Knockdown of UBD Ameliorates Experimental Rheumatoid Arthritis by Suppressing TLR4/Myd88/NF-κB and P38/MAPK Pathway
bDepartment of Preclinica Sciences, Faculty of Dentistry, MAHSA University, Bandar Saujana Putra, Jeniarom, Selangor, Malaysia,
cDepartment of General Practice, Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, China
Keywords
Abstract
Background/Aims:
Ubiquitin D (UBD), a member of the ubiquitin-like modifier (UBL) family, is significantly overexpressed in various cancers and is positively correlated with tumor progression. However, the role and underlying mechanisms of UBD in rheumatoid arthritis (RA) remain poorly understood. This study aimed to investigate the effects of UBD knockdown on the progression of RA.Materials:
We employed the type II collagen and incomplete Freund’s adjuvant (CIA) rat model. A variety of analytical techniques were employed, including hematoxylin and eosin (H&E) staining, Safranin O and Fast Green staining, tartrate-resistant acid phosphatase (TRAP) staining, enzyme-linked immunosorbent assay (ELISA), and Western blot analysis, to elucidate the mechanisms involved.Results:
UBD knockdown correlated with diminished cartilage and bone erosion, reduced counts of TRAP-positive osteoclasts, and enhanced Safranin O staining of the cartilage. Additionally, the knockdown significantly reduced serum levels of PGE2, TNF-α, TIMP-1, IL-1β, MMP-9, and IL-6 in CIA rats. Furthermore, UBD knockdown markedly suppressed the expression levels of phosphorylated p38, TLR4, MyD88, and phosphorylated p65, suggesting a critical role in modulating inflammatory signaling pathways in RA.Conclusion:
Collectively, these results suggested that knockdown of UBD significantly alleviated arthritis progression in the CIA rat model, highlighting UBD as a potential therapeutic target and a promising prognostic biomarker for RA.Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disorder characterized by inflammation of the synovial joints, leading to progressive joint destruction and subsequent disability. The global prevalence of RA is estimated to range from 0.5% to 1% [1-3]. Beyond joint involvement, RA can also cause systemic complications such as cardiovascular diseases, osteoporosis, and infections, which significantly affect the quality of life of those affected [4]. The pathogenesis of RA is driven by complex interactions between genetic susceptibility and environmental triggers, resulting in dysregulated immune responses, particularly involving T and B lymphocytes, macrophages, and synovial fibroblasts [5, 6].
Current treatments for RA focus on reducing inflammation, managing symptoms, and preventing joint damage. Traditional disease-modifying antirheumatic drugs (DMARDs), like methotrexate (MTX), are typically the first-line treatment, often used in combination with biologic DMARDs (bDMARDs) or targeted synthetic DMARDs (tsDMARDs) [2, 7]. Despite advances in therapy, there is a critical need for new therapeutic targets, especially for patients who fail to adequately respond to existing treatments or suffer from notable side effects [4, 8, 9]. Drug resistance also remains a significant challenge in RA treatment [10]. Therefore, further research is essential to better understand the mechanisms driving RA pathogenesis and identify novel therapeutic targets to improve disease control and long-term prognosis.
Proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), are crucial to the development of RA [11]. Elevated levels of IL-1β or TNF-α prompt IL-6 production, triggering an inflammatory cascade that leads to bone damage and the development of pannus [12]. Furthermore, matrix metalloproteinases (MMPs), zinc-dependent enzymes that degrade the extracellular matrix, facilitate angiogenesis and pannus formation, exacerbating symptoms such as joint pain, swelling, and the erosion of bone and cartilage [13]. Upon activation, MMP activity is regulated by a combination of broad-spectrum and specific tissue inhibitors of metalloproteinases (TIMPs) [14]. Additionally, inflammatory cytokines like TNF-α, IL-1β, and IL-6 are recognized for their role in activating MMPs, promote their release, and increase their expression within the context of RA [15]. Prostaglandin E2 (PGE2) acts as a principal mediator of inflammation and is vital in regulating the activities of osteoblasts and osteoclasts [16]. Interventions targeting the prevention or reduction of these proinflammatory cytokines are expected to significantly attenuate the progression of RA.
Ubiquitin D (UBD), also recognized as FAT10, is a prominent ubiquitin-like modifier implicated in various disease contexts [17]. UBD plays a crucial role in immunomodulation, including antigen presentation, immune response, and combating viral infections [18]. Elevated levels of UBD have been observed in several tumor types, including liver cancer [19], colorectal cancer [20] and breast cancer [21]. UBD significantly influences tumorigenesis and therapeutic resistance by modulating key signaling pathways. For example, in pancreatic cancer, UBD promotes chemoresistance by stabilizing FOXM1, thereby facilitating epithelial-mesenchymal transition (EMT) [22]. Moreover, UBD has been implicated in osteosarcoma development through regulation of the JAK/STAT signaling pathway, underscoring its multifaceted role in cancer progression [23].
In RA, the role of UBD remains largely underexplored but holds potential significance. Recent studies indicate that UBD may contribute to RA development by triggering the p38 MAPK pathway, thereby increasing inflammation and joint damage [24]. Given its multifaceted roles, further in vivo investigations are necessary to clarify the precise mechanisms of UBD and assess its therapeutic potential [24]. Such research would not only deepen our understanding of UBD's role in RA but also pave the way for developing targeted therapies aimed at mitigating its harmful effects, offering new opportunities for therapeutic intervention in RA.
Materials and Methods
Animals and CIA model
Six-week-old Wistar rats (n=30) were obtained from Shanghai Silaike Experimental Animal
Limited Liability Company (Shanghai, China). The animals were housed in a controlled environment at 24 ± 1
°C
(mean ± SD) and a 12-hour light/dark cycle. They were provided unlimited access to food and water. All
animal
procedures and care protocols were approved by the Medical Ethics Committee of the Affiliated Hospital of
Youjiang Medical College for Nationalities (Approval No. YYFY-LL-2022-96).
The rats were randomly allocated into two groups: a Control group (n=6) and a CIA model group (n=24). In
the CIA
model group, a solution of type II collagen (No. 20022, Chondrex) was mixed with Freund’s incomplete
adjuvant
(No. F5506, Sigma) in a 1:1 volume ratio and homogenized in a mortar until fully emulsified. Each rat in
this
group received a 200 µL intradermal injection of the emulsion at the tail base, 0.5 cm from the root. On
day 7
post-injection, a booster of the same emulsion was administered 1.5 cm from the tail base to intensify the
immunization. The Control group rats were injected with a comparable volume of PBS.
Two weeks later, the CIA rats were randomly divided into four distinct groups: a CIA model group, a
negative
control group, a UBD knockdown group and a MTX group (positive control, 7.6 mg/kg, No. 1414003, Sigma).
