Macrophage Migration Inhibitory Factor: Its Multifaceted Role in Inflammation and Immune Regulation Across Organ Systems
bAssociate professor of Department of Cytology, Embryology and Histology of Azerbaijan Medical University, Baku,
cAssociate professor of Department of Pathological Anatomy of Azerbaijan Medical University, Baku,
dAssistant of Department of Medical Biology and Genetics of Azerbaijan Medical University, Baku
Keywords
Abstract
Macrophage Migration Inhibitory Factor (MIF) is a pleiotropic cytokine that acts as a central regulator of inflammation and immune responses across diverse organ systems. Functioning upstream in immune activation cascades, MIF influences macrophage polarization, T and B cell differentiation, and cytokine expression through CD74, CXCR2/4/7, and downstream signaling via NF-κB, ERK1/2, and PI3K/AKT pathways. This review provides a comprehensive analysis of MIF’s mechanistic functions under both physiological and pathological conditions, highlighting its dual role as a protective mediator during acute stress and as a pro-inflammatory amplifier in chronic disease. MIF’s involvement in autoimmune disorders, neurodegeneration, metabolic syndromes, infectious diseases, and oncogenesis is examined, with particular attention to its contribution to immune dysregulation, immune escape, and the shaping of inflammatory microenvironments. Its clinical relevance as a biomarker is underscored by associations between elevated serum levels, polymorphic variants such as the -173 G>C SNP, and disease susceptibility, progression, and therapeutic response. Advances in therapeutic strategies are also discussed, including the development of small-molecule inhibitors, MIF-2-specific antagonists, CD74-targeted therapies, and gene-based interventions. Taken together, emerging evidence positions MIF as both a diagnostic indicator and a therapeutic target, with its broad regulatory functions across immune, vascular, and metabolic pathways emphasizing its relevance in precision immunotherapy and its potential to serve as a strategic axis in the future of translational medicine.
Introduction
Macrophage Migration Inhibitory Factor (MIF) was found in the 1960s, a time that changed immunologists' thoughts [1]. At its earliest, MIF was defined as something that came from activated lymphocytes, stopped macrophages from moving, and helped control inflammation [2]. MIF was one of the first lymphokines ever found, even before the cytokine classification system was developed [3, 4]. In the past five decades, MIF has moved from being a basic migration blocker to an important, single- functional, and persistent cytokine involved in the immune system, tissue injury responses, and chronic diseases [5, 6]. Unlike inducible cytokines, MIF is preformed and stored within cells, so it can be released quickly when the cells detect hypoxia, infection or glucocorticoids. MIF acts as a first responder cytokine that comes before and strengthens other inflammatory events. MIF is also unique in that it can reduce the effects of glucocorticoids, which normally reduce inflammation, and thus helps inflammation to continue under stressful conditions [7, 8]. MIF persists in promoting inflammation and also resists the action of glucocorticoids; it is involved in many diseases for a long time. MIF binds to CD74 and co-receptors CXCR2, CXCR4, and CXCR7 at the molecular level, which helps to control it signaling. As a result of these pathways, MIF turns on signaling cascades such as MAPK/ERK1/2, PI3K-Akt, and NF-κB, which increase the release of proinflammatory cytokines (such as IL-6 and TNF-α), help cells survive, promote blood vessel formation, and prevent cell death [9, 10, 11]. As a result, MIF is positioned between immunity, inflammation, and cellular remodeling. Its ability to activate the NLRP3 inflammasome means MIF is important for sterile inflammation, infectious diseases, and systemic cytokine responses. MIF is known to be overproduced in many different clinical conditions. In rheumatoid arthritis and systemic lupus erythematosus, MIF helps create Th1/Th17 cells, increases the survival of macrophages, and contributes to damage in the body [12]. In these disorders, it weakens the blood- brain barrier and encourages microglial cells to become active, leading to inflammation in the brain and a decrease in cognitive function [13]. MIF plays a role in increasing inflammatory cardiomyopathy and atherosclerosis by affecting the function of the endothelium and recruiting monocytes [14]. MIF gene polymorphisms, especially the - 173G/C SNP, have been connected to higher disease risk, lower response to glucocorticoids, and different reactions to treatments [7].
More attention is being given to MIF helps tumors escape immune detection, form new blood vessels, and adapt their metabolism in the TME. Different MIF receptors present on TAMs and MDSCs allow MIF to shape the immune environment in a way that supports tumor growth and makes tumors resistant to checkpoint inhibitors [15, 16, 17]. In studies of ovarian, colorectal, and glioblastoma, MIF has been found to play a role in chemoresistance and the reorganization of stromal cells, usually acting as a negative prognostic marker [18]. It is also known to support the protection of tissues in situations of acute stress. Studies have concluded that the MIF/CD74 system can reduce kidney damage after ischemia and help heal kidney epithelial cells [19, 20]. Since MIF may be protective or harmful in different situations, every therapy for MIF should be custom-made for each tissue. The numerous functions of MIF imply that it is currently considered a biomarker, an object of therapy, and a novel perspective in grasping inflammatory and immune conditions. Nevertheless, some aspects of how receptors and ligands interact and how they influence one another are still not exactly comprehended by researchers and how tissues differ in signaling as well as how TNF may have various beneficial or harmful properties. To explain how basic immunology is utilized, this review attempts to describe how or why MIF was discovered, its molecular characteristics, the mechanisms it employs, effects of its action on a variety of organs as well as how the discovery of MIF is studied clinically and medically treated.
Review Objectives
1. The review will be arranged in light of the following objectives in order to address a full picture of the recent development of Macrophage Migration Inhibitory Factor (MIF) within immunology and diseases:
2. To give a comprehensive study of the molecular framework, signaling pathways and regulatory processes of MIF in inflammation and immune regulation
3. To review the organized assessment of organ-specific functioning of MIF and its role in pathogenesis of autoimmune, infectious, metabolic, neurodegenerative, and malignant diseases to assess MIF’s potential as a diagnostic biomarker and therapeutic target, while identifying gaps in current knowledge and translational challenges
Historical Evolution and Cross-Species Conservation of MIF
From Discovery to Molecular Reclassification
Macrophage Migration Inhibitory Factor (MIF) was initially discovered in the 1960s as being among the
first
soluble mediators with the capability to regulate immune cell activity. Originally classified as a
lymphokine
due to its property to restrain macrophage movement, MIF's utility scope was merely appreciated in part
before
the establishment of the cytokine paradigm. Later studies, especially after it was rediscovered in the
1990s,
restyled MIF as a multifunctional cytokine capable of resisting glucocorticoid-induced suppression,
sustaining
pro-inflammatory signaling, and coordinating the recruitment of immune cells [5]. MIF is now defined not
only by
its cytokine role but also by its chemokine-like behavior, interacting with classical cytokine receptor
CD74 and
non-cognate chemokine receptors including CXCR2, CXCR4, and CXCR7. Although the functional interaction of
MIF
with its main receptor CD74 and chemokine co-receptors such as CXCR2, CXCR4, and CXCR7 is supported by a
wide
range of experimental evidence, the precise structural description of these interactions is still poor.
