and fibrosis. Another crucial aspect of the role of the JAK/STAT pathway in immune dysregulation is its relevance in assessing the health effects of pollution [244]. Liu et al. investigated how hydrogen sulphide attenuated myocardial fibrosis via JAK/STAT signalling, demonstrating the potential of the pathway for therapeutic targeting [245]. Similarly, Ji et al. found that nitrogen dioxide exposure exacerbated airway inflammation via JAK/STAT activation, highlighting its role in systemic inflammation and respiratory pathophysiology [246]. In addition, Mobasher et al. reported that Annona squamosa extract exhibited protective effects against testicular injury by modulating JAK-1/STAT-3/SOCS-1 signalling, suggesting a potential pharmacological intervention against contaminant-induced toxicity [247]. Furthermore, Kim et al. provided evidence that prenatal exposure to bisphenol compounds induces epigenetic modifications, specifically DNA methylation, which may alter immune function via JAK/STAT signalling [248]. These findings highlight the need for further research into epigenetic regulation as a mediating factor in contaminant-induced immune dysregulation.
In conclusion, these studies highlight the importance of understanding the effect of pollutants on JAK/STAT signalling in order to assess their impact on gut microbiome health and immune dysregulation. Given the increasing prevalence of pollution-related diseases, targeted therapeutic strategies aimed at restoring microbial homeostasis and modulating JAK/STAT activity may offer novel approaches to mitigate pollutant-induced inflammatory and metabolic disorders.
Role of the TLR4/MyD88 pathway in environmental pollutant-induced immune dysregulation and inflammatory disorders. The Toll-like receptor 4 (TLR4)/myeloid differentiation primary response gene 88 (MyD88) signalling pathway has been extensively documented as a critical mediator in microbial sensing and regulation of inflammatory responses through innate immune activation [249]. These functions are closely linked to the gut microbiome and its interactions with environmental contaminants, which can alter the microbial composition and immune homeostasis. TLR4, a well-characterised pattern recognition receptor (PRR), is responsible for recognising microbial components such as lipopolysaccharides (LPS) from Gram-negative bacteria, triggering downstream immune signalling via the MyD88 adaptor protein, ultimately leading to nuclear factor kappa B (NF-κB) activation and pro-inflammatory cytokine release [250]. Within the gastrointestinal tract, this pathway plays a fundamental role in maintaining immune balance, regulating microbial diversity and modulating inflammatory responses to external stressors [185].
Exposure to environmental pollutants, including fine PM2.5, heavy metals, POPs, and EDCs, has been shown to disrupt the gut microbiome, leading to dysbiosis and aberrant immune activation [13, 109]. Dysbiotic changes, characterised by an increased abundance of pro-inflammatory bacterial taxa and a reduction in beneficial microbial populations, have been shown to lead to overactivation of the TLR4/MyD88 signalling cascade, triggering chronic low-grade inflammation and oxidative stress [251]. This inflammatory state has been implicated in the pathogenesis of numerous diseases, including gastrointestinal disorders (e.g. inflammatory bowel disease and colorectal cancer) [252, 253], metabolic syndromes (e.g. type 2 diabetes and obesity) [254], and systemic inflammatory conditions (e.g. cardiovascular disease and neurodegenerative disorders) [255, 256]. In addition, pollutants have been shown to increase intestinal permeability by compromising tight junction integrity, facilitating endotoxin translocation and systemic immune activation, further exacerbating disease progression [190].
Recent research highlights the central role of the TLR4/MyD88 pathway in mediating the inflammatory effects of environmental pollutants, particularly PM2.5, heavy metal contaminants, and wastewater-derived pollutants, which have been shown to have profound effects on both gastrointestinal and respiratory health [257-259]. Specifically, Wu et al. reported that PM2.5 exposure-induced lung epithelial injury was exacerbated by TLR4/MyD88-mediated autophagy dysregulation, linking air pollutants to lung inflammation [258]. Similarly, Liu et al. found that exposure to reclaimed water induced acute lung inflammation via the TLR4/MyD88 pathway, further highlighting the role of microbial sensing in pollution-induced respiratory disease [259]. In addition, Wu et al. demonstrated that astragaloside IV, a bioactive compound, alleviated PM2.5-induced lung injury by modulating the TLR4/MyD88/NF-κB pathway, offering potential therapeutic interventions for pollution-induced inflammatory diseases [258]. Further investigations have extended these findings, highlighting the role of the TLR4/MyD88 axis in pollutant-induced immune dysregulation across multiple organ systems. He et al. found that urban PM2.5 exacerbates allergic lung inflammation via the TLR2/TLR4/MyD88 pathway, leading to airway hyperresponsiveness, a hallmark of asthma [257]. In addition, Wang et al. demonstrated that PM2.5 exposure exacerbated airway inflammation in asthmatic mice by activating NF-κB through MyD88 signalling, further supporting the involvement of TLR4/MyD88 in pollution-induced lung pathology [260].
Emerging evidence suggests that targeting the TLR4/MyD88 pathway may serve as a potential therapeutic approach to mitigate pollutant-induced immune dysregulation. For example, phytochemicals, such as curcumin, resveratrol, and epigallocatechin gallate (EGCG), have been shown to downregulate TLR4/MyD88-mediated inflammation, offering promising avenues for clinical intervention [261-263]. In addition, probiotic supplementation aimed at restoring gut microbial homeostasis has been explored as a strategy to reduce pollutant-induced activation of TLR4/MyD88 signalling, thereby modulating immune responses and alleviating inflammation-related diseases [264].
Collectively, these studies underscore the critical role of the TLR4/MyD88 pathway in environmental pollutant-induced inflammation and highlight its involvement in a range of health disorders, from gastrointestinal and respiratory diseases to metabolic and hepatic dysfunction. Future research should focus on identifying novel biomarkers of pollutant-induced immune activation and developing targeted interventions to modulate TLR4/MyD88 signalling, ultimately improving public health outcomes in polluted environments.
Role of PPAR-γ in gut homeostasis and immune regulation under environmental pollutant-induced suppression. Peroxisome proliferator-activated receptor gamma (PPAR-γ) is a nuclear receptor that serves as a critical regulator of numerous physiological processes, including immune modulation, regulation of inflammatory responses, lipid and glucose metabolism, and maintenance of gut homeostasis [265]. In particular, its role in promoting anti-inflammatory pathways and modulating the gut microbial composition is essential for maintaining gut health and immune balance. PPAR-γ exerts its effects by controlling the differentiation of regulatory T cells (Tregs), suppressing the production of pro-inflammatory cytokines, and promoting the growth of beneficial gut bacteria, such as those involved in the production of SCFAs, including butyrate [266].
The downregulation of PPAR-γ by contaminant exposure has profound implications for gut health, particularly through its effects on intestinal immune homeostasis and epithelial barrier integrity [267]. PPAR-γ plays a central role in modulating the immune system by regulating the balance between pro- and anti-inflammatory responses within gut-associated lymphoid tissue (GALT), preserving epithelial integrity, and inhibiting excessive immune activation in response to microbial antigens [268]. When PPAR-γ activity is suppressed, the resulting immune dysregulation predisposes individuals to chronic low-grade inflammation, which can drive changes in the gut microbiome and promote dysbiosis [269]. This dysbiotic state is often characterised by an overgrowth of pathobionts (Escherichia coli, Clostridium difficile) and a reduction in beneficial microbes, such as Bifidobacterium and Faecalibacterium, which produce SCFAs that are essential for maintaining gut barrier function and immune homeostasis [270]. SCFAs, particularly butyrate, serve as energy substrates for intestinal epithelial cells, strengthen tight junction proteins (occludin, claudin-1), and suppress inflammation via epigenetic modifications affecting NF-κB signalling [271, 272].
In addition to altering the composition of the gut microbiota, PPAR-γ downregulation impairs immune tolerance mechanisms, rendering the gut more susceptible to chronic inflammation, increased intestinal permeability (also known as "leaky gut"), and gastrointestinal disorders, such as inflammatory bowel disease and irritable bowel syndrome [273, 274]. Furthermore, disruption of PPAR-γ signalling has been shown to increase intestinal permeability by compromising tight junction integrity, leading to endotoxaemia, a condition in which bacterial endotoxins such as lipopolysaccharides translocate from the intestinal lumen into the systemic circulation, exacerbating immune activation and systemic inflammation [275, 276]. This process has been implicated in the pathogenesis of metabolic disorders, such as obesity, type 2 diabetes mellitus, and non-alcoholic fatty liver disease (NAFLD), as well as neuroinflammatory diseases, such as Parkinson's and Alzheimer's [277].
In summary, accumulating evidence underscores the central role of PPAR-γ in regulating immune responses, maintaining gut microbiota homeostasis, and preserving gut barrier integrity. The pollutant-induced downregulation of PPAR-γ represents a critical molecular mechanism underlying dysbiosis, chronic inflammation, and increased disease susceptibility in multiple organ systems. Further research is warranted to identify novel biomarkers of PPAR-γ suppression and develop targeted interventions to mitigate environmental pollutant-induced gut and systemic inflammation, ultimately improving public health outcomes.
Interplay between gut dysbiosis, oxidative stress, and immune dysregulation
Dysbiosis, defined as an imbalance in the composition and function of the gut microbiome, has been shown to trigger a number of interrelated mechanisms that contribute to oxidative stress, chronic inflammation, and metabolic dysfunction [18]. Changes in microbial populations have been observed to result in the overproduction of potentially harmful metabolites, including trimethylamine N-oxide (TMAO), hydrogen sulphide (H₂S), and secondary bile acids, which have the ability to stimulate ROS production in epithelial cells [278, 279]. Table 2 considers the impact of environmental pollutants on the gut microbiota, with particular reference to the mechanisms of dysbiosis and the resulting health effects observed in both animal models and human studies.

Table 2: Effects of environmental pollutants on the gut microbiota: mechanisms of dysbiosis and health effects in animal models and human studies
The development of chronic inflammation is often associated with persistent oxidative stress and dysbiosis, with pro-inflammatory cytokines (e.g. IL-6, IL-1β, TNF-α) playing a central role in immune dysregulation [293]. Exposure to environmental pollutants, such as PM2.5, toxic metals (e.g. cadmium, lead, mercury), and EDCs (e.g. bisphenol A, dioxins, phthalates), activates intracellular signalling pathways, including NF-κB, JAK/STAT, and MAPK, leading to upregulation of cytokines and inflammatory mediators [294]. This sustained inflammatory response promotes immune dysregulation, establishing a cycle of tissue damage, oxidative stress, and chronic immune activation [294]. Dysbiosis exacerbates this effect by altering antigen presentation, reducing regulatory T-cell (Treg) activity, and impairing immune tolerance, thereby increasing susceptibility to autoimmune disease [295].
Mitochondrial dysfunction is a major consequence of oxidative stress and contributes to systemic metabolic disorders, such as obesity, type 2 diabetes, and non-alcoholic fatty liver disease, through impaired electron transport chain function, impaired ATP production, and increased ROS accumulation. This inefficiency affects cellular energy requirements and contributes to insulin resistance, lipid dysregulation, and altered energy homeostasis [296-298].
The intestinal barrier is critical for maintaining intestinal homeostasis by preventing the translocation of harmful substances and pathogens into the systemic circulation. This barrier is primarily maintained by tight junction proteins, including occludin, claudins, and zonula occludens-1 (ZO-1), which form a selective seal between epithelial cells [190, 299]. However, disruption of these tight junctions, often triggered by oxidative stress, pro-inflammatory cytokines, microbial dysbiosis, and exposure to contaminants, leads to increased intestinal permeability, commonly referred to as 'leaky gut' [190, 275]. When the epithelial barrier is compromised, endotoxins such as LPS gain access to the bloodstream, triggering immune activation, endotoxaemia, and systemic inflammation. This persistent state of inflammation in turn exacerbates epithelial damage, reinforcing a damaging cycle of gut barrier dysfunction and chronic disease development [300, 301].
The systemic effects of gut barrier dysfunction have been extensively studied. Increased intestinal permeability and gut-derived inflammation have been directly linked to metabolic disorders, neurodegenerative diseases, and immune dysregulation [183]. Chronic low-grade inflammation resulting from impaired gut integrity disrupts insulin signalling pathways and lipid metabolism, thereby contributing to the pathogenesis of obesity, insulin resistance, and metabolic syndrome [302]. In addition, translocated bacterial endotoxins and circulating pro-inflammatory mediators have been implicated in the development of neuroinflammatory conditions, including Alzheimer's disease and Parkinson's disease, through mechanisms involving the gut-brain axis, microglial activation, and oxidative damage in neuronal cells [183, 303]. In addition, dysregulation of immune responses by gut-derived antigens promotes immune over-activation and loss of self-tolerance, increasing the risk of autoimmune diseases, e.g. inflammatory bowel disease, rheumatoid arthritis, and multiple sclerosis [304, 305]. Gut-derived inflammation also compromises the effectiveness of the host's immune defences, increasing susceptibility to systemic infections and chronic inflammatory conditions [306].
Thus, gut barrier dysfunction serves as a critical link between environmental exposures, gut dysbiosis, and the onset of systemic disease. Understanding the interplay between microbial imbalances, oxidative stress, and immune dysregulation is essential for the development of targeted therapeutic strategies, including microbiome-modulating interventions, antioxidant therapies, and dietary approaches, aimed at restoring gut homeostasis and mitigating pollutant-induced health risks.
Therapeutic strategies to restore gut homeostasis
Therapeutic strategies aimed at restoring gut homeostasis involve a multifaceted approach that includes microbiota modulation through probiotics, prebiotics, synbiotics, faecal microbiota transplantation (FMT), dietary interventions, immune modulation, and targeted antimicrobial therapies [180]. These interventions target the microbial composition, immune function, oxidative stress, and gut barrier integrity to counteract dysbiosis and its associated pathological consequences [307].
Probiotics, prebiotics, and synbiotics are key therapeutic agents that act synergistically to modulate the gut microbiota composition, enhance SCFA production, regulate inflammatory pathways, and strengthen epithelial barrier function [308]. Probiotics, which are live beneficial microorganisms (e.g. Bifidobacterium, Lactobacillus), facilitate microbial competition by colonising the gut and outcompeting pathogenic species [36, 309]. Prebiotics, including inulin and fructooligosaccharides, are non-digestible dietary fibres that selectively stimulate the growth of beneficial microbes and promote SCFA synthesis [310, 311]. SCFAs, particularly butyrate, acetate, and propionate, play a critical role in maintaining gut health by serving as an energy source for colonocytes, strengthening tight junctions, and modulating the immune response through inhibition of NF-κB signalling [31, 312].
In addition to their role in microbial modulation, probiotics and prebiotics contribute to the reduction of oxidative stress by enhancing antioxidant defence mechanisms [313]. For example, butyrate has been shown to suppress NF-κB activation and promote the expression of antioxidant enzymes, such as superoxide dismutase and catalase, thereby mitigating oxidative damage [314]. Probiotic strains such as Lactobacillus rhamnosus have also been shown to stimulate the production of glutathione, an important intracellular antioxidant that neutralises ROS and protects against inflammation-induced cell damage [315].
Polyphenols, i.e. naturally occurring antioxidants in fruits, vegetables, and tea, play a critical role in gut health by modulating microbial diversity and reducing oxidative stress [316]. These bioactive compounds are metabolised by the gut microbiota into secondary metabolites, which act as signalling molecules strengthening the integrity of the gut barrier and influencing immune responses [317]. Postbiotics, i.e. bioactive microbial-derived compounds (SCFAs, peptides, and extracellular vesicles), have emerged as promising therapeutic agents that regulate inflammatory pathways, improve epithelial barrier function, and modulate mitochondrial activity [318].
Faecal microbiota transplantation (FMT) is an advanced therapeutic strategy designed to restore gut homeostasis by reintroducing a diverse and functional microbiome from a healthy donor to a recipient [319]. This intervention has shown clinical efficacy in certain conditions, such as recurrent Clostridioides difficile infection and inflammatory bowel disease, by replenishing microbial diversity, reducing pathogen overgrowth, and enhancing SCFA production [320].
Finally, selective antibiotics and postbiotics play a role in modulating the microbial composition by reducing pathogenic bacterial loads while promoting beneficial microbial interactions. Precision antibiotics such as rifaximin have been used to selectively target harmful bacteria without significant disruption of the commensal microbiota, thereby preserving gut homeostasis [321, 322]. Emerging therapeutic avenues, including microbiome-based personalised medicine and engineered bacterial consortia, hold promise for optimising gut health and mitigating microbiota-associated diseases in the future [323].
These therapeutic interdependencies are illustrated in Fig. 7, highlighting the interrelated roles of probiotics, prebiotics, dietary components, and immunomodulatory agents in maintaining gut homeostasis. Future research should focus on refining these interventions to improve their clinical applicability, safety, and long-term efficacy in microbiome-associated disorders.