MTX, a
disease-modifying antirheumatic drug, is widely used in the treatment of RA [25]. Its primary function is
to
suppress the abnormal immune response, reduce joint inflammation and pain, and ultimately enhance the
patient's
quality of life. In this study, MTX was employed as the positive control group [26]. Rats in the UBD
knockdown
group were injected via the caudal vein with 2.5 × 10^10 plaque-forming units of an adenovirus carrying
the UBD
gene (UBD-siRNA, sequence: GGTTCCTGTGCAGGACCAGGT), while negative control rats received an injection of an
empty
virus.
Assessment of the arthritis severity and effects of the treatment
To rigorously assess the severity and progression of arthritis, hind paw volumes
were quantified post-arthritis induction using a plethysmometer chamber (Jinan, China), and body weight
was
recorded with a precision balance (accuracy: 0.1 g, Sartorius AG, Germany). The severity of arthritis
across all
four paws was evaluated by two independent assessors employing a semi-quantitative scale established by
van Eden
et al. (2001). The scoring criteria were as follows: 0 (no observable signs of arthritis), 4-8 (mild
changes),
8-12 (moderate changes), and 12-16 (severe changes). Additionally, the erythrocyte sedimentation rate
(ESR) was
measured on day 42 using a method approved by the International Council for Standardization in Hematology
(ICSH), with minor modifications. Four weeks after adenovirus administration, the rats were euthanized,
and
their hind paws were collected for subsequent analyses. Hematoxylin and eosin
(H&E) staining
H&E staining was performed using a kit from Solarbio (No. G1120). In brief, hind paw sections were
first
washed in distilled water, then stained with a hematoxylin solution for 5 min, followed by a 10-minute
rinse
under running tap water. The sections were then differentiated in 1% acid alcohol for 10 sec and washed
again
under running tap water for 10 min. Next, the sections were counterstained with eosin solution for 30 sec
and
subjected to another 5-minute rinse in running tap water. Dehydration and clearing were performed using
95%
ethyl alcohol, then absolute ethyl alcohol, and finally xylene, with each step repeated twice for two min.
The
sections were ultimately mounted in a resin-based medium. Safranin O and fast green
staining
Safranin O and Fast Green staining was conducted as previously described [27]. The hind paw sections were
initially hydrated in distilled water and stained with Weigert’s Iron Hematoxylin for 5 min, followed by
thorough washing in distilled water to remove any excess dye. The sections were then differentiated in 1%
acid-alcohol for 3 sec and gently rinsed in distilled water. Subsequently, the sections were stained with
0.2%
Fast Green (No. C500016, Shanghai Sangon Biology Co., Ltd) for 5 min, quickly rinsed with 1% acetic acid
solution for 10 sec, and then stained with 0.1% safranin O solution (No. A600815, Shanghai Sangon Biology
Co.,
Ltd) for 5 min. Dehydration and clearing were achieved using 95% ethyl alcohol, absolute ethyl alcohol,
and
xylene, with each solution applied for 2 min in two successive rounds. The sections were then mounted in a
resinous medium.
TRAP Staining
The TRAP incubation solution was prepared according to the instructions provided with the TRAP staining
kit (No.
HR0561, Beijing Baiaolaibo Technology Co., Ltd). Hind paw sections were first deparaffinized in water,
then
coated with the ready-to-use TRAP staining solution and incubated at 37°C for 30 min. After incubation,
the
sections were washed with distilled water and treated with a 2-amino-2-methyl-1, 3-propanediol (AMPD)-HCl
solution (pH 9.4) for 10 min. The sections were then re-stained with methyl green, washed again with
distilled
water, dried, and mounted. An inverted optical microscope (40× magnification) was utilized to identify
fields of
view, from which images were captured and analyzed. The density of osteoclasts in each TRAP-stained slide
was
quantified using ImageJ software.
Enzyme linked immunosorbent assay (ELISA) Assay
Serum concentrations of PGE2, TNF-α, TIMP-1, IL-1β, MMP-9, and IL-6 were determined using ELISA kits,
following
the manufacturers' protocols. The ELISA kits for PGE2 (cat. no. 514010) and IL-1β (cat. no. 37810) were
obtained
from Cayman Chemicals, while the kits for TNF-α (cat. no. BMS223HS), TIMP-1 (cat. no. ERTIMP1), MMP9 (cat.
no.
EEL130), and IL-6 (cat. no. ERA31RB) were sourced from Thermo Fisher Scientific. Calibration curves were
constructed on semi-logarithmic paper, and the optical density values for the samples were calculated from
these
standard curves across three separate assays.
Western blot
Proteins were extracted from hind paw tissues and quantified via the BCA method (No. 23225, Thermo
Fisher). Each
sample, containing 5 μg of protein, was mixed with 5 × SDS sample buffer and subjected to electrophoresis
on 12%
SDS-acrylamide gels, followed by transfer onto PVDF membranes. The membranes were blocked with 5% non-fat
milk
and incubated overnight at 4°C with primary antibodies: mouse anti-phospho-p38 (No. 9216; Cell Signaling
Technology), rabbit anti-p38 (No. bs-0637R; Bioss), rabbit anti-TLR4 (No. 19811-1-AP; Proteintech), rabbit
anti-MyD88 (No. 4283; Cell Signaling Technology), rabbit anti-phospho-p65 (No. 3033T; Cell Signaling
Technology), rabbit anti-p65 (No. 4764T; Cell Signaling Technology), and mouse anti-GAPDH (No. 10068-1-AP;
Proteintech). Following primary antibody incubation, the membranes were washed with TBST and then
incubated for
1 h with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse secondary antibodies (No.
074-1506 and
074-1807; KPL, Inc.). Immunoreactive bands was detected using an ECL hypersensitive chemiluminescence kit
(cat.
no. P0018M; Beyotime) and an Odyssey Scanning System (version 3.0, LI-COR Biosciences).
Statistical analysis
The data collected from the
experiments were analyzed using GraphPad Prism software (Version 8.0; California, USA). Data are presented
as
the mean ± standard deviation. For analyses involving comparisons across three or more groups, a one-way
ANOVA
was performed to assess group variations, followed by Tukey’s post hoc test to identify specific
differences
between groups. This statistical approach was chosen to minimize the risk of Type I errors in multiple
comparisons. A p-value of less than 0.05 was considered statistically significant.
Results
UBD knockdown mitigated rheumatoid arthritis progression
To evaluate the therapeutic efficacy of UBD knockdown in alleviating arthritis
symptoms, a CIA model was meticulously developed by administering Type II collagen and incomplete Freund’s
adjuvant via intra-tail injection. A notable decrease in body weight was noted in the CIA rats from day 9
to day
42 after the injection. However, treatment with methotrexate (MTX, 7.6 mg/kg, serving as a positive
control) led
to an additional reduction in body weight, whereas silencing UBD significantly attenuated the weight loss
in CIA
rats (Fig. 1A). From day 9 to day 42, CIA rats exhibited a marked increase in paw swelling volume and
arthritis
scores compared to normal controls (Fig. 1B-C). However, UBD knockdown or MTX treatment effectively
attenuated
both paw edema and arthritis scores (Fig. 1B-C). Additionally, the ESR, which significantly elevated in
CIA
rats, was substantially decreased following UBD knockdown or MTX treatment (Fig. 1D).