Currently, no crystallographic or cryo-EM studies have provided the exact molecular conformation of MIF
complexed to these receptors. This limitation inhibits a thorough appreciation of how MIF acts on its
targets at
the atomic scale. Key factors, receptor binding geometry, possible structural changes on ligand binding,
and the
co-receptor cooperation dynamics are unknown. Most is learned through indirect evidence, such as
mutational
analysis, molecular modelling, and biochemical assays, which offer only partial insight into mechanisms of
MIF
receptor specificity and activation of downstream signaling. These receptor interactions trigger MAPK/ERK,
PI3K-Akt, and Src kinase signaling cascades, situating MIF at the intersection of immune activation and
inflammatory homeostasis [9, 21]. Unlike traditional cytokines, MIF is preformed, stored intracellularly,
and
rapidly released upon cellular stress, giving it the unique biological function of an immediate-response
amplifier in both innate and adaptive immunity.
Evolutionary Conservation and Structural Integrity
At the molecular level, MIF is among the most phylogenetically conserved cytokines. Comparative genomics
reveals
that MIF orthologs exist across protists, plants, invertebrates, and vertebrates, reflecting a highly
conserved
primary structure, particularly in the tautomerase active site, the N-terminal proline (Pro1), and Lys32,
which
are essential for redox signaling [22]. Although Pro1 and Lys32 are conserved residues, their key
functional
significance goes beyond redox signaling. Pro1 is the catalytic nucleophile for the tautomerase active
site, and
its position in the structure is important for enzymatic function. The biological relevance of MIF
tautomerase
activity is not known but studies by Pantouris and his colleagues [23] suggested that point
mutations of
Pro1 or other proximal residues strongly impair MIF cytokine activity, therefore enzymatic integrity is
needed
to perform its immunomodulatory functions. The disulfide bonded Cys 56-Ala-Cys59 motif that is outside the
scope
of the current discussion, is also critical to redox sensitivity. This motif undergoes a conformational
change
in reaction to oxidative or other pressures as well, altering the structural packaging of MIF and its
ability to
bind its receptors. These sites as a combination also enhance catalytic activity in this combined form
hence
responding further to the role of MIF under pathological conditions. Functional parts, such as
enzyme-binding
areas and receiver binding sites, are preserved levels over 85 portions in the eukaryotic taxa, despite a
specified divergence in the loop regions or surface-open residues. Such conservation implies powerful
evolutionary constraint so that the combinatorial biology of both MIF present inside cells as an
intra-redox
therapeutic protein and outside cells as a pro-inflammatory mediator are vital to host defense and
cellular
homeostasis. Stereospecific tautomerase activity in simpler organisms is found in MIF, and is probably the
driving force behind cellular redox equilibrations. In mammals tautomerase activity is reduced, but it
serves a
functional purpose in the presence of oxidative stress.
The expediency of research on MIF redox chemistry has been followed by recent studies that have
contributed to
the know-how with the highlighting of the distinct biochemical peculiarities of the oxidized form of MIF.
The
tertiary structure of oxidized MIF (oxMIF) is altered and its receptor binding is also altered relative to
reduced MIF. Schinagl and his team [24] demonstrated that oxMIF assumes a comparative configuration that
is
neither totally catalytically true-blue nor does it rebuild extraordinary pro-inflammatory properties.
Sajko
with colleagues [25] further established that MIF also suffers redox alterations that influence its
affinity to
its receptors, which influence CD74-mediated signaling in conditions of oxidative tissue environments.
Furthermore, Thiele and colleagues [26] have already identified redox dependent residues in MIF that alter
their
structure and determine stability and cytokine-like activity of MIF in pathological conditions of stress.
These
findings point at the fact that the redox modulation is maintained as a regulatory mechanism, and MIF is
able to
modify its activities as a reaction to tissue redox state. These biochemical features underscore MIF as a
primordial immunotransducer, to sense metabolic stress and couple its response to the watchdog immune
system,
well before the origin of the professional immune system cells.
Functional Validation in Cross-Kingdom Models
Non-mammalian model systems support the universality of MIF function. In a basal chordate Ciona robusta,
whose
immune system is highly primitive, MIF, stimulated with lipopolysaccharide (LPS), activates Toll-like
receptor
(TLR) signaling cascades and, consequently, NF-x B activity and secretion of cytokines [27]. This
observation
highlights that MIF immunoregulatory activities have co-evolved with the most ancient elements of the
innate
immune recognition [28]. The removal of the MIF homolog of live-attenuated parasites of Leishmania major
showed
a marked increase in CD4+ T cell mediated protective immunity, identifying the factors of pathogen-derived
MIF
homologs that could therefore be considered as immune-suppressive molecules with the ability to
sub-optimize the
host response. On the same note, during African trypanosome infection, MIF interplays with IL-10 in a
Yin-Yang
relationship binding host immunity and parasitism survival by the action on dendritic cells and
suppression of
T-cells [29]. Such studies not only affirm the ancient immunomodulatory utility of MIF but also reveal
that
immune evasion mechanisms in parasites and tumors may co-opt MIF signaling, a critical insight for
translational
immunotherapy.
Evolutionary Function in Modern Pathologies
The basic functions of MIF are still associated with disease in today’s world. In RA and SLE, MIF helps
synovial
fibroblasts live longer, supports the growth of Th17 cells, and resists the effects of glucocorticoids,
making
inflammation and damage worse [30]. In atherosclerosis and myocardial infarction, MIF promotes the
recruitment
of inflammatory macrophages, endothelial dysfunction, and fibroblast activation, thereby shaping both
acute and
chronic cardiovascular responses [31, 32]. Functionally, MIF’s ability to operate via CD74-Src-PI3K-Myosin
II
signaling supports dendritic cell migration, critical for immune surveillance and antigen presentation
[21].
This ancient migratory role, retained from invertebrate immunity, reemerges in tumor-associated macrophage
dynamics, where MIF orchestrates myeloid-derived suppressor cell (MDSC) recruitment and tumor immune
evasion
[33, 34].