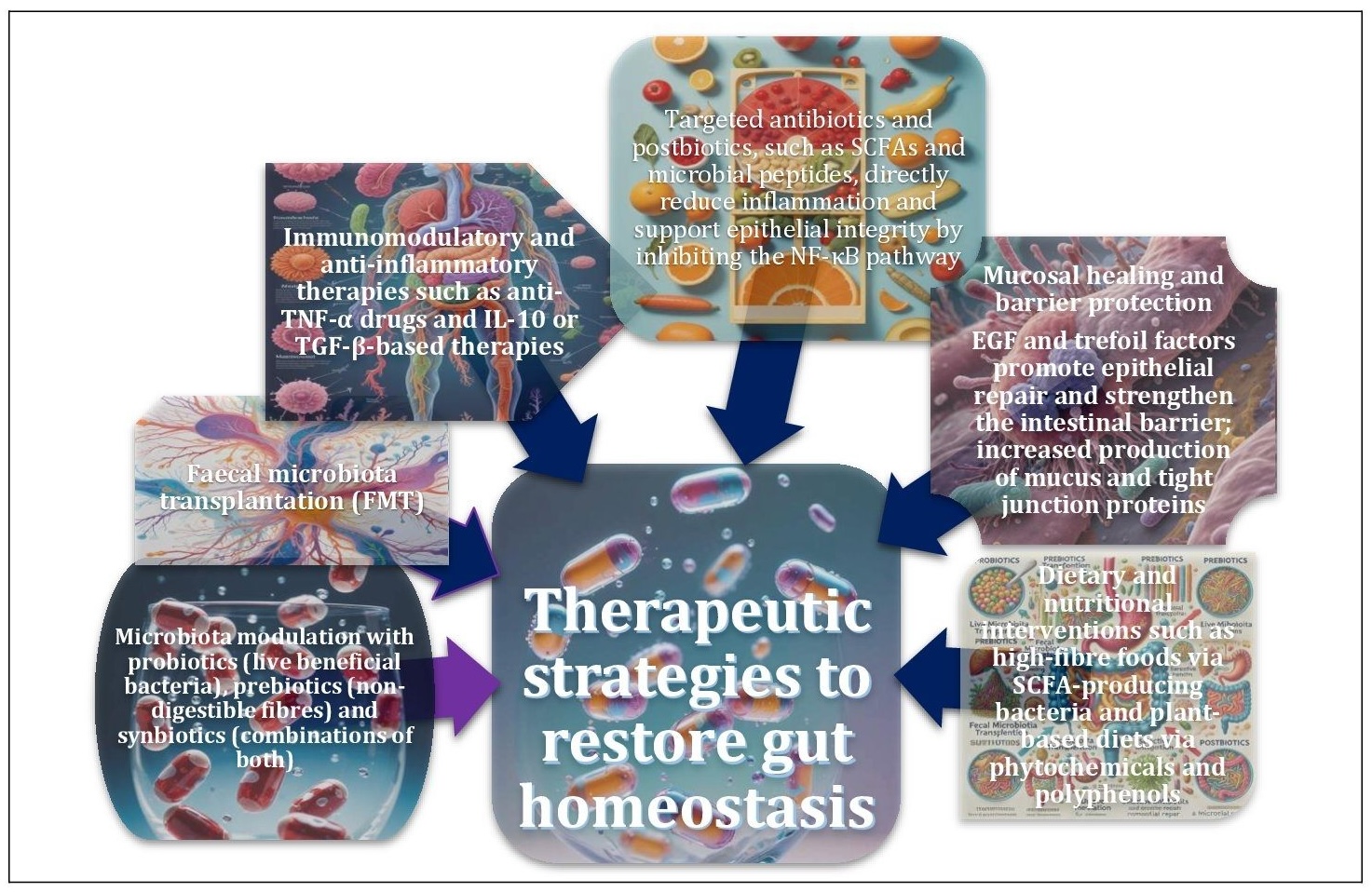
Fig. 7: Therapeutic strategies to restore intestinal homeostasis in the presence of contamination. Therapeutic strategies to restore gut homeostasis include probiotics, prebiotics, synbiotics, FMT, dietary interventions, immune modulation and targeted antibiotics. These strategies work in synergy to balance the microbiota, reduce inflammation and promote gut health.
Role of antioxidants in protecting gut microbiota from oxidative stress induced by environmental pollutants
Antioxidants play a critical role in protecting the gut microbiota from oxidative stress induced by environmental pollutants [324]. Antioxidants exert their protective effects on gut microbiota health through several molecular pathways. One key pathway is the activation of Nrf2, which induces the expression of antioxidant enzymes, such as superoxide dismutase and glutathione peroxidase. These enzymes play an important role in alleviating oxidative stress caused by pollutants [325, 326]. In addition, antioxidants have been shown to suppress the NF-κB pathway, thereby reducing the production of pro-inflammatory cytokines, such as TNF-α and IL-6, which can disrupt the gut microbiome [327, 328]. Antioxidants support the phosphoinositide 3-kinase/Akt (PI3K/Akt) pathway, which promotes cell survival and resistance to oxidative damage by stabilising tight junction proteins and maintaining the integrity of the intestinal barrier, thereby preventing microbial dysbiosis [329]. By preserving gut barrier function and microbial diversity, antioxidants promote the production of SCFAs, which are essential for gut health [312]. These processes not only reduce oxidative stress and inflammation but also help maintain the balance of beneficial bacteria within the gut microbiota, improving the overall stability of the gut ecosystem. Table 3 provides a detailed summary of experimental models, key findings, and molecular mechanisms from recent studies showing the biological effects of environmental and dietary exposures on gut microbiota health.
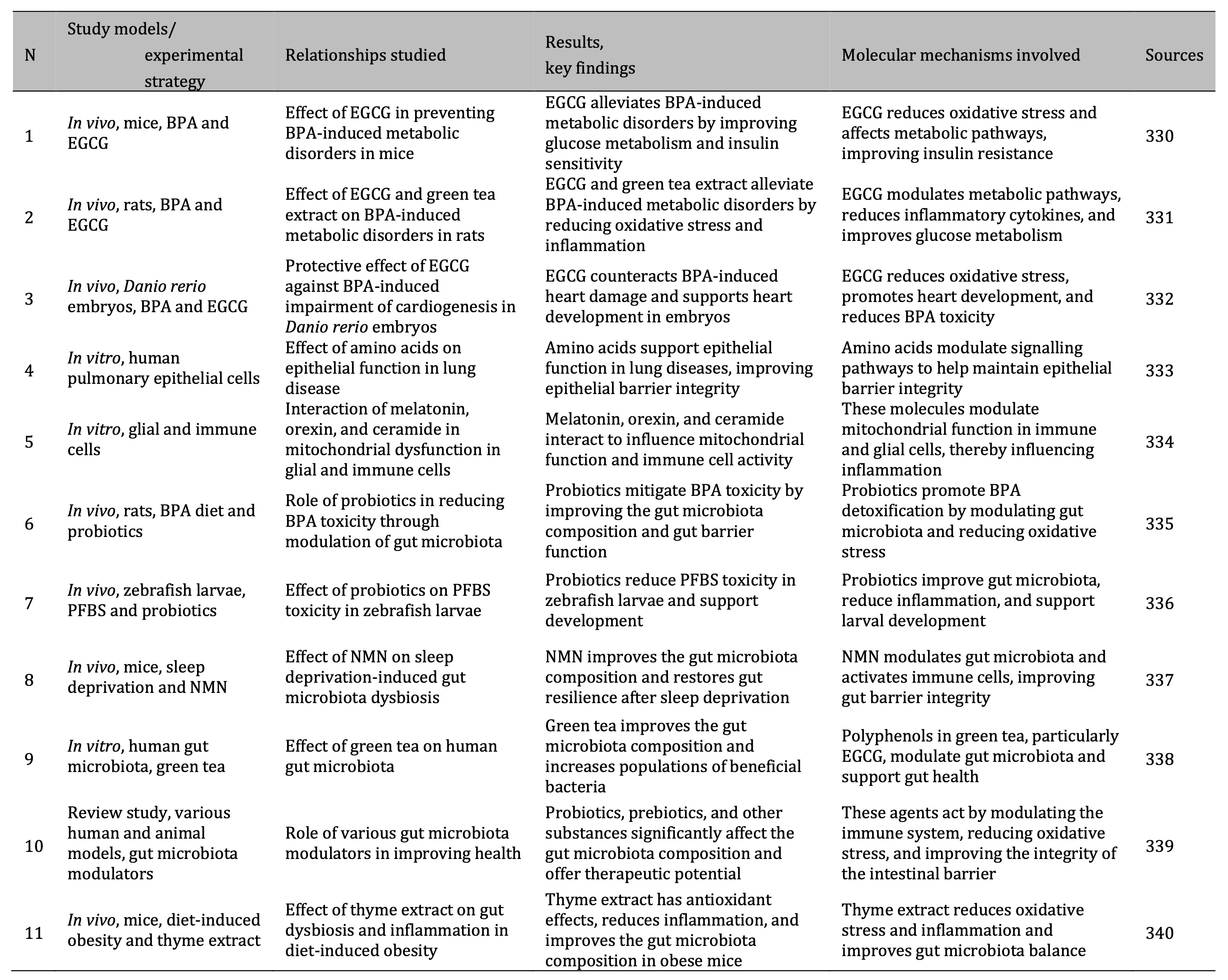
Table 3: Summary of research on environmental toxins, gut microbiota, protective interventions, key findings, and mechanisms in animal models and human studies. Notes: EGCG – epigallocatechin gallate; TNF-α – tumour necrosis factor-alpha; BPA – bisphenol A; PPAR – peroxisome proliferator-activated receptor; 16S rRNA – 16S ribosomal RNA; MS – multiple sclerosis; NMN – Nicotinamide mononucleotide, a precursor of NAD+ which plays a role in cellular energy metabolism and repair processes; CCA – Canonical Correlation Analysis; PFBS – Perfluorobutane sulphonate, perfluoroalkyl substance.
Synthetic antioxidants, such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), are commonly used in food preservation and have been shown to reduce oxidative stress in a similar way to natural antioxidants [341]. In addition, N-acetylcysteine (NAC), a precursor of glutathione, enhances the body's intrinsic antioxidant defences and supports gut microbiota health under contaminant exposure [342, 343]. Polyphenols also promote the growth of beneficial microbes, such as Bifidobacterium and Lactobacillus, which contribute to antioxidant defences through the production of beneficial metabolites [344]. By reducing oxidative stress and inflammation, antioxidants positively impact gut barrier integrity, promote microbial diversity, and counteract the adverse effects of environmental pollutants on gut health [141, 324].
Individual variability in microbiota responses to environmental contaminants
A critical area for future research is to understand the individual variability in microbiota responses to environmental contaminants. Genetic factors, including polymorphisms in detoxification enzymes and genes involved in the regulation of immune responses, play a central role in determining the resilience or susceptibility of the microbiome to such environmental insults [345, 346]. These genetic variations can significantly influence the ability of the microbiota to cope with oxidative stress and other forms of damage caused by pollutants [346]. In addition, lifestyle factors (diet, physical activity, and stress levels) have been shown to modulate the microbial composition and functionality, further influencing the microbiota response to pollutant exposure [30]. For example, the diet can either enhance or impair the gut's ability to detoxify pollutants [109], while physical activity and stress can alter gut permeability and immune system function, affecting microbial diversity and activity [347, 348].
To fully understand the mechanisms underlying these responses, it is essential to unravel the complex interplay between genetic and environmental determinants, as this will allow the identification of vulnerable populations who may require targeted interventions. Personalised healthcare approaches that take into account both genetic predisposition and environmental exposures will be crucial to improve the efficacy of interventions aimed at protecting gut microbiota health [349]. Emerging multi-omics techniques, such as metagenomics, transcriptomics, and metabolomics, hold great promise for advancing our understanding of these interactions. These cutting-edge approaches allow comprehensive analysis of the microbiome at multiple levels (DNA, RNA, and metabolites) and have the potential to provide new insights into influence of environmental factors on microbial ecosystems and associated health outcomes [350, 351]. As these technologies evolve, they are likely to uncover novel biomarkers and therapeutic targets that can inform the development of more effective personalised strategies to mitigate the health effects of pollutant exposure.
Innovations in microbiota-targeted therapies
Innovations in microbiota-targeted therapies represent a promising approach to mitigate pollutant-induced dysbiosis. Precision-based interventions, such as personalised probiotics and prebiotics, aim to restore microbial balance by tailoring treatments to individual microbiome profiles. These personalised strategies aim to optimise the microbial composition, enhance gut barrier integrity, and improve metabolic functions based on the unique characteristics of the individual's microbiota [352, 353]. In addition, synthetic biology approaches, which involve the development of engineered microbes designed to detoxify pollutants or produce specific metabolites, offer novel therapeutic opportunities. These engineered microbes can potentially be used to degrade environmental toxins or enhance the production of beneficial metabolites, e.g. short-chain fatty acids, which support gut health and reduce inflammatory responses [354].
However, despite the promise of these interventions, the long-term effects of pollutant exposure on both the microbiota and host health remain poorly understood. The chronic effects of pollutant exposure on microbial dynamics, host immunity, and metabolic processes are not fully understood, and existing studies often focus on short-term outcomes. Longitudinal studies are therefore essential to assess the impact of prolonged exposure to environmental pollutants on microbial diversity, functionality, and resulting health outcomes over time. Such studies would provide critical insights into the cumulative effects of pollutants on the microbiome and its role in disease development, paving the way for the development of more effective and sustainable microbiota-targeted therapies.
Conclusion
This review emphasises the significant impact of anthropogenic factors on human health and well-being by exploring the complex relationship between the gut microbiota and environmental pollutants. It highlights how exposure to environmental pollutants, such as toxic metals and microplastics, disrupts microbial homeostasis, with consequences that extend beyond the gastrointestinal tract, affecting systemic inflammation, cardiovascular health, and neurodegenerative diseases. These pollutants can alter the microbial composition and functionality, leading to an imbalance that can lead to chronic health conditions. A particularly worrying factor discussed in the review is the role of antibiotic resistance. The environmental spread of antibiotic resistance genes through mobile genetic elements exacerbates the negative effects of pollutants on microbial ecosystems, potentially leading to the proliferation of resistant pathogens. Furthermore, the interplay between pollutant-induced redox imbalances and gut dysbiosis further complicates health risks. This disruption compromises the integrity of the gut barrier, promotes chronic inflammation, and induces oxidative stress, all of which are central to the development of systemic disease. Alterations in microbial populations can disrupt gut immune functions, leading to compromised defence against both environmental toxins and pathogens.
The key molecular pathways, including NF-κB, Nrf2/Keap1, PI3K/AKT, p38-MAPK, JAK/STAT, and TLR4/MyD88, have been identified as central regulators in these processes. These pathways are crucial in modulating inflammation, immune responses, and antioxidant mechanisms. They provide valuable insights into the molecular mechanisms underlying the effects of pollutant-induced oxidative stress and inflammation. Understanding these pathways is essential for the development of effective therapeutic interventions that target molecular and cellular processes that contribute to microbial dysbiosis and associated health problems.
Therapeutic strategies aimed at mitigating the adverse effects of dysbiosis and oxidative stress have been shown to focus on antioxidants, microbial modulation, and advanced therapeutic approaches aimed at restoring gut homeostasis. Such strategies may include the use of prebiotics, probiotics, and bioactive compounds to support beneficial microbiota, alongside targeted antioxidant treatments to reduce oxidative damage. However, significant knowledge gaps remain, particularly regarding the variability of individual microbiota responses to pollutants and the long-term efficacy of proposed interventions. Individualised treatment plans based on personalised microbiome analysis may prove essential in optimising therapeutic outcomes. By investigating the interactions of pollutants with the microbiome at both the individual and ecological level, researchers can gain critical insights into the broader health effects of environmental pollution. There is also an urgent need to develop targeted therapeutics tailored to individual microbiota profiles. This comprehensive approach is critical to mitigating the health risks of pollution and maintaining systemic well-being in an increasingly polluted world.
Acknowledgements
Natalia Kurhaluk, ORCID: https://orcid.org/0000-0002-4669-1092
Piotr Kamiński, ORCID: https://orcid.org/0000-0003-1978-6018
Halina Tkaczenko, ORCID: https://orcid.org/0000-0003-3951-9005
Disclosure Statement
The author have no competing interests to declare.
References
| 1 | Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity
and the environment. Exp. Suppl. 2012, 101, 133-164
https://doi.org/10.1007/978-3-7643-8340-4_6 |
| 2 | Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic
Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic.
Front. Pharmacol. 2021, 12, 643972
https://doi.org/10.3389/fphar.2021.643972 |
| 3 | Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H.
Persistent Organic Pollutants in Food: Contamination Sources, Health Effects and
Detection Methods. Int. J. Environ. Res. Public Health 2019, 16(22), 4361
https://doi.org/10.3390/ijerph16224361 |
| 4 | Montano, L.; Pironti, C.; Pinto, G.; Ricciardi, M.; Buono, A.; Brogna, C.;
Venier, M.; Piscopo, M.; Amoresano, A.; Motta, O. Polychlorinated Biphenyls
(PCBs) in the Environment: Occupational and Exposure Events, Effects on Human
Health and Fertility. Toxics 2022, 10(7), 365
https://doi.org/10.3390/toxics10070365 |
| 5 | Ziani, K.; Ioniță-Mîndrican, C.B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.;
Moroșan, E.; Drăgănescu, D.; Preda, O.T. Microplastics: A Real Global Threat for
Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15(3),
617
https://doi.org/10.3390/nu15030617 |
| 6 | Ghosh, S.; Sinha, J.K.; Ghosh, S.; Vashisth, K.; Han, S.; Bhaskar, R.
Microplastics as an Emerging Threat to the Global Environment and Human Health.
Sustainability 2023, 15, 10821
https://doi.org/10.3390/su151410821 |
| 7 | Zhang, W.; Guo, R.; Yang, Y.; Ding, J.; Zhang, Y. Long-term effect of
heavy-metal pollution on diversity of gastrointestinal microbial community of
Bufo raddei. Toxicol. Lett. 2016, 258, 192-197
https://doi.org/10.1016/j.toxlet.2016.07.003 |
| 8 | Richardson, J.B.; Dancy, B.C.R.; Horton, C.L.; Lee, Y.S.; Madejczyk, M.S.; Xu,
Z.Z.; Ackermann, G.; Humphrey, G.; Palacios, G.; Knight, R.; Lewis, J.A.
Exposure to toxic metals triggers unique responses from the rat gut microbiota.
Sci. Rep. 2018, 8(1), 6578
https://doi.org/10.1038/s41598-018-24931-w |
| 9 | Assefa, S.; Köhler, G. Intestinal Microbiome and Metal Toxicity. Curr. Opin.
Toxicol. 2020, 19, 21-27
https://doi.org/10.1016/j.cotox.2019.09.009 |
| 10 | Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ştefan, M.G.; Loghin, F. Antibiotics
in the environment: causes and consequences. Med. Pharm. Rep. 2020, 93(3),
231-240
https://doi.org/10.15386/mpr-1742 |
| 11 | Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali,
S.; Yadav, M.; Kumari, R.; Singh, S.; Mohapatra, A.; Pandey, V.; Rana, N.;
Cunill, J.M. Current status of pesticide effects on environment, human health
and it's eco-friendly management as bioremediation: A comprehensive review.