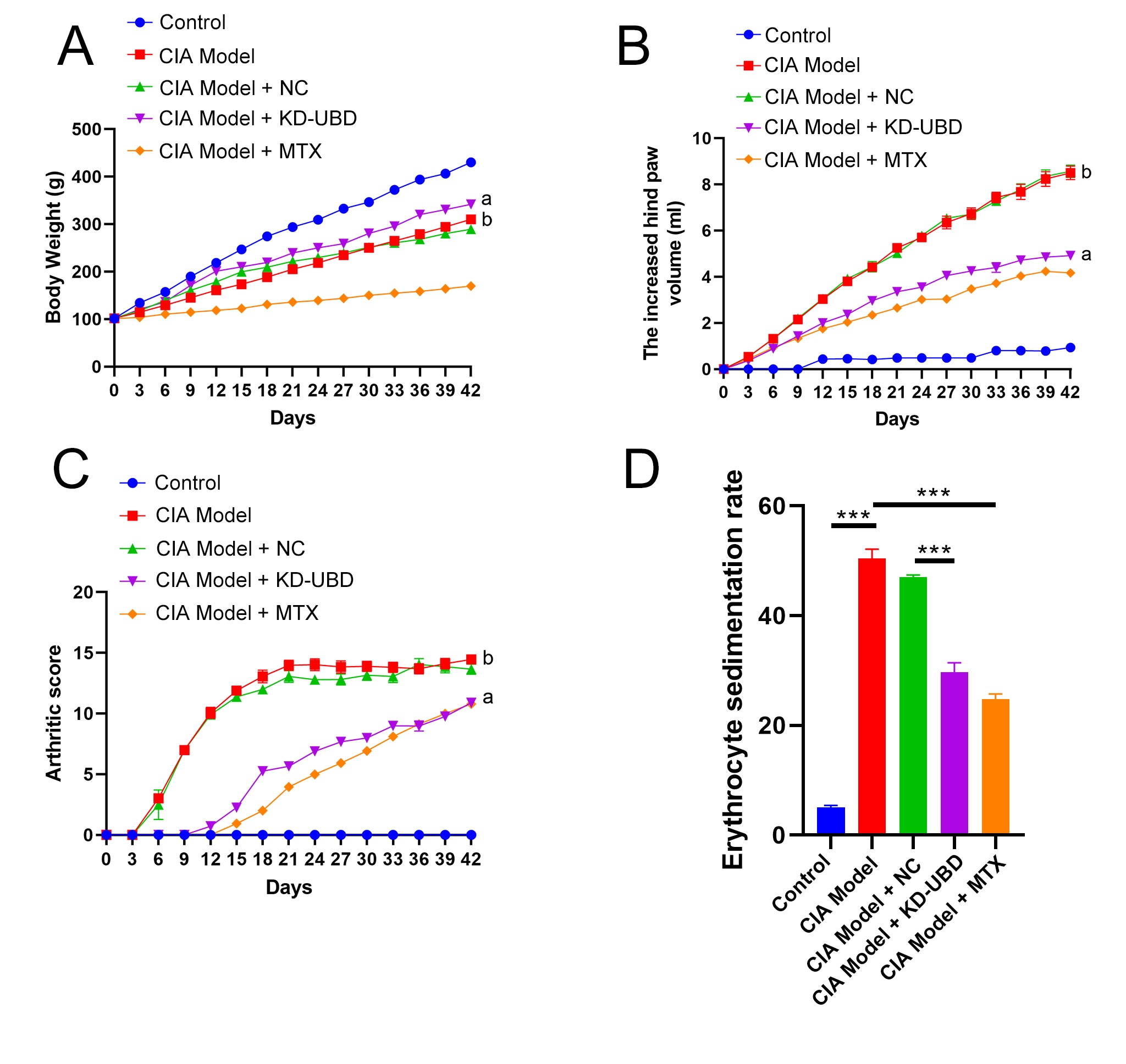
Fig. 1: UBD knockdown mitigates rheumatoid arthritis progression .A solution of type II collagen mixed with Freund’s incomplete adjuvant was used to established CIA rats. Two weeks later, CIA rats were randomly assigned to four groups: vehicle, negative control, UBD knockdown, or MTX (n=6). Four weeks after the adenovirus injection, the rats were euthanized. Hind paws were excised for the next assessment. (A) The body weight in CIA rats was recorded. (B) The hind paw volumes in CIA rats were calculated. (C) The arthritic scores in CIA rats were calculated. a: CIA Model + KD-UBD group compared to CIA Model + NC group, p < 0.001. b: CIA model group compared to Control group, p < 0.001. (D) Plasma ESR in CIA rats was measured. ***p < 0.001, n=6.
UBD knockdown prevented bone destruction in CIA rats
Histological examination of CIA rats treated with vehicle revealed a notably thickened and enlarged
synovium,
characterized by extensive infiltration of inflammatory cells, hyperplasia, conspicuous cartilage, and
narrowing
of joint spaces, confirming successful establishment of the CIA model (Fig. 2A). Knockdown of UBD or
treatment
with MTX reduced inflammatory cell infiltration and synovial hyperplasia, along with limited cartilage and
bone
erosion, implying that UBD knockdown effectively mitigates arthritis progression in CIA rats (Fig. 2A).
TRAP staining was performed to assess osteoclast activity, which plays a key role in bone erosion
associated
with RA. The results showed an increased number of TRAP-positive osteoclasts in the ankle region of CIA
rats,
indicating enhanced bone resorption or damage (Fig. 2B). Importantly, knockdown of UBD or treatment with
MTX
significantly reduced the number of TRAP-positive osteoclasts, suggesting that UBD knockdown effectively
inhibits bone resorption activity in CIA rats (Fig. 2B).
The results of Safranin O and fast green staining revealed a decrease in Safranin O staining intensity in
the
cartilage of CIA rats, indicating a reduction in proteoglycan content (Fig. 2C). Interestingly, knockdown
of UBD
or treatment with MTX prominently enhanced Safranin O staining in the cartilage, suggesting a potential
improvement in cartilage health due to UBD knockdown in CIA rats (Fig. 2C).