Neuroimmune Interface and Behavioral Evolution
Evolutionary psychoneuroimmunology is a new field, and here, MIF’s lasting presence in the CNS could link
inflammation to behavior. Yirmiya [35] proposes that MIF contributes to understanding why depression is
inflammatory and might influence mood, thinking and responses to stress. As a result, MIF now plays a part
in
evolutionary behavior and in immunity.
Molecular Structure, Composition, and Genetic Regulation of MIF
Structural Architecture and Functional Interfaces
Macrophage Migration Inhibitory Factor (MIF) is a well-kept protein weighing 12.5 kDa, is formed by three
identical subunits, and each subunit is involved in forming the main channel that determines its structure
and
function [36]. Hydrophobic and hydrogen bonds give this shape stability, allowing the structure to perform
as an
enzyme and receptor. Pro1 is the main role of MIF, which works to turn one chemical into another, but this
is
not important in humans, according to Chen and colleagues [37]. On top of being a tautomerase, MIF also
acts as
an oxidoreductase whenever cells deal with oxidative stress, so it could have once played a role in
managing
oxygen levels for early life. Alpha Fold multimer modelling supports the idea that MIF forms strong
complexes
with CD74 and JAK2, which activate the MAPK and PI3K-Akt pathways [38]. In addition, the Arg-Leu-Arg (RLR)
area
at the MIF-CXCR4 site lets MIF communicate with the chemokine receptor, proving it has a chemokine
function and
can regulate the white blood cells that are recruited and move.
MIF's receptor-mediated signaling and its binding to CD74 and related chemokine
receptors trigger downstream intracellular processes in immune and inflammatory control, as shown in Fig.
1.
Schematic illustration of MIF initiated signaling pathways: The diagram demonstrates that Macrophage
Migration
Inhibitory Factor (MIF) can interact with the cell surface receptor CD74 and with co-receptors, (CXCR2,
CXCR4,
and CXCR7). These events cause an intracellular cascade of signaling events which include MAPK/ERK
signaling
pathways, PI3K/AKT pathways and NF- KB signaling pathways. These molecular networks have the effect of
controlling different cell functions including generation of cytokines, surviving signals, angiogenesis
and
blocking apoptotic pathways. Besides, MIF has nuclear regulatory actions with the intracellular proteins
CSN5
and JAB1. Collectively, it is possible to note the integrative role of MIF in linking extracellular
signals
toward both inflammatory and metabolic regulation of gene expression as outlined by this figure.
Interactions with CD74, CXCRs, and Downstream Regulation of Proliferation,
Apoptosis, and Angiogenesis [39].Signals of MIF commence when it becomes stimulated. Upon binding with
CD74 and
CD44 and CXCR 2/4/7 MIF leads to activation of SRC-kinase and transmission of mapping through MAPK/ERK and
PI3K
/AKT pathways. They manage transcription factors, encourage cell growth, and stop cells from dying by
reducing
p53 and turning on Bcl-2 and Bcl-xL, as shown in Fig. 2. By regulating HIF-1α, MIF causes cells to make
angiogenic factors, including VEGF and IL-8. MIF also works within the cell by interacting with CSN5 and
JAB1 to
bring about more effects in the nucleus. MIF signals help reveal its role in inflammation, escaping
recognition
by the immune system, promoting cancer, and influencing tissue alterations.
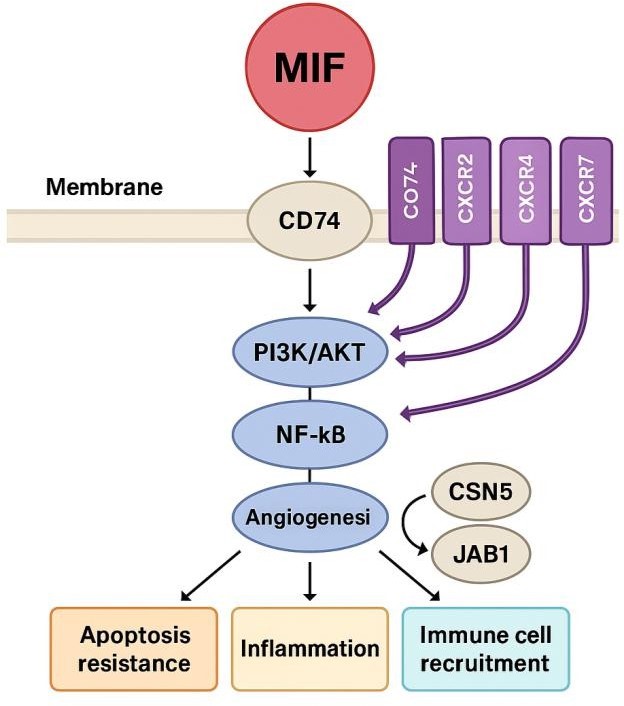
Fig. 1: MIF Signalling Pathways and Functional Outcomes.
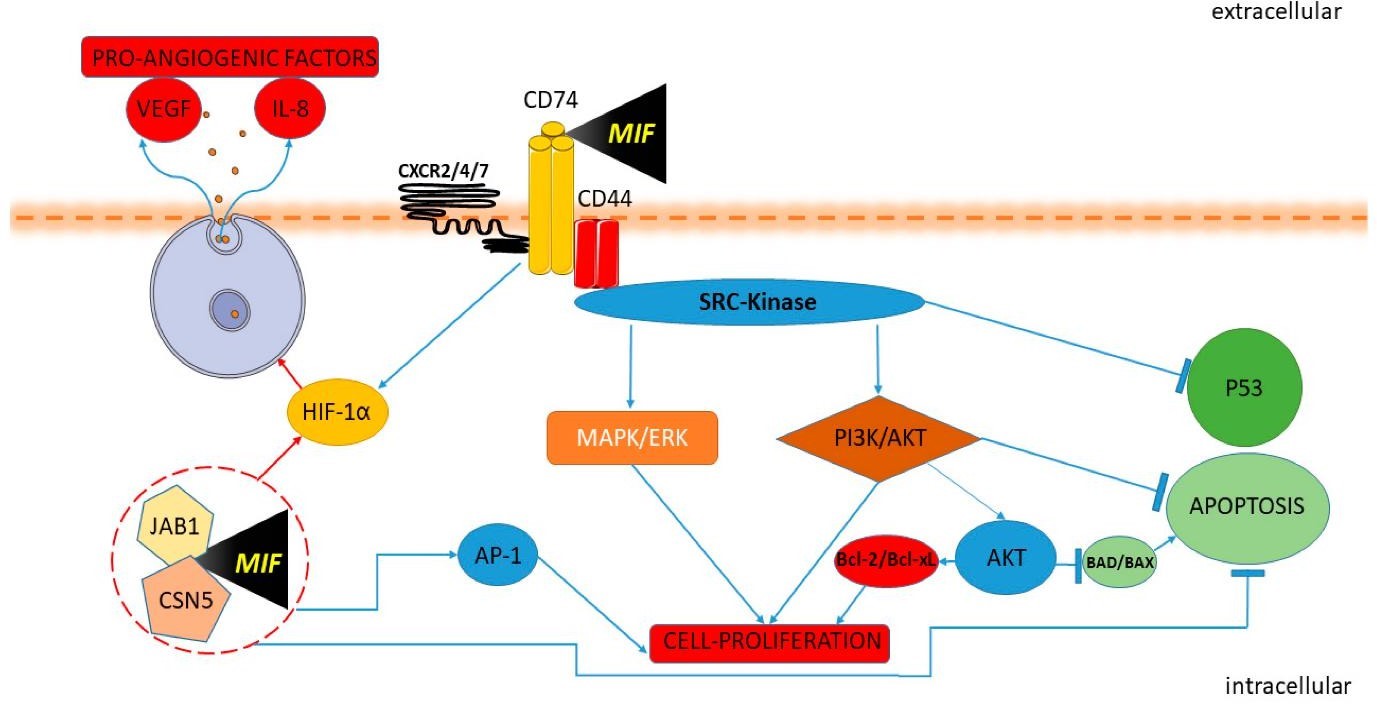
Fig. 2: MIF-Mediated Signalling Cascade: Interactions with CD74, CXCRs, and Downstream Regulation of Proliferation, Apoptosis, and Angiogenesis (Cavalli et al., 2020).