Front. Microbiol. 2022, 13, 962619
https://doi.org/10.3389/fmicb.2022.962619 |
| 12 | Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture
and Its Consequential Resistance in Environmental Sources: Potential Public
Health Implications. Molecules 2018, 23(4), 795
https://doi.org/10.3390/molecules23040795 |
| 13 | Singh, S.; Sharma, P.; Pal, N.; Kumawat, M.; Shubham, S.; Sarma, D.K.; Tiwari,
R.R.; Kumar, M.; Nagpal, R. Impact of Environmental Pollutants on Gut Microbiome
and Mental Health via the Gut-Brain Axis. Microorganisms 2022, 10(7), 1457
https://doi.org/10.3390/microorganisms10071457 |
| 14 | Rio, P.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Pollutants, microbiota and
immune system: frenemies within the gut. Front. Public Health 2024, 12, 1285186
https://doi.org/10.3389/fpubh.2024.1285186 |
| 15 | Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy,
K. Gut microbiota functions: metabolism of nutrients and other food components.
Eur. J. Nutr. 2018, 57(1), 1-24
https://doi.org/10.1007/s00394-017-1445-8 |
| 16 | Afzal, S.; Abdul, Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.;
Alhojaily, S.M. From imbalance to impairment: the central role of reactive
oxygen species in oxidative stress-induced disorders and therapeutic
exploration. Front. Pharmacol. 2023, 14, 1269581
https://doi.org/10.3389/fphar.2023.1269581 |
| 17 | Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen
species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20(7),
1126-1167
https://doi.org/10.1089/ars.2012.5149 |
| 18 | Mostafavi Abdolmaleky, H.; Zhou, J.-R. Gut Microbiota Dysbiosis, Oxidative
Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases.
Antioxidants 2024, 13, 985
https://doi.org/10.3390/antiox13080985 |
| 19 | Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World
J. Gastroenterol. 2021, 27(25), 3837-3850
https://doi.org/10.3748/wjg.v27.i25.3837 |
| 20 | Asadi, A.; Shadab Mehr, N.; Mohamadi, M.H.; Shokri, F.; Heidary, M.;
Sadeghifard, N.; Khoshnood, S. Obesity and gut-microbiota-brain axis: A
narrative review. J. Clin. Lab. Anal. 2022, 36(5), e24420
https://doi.org/10.1002/jcla.24420 |
| 21 | Saad, M.J.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to
Obesity and Insulin Resistance. Physiology (Bethesda) 2016, 31(4), 283-93
https://doi.org/10.1152/physiol.00041.2015 |
| 22 | Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko,
N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020,
51, 102590
https://doi.org/10.1016/j.ebiom.2019.11.051 |
| 23 | Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut Microbiota: An Important Player in Type 2
Diabetes Mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 834485
https://doi.org/10.3389/fcimb.2022.834485 |
| 24 | Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya,
J.B.; Wei, L.; Li, J.; Chen, Z.S. Microbiota in health and diseases. Signal
Transduct. Target. Ther. 2022, 7(1), 135
https://doi.org/10.1038/s41392-022-00974-4 |
| 25 | Yoo, J.Y.; Sniffen, S.; McGill Percy, K.C.; Pallaval, V.B.; Chidipi, B. Gut
Dysbiosis and Immune System in Atherosclerotic Cardiovascular Disease (ACVD).
Microorganisms 2022, 10, 108
https://doi.org/10.3390/microorganisms10010108 |
| 26 | Nesci, A.; Carnuccio, C.; Ruggieri, V.; D'Alessandro, A.; Di Giorgio, A.;
Santoro, L.; Gasbarrini, A.; Santoliquido, A.; Ponziani, F.R. Gut Microbiota and
Cardiovascular Disease: Evidence on the Metabolic and Inflammatory Background of
a Complex Relationship. Int. J. Mol. Sci. 2023, 24(10), 9087
https://doi.org/10.3390/ijms24109087 |
| 27 | Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E.
Environmental and Health Impacts of Air Pollution: A Review. Front. Public
Health 2020, 8, 14
https://doi.org/10.3389/fpubh.2020.00014 |
| 28 | Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.;
Nageshwar Reddy, D. Role of the normal gut microbiota. World J Gastroenterol.
2015, 21(29), 8787-8803
https://doi.org/10.3748/wjg.v21.i29.8787 |
| 29 | Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight,
R. Current understanding of the human microbiome. Nat. Med. 2018, 24(4), 392-400
https://doi.org/10.1038/nm.4517 |
| 30 | Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and
human health. Nutrients 2014, 7(1), 17-44
https://doi.org/10.3390/nu7010017 |
| 31 | Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.;
Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty
Acids and Human Health: From Metabolic Pathways to Current Therapeutic
Implications. Life 2024, 14, 559
https://doi.org/10.3390/life14050559 |
| 32 | Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.;
Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; Cammarota, G.; Ianiro, G.
Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut
Microbiota. Nutrients 2023, 15(9), 2211
https://doi.org/10.3390/nu15092211 |
| 33 | Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the
Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49
https://doi.org/10.3389/fnins.2018.00049 |
| 34 | Bonaz, B.; Sinniger, V.; Pellissier, S. The Vagus Nerve in the Neuro-Immune
Axis: Implications in the Pathology of the Gastrointestinal Tract. Front.
Immunol. 2017, 8, 1452
https://doi.org/10.3389/fimmu.2017.01452 |
| 35 | Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.;
Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; Lugli,
G.A.; Rodriguez, J.M.; Bode, L.; de Vos, W.; Gueimonde, M.; Margolles, A.; van
Sinderen, D.; Ventura, M. The First Microbial Colonizers of the Human Gut:
Composition, Activities, and Health Implications of the Infant Gut Microbiota.
Microbiol. Mol. Biol. Rev. 2017, 81(4), e00036-17
https://doi.org/10.1128/MMBR.00036-17 |
| 36 | Turroni, F.; Milani, C.; Duranti, S.; Lugli, G.A.; Bernasconi, S.; Margolles,
A.; Di Pierro, F.; van Sinderen, D.; Ventura, M. The infant gut microbiome as a
microbial organ influencing host well-being. Ital. J. Pediatr. 2020, 46(1), 16
https://doi.org/10.1186/s13052-020-0781-0 |
| 37 | Mayer, E.A. Gut feelings: the emerging biology of gut-brain communication. Nat.
Rev. Neurosci. 2011, 12(8), 453-466
https://doi.org/10.1038/nrn3071 |
| 38 | Taylor, S.L.; Simpson, J.L.; Rogers, G.B. The influence of early-life microbial
exposures on long-term respiratory health. Paediatr. Respir. Rev. 2021, 40,
15-23
https://doi.org/10.1016/j.prrv.2021.05.003 |
| 39 | Dera, N.; Kosińska-Kaczyńska, K.; Żeber-Lubecka, N.; Brawura-Biskupski-Samaha,
R.; Massalska, D.; Szymusik, I.; Dera, K.; Ciebiera, M. Impact of Early-Life
Microbiota on Immune System Development and Allergic Disorders. Biomedicines
2025, 13, 121
https://doi.org/10.3390/biomedicines13010121 |
| 40 | Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy,
N.C. Regulation of tight junction permeability by intestinal bacteria and
dietary components. J. Nutr. 2011, 141(5), 769-776
https://doi.org/10.3945/jn.110.135657 |
| 41 | Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current
and Future Perspectives. Front. Immunol. 2022, 13, 840245
https://doi.org/10.3389/fimmu.2022.840245 |
| 42 | Abdulqadir, R.; Engers, J.; Al-Sadi, R. Role of Bifidobacterium in Modulating
the Intestinal Epithelial Tight Junction Barrier: Current Knowledge and
Perspectives. Curr. Dev. Nutr. 2023, 7(12), 102026
https://doi.org/10.1016/j.cdnut.2023.102026 |
| 43 | Mo, C.; Lou, X.; Xue, J.; Shi, Z.; Zhao, Y.; Wang, F.; Chen, G. The influence of
Akkermansia muciniphila on intestinal barrier function. Gut Pathog. 2024, 16(1),
41
https://doi.org/10.1186/s13099-024-00635-7 |
| 44 | Ferreira-Halder, C.V.; Faria, A.V.S.; Andrade, S.S. Action and function of
Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin.
Gastroenterol. 2017, 31(6), 643-648
https://doi.org/10.1016/j.bpg.2017.09.011 |
| 45 | Mukherjee, A,.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the
phylogenetically diverse genus Eubacterium and their various contributions to
gut health. Gut Microbes 2020, 12(1), 1802866
https://doi.org/10.1080/19490976.2020.1802866 |
| 46 | Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact
of antibiotics on the human microbiome and consequences for host health.
Microbiologyopen 2022, 11(1), e1260
https://doi.org/10.1002/mbo3.1260 |
| 47 | Dahiya, D.; Nigam, P.S. Antibiotic-Therapy-Induced Gut Dysbiosis Affecting Gut
Microbiota-Brain Axis and Cognition: Restoration by Intake of Probiotics and
Synbiotics. Int. J. Mol. Sci. 2023, 24(4), 3074
https://doi.org/10.3390/ijms24043074 |
| 48 | Hookman, P.; Barkin, J.S. Clostridium difficile associated infection, diarrhea
and colitis. World J. Gastroenterol. 2009, 15(13), 1554-1580
https://doi.org/10.3748/wjg.15.1554 |
| 49 | Mirsepasi-Lauridsen, H.C.; Vallance, B.A.; Krogfelt, K.A.; Petersen, A.M.
Escherichia coli Pathobionts Associated with Inflammatory Bowel Disease. Clin.
Microbiol. Rev. 2019, 32(2), e00060-18
https://doi.org/10.1128/CMR.00060-18 |
| 50 | Zhang, Q.; Su, X.; Zhang, C.; Chen, W.; Wang, Y.; Yang, X.; Liu, D.; Zhang, Y.;
Yang, R. Klebsiella pneumoniae Induces Inflammatory Bowel Disease Through
Caspase-11-Mediated IL18 in the Gut Epithelial Cells. Cell. Mol. Gastroenterol.
Hepatol. 2023, 15(3), 613-632
https://doi.org/10.1016/j.jcmgh.2022.11.005 |
| 51 | Popa, G.L.; Papa, M.I. Salmonella spp. infection - a continuous threat
worldwide. Germs 2021, 11(1), 88-96
https://doi.org/10.18683/germs.2021.1244 |
| 52 | Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial
dysbiosis in the gut. Mucosal Immunol. 2017, 10(1), 18-26
https://doi.org/10.1038/mi.2016.75 |
| 53 | Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of
Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases.
Microorganisms 2021, 9(4), 697
https://doi.org/10.3390/microorganisms9040697 |
| 54 | Konstantinidis, T.; Tsigalou, C.; Karvelas, A.; Stavropoulou, E.; Voidarou, C.;
Bezirtzoglou, E. Effects of Antibiotics upon the Gut Microbiome: A Review of the
Literature. Biomedicines 2020, 8(11), 502
https://doi.org/10.3390/biomedicines8110502 |
| 55 | Porru, S.; Esplugues, A.; Llop, S.; Delgado-Saborit, J.M. The effects of heavy
metal exposure on brain and gut microbiota: A systematic review of animal
studies. Environ. Pollut. 2024, 348, 123732
https://doi.org/10.1016/j.envpol.2024.123732 |
| 56 | Zhu, Q.; Chen, B.; Zhang, F.; Zhang, B.; Guo, Y.; Pang, M.; Huang, L.; Wang, T.
Toxic and essential metals: metabolic interactions with the gut microbiota and
health implications. Front. Nutr. 2024, 11, 1448388
https://doi.org/10.3389/fnut.2024.1448388 |
| 57 | Feng, P.; Ye, Z.; Kakade, A.; Virk, A.K.; Li, X.; Liu, P. A Review on Gut
Remediation of Selected Environmental Contaminants: Possible Roles of Probiotics
and Gut Microbiota. Nutrients 2018, 11(1), 22
https://doi.org/10.3390/nu11010022 |
| 58 | Duan, H.; Yu, L.; Tian, F.; Zhai, Q.; Fan, L.; Chen, W. Gut microbiota: A target
for heavy metal toxicity and a probiotic protective strategy. Sci. Total
Environ. 2020, 742, 140429
https://doi.org/10.1016/j.scitotenv.2020.140429 |
| 59 | Djurasevic, S.; Jama, A.; Jasnic, N.; Vujovic, P.; Jovanovic, M.; Mitic-Culafic,
D.; Knezevic-Vukcevic, J.; Cakic-Milosevic, M.; Ilijevic, K.; Djordjevic, J. The
Protective Effects of Probiotic Bacteria on Cadmium Toxicity in Rats. J. Med.
Food 2017, 20(2), 189-196
https://doi.org/10.1089/jmf.2016.0090 |
| 60 | Majlesi, M.; Shekarforoush, S.S.; Ghaisari, H.R.; Nazifi, S.; Sajedianfard, J.;
Eskandari, M.H. Effect of Probiotic Bacillus Coagulans and Lactobacillus
Plantarum on Alleviation of Mercury Toxicity in Rat. Probiotics Antimicrob.
Proteins 2017, 9(3), 300-309
https://doi.org/10.1007/s12602-016-9250-x |
| 61 | Arun, K.B.; Madhavan, A.; Sindhu, R.; Emmanual, S.; Binod, P.; Pugazhendhi, A.;
Sirohi, R.; Reshmy, R.; Awasthi, M.K.; Gnansounou, E.; Pandey, A. Probiotics and
gut microbiome - Prospects and challenges in remediating heavy metal toxicity.
J. Hazard. Mater. 2021, 420, 126676
https://doi.org/10.1016/j.jhazmat.2021.126676 |
| 62 | Santiago, M.S.A.; Avellar, M.C.W.; Perobelli, J.E. Could the gut microbiota be
capable of making individuals more or less susceptible to environmental
toxicants? Toxicology 2024, 503, 153751
https://doi.org/10.1016/j.tox.2024.153751 |
| 63 | Teffera, M.; Veith, A.C.; Ronnekleiv-Kelly, S.; Bradfield, C.A.; Nikodemova, M.;
Tussing-Humphreys, L.; Malecki, K. Diverse mechanisms by which chemical
pollutant exposure alters gut microbiota metabolism and inflammation. Environ.
Int. 2024, 190, 108805
https://doi.org/10.1016/j.envint.2024.108805 |
| 64 | Bist, P.; Choudhary, S. Impact of Heavy Metal Toxicity on the Gut Microbiota and
Its Relationship with Metabolites and Future Probiotics Strategy: a Review.
Biol. Trace Elem. Res. 2022, 200(12), 5328-5350
https://doi.org/10.1007/s12011-021-03092-4 |
| 65 | Hills, R.D. Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.;
Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease.
Nutrients 2019, 11(7), 1613
https://doi.org/10.3390/nu11071613 |
| 66 | Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in
pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev.
2017, 279(1), 70-89
https://doi.org/10.1111/imr.12567 |
| 67 | Liu, H.; Zhang, H.; Yu, Q.; Zhang, S.; Tu, X.; Zhuang, F.; Fu, S. Lead induced
structural and functional damage and microbiota dysbiosis in the intestine of
crucian carp (Carassius auratus). Front. Microbiol. 2023, 14, 1239323
https://doi.org/10.3389/fmicb.2023.1239323 |
| 68 | Liu, H.; Qian, K.; Zhang, S.; Yu, Q.; Du, Y.; Fu, S. Lead exposure induces
structural damage, digestive stress, immune response and microbiota dysbiosis in
the intestine of silver carp (Hypophthalmichthys molitrix). Comp. Biochem.
Physiol. C Toxicol. Pharmacol. 2022, 262, 109464
https://doi.org/10.1016/j.cbpc.2022.109464 |
| 69 | Liu, Y.; Kang, W.; Liu, S.; Li, J.; Liu, J.; Chen, X.; Gan, F.; Huang, K. Gut
microbiota-bile acid-intestinal Farnesoid X receptor signaling axis orchestrates
cadmium-induced liver injury. Sci. Total Environ. 2022, 849, 157861
https://doi.org/10.1016/j.scitotenv.2022.157861 |
| 70 | Liu, X.; Kang, W.; Li, J.; Li, X.; Yang, P.; Shi, M.; Wang, Z.; Wang, Y.;
Medina, A.D.P.A.; Liu, D.; Zhu, F.; Shen, H.; Huang, K.; Chen, X.; Liu, Y.
Melatonin Ameliorates Cadmium-Induced Liver Fibrosis Via Modulating Gut
Microbiota and Bile Acid Metabolism. J. Pineal Res. 2024, 76(8), e70005
https://doi.org/10.1111/jpi.70005 |
| 71 | Bidashimwa, D.; Hoke, T.; Huynh, T.B.; Narkpitaks, N.; Priyonugroho, K.; Ha,
T.T.; Burns, A.; Weissman, A. Plastic pollution: how can the global health
community fight the growing problem? BMJ Glob. Health 2023, 8(Suppl. 3), e012140
https://doi.org/10.1136/bmjgh-2023-012140 |
| 72 | Fayshal, M.A. Current practices of plastic waste management, environmental
impacts, and potential alternatives for reducing pollution and improving
management. Heliyon 2024, 10(23), e40838
https://doi.org/10.1016/j.heliyon.2024.e40838 |
| 73 | Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres
from domestic washing machines: Effects of fabric type and washing conditions.
Mar. Pollut. Bull. 2016, 112(1-2), 39-45
https://doi.org/10.1016/j.marpolbul.2016.09.025 |
| 74 | Kibria, M.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic
Waste: Challenges and Opportunities to Mitigate Pollution and Effective
Management. Int. J. Environ. Res. 2023, 17(1), 20
https://doi.org/10.1007/s41742-023-00507-z |
| 75 | Lakhiar, I.A.; Yan, H.; Zhang, J.; Wang, G.; Deng, S.; Bao, R.; Zhang, C.; Syed,
T.N.; Wang, B.; Zhou, R.; et al. Plastic Pollution in Agriculture as a Threat to
Food Security, the Ecosystem, and the Environment: An Overview. Agronomy 2024,
14, 548
https://doi.org/10.3390/agronomy14030548 |
| 76 | Lin, Y.D.; Huang, P.H.; Chen, Y.W.; Hsieh, C.W.; Tain, Y.L.; Lee, B.H.; Hou,
C.Y.; Shih, M.K. Sources, Degradation, Ingestion and Effects of Microplastics on
Humans: A Review. Toxics 2023, 11(9), 747
https://doi.org/10.3390/toxics11090747 |
| 77 | Dube, E.; Okuthe, G.E. Plastics and Micro/Nano-Plastics (MNPs) in the
Environment: Occurrence, Impact, and Toxicity. Int. J. Environ. Res. Public
Health 2023, 20(17), 6667
https://doi.org/10.3390/ijerph20176667 |
| 78 | Tursi, A.; Baratta, M.; Easton, T.; Chatzisymeon, E.; Chidichimo, F.; De Biase,
M.; De Filpo, G. Microplastics in aquatic systems, a comprehensive review:
origination, accumulation, impact, and removal technologies. RSC Adv. 2022,
12(44), 28318-28340
https://doi.org/10.1039/D2RA04713F |
| 79 | Bora, S.S.; Gogoi, R.; Sharma, M.R.; Anshu, Borah, M.P.; Deka, P.; Bora, J.;
Naorem, R.S.; Das, J.; Teli, A.B. Microplastics and human health: unveiling the
gut microbiome disruption and chronic disease risks. Front. Cell. Infect.