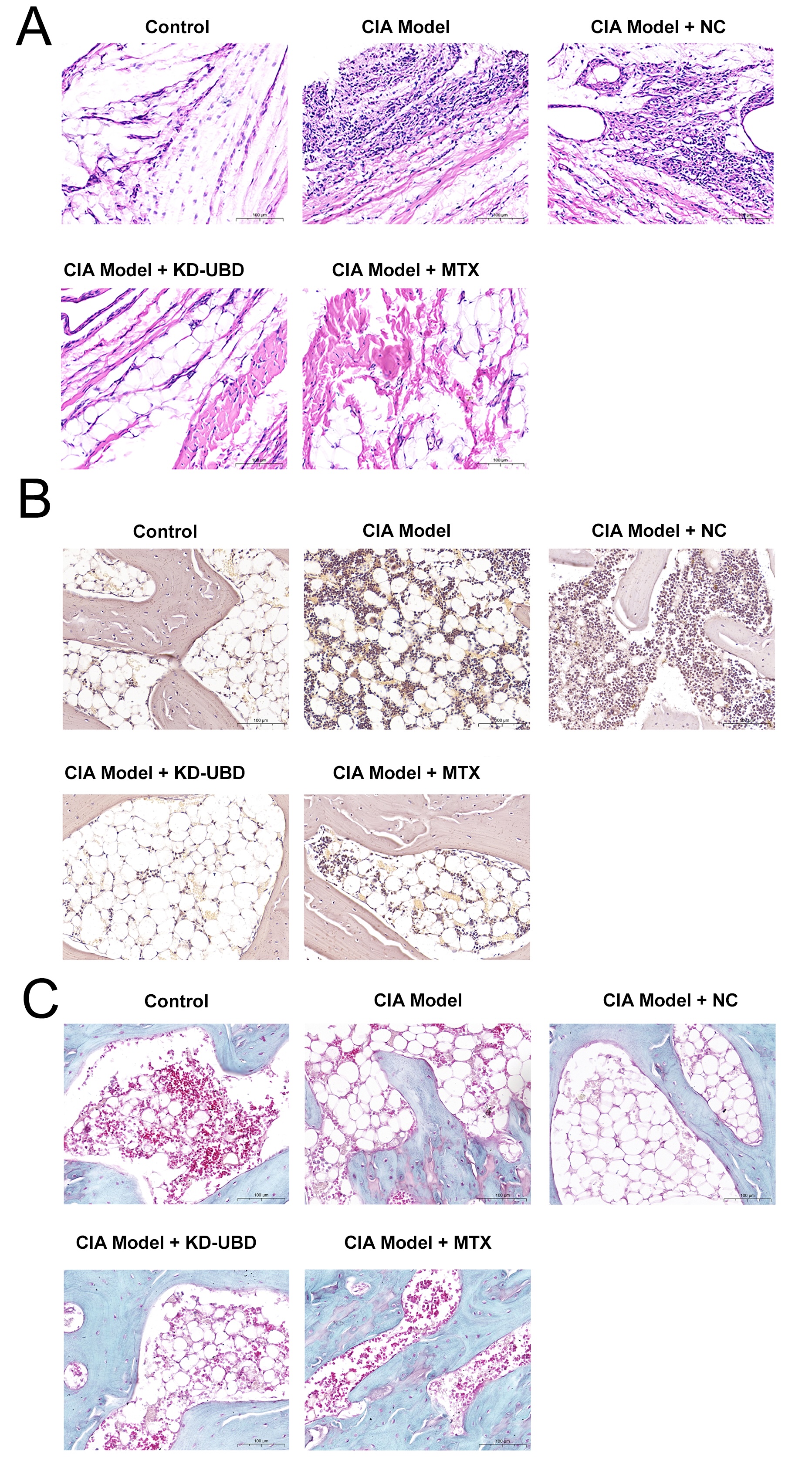
Fig. 2: UBD knockdown prevents bone destruction in CIA rats. Four weeks after adenovirus administration, the rats were euthanized, and their hind paws were harvested for subsequent analyses. (A) Representative H&E staining images of the hind paws. (B) Representative TRAP staining images of the hind paws. (C) Representative Safranin O and fast green staining images of the hind paws. (magnification × 40, n=3).
UBD knockdown inhibited chemokines and cytokines in CIA rats
Cytokines and chemokines are pivotal mediators in the pathogenesis of RA [11]. ELISA results showed
significantly elevated serum levels of PGE2, TNF-α, TIMP-1, IL-1β, MMP-9, and IL-6 in CIA rats compared to
normal controls (Fig. 3A-F). In contrast, knockdown of UBD or treatment with MTX resulted in a significant
decrease in the levels of these cytokines and chemokines (Fig. 3A-F). Collectively, these findings suggest
that
UBD knockdown effectively mitigates the progression of arthritis in CIA rats.
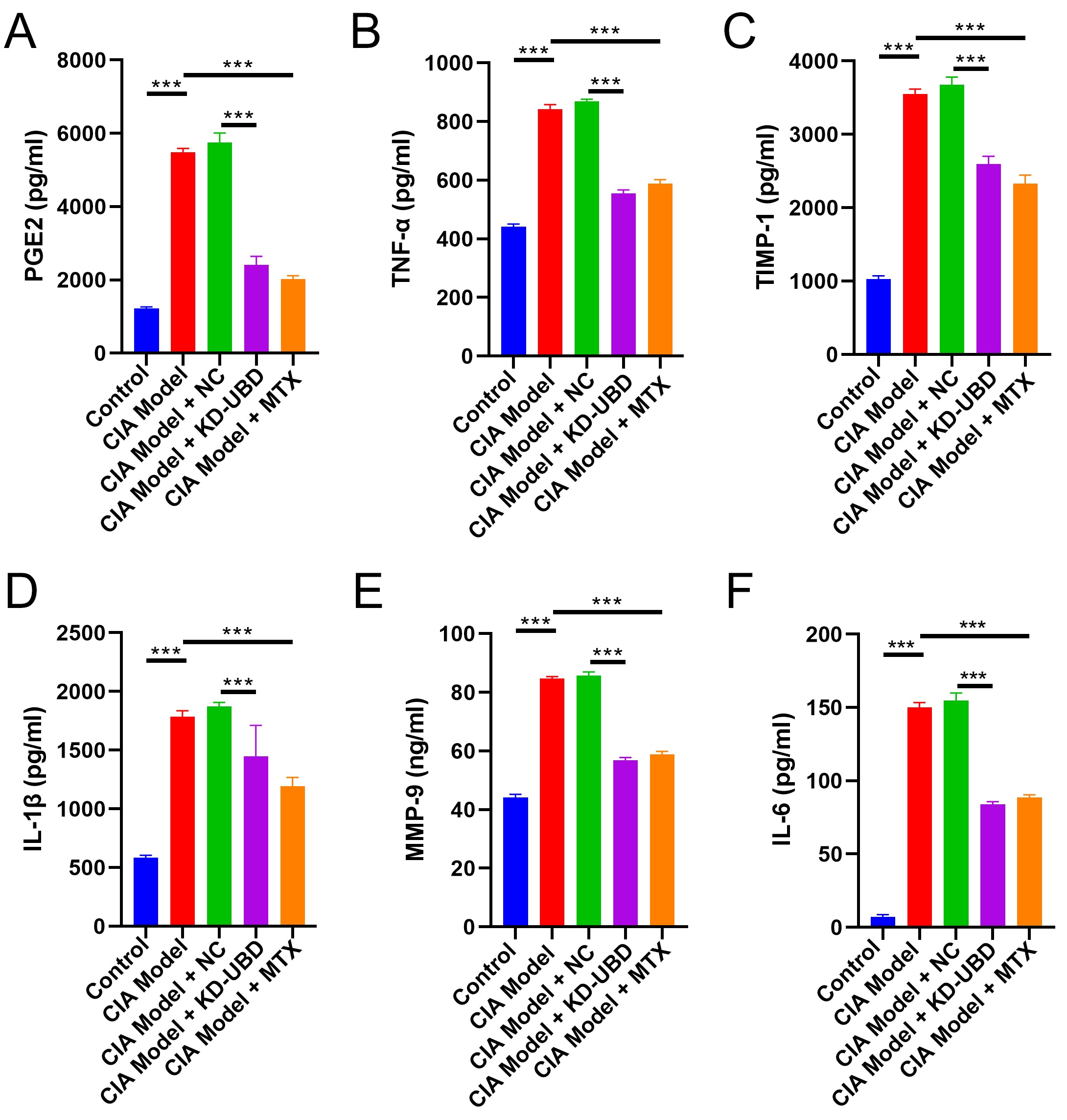
Fig. 3: UBD knockdown inhibited chemokines and cytokines in CIA rats. Four weeks after adenovirus administration, the rats were euthanized and the serum was collected for ELISA assay. The serum concentration of PGE2 (A), TNF-α (B), TIMP-1 (C), IL-1β (D), MMP-9 (E) and IL-6 (F) were measured by ELISA. (n=3, ***p < 0.001).