Regulatory Polymorphisms and Transcriptional Control
The gene for MIF in humans is located on chromosome 22q11.2 and is managed by stress and
inflammation-sensitive
elements such as AP-1, NF-κB, and CREB [6]. Recent studies found that the presence of transcription
factors at
enhancers can switch on or off gene expression, with only minor changes in enhancer accessibility causing
a big
increase in MIF expression [40]. Especially, the -173G/C SNP (rs755622) in the MIF promoter region is
associated
with higher MIF levels and a greater risk of disease. Meta- analyses have found that the C allele raises
the
risk of inflammatory diseases by 1.6-2.4 times and leads to poorer outcomes in hepatocellular carcinoma
and
rheumatoid arthritis [41, 42]. There are other variants, including rs3063368, that have been linked to
worse
results in acute kidney injury and pemphigus vulgaris [43, 44]. Taken together, these polymorphisms
suggest that
host genetics can influence MIF-related diseases and their responses to treatment.
Epigenetic and Non-Coding RNA Modulation
Apart from genetics, MIF expression can be controlled by epigenetic means, including changes to chromatin
and
histones, and non-coding RNAs. Tumors that have metabolic problems show lipid-related changes in histones
that
support high MIF levels and lead to immune cell dysfunction, mainly in the natural killer (NK) cell
exhaustion
setting [45]. In gastric cancer, chromatin accessibility profiling confirmed that MIF is found in regions
of
open chromatin that are rich in immune cells, which may explain why MIF is linked to tumor aggressiveness
[46].
MIF-AS1 and other regulatory lncRNAs have been found to induce EMT in breast cancer by removing
miR-1249-3p from
the system, which then dysregulates MIF’s downstream targets [47]. Furthermore, blocking enhancer- bound
long
non-coding RNAs or transcription factor groups using CRISPR/Cas9 has been successful in controlling MIF
expression, which may help with future epigenome-based treatments [48, 49].
Pharmacological Inhibition and Allosteric Targeting
Pharmacological inhibition of MIF has advanced considerably through the development of allosteric
modulators
that interfere with MIF function without disrupting its trimeric structure. Early compounds like ISO-1
target
the Pro1-dependent tautomerase pocket, while newer agents like Iguratimod, picolinoyl peptidomimetics, and
solvent channel blockers modulate MIF activity via structurally preserved but catalytically independent
regions
[50, 51]. These agents were shown to have effectiveness in an array of preclinical conditions. In liver
injury
and acetaminophen, Iguratimod has been shown to reduce mortality by simultaneously reducing oxidative
stress and
managing inflammation, and selective MIF inhibition in acute myeloid leukemia ends the leukemia process by
arresting the cell cycle [52]. There is also a structural similar homolog of MIF known as MIF-2 (D-DT)
which
also is a potential drug target and selective inhibitors have shown positive outcomes regarding
inflammatory and
autoimmune diseases [53]. The two are similar in that both MIF and MIF-2 employ identical receptors, but
these
two share different functions, and this helps them distinguish between them in effective drug design.
Mechanisms of Action and Signaling Pathways of MIF
MIF is the most important component of connectivity of inflammation, stress and immune signaling that user to restrain the action of a cell. MIF produces distinct tasks with the help of binding the receptors of the cell surface and catalyzing inside processes like sustaining life, enhancing immune system, causing chronic inflammation and reducing responsiveness to treatment. This section explores the primary pathways and modes through which MIF performs all its functions as well as notes that the various actions may change according to the circumstances.
Receptor Binding and Upstream Activation
MIF begins its work by binding with a protein called CD74 which is found within plasma membranes and
usually
co-clusters with CXCR2, CXCR4, CXCR7. When activated by outside of the cell signals, this receptor
multiplex
entrusts transcription activation by making JAK2 phosphorylated, bringing in PI3K, and activating Src
kinases
[11, 13]. MIF behaves as a preformed cytokine; these occurrences are swift and vigorous. MIF-CXCR7 binding
turns
on the PI3K/AKT pathway in prostate and biliary tract cancers, helping the cancer cells become
drug-resistant
and divide more rapidly under cytotoxic stress [54, 55]. MIF in the kidney regulates integrin-β1 and
cyclin D1
by activating ERK1/2, which helps the kidney cells lose their special function and causes kidney damage
[56].
Importantly, MIF engagement with these receptors helps control both inflammation and the survival and
repair of
cells at the right time.