Microbiol. 2024, 14, 1492759
https://doi.org/10.3389/fcimb.2024.1492759 |
| 80 | Zhang, K.; Yang, J.; Chen, L.; He, J.; Qu, D.; Zhang, Z.; Liu, Y.; Li, X.; Liu,
J.; Li, J.; Xie, X.; Wang, Q. Gut Microbiota Participates in Polystyrene
Microplastics-Induced Hepatic Injuries by Modulating the Gut-Liver Axis. ACS
Nano 2023, 17(15), 15125-15145
https://doi.org/10.1021/acsnano.3c04449 |
| 81 | Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative
Stress-Current Problems and Prospects. Antioxidants (Basel) 2024, 13(5), 579
https://doi.org/10.3390/antiox13050579 |
| 82 | Lang, T.; Jelić, F.; Wechselberger, C. From Cradle to Grave: Microplastics - A
Dangerous Legacy for Future Generations. Environments 2024, 11, 263
https://doi.org/10.3390/environments11120263 |
| 83 | Ke, D.; Zheng, J.; Liu, X.; Xu, X.; Zhao, L.; Gu, Y.; Yang, R.; Liu, S.; Yang,
S.; Du, J.; Chen, B.; He, G.; Dong, R. Occurrence of microplastics and
disturbance of gut microbiota: a pilot study of preschool children in Xiamen,
China. EBioMedicine 2023, 97, 104828
https://doi.org/10.1016/j.ebiom.2023.104828 |
| 84 | Amran, N.H.; Zaid, S.S.M.; Mokhtar, M.H.; Manaf, L.A.; Othman, S. Exposure to
Microplastics during Early Developmental Stage: Review of Current Evidence.
Toxics 2022, 10(10), 597
https://doi.org/10.3390/toxics10100597 |
| 85 | Zaheer, J.; Kim, H.; Ko, I.O.; Jo, E.K.; Choi, E.J.; Lee, H.J.; Shim, I.; Woo,
H.J.; Choi, J.; Kim, G.H.; Kim, J.S. Pre/post-natal exposure to microplastic as
a potential risk factor for autism spectrum disorder. Environ. Int. 2022, 161,
107121
https://doi.org/10.1016/j.envint.2022.107121 |
| 86 | Jing, J.; Zhang, L.; Han, L.; Wang, J.; Zhang, W.; Liu, Z.; Gao, A. Polystyrene
micro-/nanoplastics induced hematopoietic damages via the crosstalk of gut
microbiota, metabolites, and cytokines. Environ. Int. 2022, 161, 107131
https://doi.org/10.1016/j.envint.2022.107131 |
| 87 | Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on
the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019,
649, 308-317
https://doi.org/10.1016/j.scitotenv.2018.08.353 |
| 88 | Arias-Borrego, A.; Selma-Royo, M.; Collado, M.C.; Abril, N.; García-Barrera, T.
Impact of "chemical cocktails" exposure in shaping mice gut microbiota and the
role of selenium supplementation combining metallomics, metabolomics, and
metataxonomics. J. Hazard. Mater. 2022, 438, 129444
https://doi.org/10.1016/j.jhazmat.2022.129444 |
| 89 | Parra-Martínez, C.; Selma-Royo, M.; Callejón-Leblic, B.; Collado, M.C.; Abril,
N.; García-Barrera, T. Mice brain metabolomics after the exposure to a "chemical
cocktail" and selenium supplementation through the gut-brain axis. J. Hazard.
Mater. 2022, 438, 129443
https://doi.org/10.1016/j.jhazmat.2022.129443 |
| 90 | Sarubbo, F.; Cavallucci, V.; Pani, G. The Influence of Gut Microbiota on
Neurogenesis: Evidence and Hopes. Cells 2022, 11(3), 382
https://doi.org/10.3390/cells11030382 |
| 91 | Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan,
C.; Deng, J.; Yin, Y.; Deng, B. Impact of Gallic Acid on Gut Health: Focus on
the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol.
2020, 11, 580208
https://doi.org/10.3389/fimmu.2020.580208 |
| 92 | Zheng, P.; Zhang, B.; Zhang, K.; Lv, X.; Wang, Q.; Bai, X. The Impact of Air
Pollution on Intestinal Microbiome of Asthmatic Children: A Panel Study. Biomed.
Res. Int. 2020, 2020, 5753427
https://doi.org/10.1155/2020/5753427 |
| 93 | Naomi, R.; Yazid, M.D.; Bahari, H.; Keong, Y.Y.; Rajandram, R.; Embong, H.;
Teoh, S.H.; Halim, S.; Othman, F. Bisphenol A (BPA) Leading to Obesity and
Cardiovascular Complications: A Compilation of Current In vivo Study. Int. J.
Mol. Sci. 2022, 23(6), 2969
https://doi.org/10.3390/ijms23062969 |
| 94 | Zhou, Y.; Gui, L.; Wei, W.; Xu, E.G.; Zhou, W.; Sokolova, I.M.; Li, M.; Wang, Y.
Low particle concentrations of nanoplastics impair the gut health of medaka.
Aquat. Toxicol. 2023, 256, 106422
https://doi.org/10.1016/j.aquatox.2023.106422 |
| 95 | He, S.; Li, X.; Li, C.; Deng, H.; Shao, Y.; Yuan, L. Isoorientin attenuates
benzo [a]pyrene-induced colonic injury and gut microbiota disorders in mice.
Food Res. Int. 2019, 126, 108599
https://doi.org/10.1016/j.foodres.2019.108599 |
| 96 | Termite, F.; Archilei, S.; D'Ambrosio, F.; Petrucci, L.; Viceconti, N.;
Iaccarino, R.; Liguori, A.; Gasbarrini, A.; Miele, L. Gut Microbiota at the
Crossroad of Hepatic Oxidative Stress and MASLD. Antioxidants (Basel) 2025,
14(1), 56
https://doi.org/10.3390/antiox14010056 |
| 97 | Cheng, J.; Meistertzheim, A.L.; Leistenschneider, D.; Philip, L.; Jacquin, J.;
Escande, M.L.; Barbe, V.; Ter Halle, A.; Chapron, L.; Lartaud, F.; Bertrand, S.;
Escriva, H.; Ghiglione, J.F. Impacts of microplastics and the associated
plastisphere on physiological, biochemical, genetic expression and gut
microbiota of the filter-feeder amphioxus. Environ. Int. 2023, 172, 107750
https://doi.org/10.1016/j.envint.2023.107750 |
| 98 | Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji,
X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut
microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492
https://doi.org/10.1016/j.chemosphere.2019.125492 |
| 99 | Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and
Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020,
17(5), 1509
https://doi.org/10.3390/ijerph17051509 |
| 100 | Yuan, X.; Pan, Z.; Jin, C.; Ni, Y.; Fu, Z.; Jin, Y. Gut microbiota: An
underestimated and unintended recipient for pesticide-induced toxicity.
Chemosphere 2019, 227, 425-434
https://doi.org/10.1016/j.chemosphere.2019.04.088 |
| 101 | Giambò, F.; Teodoro, M.; Costa, C.; Fenga, C. Toxicology and Microbiota: How Do
Pesticides Influence Gut Microbiota? A Review. Int. J. Environ. Res. Public
Health 2021, 18, 5510
https://doi.org/10.3390/ijerph18115510 |
| 102 | Ueyama, J.; Hayashi, M.; Hirayama, M.; Nishiwaki, H.; Ito, M.; Saito, I.;
Tsuboi, Y.; Isobe, T.; Ohno, K. Effects of Pesticide Intake on Gut Microbiota
and Metabolites in Healthy Adults. Int. J. Environ. Res. Public Health 2022,
20(1), 213
https://doi.org/10.3390/ijerph20010213 |
| 103 | Ali, A.; AlHussaini, K.I. Pesticides: Unintended Impact on the Hidden World of
Gut Microbiota. Metabolites 2024, 14(3), 155
https://doi.org/10.3390/metabo14030155 |
| 104 | Meng, Z.; Liu, L.; Yan, S.; Sun, W.; Jia, M.; Tian, S.; Huang, S.; Zhou, Z.;
Zhu, W. Gut Microbiota: A Key Factor in the Host Health Effects Induced by
Pesticide Exposure? J. Agric. Food Chem. 2020, 68(39), 10517-10531
https://doi.org/10.1021/acs.jafc.0c04678 |
| 105 | Kulcsarova, K.; Bang, C.; Berg, D.; Schaeffer, E. Pesticides and the
Microbiome-Gut-Brain Axis: Convergent Pathways in the Pathogenesis of
Parkinson's Disease. J. Parkinsons Dis. 2023, 13(7), 1079-1106
https://doi.org/10.3233/JPD-230206 |
| 106 | Sharma, T.; Sirpu Natesh, N.; Pothuraju, R.; Batra, S.K.; Rachagani, S. Gut
microbiota: a non-target victim of pesticide-induced toxicity. Gut Microbes
2023, 15(1), 2187578
https://doi.org/10.1080/19490976.2023.2187578 |
| 107 | Bamicha, V.; Pergantis, P.; Drigas, A. The Effect of Gut Microbiome,
Neurotransmitters, and Digital Insights in Autism. Appl. Microbiol. 2024, 4,
1677-1701
https://doi.org/10.3390/applmicrobiol4040114 |
| 108 | Abou Diwan, M.; Djekkoun, N.; Boucau, M.C.; Corona, A.; Dehouck, L.; Biendo, M.;
Gosselet, F.; Bach, V.; Candela, P.; Khorsi-Cauet, H. Maternal exposure to
pesticides induces perturbations in the gut microbiota and blood-brain barrier
of dams and the progeny, prevented by a prebiotic. Environ. Sci. Pollut. Res.
Int. 2024, 31(49), 58957-58972
https://doi.org/10.1007/s11356-024-34969-1 |
| 109 | Chiu, K.; Warner, G.; Nowak, R.A.; Flaws, J.A.; Mei, W. The Impact of
Environmental Chemicals on the Gut Microbiome. Toxicol. Sci. 2020, 176(2),
253-284
https://doi.org/10.1093/toxsci/kfaa065 |
| 110 | Tu, P.; Xue, J.; Niu, H.; Tang, Q.; Mo, Z.; Zheng, X.; Wu, L.; Chen, Z.; Cai,
Y.; Wang, X. Deciphering Gut Microbiome Responses upon Microplastic Exposure via
Integrating Metagenomics and Activity-Based Metabolomics. Metabolites 2023,
13(4), 530
https://doi.org/10.3390/metabo13040530 |
| 111 | Peng, Z.; Liao, Y.; Yang, W.; Liu, L. Metal(loid)-gut microbiota interactions
and microbiota-related protective strategies: A review. Environ. Int. 2024, 192,
109017
https://doi.org/10.1016/j.envint.2024.109017 |
| 112 | Sofield, C.E.; Anderton, R.S.; Gorecki, A.M. Mind over Microplastics: Exploring
Microplastic-Induced Gut Disruption and Gut-Brain-Axis Consequences. Curr.
Issues Mol. Biol. 2024, 46(5), 4186-4202
https://doi.org/10.3390/cimb46050256 |
| 113 | Meli, R.; Monnolo, A.; Annunziata, C.; Pirozzi, C.; Ferrante, M.C. Oxidative
Stress and BPA Toxicity: An Antioxidant Approach for Male and Female
Reproductive Dysfunction. Antioxidants (Basel) 2020, 9(5), 405
https://doi.org/10.3390/antiox9050405 |
| 114 | Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome
and its metabolites in metabolic diseases. Protein Cell 2021, 12(5), 360-373
https://doi.org/10.1007/s13238-020-00814-7 |
| 115 | Larabi, A.B.; Masson, H.L.P.; Bäumler, A.J. Bile acids as modulators of gut
microbiota composition and function. Gut Microbes 2023, 15(1), 2172671
https://doi.org/10.1080/19490976.2023.2172671 |
| 116 | Safari, Z.; Gérard, P. The links between the gut microbiome and non-alcoholic
fatty liver disease (NAFLD). Cell. Mol. Life Sci. 2019, 76(8), 1541-1558
https://doi.org/10.1007/s00018-019-03011-w |
| 117 | Tilg, H.; Adolph, T.E.; Dudek, M.; Knolle, P. Non-alcoholic fatty liver disease:
the interplay between metabolism, microbes and immunity. Nat. Metab. 2021,
3(12), 1596-1607
https://doi.org/10.1038/s42255-021-00501-9 |
| 118 | Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2
Diabetes. Nutrients 2021, 14(1), 166
https://doi.org/10.3390/nu14010166 |
| 119 | Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the
Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7(6),
180
https://doi.org/10.3390/microorganisms7060180 |
| 120 | Dickinson, A.W.; Power, A.; Hansen, M.G.; Brandt, K.K.; Piliposian, G.; Appleby,
P.; O'Neill, P.A.; Jones, R.T.; Sierocinski, P.; Koskella, B.; Vos, M. Heavy
metal pollution and co-selection for antibiotic resistance: A microbial
palaeontology approach. Environ. Int. 2019, 132, 105117
https://doi.org/10.1016/j.envint.2019.105117 |
| 121 | Tuvo, B.; Scarpaci, M.; Bracaloni, S.; Esposito, E.; Costa, A.L.; Ioppolo, M.;
Casini, B. Microplastics and Antibiotic Resistance: The Magnitude of the Problem
and the Emerging Role of Hospital Wastewater. Int. J. Environ. Res. Public
Health 2023, 20(10), 5868
https://doi.org/10.3390/ijerph20105868 |
| 122 | Tang, K.H.D.; Li, R. Aged Microplastics and Antibiotic Resistance Genes: A
Review of Aging Effects on Their Interactions. Antibiotics (Basel) 2024, 13(10),
941
https://doi.org/10.3390/antibiotics13100941 |
| 123 | Anedda, E.; Farrell, M.L.; Morris, D.; Burgess, C.M. Evaluating the impact of
heavy metals on antimicrobial resistance in the primary food production
environment: A scoping review. Environ. Pollut. 2023, 320, 121035
https://doi.org/10.1016/j.envpol.2023.121035 |
| 124 | Gillieatt, B.F.; Coleman, N.V. Unravelling the mechanisms of antibiotic and
heavy metal resistance co-selection in environmental bacteria. FEMS Microbiol.
Rev. 2024, 48(4), fuae017
https://doi.org/10.1093/femsre/fuae017 |
| 125 | Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic
resistance and persistence-Implications for human health and treatment
perspectives. EMBO Rep. 2020, 21(12), e51034
https://doi.org/10.15252/embr.202051034 |
| 126 | Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.;
Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The
challenges and some emerging strategies for tackling a global menace. J. Clin.
Lab. Anal. 2022, 36(9), e24655
https://doi.org/10.1002/jcla.24655 |
| 127 | Woerther, P.L.; Burdet, C.; Chachaty, E.; Andremont, A. Trends in human fecal
carriage of extended-spectrum β-lactamases in the community: toward the
globalization of CTX-M. Clin. Microbiol. Rev. 2013, 26(4), 744-58
https://doi.org/10.1128/CMR.00023-13 |
| 128 | Liu, L.; Guang, S.B.; Xin, Y.; Li, J.; Lin, G.F.; Zeng, L.Q.; He, S.Q.; Zheng,
Y.M.; Chen, G.Y.; Zhao, Q.B. Antibiotic resistant genes profile in the surface
water of subtropical drinking water river-reservoir system. Environ. Pollut.
2023, 337, 122619
https://doi.org/10.1016/j.envpol.2023.122619 |
| 129 | Shao, S.; Hu, Y.; Cheng, J.; Chen, Y. Research progress on distribution,
migration, transformation of antibiotics and antibiotic resistance genes (ARGs)
in aquatic environment. Crit. Rev. Biotechnol. 2018, 38(8), 1195-1208
https://doi.org/10.1080/07388551.2018.1471038 |
| 130 | Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev.
Microbiol. 2022, 20(5), 257-269
https://doi.org/10.1038/s41579-021-00649-x |
| 131 | Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.;
Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global
Public Health. Healthcare (Basel) 2023, 11(13), 1946
https://doi.org/10.3390/healthcare11131946 |
| 132 | Czatzkowska, M.; Wolak, I.; Harnisz, M.; Korzeniewska, E. Impact of
Anthropogenic Activities on the Dissemination of ARGs in the Environment-A
Review. Int. J. Environ. Res. Public Health 2022, 19(19), 12853
https://doi.org/10.3390/ijerph191912853 |
| 133 | Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Yang, L.; Huang, Y.; Jia, J.; Dou,
T. Antibiotic resistance genes in bacteria: Occurrence, spread, and control. J.
Basic Microbiol. 2021, 61(12), 1049-1070
https://doi.org/10.1002/jobm.202100201 |
| 134 | Bhat, B.A.; Mir, R.A.; Qadri, H.; Dhiman, R.; Almilaibary, A.; Alkhanani, M.;
Mir, M.A. Integrons in the development of antimicrobial resistance: critical
review and perspectives. Front. Microbiol. 2023, 14, 1231938
https://doi.org/10.3389/fmicb.2023.1231938 |
| 135 | Flores-Vargas, G.; Bergsveinson, J.; Lawrence, J.R.; Korber, D.R. Environmental
Biofilms as Reservoirs for Antimicrobial Resistance. Front. Microbiol. 2021, 12,
766242
https://doi.org/10.3389/fmicb.2021.766242 |
| 136 | Nguyen, A.Q.; Vu, H.P.; Nguyen, L.N.; Wang, Q.; Djordjevic, S.P.; Donner, E.;
Yin, H.; Nghiem, L.D. Monitoring antibiotic resistance genes in wastewater
treatment: Current strategies and future challenges. Sci. Total Environ. 2021,
783, 146964
https://doi.org/10.1016/j.scitotenv.2021.146964 |
| 137 | Thiang, E.L.; Lee, C.W.; Takada, H.; Seki, K.; Takei, A.; Suzuki, S.; Wang A.;
Bong, C.W. Antibiotic residues from aquaculture farms and their ecological risks
in Southeast Asia: a case study from Malaysia. Ecosystem Health and
Sustainability, 2021, 7(1).
https://doi.org/10.1080/20964129.2021.1926337 |
| 138 | Yuan, X.; Lv, Z.; Zhang, Z.; Han, Y.; Liu, Z.; Zhang, H. A Review of
Antibiotics, Antibiotic Resistant Bacteria, and Resistance Genes in Aquaculture:
Occurrence, Contamination, and Transmission. Toxics 2023, 11(5), 420
https://doi.org/10.3390/toxics11050420 |
| 139 | Kim, M.; Park, J.; Kang, M.; Yang, J.; Park, W. Gain and loss of antibiotic
resistant genes in multidrug resistant bacteria: One Health perspective. J.