UBD knockdown suppressed p38/MAPK and TLR4/MyD88/NF-κB pathway
Knockdown of UBD significantly reduced the protein expression of p-p38 without
affecting the expression of p38(Fig. 4A-B). Notably, UBD knockdown also inhibited the protein level of
TLR4,
MyD88, and p-p65, while showing no obvious effect on p65 expression (Fig. 4A-B). These findings indicate
that
UBD knockdown suppresses the activation of the p38/MAPK and TLR4/MyD88/NF-κB pathways, which may
contribute to
its inhibitory effect on the progression of RA.
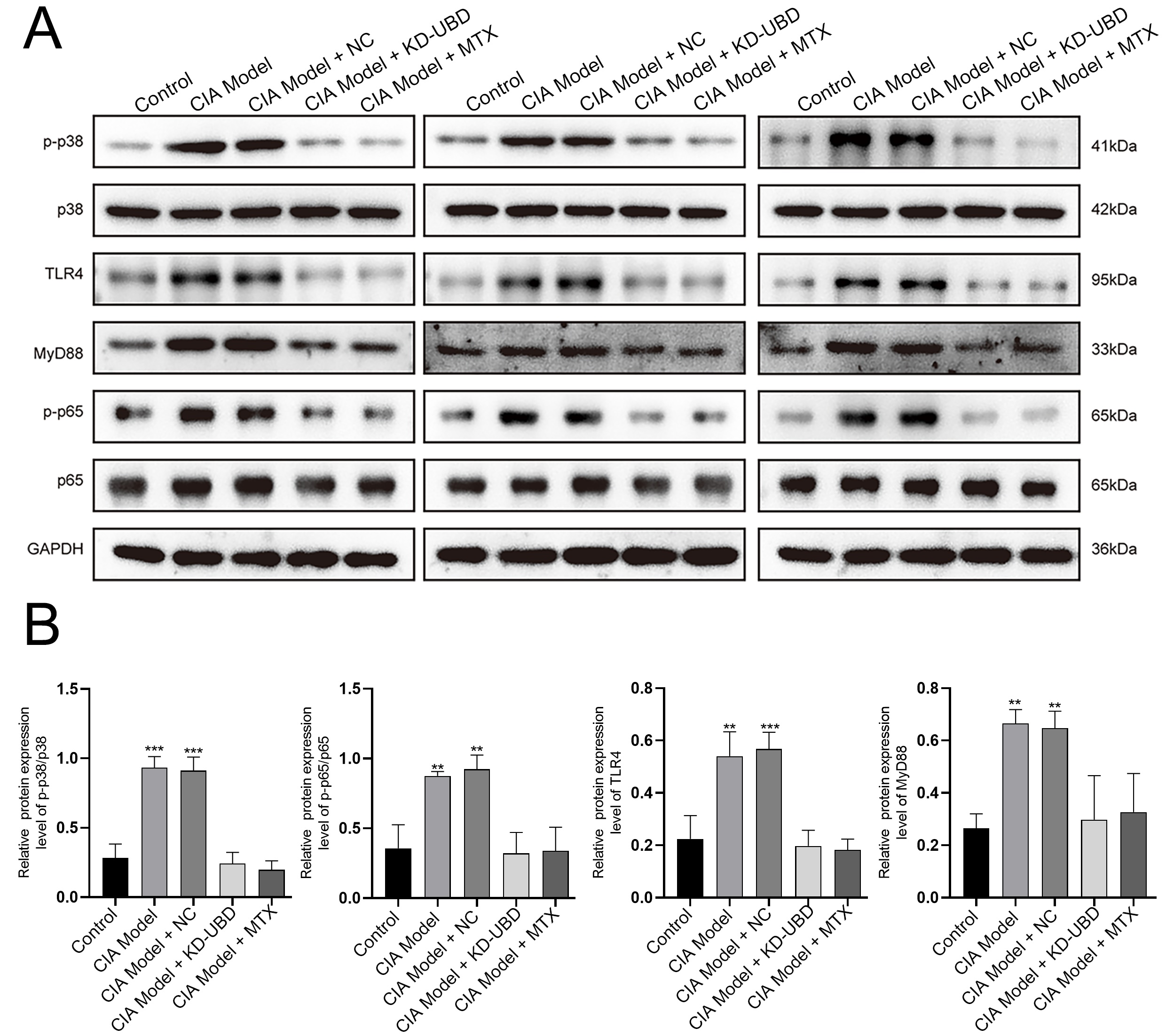
Fig. 4: UBD knockdown suppressed p38/MAPK and TLR4/MyD88/NF-κB pathway. Proteins were extracted from hind paw tissues for Western blot analysis. (A) The protein level of p-p38, p38, TLR4, MyD88, p-p65 and p65 were examined by Western blot. (B) Quantification of gene expression was normalized to GAPDH. Means ± SD (n=3, **p < 0.01, ***p < 0.001).
Discussion
Deregulated gene expression critically influences the development of RA by interacting with the immune system to amplify inflammatory processes [8, 28]. Previous studies from our group have demonstrated that UBD accelerates RA progression through activation of the p38 MAPK pathway [24]. However, the specific roles of UBD in RA development remain to be fully elucidated. This study demonstrates that targeted knockdown of UBD significantly attenuates RA progression by suppressing both the p38/MAPK and the TLR4/MyD88/NF-κB signaling pathways, highlighting potential new targets for therapeutic intervention and management of RA.