Core Pathways: PI3K/AKT, NF-κB, and ERK Signaling
Most of the time, receptor binding by MIF causes a chain of events through the PI3K/AKT pathway that
supports
cell survival, energy use and avoids detection by the immune system. Researchers Wei et al.
found that exosomes carrying MIF in gliomas make it possible for tumor cells to become resistant to
chemotherapy
with temozolomide by acting on the TIMP3/PI3K/AKT pathway [57]. MIF also helps preserve the youth of
mesenchymal
stem cells from ageing due to doxorubicin by using AKT signaling [58]. The p65/p50 arm of NF-κB is an
important
downstream pathway activated by MIF. In women with PCOS, MIF increases NF-κB activity in granulosa cells,
which
affects the ovaries and disrupts hormone balance [59]. This mechanism is aided by long non- coding RNA
LRNA9884,
which increases MIF production by activating NF-κB during acute kidney injury [60]. These signaling
cascades act
together: ERK1/2 controls cell growth in renal and immune cells, PI3K/AKT helps cells adapt to metabolic
changes, and NF-κB maintains inflammation, all showing MIF’s ability to influence cellular balance and
disease
development.
MIF binds to CD74 and co-receptors CXCR2, CXCR4, and CXCR7 to trigger intracellular
cascades, such as PI3K/AKT, NF-κB, and MAPK/ERK pathways, as shown in Fig. 3. These signaling pathways
control
pro-inflammatory cytokine production, resistance to apoptosis, angiogenesis, and immune cell recruitment,
leading to tissue repair or chronic inflammation depending on the situation.
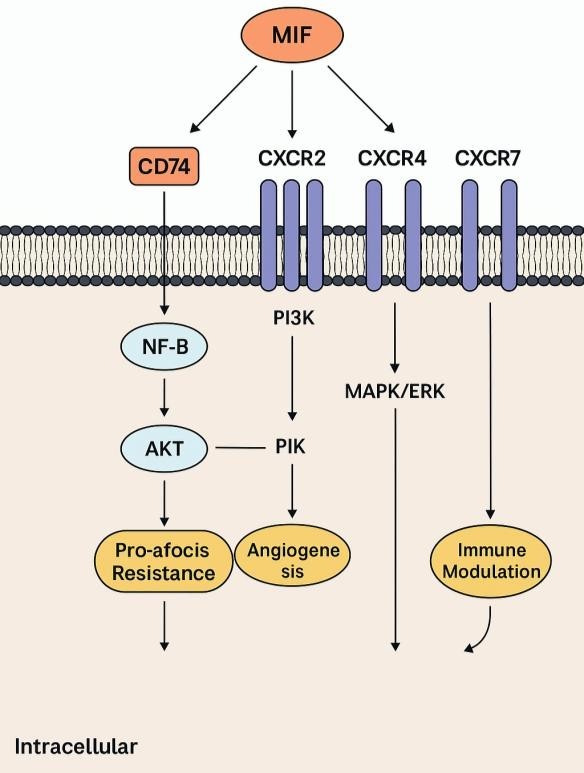
Fig. 3: Receptor-Mediated Signalling Cascades of MIF.
Immunomodulation, Inflammation, and Resistance Mechanisms
MIF strongly affects the behavior of immune cells, mainly by influencing the way macrophages are
polarized. In
adipose tissue, it helps M1 macrophages become active by increasing COX-2 and stops M2 macrophages from
becoming
active, which causes insulin resistance and ongoing metabolic inflammation [61]. Blocking CD74-MIF
signaling has
been shown to reverse this polarization, indicating that MIF acts as a molecular switch in inflammatory
environments [62]. Beyond innate immune cells, MIF affects T cells, monocytes, and dendritic cells,
modulating
survival, cytokine secretion, and antigen presentation [63]. It also functions as a counter-regulator of
glucocorticoids, antagonizing their anti-inflammatory effects. Genetic variation at the MIF immune
susceptibility locus, particularly involving the regulator ICBP90, impacts glucocorticoid sensitivity and
apoptosis thresholds [64]. Resistance to therapy, especially in cancers, is reinforced by MIF’s
integration with
non-coding RNAs. For example, microRNA-451a suppresses the MIF-PI3K/AKT pathway in biliary cancers, while
its
downregulation enables chemoresistance and unchecked proliferation [55]. These interactions underscore the
importance of MIF not just in inflammation, but in transcriptional resilience and stress adaptation.
MIF in Cytokine Storms and Systemic Inflammatory States
MIF plays a critical role in the pathogenesis of cytokine storms, particularly in conditions like sepsis,
multi-organ failure, and severe viral infections. It is found early in the response, coming before TNF-α
and
IL-6 increase, and it helps sustain the amount of cytokine mRNA, prolonging the body’s systemic
inflammation
[65]. In this situation, MIF increases the breaking of blood vessels, attracts neutrophils into tissues
and
damages the endothelium, which are important parts of multi-organ dysfunction syndrome (MODS). MIF also
increases COX-2 and PGE2 in tissues other than the brain, which leads to a more general increase in
inflammation
[66]. Mild inflammation occurs in metabolic disorders; in critical cases, the body responds to danger with
immune reactions that do not react to glucocorticoids, which only makes the disease worse [30, 61]. MIF
uses
both types of immune cells and is not stopped by usual restraints; it allows inflammation to continue,
making it
a valuable, but difficult, target for therapy.
Role of MIF in Inflammatory Processes
MIF is a main cytokine responsible for organizing the inflammation-related actions of both immune and non-immune cells. It turns on, sustains, and increases inflammation, and it helps the body cope with unexpected stress, although it can also lead to persistent immune issues. In this section, MIF affects macrophage polarization, the development of T-helper and B-cells, the rebuilding of tissues, and the body’s inflammatory response, particularly its ability to resist being managed by the immune system.
MIF is well known for shifting macrophages toward the M1 pro-inflammatory type, which increases the secretion of TNF-α, IL-1β, IL-6, and nitric oxide [67]. When the effect is controlled, it helps clear microbes, but when it is out of control, it can harm tissues and lead to autoimmunity [68]. MIF also stops the process by which M2 macrophages differentiate, since these cells help heal and resolve wounds. In detail, MIF helps TLR4 function by keeping it in the membrane and increasing the activation of MyD88-dependent NF- κB, which causes a greater release of cytokines [69]. In marine invertebrate models like Ciona robusta, MIF-TLR co-expression during lipopolysaccharide challenge confirms its evolutionarily conserved role in innate immune priming [27]. Importantly, CD74 blockade can reverse this polarization, restoring immune balance and bolstering anti-tumor immunity [70]. Current evidence points to the possibility that MIF interaction with CD74 can be highly regulated by oxidative conditions. In inflamed tissues, where redox imbalance is prevalent, oxidation of MIF seems to drive its conformational modifications and increase receptor binding affinity. This redox-sensitive transition has been suggested as an important initiator of MIF-CD74 interaction and resultant signaling in immune cells. Sajko and group of researches [25] showed that the oxidized state of MIF exhibits modified receptor behavior, which can be used therapeutically to target selectively pathological inflammation. These results crosslink MIF's redox biology and pro-inflammatory function and indicate that oxidative stress not only activates MIF but also enhances it signaling capacity in chronic disease settings.