Microbiol. 2021, 59(6), 535-545
https://doi.org/10.1007/s12275-021-1085-9 |
| 140 | Ajayi, A.O.; Odeyemi, A.T.; Akinjogunla, O.J.; Adeyeye, A.B.; Ayo-Ajayi, I.
Review of antibiotic-resistant bacteria and antibiotic resistance genes within
the one health framework. Infect. Ecol. Epidemiol. 2024, 14(1), 2312953
https://doi.org/10.1080/20008686.2024.2312953 |
| 141 | Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.;
Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune
System. Biomedicines 2023, 11, 1388
https://doi.org/10.3390/biomedicines11051388 |
| 142 | Anetor, G.O.; Nwobi, N.L.; Igharo, G.O.; Sonuga, O.O.; Anetor, J.I.
Environmental Pollutants and Oxidative Stress in Terrestrial and Aquatic
Organisms: Examination of the Total Picture and Implications for Human Health.
Front. Physiol. 2022, 13, 931386
https://doi.org/10.3389/fphys.2022.931386 |
| 143 | Itziou, A.; Balis, V.; Lakioti, E.; Karayannis, V.; Tsanaktsidis, C.
Environmental Pollution and Oxidative Stress: Health Effects During Pregnancy: A
Review. Appl. Sci. 2024, 14, 9884
https://doi.org/10.3390/app14219884 |
| 144 | Poljšak, B.; Fink, R. The protective role of antioxidants in the defence against
ROS/RNS-mediated environmental pollution. Oxid. Med. Cell. Longev. 2014, 2014,
671539
https://doi.org/10.1155/2014/671539 |
| 145 | Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.;
Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for
Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763
https://doi.org/10.1155/2017/8416763 |
| 146 | Marynowski, M.; Likońska, A.; Zatorski, H.; Fichna, J. Role of environmental
pollution in irritable bowel syndrome. World J. Gastroenterol. 2015, 21(40),
11371-8
https://doi.org/10.3748/wjg.v21.i40.11371 |
| 147 | Tenailleau, Q.M.; Lanier, C.; Gower-Rousseau, C.; Cuny, D.; Deram, A.; Occelli,
F. Crohn's disease and environmental contamination: Current challenges and
perspectives in exposure evaluation. Environ. Pollut. 2020, 263(Pt. B), 114599
https://doi.org/10.1016/j.envpol.2020.114599 |
| 148 | Fu, C.; Wang, Q.; Chen, Y.; Zhang, Y. Exploring the causal relationship between
airborne particulate matter and ulcerative colitis: A two-sample mendelian
randomization study. PLoS One 2024, 19(3), e0300066
https://doi.org/10.1371/journal.pone.0300066 |
| 149 | Hectors, T.L.; Vanparys, C.; van der Ven, K.; Martens, G.A.; Jorens, P.G.; Van
Gaal, L.F.; Covaci, A.; De Coen, W.; Blust, R. Environmental pollutants and type
2 diabetes: a review of mechanisms that can disrupt beta cell function.
Diabetologia 2011, 54(6), 1273-1290
https://doi.org/10.1007/s00125-011-2109-5 |
| 150 | Limaye, S.; Salvi, S. Obesity and asthma: the role of environmental pollutants.
Immunol. Allergy Clin. North Am. 2014, 34(4), 839-855
https://doi.org/10.1016/j.iac.2014.07.005 |
| 151 | Hectors, T.L.; Vanparys, C.; Van Gaal, L.F.; Jorens, P.G.; Covaci, A.; Blust, R.
Insulin resistance and environmental pollutants: experimental evidence and
future perspectives. Environ. Health Perspect. 2013, 121(11-12), 1273-1281
https://doi.org/10.1289/ehp.1307082 |
| 152 | Alpízar-Rodríguez, D.; Finckh, A. Environmental factors and hormones in the
development of rheumatoid arthritis. Semin. Immunopathol. 2017, 39(4), 461-468
https://doi.org/10.1007/s00281-017-0624-2 |
| 153 | Mak, A.; Tay, S.H. Environmental factors, toxicants and systemic lupus
erythematosus. Int. J. Mol. Sci. 2014, 15(9), 16043-15056
https://doi.org/10.3390/ijms150916043 |
| 154 | Zhang, Y.; Li, K.; Kong, A.; Zhou, Y.; Chen, D.; Gu, J.; Shi, H. Dysregulation
of autophagy acts as a pathogenic mechanism of non-alcoholic fatty liver disease
(NAFLD) induced by common environmental pollutants. Ecotoxicol. Environ. Saf.
2021, 217, 112256
https://doi.org/10.1016/j.ecoenv.2021.112256 |
| 155 | Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.;
Püschel, K.; Huber, S.; Fischer, E.K. Microplastics detected in cirrhotic liver
tissue. EBioMedicine 2022, 82, 104147
https://doi.org/10.1016/j.ebiom.2022.104147 |
| 156 | Pulliero, A.; Godschalk, R.; Andreassi, M.G.; Curfs, D.; Van Schooten, F.J.;
Izzotti, A. Environmental carcinogens and mutational pathways in
atherosclerosis. Int. J. Hyg. Environ. Health 2015, 218(3), 293-312
https://doi.org/10.1016/j.ijheh.2015.01.007 |
| 157 | Salamanca-Fernández, E.;, Vela-Soria, F.; Rodríguez-Barranco, M.;
Arrebola-Moreno, A.; Iribarne-Durán, L.M.; Olea, N.; Sánchez, M.J.; Arrebola,
J.P. Serum levels of non-persistent environmental pollutants and risk of
incident hypertension in a sub-cohort from the EPIC study. Environ. Res. 2021,
193, 110491
https://doi.org/10.1016/j.envres.2020.110491 |
| 158 | Claeys, M.J.; Rajagopalan, S.; Nawrot, T.S.; Brook, R.D. Climate and
environmental triggers of acute myocardial infarction. Eur. Heart J. 2017,
38(13), 955-960
|
| 159 | Mir, R.H.; Sawhney, G.; Pottoo, F.H.; Mohi-Ud-Din, R.; Madishetti, S.; Jachak,
S.M.; Ahmed, Z.; Masoodi, M.H. Role of environmental pollutants in Alzheimer's
disease: a review. Environ. Sci. Pollut. Res. Int. 2020, 27(36), 44724-44742
https://doi.org/10.1007/s11356-020-09964-x |
| 160 | Goldman, S.M. Environmental toxins and Parkinson's disease. Annu. Rev.
Pharmacol. Toxicol. 2014, 54, 141-164
https://doi.org/10.1146/annurev-pharmtox-011613-135937 |
| 161 | Swash, M.; Eisen, A. Hypothesis: amyotrophic lateral sclerosis and environmental
pollutants. Muscle Nerve 2020, 62(2), 187-191
https://doi.org/10.1002/mus.26855 |
| 162 | Gulis, G.; Fitz, O.; Wittgruber, J.; Suchanová, G. Colorectal cancer and
environmental pollution. Cent. Eur. J. Public Health 1998, 6(3), 188-191.
|
| 163 | Gan, T.; Bambrick, H.; Tong, S.; Hu, W. Air pollution and liver cancer: A
systematic review. J. Environ. Sci. (China) 2023, 126, 817-826
https://doi.org/10.1016/j.jes.2022.05.037 |
| 164 | Moore, E.; Chatzidiakou, L.; Kuku, M.O.; Jones, R.L.; Smeeth, L.; Beevers, S.;
Kelly, F.J.; Barratt, B.; Quint, J.K. Global Associations between Air Pollutants
and Chronic Obstructive Pulmonary Disease Hospitalizations. A Systematic Review.
Ann. Am. Thorac. Soc. 2016, 13(10), 1814-1827
https://doi.org/10.1513/AnnalsATS.201601-064OC |
| 165 | Paun, A.; Danska, J.S. Modulation of type 1 and type 2 diabetes risk by the
intestinal microbiome. Pediatr. Diabetes 2016, 17(7), 469-477
https://doi.org/10.1111/pedi.12424 |
| 166 | Zhao, L.; Fang, J.; Tang, S.; Deng, F.; Liu, X.; Shen, Y.; Liu, Y.; Kong, F.;
Du, Y.; Cui, L.; Shi, W.; Wang, Y.; Wang, J.; Zhang, Y.; Dong, X.; Gao, Y.;
Dong, L.; Zhou, H.; Sun, Q.; Dong, H.; Peng, X.; Zhang, Y.; Cao, M.; Wang, Y.;
Zhi, H.; Du, H.; Zhou, J.; Li, T.; Shi, X. PM2.5 and Serum Metabolome and
Insulin Resistance, Potential Mediation by the Gut Microbiome: A
Population-Based Panel Study of Older Adults in China. Environ. Health Perspect.
2022, 130(2), 27007
https://doi.org/10.1289/EHP9688 |
| 167 | Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M.
Several lines of antioxidant defense against oxidative stress: antioxidant
enzymes, nanomaterials with multiple enzyme-mimicking activities, and
low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98(5), 1323-1367
https://doi.org/10.1007/s00204-024-03696-4 |
| 168 | Alengebawy, A.;Abdelkhalek, S.T.;Qureshi, S.R.; Wang, M.Q. Heavy Metals and
Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human
Health Implications. Toxics 2021, 9(3), 42
https://doi.org/10.3390/toxics9030042 |
| 169 | Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.;
Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants:
chronic diseases and aging. Arch. Toxicol. 2023, 97(10), 2499-2574
https://doi.org/10.1007/s00204-023-03562-9 |
| 170 | Hou, L.; Zhang, X.; Wang, D.; Baccarelli, A. Environmental chemical exposures
and human epigenetics. Int. J. Epidemiol. 2012, 41(1), 79-105
https://doi.org/10.1093/ije/dyr154 |
| 171 | Ho, S.M.; Johnson, A.; Tarapore, P.; Janakiram, V.; Zhang, X.; Leung, Y.K.
Environmental epigenetics and its implication on disease risk and health
outcomes. ILAR J. 2012, 53(3-4), 289-305
https://doi.org/10.1093/ilar.53.3-4.289 |
| 172 | Omar, N.N.; Mosbah, R.A.; Sarawi, W.S.; Rashed, M.M.; Badr, A.M. Rifaximin
Protects against Malathion-Induced Rat Testicular Toxicity: A Possible Clue on
Modulating Gut Microbiome and Inhibition of Oxidative Stress by Mitophagy.
Molecules 2022, 27(13), 4069
https://doi.org/10.3390/molecules27134069 |
| 173 | Love, C.; Sominsky, L.; O'Hely, M.; Berk, M.; Vuillermin, P.; Dawson, S.L.
Prenatal environmental risk factors for autism spectrum disorder and their
potential mechanisms. BMC Med. 2024, 22(1), 393
https://doi.org/10.1186/s12916-024-03617-3 |
| 174 | Klimkaite, L.; Liveikis, T.; Kaspute, G.; Armalyte, J.; Aldonyte, R. Air
pollution-associated shifts in the human airway microbiome and
exposure-associated molecular events. Future Microbiol. 2023, 18, 607-623
https://doi.org/10.2217/fmb-2022-0258 |
| 175 | Gao, Y.; Yu, T.; Wu, Y.; Huang, X.; Teng, J.; Zhao, N.; Zheng, X.; Yan, F.
Bacillus coagulans (Weizmannia coagulans) XY2 attenuates Cu-induced oxidative
stress via DAF-16/FoxO and SKN-1/Nrf2 pathways and gut microbiota regulation. J.
Hazard. Mater. 2023, 457, 131741
https://doi.org/10.1016/j.jhazmat.2023.131741 |
| 176 | Salim, S.Y.; Kaplan, G.G.; Madsen, K.L. Air pollution effects on the gut
microbiota: a link between exposure and inflammatory disease. Gut Microbes 2014,
5(2), 215-9
https://doi.org/10.4161/gmic.27251 |
| 177 | Liu, W.; Feng, H.; Zheng, S.; Xu, S.; Massey, I.Y.; Zhang, C.; Wang, X.; Yang,
F. Pb Toxicity on Gut Physiology and Microbiota. Front. Physiol. 2021, 12,
574913
https://doi.org/10.3389/fphys.2021.574913 |
| 178 | Gupta, N.; Yadav, V.K.; Gacem, A.; Al-Dossari, M.; Yadav, K.K.; Abd El-Gawaad,
N.S.; Ben Khedher, N.; Choudhary, N.; Kumar, P.; Cavalu, S. Deleterious Effect
of Air Pollution on Human Microbial Community and Bacterial Flora: A Short
Review. Int. J. Environ. Res. Public Health 2022, 19(23), 15494
https://doi.org/10.3390/ijerph192315494 |
| 179 | Gao, B.; Chen, L.; Wu, L.; Zhang, S.; Zhao, S,.; Mo, Z.; Chen, Z.; Tu, P.
Association between microplastics and the functionalities of human gut
microbiome. Ecotoxicol. Environ. Saf. 2025, 290, 117497
https://doi.org/10.1016/j.ecoenv.2024.117497 |
| 180 | Ugwu, O.P.; Alum, E.U.; Okon, M.B.; Obeagu, E.I. Mechanisms of microbiota
modulation: Implications for health, disease, and therapeutic interventions.
Medicine (Baltimore) 2024, 103(19), e38088
https://doi.org/10.1097/MD.0000000000038088 |
| 181 | Zhang, R.; Ding, N.; Feng, X.; Liao, W. The gut microbiome, immune modulation,
and cognitive decline: insights on the gut-brain axis. Front. Immunol. 2025, 16,
1529958
https://doi.org/10.3389/fimmu.2025.1529958 |
| 182 | Wang, B.; Shen, J. NF-κB Inducing Kinase Regulates Intestinal Immunity and
Homeostasis. Front. Immunol. 2022, 13, 895636
https://doi.org/10.3389/fimmu.2022.895636 |
| 183 | Kearns, R. Gut-Brain Axis and Neuroinflammation: The Role of Gut Permeability
and the Kynurenine Pathway in Neurological Disorders. Cell. Mol. Neurobiol.
2024, 44(1), 64
https://doi.org/10.1007/s10571-024-01496-z |
| 184 | Hug, H.; Mohajeri, M.H.; La Fata, G. Toll-Like Receptors: Regulators of the
Immune Response in the Human Gut. Nutrients 2018, 10, 203
https://doi.org/10.3390/nu10020203 |
| 185 | Chen, L.; Zhang, L.; Hua, H.; Liu, L.; Mao, Y.; Wang, R. Interactions between
toll-like receptors signaling pathway and gut microbiota in host homeostasis.
Immun. Inflamm. Dis. 2024, 12(7), e1356
https://doi.org/10.1002/iid3.1356 |
| 186 | Rivas-Arancibia, S.; Hernández-Orozco, E.; Rodríguez-Martínez, E.;
Valdés-Fuentes, M.; Cornejo-Trejo, V.; Pérez-Pacheco, N.; Dorado-Martínez, C.;
Zequeida-Carmona, D.; Espinosa-Caleti, I. Ozone Pollution, Oxidative Stress,
Regulatory T Cells and Antioxidants. Antioxidants 2022, 11, 1553
https://doi.org/10.3390/antiox11081553 |
| 187 | Toor, D.; Wsson, M.K,.; Kumar, P.; Karthikeyan, G.; Kaushik, N.K.; Goel, C.;
Singh, S.; Kumar, A.; Prakash, H. Dysbiosis Disrupts Gut Immune Homeostasis and
Promotes Gastric Diseases. Int. J. Mol. Sci. 2019, 20(10), 2432
https://doi.org/10.3390/ijms20102432 |
| 188 | Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.;
Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines Il-6 and TNF-α and
the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15
Suppl. 2(Suppl. 2), 120-122
https://doi.org/10.1186/2047-783X-15-S2-120 |
| 189 | Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal
Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol.
2021, 11(5), 1463-1482
https://doi.org/10.1016/j.jcmgh.2021.02.007 |
| 190 | Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal
Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289
https://doi.org/10.3390/biomedicines10020289 |
| 191 | Olivo-Martínez, Y.; Bosch, M.; Badia, J.; Baldomà, L. Modulation of the
Intestinal Barrier Integrity and Repair by Microbiota Extracellular Vesicles
through the Differential Regulation of Trefoil Factor 3 in LS174T Goblet Cells.
Nutrients 2023, 15, 2437
https://doi.org/10.3390/nu15112437 |
| 192 | Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites
Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12(5), 793
https://doi.org/10.3390/cells12050793 |
| 193 | Zheng, F.; Gonçalves, F.M.; Abiko, Y.; Li, H.; Kumagai, Y.; Aschner, M. Redox
toxicology of environmental chemicals causing oxidative stress. Redox Biol.
2020, 34, 101475
https://doi.org/10.1016/j.redox.2020.101475 |
| 194 | Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in
Xenobiotic Metabolism - A Brief Review on a Fascinating Enzyme Family. J.