UBD is closely associated with the immune system and is significantly upregulated by pro-inflammatory cytokines [29]. Notably, UBD is the only ubiquitin-like modifier (ULMs) that independently triggers degradation through the 26S proteasome, a mechanism that does not require ubiquitin [30]. Predominantly triggered by pro-inflammatory cytokines within the tumor microenvironment, substantial research indicates that the oncogenic potential of UBD plays a significant role in its elevated expression in tumor tissues [17, 31]. Numerous studies have documented the overexpression of UBD across various cancer types, where it promotes cell migration, invasion, and metastasis development [32-34]. Additionally, UBD is among the most highly upregulated genes in RA according to GEO dataset analyses [24]. However, further investigation into UBD expression in RA clinical samples was hindered by the lack of available specimens. In this study, we investigated the role of UBD in RA using a CIA model, which partially reflects the in vivo situation. Our findings show that UBD knockdown reduces inflammatory cell infiltration and synovial hyperplasia. Additionally, this knockdown is associated with decreased cartilage and bone erosion, a reduction in TRAP-positive osteoclasts, and enhanced Safranin O staining of the cartilage. Collectively, these results suggest that UBD knockdown significantly alleviates the progression of arthritis in CIA rats.
Potential triggers of RA encompass cytokines, and dysregulation of the cytokine network can lead to uncontrolled inflammation culminating in RA [11]. Elevated expressions of IL-1β or TNF-α trigger the generation of IL-6, which then initiates an inflammatory cascade, resulting in bone degradation and pannus formation [35]. Targeted inhibition of TNF-α and IL-6 has been shown to be effective in treating resistant RA cases [36]. TNF-α, IL-1β, and IL-6 are known to activate MMPs, promote MMP release, and increase their expression in RA [15]. TIMPs counteract the proteolytic activity of MMPs, with both MMPs and TIMPs playing pivotal roles in the autoregulation of inflammation in RA [37]. PGE2 is another key mediator of inflammation in diseases such as RA, and suppression of PGE2 synthesis can alleviate the inflammation characteristic of RA [38, 39]. Therefore, reducing the expression levels of PGE2, TNF-α, TIMP-1, IL-1β, MMP-9, and IL-6 can significantly ameliorate the symptoms of RA. In this study, UBD knockdown led to a marked decrease in the serum concentrations of PGE2, TNF-α, TIMP-1, IL-1β, MMP-9, and IL-6, which were notably elevated in the serum of CIA rats. These findings robustly substantiate the therapeutic potential of UBD knockdown in the management of RA.
UBD influences various signaling pathways critical to tumor growth. For example, UBD promotes the progression of oral squamous cell carcinoma through NF-κB signaling [40] and facilitates the invasion and metastasis of hepatocellular carcinoma cells by interacting with β-catenin, inhibiting its ubiquitylation and subsequent degradation [41]. The p38/MAPK pathway plays a central role in the inflammatory response in RA, regulating the release of inflammatory cytokines and contributing to the proliferation and migration of synovial fibroblasts, which are key players in the pathogenesis of RA [42, 43]. Similarly, the TLR4/MyD88/NF-κB signaling pathway is a critical mediator of inflammation in RA. Activation of TLR4 triggers the MyD88-dependent pathway, leading to NF-κB activation, which subsequently enhances the generation of pro-inflammatory cytokines and mediates immune responses [44-46]. Increased protein levels of p-p38, TLR4, MyD88, and p-p65 have been observed in CIA rats. Notably, UBD knockdown significantly reduces the protein expression of p-p38, TLR4, MyD88 and p-p65, without markedly affecting the levels of p38 and p65. This suggests that UBD knockdown suppresses both p38/MAPK and TLR4/MyD88/NF-κB pathways in RA progression. Overall, these findings indicate that UBD could be a key pathogenic factor in RA through these pathways, thus presenting additional opportunities for therapeutic intervention in RA.
While our findings provide promising insights into UBD as a key pathogenic factor in RA through its interaction with the p38/MAPK and TLR4/MyD88/NF-κB pathways, the study has certain limitations. Future studies should investigate the regulatory role of UBD in other relevant pathways, including JAK/STAT, NFAT, and the NLRP3 inflammasome, which are also integral to the immune and inflammatory processes of RA. A multi-pathway approach will provide a more comprehensive understanding of UBD's role in RA and support the development of multi-target therapeutic strategies. Additionally, further investigation into the expression of downstream targets within the p38/MAPK or TLR4/MyD88/NF-κB pathways could offer further confirmation of UBD’s regulatory role. Our conclusions were primarily based on the CIA animal model, and thus, the lack of clinical data limits the generalizability of the results. Future research should prioritize the collection and analysis of clinical samples from RA patients—including synovial fluid, peripheral blood, and tissue biopsies—to validate UBD expression levels. Furthermore, investigating the relationship between UBD and disease progression, alongside key clinical indicators such as C-reactive protein, erythrocyte sedimentation rate, and rheumatoid factor, will be essential to confirm its potential as a biomarker for RA.
Conclusion
Knockdown of UBD may mitigate the progression of RA by inhibiting the p38/MAPK and TLR4/MyD88/NF-κB pathways. This provides novel insights into the pathogenesis of RA and underscores the potential of UBD as a therapeutic target for combating this disease.
Acknowledgements
PI and HW contributed to study conception and design. Data collection and analysis were performed by HC, SL and XRD. The manuscript was drafted by HC and revised by HW. PI and HW confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
This work was supported by Research Project of High-Level and Middle-aged and Young Backbone Talents of Affiliated Hospital of Youjiang Medical College for Nationalities in 2021 (Y20213003), the Natural Science Foundation of Scientific research project of Guangxi Zhuang Autonomous Region Administration of traditional Chinese medicine (gzzc2019145) and scientific research project of Guangxi Zhuang Autonomous Region Health Committee (z20200163).
The authors utilized ChatGPT during the preparation of this work to enhance language clarity and readability. All content was subsequently reviewed and edited as needed by the authors, who take full responsibility for the final version of the manuscript.
All protocols for animal handling and care used in the experiments received approval from the Medical Ethics Committee of the Affiliated Hospital of Youjiang Medical College for Nationalities (Approval No. YYFY-LL-2022-96).
Disclosure Statement
Authors state no Disclosure Statement.