Modulation of T-Helper Cell Differentiation
and Cytokine Imbalance
MIF is important for adaptive immunity, as it mainly controls the T-helper cell subsets' development and
function. As a result, there are more Th1 and Th17 cells, which cause inflammation important for diseases
such
as RA and SLE [30]. MIF stimulates the cultures of PBMCs and this makes SLE patients release IL-17A, IL-6,
and
TNF- alpha [71]. The study by Yan and colleagues [72] demonstrated the role of MIF in promoting the
differentiation of Th17 cells via promoting the effect of ATF6 signaling pathway connecting endoplasmic
reticulum stress to the induction of immune response to unveil an unexpected association between
inflammation
and endoplasmic reticulum stress. The results show that MIF also assists in the regulation of the
inflammatory
messages as well as the process of selecting the inflammatory cells.
B Cell Dysregulation and Autoantibody
Production
Although studies concentrate more on the effects of MIF on macrophage and T cells, MIF has an effect on B
cell
biology by aiding the survival and increased numbers of age-related B cells (ABCs). Such cells encompass
autoimmune prone and ageing cell populations and produce autoantibodies and secrete cytokines [73]. Phalke
with
his team [74] explained that there was an accumulation of ABC facilitated by the MIF, which provides a
microenvironment, which supports chronic inflammation and presentation of the autoantigens [75]. Sex- and
age-dependent variations in the prevalence of autoimmune diseases might also be outlined by this pathway
with an
increased role of MIF- mediated effect through the modulating influence of estrogen and immune senescence.
MIF in Organ-Specific Inflammatory Pathologies
MIF plays a critical role in the pathogenesis of multiple inflammatory and autoimmune diseases by acting
at the
interface of immune activation and tissue remodeling. In RA, MIF induces matrix metalloproteinases (MMPs)
and
vascular endothelial growth factor (VEGF), directly contributing to joint erosion and synovial
angiogenesis. In
SLE, elevated MIF levels correlate with disease activity, particularly nephritis, and reduced response to
corticosteroids [76]. In chronic respiratory disorders, MIF promotes leukocyte infiltration and tissue
fibrosis,
while disrupting barrier function and sustaining a cytokine loop. In these contexts, MIF functions as a
non-resolving amplifier, maintaining inflammation even in the absence of active triggers, largely due to
its
resistance to anti-inflammatory feedback, such as IL-10 or glucocorticoid signaling.
Persistence, Glucocorticoid Resistance, and
Transition to Systemic Inflammation
One of the most clinically significant features of MIF is its resistance to classical anti- inflammatory
feedback mechanisms. Unlike most cytokines that are downregulated by glucocorticoids, MIF expression is
paradoxically upregulated by glucocorticoid signaling, allowing it to bypass immune resolution checkpoints
[64].
This trait helps explain MIF’s involvement in cytokine storms, where it amplifies systemic inflammation
through
sustained IL-6 and TNF-α production. Clinically, elevated serum MIF has been linked to a twofold increase
in
renal flare frequency in SLE and a 30-50% reduction in steroid responsiveness in RA patients [77]. This
glucocorticoid resistance, combined with persistent NF-κB activation, establishes MIF as a chronic driver
of
unresolved inflammation
Immunoregulatory Functions of MIF
In addition to causing inflammation, Macrophage Migration Inhibitory Factor (MIF) plays a role in maintaining balance between tissue repair, tolerance to the immune system, and overall homeostasis. Lymphocytes respond differently to signals in various organs, using CD74 and chemokine receptors and regulate genes using NF-κB, ERK and JNK. Now, investigate the MIF supports the immune system and see that it is beneficial in acute illnesses, but can become problematic if it lasts too long.
Neuroimmune Regulation and Blood-Brain Barrier
Dynamics
MIF is involved in both the control of neuroinflammation and the condition of blood vessels in the central
nervous system. When ischemic stroke occurs, expression of MIF jumps up, which causes CD74 to be activated
and
endothelial cell permeability to rise, resulting in damage to the BBB. Liu with colleagues [78] discovered
that
blocking MIF made the BBB much less leaky and improved outcomes for injured mice. As a result of MIF, more
neurons are damaged as RIPK1 activates the death of endothelial cells, which causes more severe harm after
a
stroke and slower vascular restoration [79]. When MIF is present, it causes microglia to be more active,
and
this causes an increase in brain cell deaths from cytokines in Alzheimer’s disease [80, 13]. Blocking MIF
in
traumatic brain injury lessens the much astrocytes and lymphocytes that are activated, proving that MIF
plays a
key role in hyperactive reactions of the brain’s immune system [81, 82].
Cardiopulmonary and Hepatic Immune Regulation
During myocardial infarction, the cardiovascular system shows a large increase in MIF, which promotes the
activation of cardiac fibroblasts by NF-κB and supports the release of inflammatory cytokines, resulting
in poor
tissue remodeling [31]. In chronic lung diseases such as pulmonary fibrosis and COPD, MIF contributes to
the
transformation of lung cells and stops the repair of damage, but it surprisingly also promotes a small
amount of
new epithelial cell growth [83]. In the liver, MIF acts as a chemokine orchestrator. In alcohol-associated
hepatitis, it regulates leukocyte infiltration by coordinating CXCL1 and MCP-1 expression [84]. In
contrast, in
non-alcoholic steatohepatitis (NASH), MIF promotes fibrosis by shifting NKT cell polarization toward a
pro-fibrotic phenotype, rather than resolving inflammation [85]. In hepatocellular carcinoma (HCC), MIF
enhances
tumor immune evasion by promoting mononuclear phagocyte infiltration, shaping an immunosuppressive
microenvironment [86].
MIF in Renal, Gastrointestinal, and
Barrier Immunity
In renal tissue, MIF is implicated in both ischemia-reperfusion injury and autoimmune nephritis. It
activates
tubular epithelial cells and recruits inflammatory macrophages via NF- κB, worsening renal dysfunction
[87].
Yang and colleagues [88] demonstrated that macrophage-derived MIF contributes directly to
glomerular
damage in anti-GBM nephritis. In lupus nephritis models, therapeutic upregulation of miR-654 suppresses
MIF and
ameliorates renal pathology [89]. In the gastrointestinal tract, MIF supports epithelial cell survival
during
acute inflammation by promoting CD74-ERK signaling, thereby enhancing mucosal regeneration [90]. However,
persistent MIF expression can impair intestinal barrier function, increasing permeability and
susceptibility to
microbial translocation [91]. In colitis-associated colorectal cancer, MIF orchestrates tumor- promoting
interactions between macrophages, T cells, and epithelial cells, fostering chronic immune activation and
tumor
progression [92].