Xenobiot. 2021, 11(3), 94-114
https://doi.org/10.3390/jox11030007 |
| 195 | Srivastava, R.K.; Lutz, B.; Ruiz de Azua, I. The Microbiome and Gut
Endocannabinoid System in the Regulation of Stress Responses and Metabolism.
Front. Cell. Neurosci. 2022, 16, 867267
https://doi.org/10.3389/fncel.2022.867267 |
| 196 | Crowley, K.; Kiraga, Ł.; Miszczuk, E.; Skiba, S.; Banach, J.; Latek, U.; Mendel,
M.; Chłopecka, M. Effects of Cannabinoids on Intestinal Motility, Barrier
Permeability, and Therapeutic Potential in Gastrointestinal Diseases. Int. J.
Mol. Sci. 2024, 25(12), 6682
https://doi.org/10.3390/ijms25126682 |
| 197 | Peng, C.; Ouyang, Y.; Lu, N.; Li, N. The NF-κB Signaling Pathway, the
Microbiota, and Gastrointestinal Tumorigenesis: Recent Advances. Front. Immunol.
2020, 11, 1387
https://doi.org/10.3389/fimmu.2020.01387 |
| 198 | Xu, R.; Cao, J.W.; Lv, H.L.; Geng, Y.; Guo, M.Y. Polyethylene microplastics
induced gut microbiota dysbiosis leading to liver injury via the
TLR2/NF-κB/NLRP3 pathway in mice. Sci. Total Environ. 2024, 917, 170518
https://doi.org/10.1016/j.scitotenv.2024.170518 |
| 199 | Lin, H.; Li, X.; Gao, H.; Hu, W.; Yu, S.; Li, X.; Lei, L.; Yang, F. The role of
gut microbiota in mediating increased toxicity of nano-sized polystyrene
compared to micro-sized polystyrene in mice. Chemosphere 2024, 358, 142275
https://doi.org/10.1016/j.chemosphere.2024.142275 |
| 200 | Liu, H.; Nie, H.; Shi, Y.; Lai, W.; Bian, L.; Tian, L.; Li, K.; Xi, Z.; Lin, B.
Oil mistparticulate matter exposure induces hyperlipidemia-related inflammation
via microbiota/ SCFAs/GPR43 axis inhibition and TLR4/NF-κB activation. Environ.
Pollut. 2024, 344, 123331
https://doi.org/10.1016/j.envpol.2024.123331 |
| 201 | Lu, Z.J.; Shi, W.J.; Qiao, L.K.; Ma, D.D.; Zhang, J.G.; Yao, C.R.; Li, S.Y.;
Long, X.B.; Ying, G.G. Benzimidazole Fungicide Carbendazim Induces Gut
Inflammation through the TLR5/NF-κB Pathway in Grass Carp. Environ. Sci.
Technol. 2025, 59(5), 2473-2483
https://doi.org/10.1021/acs.est.4c12695 |
| 202 | Duan, X.; Cai, H.; Hu, T.; Lin, L.; Zeng, L.; Wang, H.; Cao, L.; Li, X.
Ginsenoside Rg3 treats acute radiation proctitis through the TLR4/MyD88/NF-κB
pathway and regulation of intestinal flora. Front. Cell. Infect. Microbiol.
2023, 12, 1028576
https://doi.org/10.3389/fcimb.2022.1028576 |
| 203 | Liu, Y.; Lin, S.; Wang, C.; Li, T.; Zheng, G.; Sun, W.; An, L.; Bai, Y.; Wu, F.
Sex-Specific Effects of Environmental Exposure to the Antimicrobial Agents
Benzalkonium Chloride and Triclosan on the Gut Microbiota and Health of
Zebrafish (Danio rerio). Environ. Sci. Technol. 2024, 58(35), 15450-15462
https://doi.org/10.1021/acs.est.4c03205 |
| 204 | Chen, F.; Shi, X. Signaling from toxic metals to NF-kappaB and beyond: not just
a matter of reactive oxygen species. Environ. Health Perspect. 2002, 110 Suppl.
5(Suppl. 5), 807-811
https://doi.org/10.1289/ehp.02110s5807 |
| 205 | Øvrevik, J.; Refsnes, M.; Låg, M.; Holme, J.A.; Schwarze, P.E. Activation of
Proinflammatory Responses in Cells of the Airway Mucosa by Particulate Matter:
Oxidant- and Non-Oxidant-Mediated Triggering Mechanisms. Biomolecules 2015, 5,
1399-1440
https://doi.org/10.3390/biom5031399 |
| 206 | Cao, J.; Xu, R.; Geng, Y.; Xu, S.; Guo, M. Exposure to polystyrene microplastics
triggers lung injury via targeting toll-like receptor 2 and activation of the
NF-κB signal in mice. Environ. Pollut. 2023, 320, 121068
https://doi.org/10.1016/j.envpol.2023.121068 |
| 207 | Wang, J.; Yan, Y.; Si, H.; Li, J.; Zhao, Y.; Gao, T.; Pi, J.; Zhang, R.; Chen,
R.; Chen, W.; Zheng, Y.; Jiang, M. The effect of real-ambient PM2.5 exposure on
the lung and gut microbiomes and the regulation of Nrf2 Ecotoxicol. Environ.
Saf. 2023, 254, 114702
https://doi.org/10.1016/j.ecoenv.2023.114702 |
| 208 | Han, B.; Li, X.; Ai, R.S.; Deng, S.Y.; Ye, Z.Q.; Deng, X.; Ma, W.; Xiao, S.;
Wang, J.Z.; Wang, L.M.; Xie, C.; Zhang, Y.; Xu, Y.; Zhang, Y. Atmospheric
particulate matter aggravates cns demyelination through involvement of
TLR-4/NF-kB signaling and microglial activation. Elife 2022, 11, e72247
https://doi.org/10.7554/eLife.72247 |
| 209 | Lopes-Ferreira, M.; Farinha, L.R.L.; Costa, Y.S.O.; Pinto, F.J.; Disner, G.R.;
da Rosa, J.G.D.S.; Lima, C. Pesticide-Induced Inflammation at a Glance. Toxics
2023, 11(11), 896
https://doi.org/10.3390/toxics11110896 |
| 210 | Poritz, L.S.; Harris, L.R. 3rd; Kelly, A.A.; Koltun, W.A. Increase in the tight
junction protein claudin-1 in intestinal inflammation. Dig. Dis. Sci. 2011,
56(10), 2802-2809
https://doi.org/10.1007/s10620-011-1688-9 |
| 211 | Bellezza, I,.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in
oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell. Res. 2018,
1865(5), 721-733
https://doi.org/10.1016/j.bbamcr.2018.02.010 |
| 212 | He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and
Beyond. Int. J. Mol. Sci. 2020, 21(13), 4777
https://doi.org/10.3390/ijms21134777 |
| 213 | Pardo, M.; Qiu, X.; Zimmermann, R.; Rudich, Y. Particulate Matter Toxicity Is
Nrf2 and Mitochondria Dependent: The Roles of Metals and Polycyclic Aromatic
Hydrocarbons. Chem. Res. Toxicol. 2020, 33(5), 1110-1120
https://doi.org/10.1021/acs.chemrestox.0c00007 |
| 214 | Park, B.; London, N.R. Jr.; Tharakan, A.; Rengasamy, P.; Rajagopalan, S.;
Biswal, S.; Pinto, J.M.; Ramanathan, M.Jr. Particulate matter air pollution
exposure disrupts the Nrf2 pathway in sinonasal epithelium via epigenetic
alterations in a murine model. Int. Forum Allergy Rhinol. 2022, 12(11),
1424-1427
https://doi.org/10.1002/alr.23010 |
| 215 | Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of
Mechanisms and Implications in Human Disease. Antioxidants (Basel) 2022, 11(12),
2345
https://doi.org/10.3390/antiox11122345 |
| 216 | Saeedi, B.J.; Liu, K.H.; Owens, J.A.; Hunter-Chang, S.; Camacho, M.C.; Eboka,
R.U.; Chandrasekharan, B.; Baker, N.F.; Darby, T.M.; Robinson, B.S.; Jones,
R.M.; Jones, D.P.; Neish, A.S. Gut-Resident Lactobacilli Activate Hepatic Nrf2
and Protect Against Oxidative Liver Injury. Cell Metab. 2020, 31(5), 956-968.e5
https://doi.org/10.1016/j.cmet.2020.03.006 |
| 217 | Wang, L.; Zhao, Z.; Zhao, L.; Zhao, Y.; Yang, G.; Wang, C.; Gao, L.; Niu, C.;
Li, S. Lactobacillus plantarum DP189 Reduces α-SYN Aggravation in MPTP-Induced
Parkinson's Disease Mice via Regulating Oxidative Damage, Inflammation, and Gut
Microbiota Disorder. J. Agric. Food Chem. 2022, 70(4), 1163-1173
https://doi.org/10.1021/acs.jafc.1c07711 |
| 218 | Xu, Y.; Li, N.; Xiang, R.; Sun, P. Emerging roles of the p38 MAPK and
PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem. Sci.
2014, 39(6), 268-276
https://doi.org/10.1016/j.tibs.2014.04.004 |
| 219 | Su, R.; Jin, X.; Zhang, W.; Li, Z.; Liu, X.; Ren, J. Particulate matter exposure
induces the autophagy of macrophages via oxidative stress-mediated PI3K/AKT/mTOR
pathway. Chemosphere 2017, 167, 444-453
https://doi.org/10.1016/j.chemosphere.2016.10.024 |
| 220 | Han, X.; Zhuang, Y. PM2.5 induces autophagy-mediated cell apoptosis via
PI3K/AKT/mTOR signaling pathway in mice bronchial epithelium cells. Exp. Ther.
Med. 2021, 21(1), 1
https://doi.org/10.3892/etm.2020.9433 |
| 221 | Guo, M.; Wang, Y.; Zhao, H.; Wang, D.; Yin, K.; Liu, Y.; Li, B.; Xing, M. Zinc
antagonizes common carp (Cyprinus carpio) intestinal arsenic poisoning through
PI3K/AKT/mTOR signaling cascade and MAPK pathway. Aquat. Toxicol. 2021, 240,
105986
https://doi.org/10.1016/j.aquatox.2021.105986 |
| 222 | Yin, H.; Zuo, Z.; Yang, Z.; Guo, H.; Fang, J.; Cui, H.; Ouyang, P.; Chen, X.;
Chen, J.; Geng, Y.; Chen, Z.; Huang, C.; Zhu, Y. Nickel induces autophagy via
PI3K/AKT/mTOR and AMPK pathways in mouse kidney. Ecotoxicol. Environ. Saf. 2021,
223, 112583
https://doi.org/10.1016/j.ecoenv.2021.112583 |
| 223 | Zhang, F.; Ma, H.; Wang, Z.L.; Li, W.H.; Liu, H.; Zhao, Y.X. The PI3K/AKT/mTOR
pathway regulates autophagy to induce apoptosis of alveolar epithelial cells in
chronic obstructive pulmonary disease caused by PM2.5 particulate matter. J.
Int. Med. Res. 2020, 48(7), 300060520927919
https://doi.org/10.1177/0300060520927919 |
| 224 | Lu, H.; Yin, K.; Su, H.; Wang, D.; Zhang, Y.; Hou, L.; Li, J.B.; Wang, Y.; Xing,
M. Polystyrene microplastics induce autophagy and apoptosis in birds lungs via
PTEN/PI3K/AKT/mTOR. Environ. Toxicol. 2023, 38(1), 78-89
https://doi.org/10.1002/tox.23663 |
| 225 | Sun, W.; Lei, Y.; Jiang, Z.; Wang, K.; Liu, H.; Xu, T. BPA and low-Se exacerbate
apoptosis and mitophagy in chicken pancreatic cells by regulating the
PTEN/PI3K/AKT/mTOR pathway. J. Adv. Res. 2025, 67, 61-69
https://doi.org/10.1016/j.jare.2024.01.029 |
| 226 | Dong, Y.; Gao, L.; Sun, Q.; Jia, L.; Liu, D. Increased levels of IL-17 and
autoantibodies following Bisphenol A exposure were associated with activation of
PI3K/AKT/mTOR pathway and abnormal autophagy in MRL/lpr mice. Ecotoxicol.
Environ. Saf. 2023, 255, 114788
https://doi.org/10.1016/j.ecoenv.2023.114788 |
| 227 | Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.;
Guida, M.; Plunk, E.; Viggiano, A.; Pierantoni, R.; Meccariello, R. Neuro-toxic
and Reproductive Effects of BPA. Curr. Neuropharmacol. 2019, 17(12), 1109-1132
https://doi.org/10.2174/1570159X17666190726112101 |
| 228 | Braun, J.M. Early-life exposure to EDCs: role in childhood obesity and
neurodevelopment. Nat. Rev. Endocrinol. 2017, 13(3), 161-173
https://doi.org/10.1038/nrendo.2016.186 |
| 229 | Chakraborty, S.; Dissanayake, M.; Godwin, J.; Wang, X.; Bhandari, R.K. Ancestral
BPA exposure caused defects in the liver of medaka for four generations. Sci.
Total Environ. 2023, 856(Pt. 1), 159067
https://doi.org/10.1016/j.scitotenv.2022.159067 |
| 230 | Zhang, Y.; Dong, T.; Hu, W.; Wang, X.; Xu, B.; Lin, Z.; Hofer, T.; Stefanoff,
P.; Chen, Y.; Wang, X.; Xia, Y. Association between exposure to a mixture of
phenols, pesticides, and phthalates and obesity: Comparison of three statistical
models. Environ. Int. 2019, 123, 325-336
https://doi.org/10.1016/j.envint.2018.11.076 |
| 231 | Sonavane, M.; Gassman, NR. Bisphenol A co-exposure effects: a key factor in
understanding BPA's complex mechanism and health outcomes. Crit Rev Toxicol.
2019, 49(5), 371-386
https://doi.org/10.1080/10408444.2019.1621263 |
| 232 | Sultan, M.; Cai, Z.X.; Bao, L.; Duan, J.J.; Liu, Y.Y.; Yang, G.; Pei, D.S.
Trophic transfer induced gut inflammation, dysbiosis, and inflammatory pathways
in zebrafish via Artemia franciscana: A differential analysis of nanoplastic
toxicity. J. Hazard. Mater. 2024, 480, 136030
https://doi.org/10.1016/j.jhazmat.2024.136030 |
| 233 | Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to polystyrene
microplastics causes reproductive toxicity through oxidative stress and
activation of the p38 MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2020,
190, 110133
https://doi.org/10.1016/j.ecoenv.2019.110133 |
| 234 | Li, J.; Weng, H.; Liu, S.; Li, F.;Xu, K.; Wen, S.; Chen, X.; Li, C.; Nie, Y.;
Liao, B.; Wu, J.; Kantawong, F.; Xie, X.; Yu, F.; Li, G. Embryonic exposure of
polystyrene nanoplastics affects cardiac development. Sci. Total Environ. 2024,
906, 167406
https://doi.org/10.1016/j.scitotenv.2023.167406 |
| 235 | Cao, X.; Fu, M.; Bi, R.; Zheng, X.; Fu, B.; Tian, S.; Liu, C.; Li, Q.; Liu, J.
Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK
signaling pathway. Chemosphere 2021, 263, 128346
https://doi.org/10.1016/j.chemosphere.2020.128346 |
| 236 | Liu, X,.; Zhang, R.; Fan, J.; Chen, Y.; Wang, H.; Ge, Y.; Liang, H.; Li, W.;
Liu, H.; Lv, Z.; Dou, W.; Jiang, H.; Li, X. The role of ROS/p38 MAPK/NLRP3
inflammasome cascade in arsenic-induced depression-/anxiety-like behaviors of
mice. Ecotoxicol. Environ. Saf. 2023, 261, 115111
https://doi.org/10.1016/j.ecoenv.2023.115111 |
| 237 | Ijomone, O.M.; Iroegbu, J.D.; Aschner, M.; Bornhorst, J. Impact of environmental
toxicants on p38- and ERK-MAPK signaling pathways in the central nervous system.
Neurotoxicology 2021, 86, 166-171
https://doi.org/10.1016/j.neuro.2021.08.005 |
| 238 | Han, L.; Haefner, V.; Yu, Y.; Han, B.; Ren, H.; Irmler, M.; Beckers, J.; Liu,
Q.; Feuchtinger, A.; Yildirim, A.O.; Adler, H.; Stoeger, T.