References
| 1 | O'Neil LJ, Barrera-Vargas A, Sandoval-Heglund D, Merayo-Chalico J, Aguirre-Aguilar E, Aponte AM,
Ruiz-Perdomo Y, Gucek M, El-Gabalawy H, Fox DA, Katz JD, Kaplan MJ, Carmona-Rivera C:
Neutrophil-mediated carbamylation promotes articular damage in rheumatoid arthritis. Sci Adv 2020;6
https://doi.org/10.1126/sciadv.abd2688 |
| 2 | Radu AF, Bungau SG: Management of Rheumatoid Arthritis: An Overview. Cells 2021;10
https://doi.org/10.3390/cells10112857 |
| 3 | Smith MH, Berman JR: What Is Rheumatoid Arthritis? JAMA 2022;327:1194.
https://doi.org/10.1001/jama.2022.0786 |
| 4 | Wu D, Luo Y, Li T, Zhao X, Lv T, Fang G, Ou P, Li H, Luo X, Huang A, Pang Y: Systemic
complications of rheumatoid arthritis: Focus on pathogenesis and treatment. Front Immunol
2022;13:1051082.
https://doi.org/10.3389/fimmu.2022.1051082 |
| 5 | Jang S, Kwon EJ, Lee JJ: Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int J Mol
Sci 2022;23
https://doi.org/10.3390/ijms23020905 |
| 6 | Lin YJ, Anzaghe M, Schulke S: Update on the Pathomechanism, Diagnosis, and Treatment Options for
Rheumatoid Arthritis. Cells 2020;9
https://doi.org/10.3390/cells9040880 |
| 7 | Cush JJ: Rheumatoid Arthritis: Early Diagnosis and Treatment. Med Clin North Am 2021;105:355-365.
https://doi.org/10.1016/j.mcna.2020.10.006 |
| 8 | Huang J, Fu X, Chen X, Li Z, Huang Y, Liang C: Promising Therapeutic Targets for Treatment of
Rheumatoid Arthritis. Front Immunol 2021;12:686155.
https://doi.org/10.3389/fimmu.2021.686155 |
| 9 | Donlin LT: Inching closer to precision treatment for rheumatoid arthritis. Nat Med
2022;28:1129-1131.
https://doi.org/10.1038/s41591-022-01857-5 |
| 10 | Lourenzi FM, Jones A, Pereira DF, Santos J, Furtado RNV, Natour J: Effectiveness of an overall
progressive resistance strength program for improving the functional capacity of patients with
rheumatoid arthritis: a randomized controlled trial. Clin Rehabil 2017;31:1482-1491.
https://doi.org/10.1177/0269215517698732 |
| 11 | Diaz-Gonzalez F, Hernandez-Hernandez MV: Rheumatoid arthritis. Med Clin (Barc) 2023;161:533-542.
https://doi.org/10.1016/j.medcle.2023.07.008 |
| 12 | Kondo N, Kuroda T, Kobayashi D: Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int
J Mol Sci 2021;22
https://doi.org/10.3390/ijms222010922 |
| 13 | Grillet B, Pereira RVS, Van Damme J, Abu El-Asrar A, Proost P, Opdenakker G: Matrix
metalloproteinases in arthritis: towards precision medicine. Nat Rev Rheumatol 2023;19:363-377.
https://doi.org/10.1038/s41584-023-00966-w |
| 14 | Behm C, Nemec M, Weissinger F, Rausch MA, Andrukhov O, Jonke E: MMPs and TIMPs Expression Levels
in the Periodontal Ligament during Orthodontic Tooth Movement: A Systematic Review of In vitro and
In vivo Studies. Int J Mol Sci 2021;22
https://doi.org/10.3390/ijms22136967 |
| 15 | Jahid M, Rehan Ul H, Jha PK, Chawla D, Avasthi R, Ahmed RS: Tumor necrosis factor-alpha -308
polymorphism in North Indian rheumatoid arthritis patients and association with mRNA and serum
TNF-alpha. Clin Rheumatol 2017;36:2209-2216.
https://doi.org/10.1007/s10067-017-3774-7 |
| 16 | Epsley S, Tadros S, Farid A, Kargilis D, Mehta S, Rajapakse CS: The Effect of Inflammation on
Bone. Front Physiol 2020;11:511799.
https://doi.org/10.3389/fphys.2020.511799 |
| 17 | Aichem A, Groettrup M: The ubiquitin-like modifier FAT10 in cancer development. Int J Biochem Cell
Biol 2016;79:451-461.
https://doi.org/10.1016/j.biocel.2016.07.001 |
| 18 | Battaglia A, Fossati M, Buzzonetti A, Scambia G, Fattorossi A: A robust immune system conditions
the response to abagovomab (anti-idiotypic monoclonal antibody mimicking the CA125 protein)
vaccination in ovarian cancer patients. Immunol Lett 2017;191:35-39.
https://doi.org/10.1016/j.imlet.2017.09.006 |
| 19 | Paijens ST, Leffers N, Daemen T, Helfrich W, Boezen HM, Cohlen BJ, Melief CJ, de Bruyn M, Nijman
HW: Antigen-specific active immunotherapy for ovarian cancer. Cochrane Database Syst Rev
2018;9:CD007287.
https://doi.org/10.1002/14651858.CD007287.pub4 |
| 20 | Kalli KR, Block MS, Kasi PM, Erskine CL, Hobday TJ, Dietz A, Padley D, Gustafson MP, Shreeder B,
Puglisi-Knutson D, Visscher DW, Mangskau TK, Wilson G, Knutson KL: Folate Receptor Alpha Peptide
Vaccine Generates Immunity in Breast and Ovarian Cancer Patients. Clin Cancer Res 2018;24:3014-3025.
https://doi.org/10.1158/1078-0432.CCR-17-2499 |
| 21 | Han T, Liu Z, Li H, Xie W, Zhang R, Zhu L, Guo F, Han Y, Sheng Y, Xie X: High expression of UBD
correlates with epirubicin resistance and indicates poor prognosis in triple-negative breast cancer.
Onco Targets Ther 2015;8:1643-1649.
https://doi.org/10.2147/OTT.S81214 |
| 22 | Zhu J, Zhao J, Luo C, Zhu Z, Peng X, Zhu X, Lin K, Bu F, Zhang W, Li Q, Wang K, Hu Z, Yu X, Chen
L, Yuan R: FAT10 promotes chemotherapeutic resistance in pancreatic cancer by inducing
epithelial-mesenchymal transition via stabilization of FOXM1 expression. Cell Death Dis 2022;13:497.
https://doi.org/10.1038/s41419-022-04960-0 |
| 23 | Shi F, Li L, Cheng Y: FAT10 stimulates the development of osteosarcoma by regulating the JAK/STAT
signaling pathway. J BUON 2021;26:2090-2096.
|
| 24 | Chen H, Tao L, Liang J, Pan C, Wei H: Ubiquitin D promotes the progression of rheumatoid arthritis
via activation of the p38 MAPK pathway. Mol Med Rep 2023;27
https://doi.org/10.3892/mmr.2023.12940 |
| 25 | Aaltonen KJ, Turunen JH, Sokka T, Puolakka K, Valleala H: A survey on the medication adherence to
methotrexate among rheumatoid arthritis patients treated with self-administered biologic drugs. Clin
Exp Rheumatol 2016;34:694-697.