Metabolic and Reproductive System
Immunoregulation
MIF’s role in metabolic regulation is increasingly appreciated. It correlates strongly with visceral fat
mass,
serum hs-CRP, and insulin resistance in prediabetic individuals [93]. It also suppresses hormone-sensitive
lipase (HSL) in adipocytes, promoting triglyceride accumulation and adipose inflammation, both key drivers
of
metabolic syndrome [94]. In the reproductive system, MIF displays a dual function. It promotes trophoblast
survival in early pregnancy under oxidative stress by regulating apoptosis and mitochondrial function
[95].
Conversely, in endometriosis, elevated serum MIF levels correlate with disease severity and have been
proposed
as diagnostic biomarkers [96]. In PCOS, MIF activates NF-κB signaling in granulosa cells, exacerbating
endocrine
dysfunction and ovarian inflammation [59].
Immune Balancing and Therapeutic Relevance
It depends on the environment to either encourage or protect the immune system functions. It is clear from
its
role as the first messenger in the immune system, its resistance to glucocorticoids, and its effects on
both
innate and adaptive immunity. MIF can help reduce inflammation in ongoing or cancerous diseases and also
support
healing and shield tissues under sudden stress. Many of the actions of the inflammatory response are
carried out
by conserved signaling pathways, NF-κB, ERK1/2, JNK, and CD74-CXCR complexes that are found in many
systems but
lead to different results in each tissue. That’s why targeting MIF in therapy can be tricky, since it
helps in
many ways and can be harmful if not used the right way. MIF is involved in multifarious physiological
functions,
and its functional consequences look exceedingly context-specific. In many organ systems, particularly the
liver, brain, and reproductive tissues, MIF exhibits protective and harmful activities, contingent upon
the
stage of disease, the immune environment, and the degree of oxidative stress. For example, MIF ensures
epithelial regeneration and tissue repair following acute hepatic insult, but the identical pathway
aggravates
fibrosis in chronic liver disease. In the CNS and MIF is beneficial to neuroprotection in ischemia, but
causes
neuroinflammation and neuronal damage in progressive diseases such as Alzheimer's disease. This dualism
has been
controversial in the literature, with a focus by some studies on its reparative and anti-apoptotic
functions,
and by others on its role in immune dysregulation and chronic inflammation. These contradictory data
highlight
the need to take into account tissue specificity, redox status, and disease timing when assessing MIF's
mechanistic functions and therapeutic targeting potential.
Clinical Relevance, Diagnostic Potential, and
Therapeutic Targeting of MIF
Macrophage Migration Inhibitory Factor (MIF) is now recognized as an important immunoregulatory molecule
in many
diseases, including autoimmune illnesses, infections, cancers, brain disorders and metabolic problems. The
active early, resists glucocorticoid interference and is located upstream in immune responses; it is a
good
target for precision medicine. In addition to contributing to disease development, MIF is now seen as a
helpful
marker for diagnosing, predicting outcomes and choosing treatments.
Clinical Significance of MIF in Inflammatory, Oncologic, and Infectious Diseases
In the medical field, MIF is associated with severe, fast, and hard many chronic and acute diseases. In
diseases
such as RA and SLE, MIF increases inflammation by activating MMPs and makes it harder for glucocorticoids
to
resolve inflammation, leading to damage in joints and flares throughout the body. Bilsborrow and group of
researches [30] pointed out that MIF controls the way macrophages function and supports inflammation in
the
joints, suggesting it could be a good drug target for RA patients who do not respond to other treatments.
In
genitourinary cancers, both MIF and its homolog MIF-2 promote growth by acting on the same cancer cells.
According to Penticuff and his team [97] high MIF levels in bladder and prostate cancers are linked to a
poor
outcome and help cancer cells avoid the immune system by recruiting macrophages and promoting blood vessel
growth. According to Nasiri and colleagues [80] in Alzheimer’s disease, microglial activation and
persistent
neuroinflammation caused by MIF lead to harm to neurons and cognitive problems. MIF is also implicated in
diseases of the blood vessels and metabolism. In atherosclerosis, MIF increases the speed of lesion
development
by encouraging monocytes to join the lesion and form foam cells [32]. In COVID-19 cases, some variations
in the
MIF gene have been connected to a higher risk of severe disease. Shin and colleagues [98] found that MIF
is a
gene linked to COVID-19 outcomes, as high-expression alleles were connected to developing symptoms and
problems
with the immune system.
MIF's functional consequences in major organs. In the CNS, MIF supports
neuroinflammation; in adipose tissue, macrophage-mediated metabolic derangement; in the liver and kidneys,
it
could cause repair or fibrosis based on disease context. Fig. 4 emphasizes the dual functions of MIF as
both a
protective and disease-causing mediator.
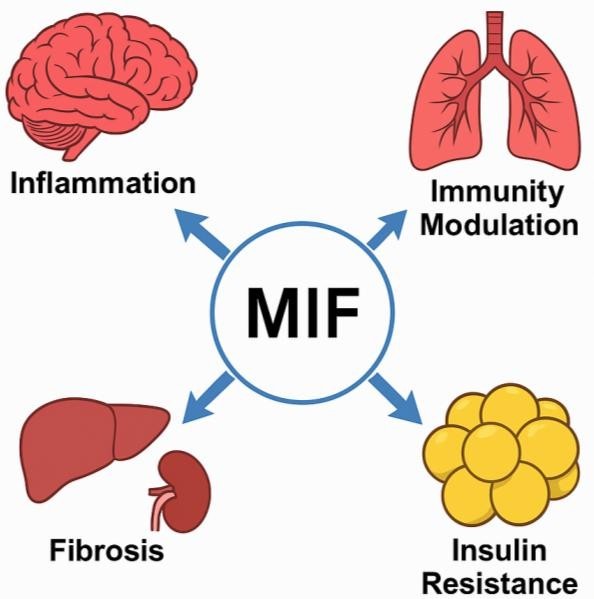
Fig. 4: Organ-Specific Functions of MIF.