Nanoparticle-Exposure-Triggered Virus Reactivation Induces Lung Emphysema in
Mice. ACS Nano 2023, 17(21), 21056-21072
https://doi.org/10.1021/acsnano.3c04111 |
| 239 | Sarapultsev, A.; Gusev, E.; Komelkova, M.; Utepova, I.; Luo, S.; Hu, D. JAK-STAT
signaling in inflammation and stress-related diseases: implications for
therapeutic interventions. Mol. Biomed. 2023, 4(1), 40
https://doi.org/10.1186/s43556-023-00151-1 |
| 240 | Hu, Q.; Bian, Q.; Rong, D.; Wang, L.; Song, J.; Huang, H.S.; Zeng, J.; Mei, J.;
Wang, P.Y. JAK/STAT pathway: Extracellular signals, diseases, immunity, and
therapeutic regimens. Front. Bioeng. Biotechnol. 2023, 11, 1110765
https://doi.org/10.3389/fbioe.2023.1110765 |
| 241 | Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: from
bench to clinic. Signal Transduct. Target Ther. 2021, 6(1), 402
https://doi.org/10.1038/s41392-021-00791-1 |
| 242 | Giovannini, S.; Onder, G.; Liperoti, R.; Russo, A.; Carter, C.; Capoluongo, E.;
Pahor, M.; Bernabei, R.; Landi, F. Interleukin-6, C-reactive protein, and tumor
necrosis factor-alpha as predictors of mortality in frail, community-living
elderly individuals. J. Am. Geriatr. Soc. 2011, 59(9), 1679-1685
https://doi.org/10.1111/j.1532-5415.2011.03570.x |
| 243 | Heinzel, S.; Jureczek, J.; Kainulainen, V.; Nieminen, A.I.; Suenkel, U.; von
Thaler, A.K.; Kaleta, C.; Eschweiler, G.W.; Brockmann, K.; Aho, V.T.E.; Auvinen,
P.; Maetzler, W.; Berg, D.; Scheperjans, F. Elevated fecal calprotectin is
associated with gut microbial dysbiosis, altered serum markers and clinical
outcomes in older individuals. Sci. Rep. 2024, 14(1), 13513
https://doi.org/10.1038/s41598-024-63893-0 |
| 244 | Jin, H.; Liu, Y.; Lei, Y.; Li, G.; Huang, L.; Zhang, Z. Hsa_circ_0004214
involved in the epithelial-mesenchymal transition induced by beryllium sulfate
through modulating JAK-STAT signaling pathway. Toxicol. Res. (Camb.) 2024,
13(3), tfae067
https://doi.org/10.1093/toxres/tfae067 |
| 245 | Liu, M.; Li, Y.; Liang, B.; Li, Z.; Jiang, Z.; Chu, C.; Yang, J. Hydrogen
sulfide attenuates myocardial fibrosis in diabetic rats through the JAK/STAT
signaling pathway. Int. J. Mol. Med. 2018, 41(4), 1867-1876
https://doi.org/10.3892/ijmm.2018.3419 |
| 246 | Ji, X.; Han, M.; Yun, Y.; Li, G.; Sang, N. Acute nitrogen dioxide (NO2) exposure
enhances airway inflammation via modulating Th1/Th2 differentiation and
activating JAK-STAT pathway. Chemosphere 2015, 120, 722-8
https://doi.org/10.1016/j.chemosphere.2014.10.039 |
| 247 | Mobasher, M.A.; Alsirhani, A.M.; Alwaili, M.A.; Baakdah, F.; Eid, T.M.;
Alshanbari, F.A.; Alzahri, R.Y.; Alkhodair, S.A.; El-Said, K.S. Annona squamosa
Fruit Extract Ameliorates Lead Acetate-Induced Testicular Injury by Modulating
JAK-1/STAT-3/SOCS-1 Signaling in Male Rats. Int. J. Mol. Sci. 2024, 25(10), 5562
https://doi.org/10.3390/ijms25105562 |
| 248 | Kim, S.H.; Yu, S.Y.; Choo, J.H.; Kim, J.; Ahn, K.; Hwang, S.Y. Epigenetic
Methylation Changes in Pregnant Women: Bisphenol Exposure and Atopic Dermatitis.
Int. J. Mol. Sci. 2024, 25(3), 1579
https://doi.org/10.3390/ijms25031579 |
| 249 | Wu, Y.F.; Li, Z.Y.; Dong, L.L.; Li, W.J.; Wu, Y.P.; Wang, J.; Chen, H.P.; Liu,
H.W.; Li, M.; Jin, C.L.; Huang, H.Q.; Ying, S.M.; Li, W.; Shen, H.H.; Chen, Z.H.
Inactivation of MTOR promotes autophagy-mediated epithelial injury in
particulate matter-induced airway inflammation. Autophagy 2020, 16(3), 435-450
https://doi.org/10.1080/15548627.2019.1628536 |
| 250 | Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its
influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021,
78(4), 1233-1261
https://doi.org/10.1007/s00018-020-03656-y |
| 251 | Rong, Z.; Huang, Y.; Cai, H.; Chen, M.; Wang, H.; Liu, G.; Zhang, Z.; Wu, J. Gut
Microbiota Disorders Promote Inflammation and Aggravate Spinal Cord Injury
Through the TLR4/MyD88 Signaling Pathway. Front. Nutr. 2021, 8, 702659
https://doi.org/10.3389/fnut.2021.702659 |
| 252 | Dai, Y.; Lu, Q.; Li, P.; Zhu, J.; Jiang, J.; Zhao, T.; Hu, Y.; Ding, K.; Zhao,
M. Xianglian Pill attenuates ulcerative colitis through TLR4/MyD88/NF-κB
signaling pathway. J. Ethnopharmacol. 2023, 300, 115690
https://doi.org/10.1016/j.jep.2022.115690 |
| 253 | Lai, X.; Liu, B.; Wan, Y.; Zhou, P.; Li, W.; Hu, W.; Gong, W. Metformin
alleviates colitis-associated colorectal cancer via inhibition of the
TLR4/MyD88/NFκB/MAPK pathway and macrophage M2 polarization. Int.
Immunopharmacol. 2025, 144, 113683
https://doi.org/10.1016/j.intimp.2024.113683 |
| 254 | Duan, Y.; Dai, H.; An, Y.; Cheng, L.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; He, C.;
Zhang, H.; Huang, Y.; Fu, W.; Meng, Y.; Zhao, B. Mulberry Leaf Flavonoids
Inhibit Liver Inflammation in Type 2 Diabetes Rats by Regulating
TLR4/MyD88/NF-κB Signaling Pathway. Evid. Based Complement. Alternat. Med. 2022,
2022, 3354062
https://doi.org/10.1155/2022/3354062 |
| 255 | Wang, L.; Yang, J.W.; Lin, L.T.; Huang, J.; Wang, X.R.; Su, X.T.; Cao, Y.;
Fisher, M.; Liu, C.Z. Acupuncture Attenuates Inflammation in Microglia of
Vascular Dementia Rats by Inhibiting miR-93-Mediated TLR4/MyD88/NF-κB Signaling
Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 8253904
https://doi.org/10.1155/2020/8253904 |
| 256 | Zhao, Z.; Ning, J.; Bao, X.Q.; Shang, M.; Ma, J.; Li, G.; Zhang, D. Fecal
microbiota transplantation protects rotenone-induced Parkinson's disease mice
via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling
pathway through the microbiota-gut-brain axis. Microbiome 2021, 9(1), 226
https://doi.org/10.1186/s40168-021-01107-9 |
| 257 | He, M.; Ichinose, T.; Yoshida, Y.; Arashidani, K.; Yoshida, S.; Takano, H.; Sun,
G.; Shibamoto, T. Urban PM2.5 exacerbates allergic inflammation in the murine
lung via a TLR2/TLR4/MyD88-signaling pathway. Sci. Rep. 2017, 7(1), 11027
https://doi.org/10.1038/s41598-017-11471-y |
| 258 | Wu, Y.; Xiao, W.; Pei, C.; Wang, M.; Wang, X.; Huang, D.; Wang, F.; Wang, Z.
Astragaloside IV alleviates PM2.5-induced lung injury in rats by modulating
TLR4/MyD88/NF-κB signalling pathway. Int. Immunopharmacol. 2021, 91, 107290
https://doi.org/10.1016/j.intimp.2020.107290 |
| 259 | Liu, G.; Lu, Y.; Shi, L.; Ren, Y.; Kong, J.; Zhang, M.; Chen, M.; Liu, W.
TLR4-MyD88 signaling pathway is responsible for acute lung inflammation induced
by reclaimed water. J. Hazard. Mater. 2020, 396, 122586
https://doi.org/10.1016/j.jhazmat.2020.122586 |
| 260 | Wang, L.; Cui, Y.; Liu, H.; Wu, J.; Li, J.; Liu, X. PM2.5 aggravates airway
inflammation in asthmatic mice: activating NF-κB via MyD88 signaling pathway.
Int. J. Environ. Health Res. 2023, 33(6), 563-574
https://doi.org/10.1080/09603123.2022.2041561 |
| 261 | Panaro, M.A.; Corrado, A.; Benameur, T.; Paolo, C.F.; Cici, D.; Porro, C. The
Emerging Role of Curcumin in the Modulation of TLR-4 Signaling Pathway: Focus on
Neuroprotective and Anti-Rheumatic Properties. Int. J. Mol. Sci. 2020, 21, 2299
https://doi.org/10.3390/ijms21072299 |
| 262 | Yang, J.Z.; Zhang, K.K.; Liu, Y.; Li, X.W.; Chen, L.J.; Liu, J.L.; Li, J.H.;
Chen, L.; Hsu, C.; Zeng, J.H.; Xie, X.L.; Wang, Q. Epigallocatechin-3-gallate
ameliorates polystyrene microplastics-induced anxiety-like behavior in mice by
modulating gut microbe homeostasis. Sci. Total Environ. 2023, 892, 164619
https://doi.org/10.1016/j.scitotenv.2023.164619 |
| 263 | Shi, Z.; Jiao, Y.; Lai, Z.; Liu, J.; Yang, B.; Hu, M.; Meng, J. Evaluation of
the protective role of resveratrol on LPS-induced septic intestinal barrier
function via TLR4/MyD88/NF-κB signaling pathways. Sci. Rep. 2025, 15(1), 828
https://doi.org/10.1038/s41598-025-85148-2 |
| 264 | Pan, I.; Umapathy, S. Probiotics an emerging therapeutic approach towards
gut-brain-axis oriented chronic health issues induced by microplastics: A
comprehensive review. Heliyon 2024, 10(11), e32004
https://doi.org/10.1016/j.heliyon.2024.e32004 |
| 265 | Sobolev, V.V.; Tchepourina, E.; Korsunskaya, I.M.; Geppe, N.A.; Chebysheva,
S.N.; Soboleva, A.G.; Mezentsev, A. The Role of Transcription Factor PPAR-γ in
the Pathogenesis of Psoriasis, Skin Cells, and Immune Cells. Int. J. Mol. Sci.
2022, 23(17), 9708
https://doi.org/10.3390/ijms23179708 |
| 266 | Posta, E.; Fekete, I.; Varkonyi, I.; Zold, E.; Barta, Z. The Versatile Role of
Peroxisome Proliferator-Activated Receptors in Immune-Mediated Intestinal
Diseases. Cells 2024, 13, 1688
https://doi.org/10.3390/cells13201688 |
| 267 | Grabacka, M.; Płonka, P.M.; Pierzchalska, M. The PPARα Regulation of the Gut
Physiology in Regard to Interaction with Microbiota, Intestinal Immunity,
Metabolism, and Permeability. Int. J. Mol. Sci. 2022, 23(22), 14156
https://doi.org/10.3390/ijms232214156 |
| 268 | Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of
peroxisome proliferator-activated receptors (PPAR) in immune responses.
Metabolism 2021, 114, 154338
https://doi.org/10.1016/j.metabol.2020.154338 |
| 269 | Hasan, A.U.; Rahman, A.; Kobori, H. Interactions between Host PPARs and Gut
Microbiota in Health and Disease. Int. J. Mol. Sci. 2019, 20, 387
https://doi.org/10.3390/ijms20020387 |
| 270 | Gagliardi, A.; Totino, V.; Cacciotti, F.; Iebba, V.; Neroni, B.; Bonfiglio, G.;
Trancassini, M.; Passariello, C.; Pantanella, F.; Schippa, S. Rebuilding the Gut
Microbiota Ecosystem. Int. J. Environ. Res. Public Health 2018, 15(8), 1679
https://doi.org/10.3390/ijerph15081679 |
| 271 | Pérez-Reytor, D.; Puebla, C.; Karahanian, E.; García, K. Use of Short-Chain
Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by
Bacterial Toxins. Front. Physiol. 2021, 12, 650313
https://doi.org/10.3389/fphys.2021.650313 |
| 272 | O'Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.;
Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain
fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell
Endocrinol. 2022, 546, 111572
https://doi.org/10.1016/j.mce.2022.111572 |
| 273 | Yuan, G.; Chen, X.; Li, D. Modulation of peroxisome proliferator-activated
receptor gamma (PPAR γ) by conjugated fatty acid in obesity and inflammatory
bowel disease. J. Agric. Food Chem. 2015, 63(7), 1883-1895
https://doi.org/10.1021/jf505050c |
| 274 | Roudsari, N.M.; Lashgari, N.A.; Zandi, N.; Pazoki, B.; Momtaz, S.; Sahebkar, A.;
Abdolghaffari, A.H. PPARγ: A turning point for irritable bowel syndrome
treatment. Life Sci. 2020, 257, 118103
https://doi.org/10.1016/j.lfs.2020.118103 |
| 275 | Massier, L.; Blüher, M.; Kovacs, P.; Chakaroun, R.M. Impaired Intestinal Barrier
and Tissue Bacteria: Pathomechanisms for Metabolic Diseases. Front. Endocrinol.
(Lausanne) 2021, 12, 616506
https://doi.org/10.3389/fendo.2021.616506 |
| 276 | Shemtov, S.J.; Emani, R.; Bielska, O.; Covarrubias, A.J.; Verdin, E.; Andersen,
J.K.; Winer, D.A. The intestinal immune system and gut barrier function in
obesity and ageing. FEBS J. 2023, 290(17), 4163-4186
https://doi.org/10.1111/febs.16558 |
| 277 | Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino,
M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability - a new target for
disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189
https://doi.org/10.1186/s12876-014-0189-7 |
| 278 | Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M.
Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or
New Therapeutic Target. Nutrients 2018, 10, 1398
https://doi.org/10.3390/nu10101398 |
| 279 | Stummer, N.; Feichtinger, R.G.; Weghuber, D.; Kofler, B.; Schneider, A.M. Role
of Hydrogen Sulfide in Inflammatory Bowel Disease. Antioxidants 2023, 12, 1570
https://doi.org/10.3390/antiox12081570 |
| 280 | Zhang, H.; Zha, X.; Zhang, B.; Zheng, Y.; Elsabagh, M.; Wang, H.; Wang, M. Gut
microbiota contributes to bisphenol A-induced maternal intestinal and placental
apoptosis, oxidative stress, and fetal growth restriction in pregnant ewe model
by regulating gut-placental axis. Microbiome 2024, 12(1), 28
https://doi.org/10.1186/s40168-024-01749-5 |
| 281 | Hong, T.; Zou, J.; He, Y.; Zhang, H.; Liu, H.; Mai, H.; Yang, J.; Cao, Z.; Chen,
X.; Yao, J.; Feng, D. Bisphenol A induced hepatic steatosis by disturbing bile
acid metabolism and FXR/TGR5 signaling pathways via remodeling the gut
microbiota in CD-1 mice. Sci. Total Environ. 2023, 889, 164307
https://doi.org/10.1016/j.scitotenv.2023.164307 |
| 282 | Wang, G.; Pan, R.; Liang, X.; Wu, X.; Wu, Y.; Zhang, H.; Zhao, J.; Chen, W.
Perfluorooctanoic acid-induced liver injury is potentially associated with gut
microbiota dysbiosis. Chemosphere 2021, 266, 129004
https://doi.org/10.1016/j.chemosphere.2020.129004 |
| 283 | Ma, N.; Ma, D.; Liu, X.; Zhao, L.; Ma, L.; Ma, D.; Dong, S. Bisphenol P exposure
in C57BL/6 mice caused gut microbiota dysbiosis and induced intestinal barrier
disruption via LPS/TLR4/NF-κB signaling pathway. Environ. Int. 2023, 175, 107949
https://doi.org/10.1016/j.envint.2023.107949 |
| 284 | Feng, D.; Zhang, H.; Jiang, X.; Zou, J.; Li, Q.; Mai, H.; Su, D.; Ling, W.;
Feng, X. Bisphenol A exposure induces gut microbiota dysbiosis and consequent
activation of gut-liver axis leading to hepatic steatosis in CD-1 mice. Environ.
Pollut. 2020, 265(Pt. A), 114880
https://doi.org/10.1016/j.envpol.2020.114880 |
| 285 | Liu, R.; Cai, D.; Li, X.; Liu, B.; Chen, J.; Jiang, X.; Li, H.; Li, Z.; Teerds,
K.; Sun, J.; Bai, W.; Jin, Y. Effects of Bisphenol A on reproductive toxicity
and gut microbiota dysbiosis in male rats. Ecotoxicol. Environ. Saf. 2022, 239,
113623
https://doi.org/10.1016/j.ecoenv.2022.113623 |
| 286 | Hong, T.; Jiang, X.; Zou, J.; Yang, J.; Zhang, H.; Mai, H.; Ling, W.; Feng, D.
Hepatoprotective effect of curcumin against bisphenol A-induced hepatic
steatosis via modulating gut microbiota dysbiosis and related gut-liver axis
activation in CD-1 mice. J. Nutr. Biochem. 2022, 109, 109103
https://doi.org/10.1016/j.jnutbio.2022.109103 |
| 287 | Wu, L.; Lv, X.; Zhang, Y.; Xin, Q.; Zou, Y.; Li, X. Tartrazine exposure results
in histological damage, oxidative stress, immune disorders and gut microbiota
dysbiosis in juvenile crucian carp (Carassius carassius). Aquat. Toxicol. 2021,
241, 105998
https://doi.org/10.1016/j.aquatox.2021.105998 |
| 288 | Tang, J.; Wang, W.; Jiang, Y.; Chu, W. Diazinon exposure produces histological
damage, oxidative stress, immune disorders and gut microbiota dysbiosis in
crucian carp (Carassius auratus gibelio). Environ. Pollut. 2021, 269, 116129
https://doi.org/10.1016/j.envpol.2020.116129 |
| 289 | Pei, X.; Heng, X.; Chu, W. Polystyrene nano/microplastics induce microbiota
dysbiosis, oxidative damage, and innate immune disruption in zebrafish. Microb.
Pathog. 2022, 163, 105387
https://doi.org/10.1016/j.micpath.2021.105387 |
| 290 | Jiang, D.; Yang, Y.; Han, X.; Li, Q.; Jiao, J.; Ma, Y.; Chao, L. Gut microbiota
combined with metabolome dissects Fluorene-9-bisphenol exposure-induced male
reproductive toxicity. Environ. Pollut. 2025, 364(Pt. 1), 125339
https://doi.org/10.1016/j.envpol.2024.125339 |
| 291 | Lin, H.; Wu, H.; Liu, F.; Yang, H.; Shen, L.; Chen, J.; Zhang, X.; Zhong, Y.;
Zhang, H.; Liu, Z. Assessing the hepatotoxicity of PFOA, PFOS, and 6:2 Cl-PFESA
in black-spotted frogs (Rana nigromaculata) and elucidating potential
association with gut microbiota. Environ. Pollut. 2022, 312, 120029
https://doi.org/10.1016/j.envpol.2022.120029 |
| 292 | López-Moreno, A.; Torres-Sánchez, A.; Acuña, I.; Suárez, A.; Aguilera, M.