|
| 26 | Romao VC, Canhao H, Fonseca JE: Old drugs, old problems: where do we stand in prediction of
rheumatoid arthritis responsiveness to methotrexate and other synthetic DMARDs? BMC Med 2013;11:17.
https://doi.org/10.1186/1741-7015-11-17 |
| 27 | Lin S, Li H, Wu B, Shang J, Jiang N, Peng R, Xing B, Xu X, Lu H: TGF-beta1 regulates chondrocyte
proliferation and extracellular matrix synthesis via circPhf21a-Vegfa axis in osteoarthritis. Cell
Commun Signal 2022;20:75.
https://doi.org/10.1186/s12964-022-00881-9 |
| 28 | Lee DM, Weinblatt ME: Rheumatoid arthritis. Lancet 2001;358:903-911.
https://doi.org/10.1016/S0140-6736(01)06075-5 |
| 29 | Raasi S, Schmidtke G, De Giuli R, Groettrup MJEJoI: A ubiquitin-like protein which is
synergistically inducible by interferon-gamma and tumor necrosis factor-alpha. 2015;29:4030-4036.
https://doi.org/10.1002/(SICI)1521-4141(199912)29:12<4030::AID-IMMU4030>3.0.CO;2-Y |
| 30 | Schmidtke G, Kalveram B, Groettrup M: Degradation of FAT10 by the 26S proteasome is independent of
ubiquitylation but relies on NUB1L. FEBS Lett 2009;583:591-594.
https://doi.org/10.1016/j.febslet.2009.01.006 |
| 31 | Xiang S, Shao X, Cao J, Yang B, He Q, Ying M: FAT10: Function and Relationship with Cancer. Curr
Mol Pharmacol 2020;13:182-191.
https://doi.org/10.2174/1874467212666191113130312 |
| 32 | Lee CG, Ren J, Cheong IS, Ban KH, Ooi LL, Yong Tan S, Kan A, Nuchprayoon I, Jin R, Lee KH, Choti
M, Lee LA: Expression of the FAT10 gene is highly upregulated in hepatocellular carcinoma and other
gastrointestinal and gynecological cancers. Oncogene 2003;22:2592-2603.
https://doi.org/10.1038/sj.onc.1206337 |
| 33 | Theng SS, Wang W, Mah WC, Chan C, Zhuo J, Gao Y, Qin H, Lim L, Chong SS, Song J, Lee CG:
Disruption of FAT10-MAD2 binding inhibits tumor progression. Proc Natl Acad Sci U S A
2014;111:E5282-5291.
https://doi.org/10.1073/pnas.1403383111 |
| 34 | Su H, Qin M, Liu Q, Jin B, Shi X, Xiang Z: Ubiquitin-Like Protein UBD Promotes Cell Proliferation
in Colorectal Cancer by Facilitating p53 Degradation. Front Oncol 2021;11:691347.
https://doi.org/10.3389/fonc.2021.691347 |
| 35 | Leyva-Lopez N, Gutierrez-Grijalva EP, Ambriz-Perez DL, Heredia JB: Flavonoids as Cytokine
Modulators: A Possible Therapy for Inflammation-Related Diseases. Int J Mol Sci 2016;17
https://doi.org/10.3390/ijms17060921 |
| 36 | Feldmann M, Maini RN: Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu
Rev Immunol 2001;19:163-196.
https://doi.org/10.1146/annurev.immunol.19.1.163 |
| 37 | He J, Qin M, Chen Y, Hu Z, Xie F, Ye L, Hui T: Epigenetic regulation of matrix metalloproteinases
in inflammatory diseases: a narrative review. Cell Biosci 2020;10:86.
https://doi.org/10.1186/s13578-020-00451-x |
| 38 | Akaogi J, Nozaki T, Satoh M, Yamada H: Role of PGE2 and EP receptors in the pathogenesis of
rheumatoid arthritis and as a novel therapeutic strategy. Endocr Metab Immune Disord Drug Targets
2006;6:383-394.
https://doi.org/10.2174/187153006779025711 |
| 39 | Takala R, Ramji DP, Andrews R, Zhou Y, Burston J, Choy E: Anti-inflammatory and immunoregulatory
effects of pinolenic acid in rheumatoid arthritis. Rheumatology (Oxford) 2022;61:992-1004.
https://doi.org/10.1093/rheumatology/keab467 |
| 40 | Song A, Wang Y, Jiang F, Yan E, Zhou J, Ye J, Zhang H, Ding X, Li G, Wu Y, Zheng Y, Song X:
Ubiquitin D Promotes Progression of Oral Squamous Cell Carcinoma via NF-Kappa B Signaling. Mol Cells
2021;44:468-480.
https://doi.org/10.14348/molcells.2021.2229 |
| 41 | Yuan R, Wang K, Hu J, Yan C, Li M, Yu X, Liu X, Lei J, Guo W, Wu L, Hong K, Shao J: Ubiquitin-like
protein FAT10 promotes the invasion and metastasis of hepatocellular carcinoma by modifying
beta-catenin degradation. Cancer Res 2014;74:5287-5300.
https://doi.org/10.1158/0008-5472.CAN-14-0284 |
| 42 | Liu C, He L, Wang J, Wang Q, Sun C, Li Y, Jia K, Wang J, Xu T, Ming R, Wang Q, Lin N:
Anti-angiogenic effect of Shikonin in rheumatoid arthritis by downregulating PI3K/AKT and MAPKs
signaling pathways. J Ethnopharmacol 2020;260:113039.
https://doi.org/10.1016/j.jep.2020.113039 |
| 43 | Achudhan D, Lai YL, Lin YY, Huang YL, Tsai CH, Ho TL, Ko CY, Fong YC, Huang CC, Tang CH: CXCL13
promotes TNF-alpha synthesis in rheumatoid arthritis through activating ERK/p38 pathway and
inhibiting miR-330-3p generation. Biochem Pharmacol 2024;221:116037.
https://doi.org/10.1016/j.bcp.2024.116037 |
| 44 | Kong XH, Shi SF, Hu HJ, Wang JX: MicroRNA-20a suppresses RANKL-modulated osteoclastogenesis and
prevents bone erosion in mice with rheumatoid arthritis through the TLR4/p38 pathway. J Biol Regul
Homeost Agents 2021;35:921-931.
https://doi.org/10.23812/20-604-A |
| 45 | Kim SH, Bang J, Son CN, Baek WK, Kim JM: Grape seed proanthocyanidin extract ameliorates murine
autoimmune arthritis through regulation of TLR4/MyD88/NF-kappaB signaling pathway. Korean J Intern
Med 2018;33:612-621.
https://doi.org/10.3904/kjim.2016.053 |
| 46 | Li Y, Xu JZ, Gu CX, Liu GL, Tian K: Carvacrol suppresses inflammatory responses in rheumatoid
arthritis fibroblast-like synoviocytes. J Cell Biochem 2019;120:8169-8176.
https://doi.org/10.1002/jcb.28098 |