Representative Bacillus sp. AM1 from Gut Microbiota Harbor Versatile Molecular
Pathways for Bisphenol A Biodegradation. Int. J. Mol. Sci. 2021, 22(9), 4952
https://doi.org/10.3390/ijms22094952 |
| 293 | DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of
Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016,
22(5), 1137-1150
https://doi.org/10.1097/MIB.0000000000000750 |
| 294 | Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological
mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A
review. Biomed. Pharmacother. 2023, 164, 114985
https://doi.org/10.1016/j.biopha.2023.114985 |
| 295 | Shim, J.A.; Ryu, J.H.; Jo, Y.; Hong, C. The role of gut microbiota in T cell
immunity and immune mediated disorders. Int. J. Biol. Sci. 2023, 19(4),
1178-1191
https://doi.org/10.7150/ijbs.79430 |
| 296 | de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial
dysfunction in obesity. Life Sci. 2018, 192, 26-32
https://doi.org/10.1016/j.lfs.2017.11.019 |
| 297 | Pinti, M.V.; Fink, G.K.; Hathaway, Q.A.; Durr, A.J.; Kunovac, A.; Hollander,
J.M. Mitochondrial dysfunction in type 2 diabetes mellitus: an organ-based
analysis. Am. J. Physiol. Endocrinol. Metab. 2019, 316(2), E268-E285
https://doi.org/10.1152/ajpendo.00314.2018 |
| 298 | Prasun, P.; Ginevic, I.; Oishi, K. Mitochondrial dysfunction in nonalcoholic
fatty liver disease and alcohol related liver disease. Transl. Gastroenterol.
Hepatol. 2021, 6, 4
https://doi.org/10.21037/tgh-20-125 |
| 299 | Alizadeh, A.; Akbari, P.; Garssen, J.; Fink-Gremmels, J.; Braber, S. Epithelial
integrity, junctional complexes, and biomarkers associated with intestinal
functions. Tissue Barriers 2022, 10(3), 1996830
https://doi.org/10.1080/21688370.2021.1996830 |
| 300 | König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.;
Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier
Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7(10), e196
https://doi.org/10.1038/ctg.2016.54 |
| 301 | Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic
Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 594150
https://doi.org/10.3389/fimmu.2020.594150 |
| 302 | Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere,
C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic
Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731
https://doi.org/10.3389/fimmu.2020.571731 |
| 303 | Kustrimovic, N.; Balkhi, S.; Bilato, G.; Mortara, L. Gut Microbiota and Immune
System Dynamics in Parkinson's and Alzheimer's Diseases. Int. J. Mol. Sci. 2024,
25, 12164
https://doi.org/10.3390/ijms252212164 |
| 304 | Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and
autoimmunity. Gut Microbes 2012, 3(1), 4-14
https://doi.org/10.4161/gmic.19320 |
| 305 | Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives
systemic autoimmune diseases. Front. Immunol. 2022, 13, 906258
https://doi.org/10.3389/fimmu.2022.906258 |
| 306 | Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The
Interplay between the Gut Microbiome and the Immune System in the Context of
Infectious Diseases throughout Life and the Role of Nutrition in Optimizing
Treatment Strategies. Nutrients 2021, 13(3), 886
https://doi.org/10.3390/nu13030886 |
| 307 | Wang, S.; Ju, D.; Zeng, X. Mechanisms and Clinical Implications of Human Gut
Microbiota-Drug Interactions in the Precision Medicine Era. Biomedicines 2024,
12, 194
https://doi.org/10.3390/biomedicines12010194 |
| 308 | Al-Habsi, N.; Al-Khalili, M.; Haque, S.A.; Elias, M.; Olqi, N.A.; Al Uraimi, T.
Health Benefits of Prebiotics, Probiotics, Synbiotics, and Postbiotics.
Nutrients 2024, 16(22), 3955
https://doi.org/10.3390/nu16223955 |
| 309 | Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on
Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25(11), 6022
https://doi.org/10.3390/ijms25116022 |
| 310 | Slavin, J. Fiber and prebiotics: mechanisms and health benefits. Nutrients 2013,
5(4), 1417-1435
https://doi.org/10.3390/nu5041417 |
| 311 | Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.;
Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types,
Sources, Mechanisms, and Clinical Applications. Foods 2019, 8(3), 92
https://doi.org/10.3390/foods8030092 |
| 312 | Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the
interplay between gut microbiota and diet in cardio-metabolic health. Gut
Microbes 2021, 13(1), 1-24
https://doi.org/10.1080/19490976.2021.1897212 |
| 313 | Sánchez Macarro, M.; Ávila-Gandía, V.; Pérez-Piñero, S.; Cánovas, F.;
García-Muñoz, A.M.; Abellán-Ruiz, M.S.; Victoria-Montesinos, D.; Luque-Rubia,
A.J.; Climent, E.; Genovés, S.; Ramon, D.; Chenoll, E.; López-Román, F.J.
Antioxidant Effect of a Probiotic Product on a Model of Oxidative Stress Induced
by High-Intensity and Duration Physical Exercise. Antioxidants (Basel) 2021,
10(2), 323
https://doi.org/10.3390/antiox10020323 |
| 314 | Chen, W.; Ma, Q.; Li, Y.; Wei, L.; Zhang, Z.; Khan, A.; Khan, M.Z.; Wang, C.
Butyrate Supplementation Improves Intestinal Health and Growth Performance in
Livestock: A Review. Biomolecules 2025, 15(1), 85
https://doi.org/10.3390/biom15010085 |
| 315 | Shah, A.B.; Baiseitova, A.; Zahoor, M.; Ahmad, I.; Ikram, M.; Bakhsh, A.; Shah,
M.A.; Ali, I.; Idress, M.; Ullah, R.; Nasr, F.A.; Al-Zharani, M. Probiotic
significance of Lactobacillus strains: a comprehensive review on health impacts,
research gaps, and future prospects. Gut Microbes 2024, 16(1), 2431643
https://doi.org/10.1080/19490976.2024.2431643 |
| 316 | Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health
and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds
2023, 3, 40-72
https://doi.org/10.3390/compounds3010005 |
| 317 | Perler, B.K.; Friedman, E.S.; Wu, G.D. The Role of the Gut Microbiota in the
Relationship Between Diet and Human Health. Annu. Rev. Physiol. 2023, 85,
449-468
https://doi.org/10.1146/annurev-physiol-031522-092054 |
| 318 | Hijová, E. Postbiotics as Metabolites and Their Biotherapeutic Potential. Int.
J. Mol. Sci. 2024, 25(10), 5441
https://doi.org/10.3390/ijms25105441 |
| 319 | Quaranta, G.; Guarnaccia, A.; Fancello, G.; Agrillo, C.; Iannarelli, F.;
Sanguinetti, M.; Masucci, L. Fecal Microbiota Transplantation and Other Gut
Microbiota Manipulation Strategies. Microorganisms 2022, 10, 2424
https://doi.org/10.3390/microorganisms10122424 |
| 320 | Cheng, Y.W.; Fischer, M. Fecal Microbiota Transplantation. Clin. Colon Rectal
Surg. 2023, 36(2), 151-156
https://doi.org/10.1055/s-0043-1760865 |
| 321 | Koo, H.L.; DuPont, H.L. Rifaximin: a unique gastrointestinal-selective
antibiotic for enteric diseases. Curr. Opin. Gastroenterol. 2010, 26(1), 17-25
https://doi.org/10.1097/MOG.0b013e328333dc8d |
| 322 | DuPont, HL. Review article: the antimicrobial effects of rifaximin on the gut
microbiota. Aliment. Pharmacol. Ther. 2016, 43 Suppl. 1, 3-10
https://doi.org/10.1111/apt.13434 |
| 323 | Schupack, D.A.; Mars, R.A.T.; Voelker, D.H.; Abeykoon, J.P.; Kashyap, P.C. The
promise of the gut microbiome as part of individualized treatment strategies.
Nat. Rev. Gastroenterol. Hepatol. 2022, 19(1), 7-25
https://doi.org/10.1038/s41575-021-00499-1 |
| 324 | Riaz Rajoka, M.S.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat,
H.F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F.J.; Barba, F.J. Role of
Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings
in Intestinal Oxidative Stress Damage and Their Impact on Host Health.
Antioxidants (Basel) 2021, 10(10), 1563
https://doi.org/10.3390/antiox10101563 |
| 325 | Kurutas, E.B. The importance of antioxidants which play the role in cellular
response against oxidative/nitrosative stress: current state. Nutr. J. 2016,
15(1), 71
https://doi.org/10.1186/s12937-016-0186-5 |
| 326 | Tkaczenko, H.; Kurhaluk, N. Antioxidant-Rich Functional Foods and Exercise:
Unlocking Metabolic Health Through Nrf2 and Related Pathways. Int. J. Mol. Sci.
2025, 26, 1098
https://doi.org/10.3390/ijms26031098 |
| 327 | Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut
microbiota, intestinal permeability, and systemic inflammation: a narrative
review. Intern. Emerg. Med. 2024, 19(2), 275-293
https://doi.org/10.1007/s11739-023-03374-w |
| 328 | Xavier, L.E.M.d.S.; Reis, T.C.G.; Martins, A.S.d.P.; Santos, J.C.d.F.; Bueno,
N.B.; Goulart, M.O.F.; Moura, F.A. Antioxidant Therapy in Inflammatory Bowel
Diseases: How Far Have We Come and How Close Are We? Antioxidants 2024, 13, 1369
https://doi.org/10.3390/antiox13111369 |
| 329 | Mohseni, A.H.; Casolaro, V.; Bermúdez-Humarán, L.G.; Keyvani, H.; Taghinezhad,
S.S. Modulation of the PI3K/Akt/mTOR signaling pathway by probiotics as a
fruitful target for orchestrating the immune response. Gut Microbes 2021, 13(1),
1-17
https://doi.org/10.1080/19490976.2021.1886844 |
| 330 | Zhang, Z.; Jia, Y.; Zhang, C.; Zhang, Z.; Jin, F.; Pan, D.; Li, D.; Wu, X.
Efficacy of epigallocatechin gallate (EGCG) and its underlying mechanism in
preventing bisphenol-A-induced metabolic disorders in mice. J. Hazard. Mater.
2024, 469, 134098
https://doi.org/10.1016/j.jhazmat.2024.134098 |
| 331 | Mohsenzadeh, M.S.; Razavi, B.M.; Imenshahidi, M.; Tabatabaee Yazdi, S.A.;
Mohajeri, S.A.; Hosseinzadeh, H. Potential role of green tea extract and
epigallocatechin gallate in preventing bisphenol A-induced metabolic disorders
in rats: Biochemical and molecular evidence. Phytomedicine 2021, 92, 153754
https://doi.org/10.1016/j.phymed.2021.153754 |
| 332 | Lombó, M.; González-Rojo, S.; Fernández-Díez, C.; Herráez, M.P. Cardiogenesis
impairment promoted by bisphenol A exposure is successfully counteracted by
epigallocatechin gallate. Environ. Pollut. 2019, 246, 1008-1019
https://doi.org/10.1016/j.envpol.2019.01.004 |
| 333 | Chen, J.; Jin, Y.; Yang, Y.; Wu, Z.; Wu, G. Epithelial Dysfunction in Lung
Diseases: Effects of Amino Acids and Potential Mechanisms. Adv. Exp. Med. Biol.
2020, 1265, 57-70
https://doi.org/10.1007/978-3-030-45328-2_4 |
| 334 | Anderson, G.; Rodriguez, M.; Reiter, R.J. Multiple Sclerosis: Melatonin, Orexin,
and Ceramide Interact with Platelet Activation Coagulation Factors and
Gut-Microbiome-Derived Butyrate in the Circadian Dysregulation of Mitochondria
in Glia and Immune Cells. Int. J. Mol. Sci. 2019, 20(21), 5500
https://doi.org/10.3390/ijms20215500 |
| 335 | Emanowicz, P.; Średnicka, P.; Wójcicki, M.; Roszko, M.; Juszczuk-Kubiak, E.
Mitigating Dietary Bisphenol Exposure Through the Gut Microbiota: The Role of
Next-Generation Probiotics in Bacterial Detoxification. Nutrients 2024, 16(21),
3757
https://doi.org/10.3390/nu16213757 |
| 336 | Sun, B.; Liu, M.; Tang, L.; Hu, C.; Huang, Z.; Zhou, X.; Chen, L. Probiotic
supplementation mitigates the developmental toxicity of perfluorobutanesulfonate
in zebrafish larvae. Sci. Total Environ. 2021, 799, 149458
https://doi.org/10.1016/j.scitotenv.2021.149458 |
| 337 | Fang, D.; Xu, T.; Sun, J.; Shi, J.; Li, F.; Yin, Y.; Wang, Z.; Liu, Y.
Nicotinamide Mononucleotide Ameliorates Sleep Deprivation-Induced Gut Microbiota
Dysbiosis and Restores Colonization Resistance against Intestinal Infections.
Adv. Sci. (Weinh) 2023, 10(9), e2207170
https://doi.org/10.1002/advs.202207170 |
| 338 | Pérez-Burillo, S.; Navajas-Porras, B.; López-Maldonado, A.; Hinojosa-Nogueira,
D.; Pastoriza, S.; Rufián-Henares, J.Á. Green Tea and Its Relation to Human Gut
Microbiome. Molecules 2021, 26(13), 3907
https://doi.org/10.3390/molecules26133907 |
| 339 | Pires, L.; González-Paramás, A.M.; Heleno, S.A.; Calhelha, R.C. Exploring
Therapeutic Advances: A Comprehensive Review of Intestinal Microbiota
Modulators. Antibiotics (Basel) 2024, 13(8), 720
https://doi.org/10.3390/antibiotics13080720 |
| 340 | Ruiz-Malagón, A.J.; Rodríguez-Sojo, M.J.; Hidalgo-García, L.; Molina-Tijeras,
J.A.; García, F.; Pischel, I.; Romero, M.; Duarte, J.; Diez-Echave, P.;
Rodríguez-Cabezas, M.E.; Rodríguez-Nogales, A.; Gálvez, J. The Antioxidant
Activity of Thymus serpyllum Extract Protects against the Inflammatory State and
Modulates Gut Dysbiosis in Diet-Induced Obesity in Mice. Antioxidants (Basel)
2022, 11(6), 1073
https://doi.org/10.3390/antiox11061073 |
| 341 | Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez,
M.; Anadón, A.; Martínez, M.A. Synthetic phenolic antioxidants: Metabolism,
hazards and mechanism of action. Food Chem. 2021, 353, 129488
https://doi.org/10.1016/j.foodchem.2021.129488 |
| 342 | Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.; Goulart,
M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants (Basel)
2021, 10(6), 967
https://doi.org/10.3390/antiox10060967 |
| 343 | Tieu, S.; Charchoglyan, A.; Paulsen, L.; Wagter-Lesperance, L.C.; Shandilya,
U.K.; Bridle, B.W.; Mallard, B.A.; Karrow, N.A. N-Acetylcysteine and Its
Immunomodulatory Properties in Humans and Domesticated Animals. Antioxidants
2023, 12, 1867
https://doi.org/10.3390/antiox12101867 |
| 344 | Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health
Benefits. Antioxidants (Basel) 2022, 11(6), 1212
https://doi.org/10.3390/antiox11061212 |
| 345 | Kim, B.; Song, A.; Son, A.; Shin, Y. Gut microbiota and epigenetic choreography:
Implications for human health: A review. Medicine (Baltimore) 2024, 103(29),
e39051
https://doi.org/10.1097/MD.0000000000039051 |
| 346 | Lin, X.; Han, H.; Wang, N.; Wang, C.; Qi, M.; Wang, J.; Liu, G. The Gut
Microbial Regulation of Epigenetic Modification from a Metabolic Perspective.
Int. J. Mol. Sci. 2024, 25, 7175
https://doi.org/10.3390/ijms25137175 |
| 347 | Sohail, M.U.; Yassine, H.M.; Sohail, A.; Thani, A.A.A. Impact of Physical
Exercise on Gut Microbiome, Inflammation, and the Pathobiology of Metabolic
Disorders. Rev. Diabet. Stud. 2019, 15, 35-48
https://doi.org/10.1900/RDS.2019.15.35 |
| 348 | Varghese, S.; Rao, S.; Khattak, A.; Zamir, F.; Chaari, A. Physical Exercise and
the Gut Microbiome: A Bidirectional Relationship Influencing Health and
Performance. Nutrients 2024, 16(21), 3663
https://doi.org/10.3390/nu16213663 |
| 349 | Shukla, V.; Singh, S.; Verma, S.; Verma, S.; Rizvi, A.A.; Abbas, M. Targeting
the microbiome to improve human health with the approach of personalized
medicine: Latest aspects and current updates. Clin. Nutr. ESPEN 2024, 63,
813-820
https://doi.org/10.1016/j.clnesp.2024.08.005 |
| 350 | Aguiar-Pulido, V.; Huang, W.; Suarez-Ulloa, V.; Cickovski, T.; Mathee, K.;
Narasimhan, G. Metagenomics, Metatranscriptomics, and Metabolomics Approaches
for Microbiome Analysis. Evol. Bioinform. Online 2016, 12(Suppl. 1), 5-16
https://doi.org/10.4137/EBO.S36436 |
| 351 | Go, D.; Yeon, G.H.; Park, S.J.; Lee, Y.; Koh, H.G.; Koo, H.; Kim, K.H.; Jin,
Y.S.; Sung, B.H.; Kim, J. Integration of metabolomics and other omics: from
microbes to microbiome. Appl. Microbiol. Biotechnol. 2024, 108(1), 538
https://doi.org/10.1007/s00253-024-13384-z |
| 352 | Yaqub, M.O.; Jain, A.; Joseph, C.E.; Edison, L.K. Microbiome-Driven
Therapeutics: From Gut Health to Precision Medicine. Gastrointest. Disord. 2025,
7, 7
https://doi.org/10.3390/gidisord7010007 |
| 353 | Hitch, T.C.A.; Hall, L.J.; Walsh, S.K.; Leventhal, G.E.; Slack, E.; de Wouters,
T.; Walter, J.; Clavel, T. Microbiome-based interventions to modulate gut
ecology and the immune system. Mucosal Immunol. 2022, 15(6), 1095-1113
https://doi.org/10.1038/s41385-022-00564-1 |
| 354 | Kang, M.; Choe, D.; Kim, K.; Cho, B.K.; Cho, S. Synthetic Biology Approaches in
The Development of Engineered Therapeutic Microbes. Int. J. Mol. Sci. 2020,
21(22), 8744
https://doi.org/10.3390/ijms21228744 |