Diagnostic and Prognostic Utility of MIF and Genetic Markers
Beyond its pathogenic role, MIF demonstrates consistent potential as a quantitative biomarker. High levels
of
serum MIF, especially above 10 ng/mL, have also been linked with further mortality, multi-organ
dysfunction and
non-response to therapy in sepsis. Meta-analysis by Toldi et al [99]. proved its diagnostic
and prognostic properties in a variety of clinical trials and may replace traditional sepsis scores. Serum
MIF
and soluble CD27 have been noted to be highly sensitive in monitoring the activity of the disease and
predicting
the relapse in vitiligo [100]. Genetic studies favor the fact that MIF is significant to diagnosis. Gehlen
et
al [101]. revealed that the -173 G>C SNP in the promoter region of MIF is linked to the increased
risk of pulmonary tuberculosis among individuals with high rates of MIF expression. In addition to that,
the
eQTL mapping studies of the lung tissue show that the expression of MIF and MIF-2 is genetically regulated
and
could play a crucial role in the context of chronic respiratory disorders and the treatment response
[102]. The
findings show that both the measurement of serum MIF level and genotype testing might contribute to the
development of individualized strategies in treating different inflammatory and infectious conditions.
Advances in Therapeutic Targeting of MIF and MIF-2
MIF treatment is done by therapists through three primary modes, and these include small- molecule
medicines,
CD74 blockers, and gene or antibody-based therapies. There are two inhibitors, ISO-1 and MIF-2, that were
developed in a laboratory and are likely to prevent the work of tautomerase, as well as the work of the
receptor
proteins. Tilstam et al [53]. discovered that when MIF-2 is prevented from forming, inflammation is
reduced, but MIF still contributes to tissue healing. The presence of MIF and MIF-2, and their receptors,
helps
improve the treatment carried out [103]. Working on the MIF-CD74 connection has been beneficial. Chan and
his
team found that decreasing CD74 in mice with obesity decreased the activity of M1 macrophages, improved
insulin
function, and lowered inflammation in fat cells, showing that MIF signaling affects metabolic problems
[104].
Precision immunotherapy is also using CRISPR, RNA interference, and monoclonal antibodies to lower the
amount or
activity of MIF. Kang and Bucala [6] believe that testing for MIF polymorphisms in patients may improve
treatment outcomes, mainly in autoimmune and cancer diseases where MIF levels are not properly regulated
[105].
At this point, scientists are testing the safety of these treatments to develop ones that can treat
chronic
inflammation early and have fewer negative effects on the immune system. Aside from agents mentioned
previously,
more diverse sets of therapeutic approaches have since been created to target MIF family proteins.
Orthosteric
inhibitors function by directly interacting within the catalytic tautomerase pocket, usually through the
conserved Pro1 residue, thus inhibiting receptor interaction. Conversely, allosteric modulators, including
Iguratimod and other solvent channel blockers, act by changing the conformation of MIF and not by filling
its
active site, providing greater selectivity with potentially fewer side effects. Covalent inhibitors also
exist,
which bind irreversibly to crucial residues to provide prolonged inhibition, especially in situations of
chronic
inflammation. Notably, the therapeutic strategies now segregate by MIF and structural homolog MIF-2, their
distinct receptor binding and tissue-restricted functions having been appreciated. Selective MIF-2
inhibitors
have exhibited anti-inflammatory effects in multiple autoimmune and metabolic disease models, underscoring
the
value of isoform-specific pharmacological logic. This larger pharmacological repertoire offers a richer
landscape to interfere with MIF-mediated pathologies and optimize interventions to the disease context and
expression profile.
Future Directions
Although important advances have been made in defining the functions of MIF and MIF-2 in immune regulation, inflammation, and disease, several key questions have to be addressed. At a structural level, the absence of high-resolution structures of MIF-receptor complexes, especially with CD74 and CXCR co-receptors, restricts knowledge regarding specific binding interfaces and receptor recognition-induced conformational changes. Mechanistically, the functional effects of MIF oxidation, dimerization status, and intracellular partner interactions like with JAB1 and CSN5 remain to be fully understood. Therapeutically, while a number of small-molecule and monoclonal antibody inhibitors of MIF have exhibited preclinical potential, there are issues regarding the attainment of isoform specificity, off-target effects, and overcoming immune compensation. The dual role of MIF, being protective in acute responses and pathogenic in chronic inflammation, represents a major obstacle for developing broadly effective therapies. The future will focus on the following important areas: definition of tissue- and context-dependent MIF functions through spatial transcriptomics and proteomics, the development of targeted delivery platforms to selectively modulate MIF activity in diseased tissues, and clarification of the genetic polymorphisms or post-translational modifications that will be known to impact MIF function across different patient groups. Resolution of these issues will further improve the translational potential of MIF-targeted therapies and define its utility as a biomarker in personalized medicine.
Conclusion
Macrophage Migration Inhibitory Factor (MIF) has evolved from a historically defined cytokine into a multifunctional immune regulator with profound relevance across physiology and pathology. This review has explained that MIF is active at the meeting point of innate and adaptive immunity, affecting many processes by signaling through CD74, CXCR4/7 and leading to the activation of NF-κB, ERK1/2, PI3K/AKT, and JNK. It is not easily suppressed by glucocorticoids; it acts as a major cause of inflammation in both sudden and long-lasting conditions. At the cellular level, MIF governs macrophage polarization, favors Th1 and Th17 lineage differentiation, sustains B cell hyperactivation, and contributes to immune evasion in cancer. These mechanisms have wide-reaching consequences in autoimmune disorders (RA, SLE), metabolic dysfunction (obesity, insulin resistance), chronic inflammatory diseases (asthma, hepatitis, nephritis), and neurodegeneration (Alzheimer’s disease, ischemic stroke). MIF's context-dependent duality, protective in controlled injury but pathogenic in unresolved inflammation, makes it a complex but crucial modulator of tissue microenvironments. Importantly, integrating MIF polymorphisms, serum level monitoring, and tissue-specific expression patterns has paved the way for its use as a diagnostic and prognostic biomarker. Elevated MIF levels have been linked to disease severity, therapeutic resistance, and relapse risk in diverse disorders, including sepsis, tuberculosis, COVID-19, vitiligo, and cancers. Genotype-phenotype associations have further demonstrated that the individual MIF expression profiles can influence susceptibility and response to treatment, advancing its utility in precision medicine. Therapeutically, MIF has become a viable intervention point for targeted drug development. Strategies such as small-molecule inhibitors, MIF-2-selective blockers, CD74 antagonists, and gene-editing tools (e.g., RNAi, CRISPR) are being investigated for their ability to neutralize MIF activity without compromising protective immune functions. These approaches have shown promise in preclinical models of inflammatory, metabolic, oncologic, and fibrotic diseases. However, the therapeutic challenge remains to discriminate pathological MIF signaling from its physiological roles in tissue protection, regeneration, and acute immune defense. MIF stands at the forefront of immunopathology as a dynamic cytokine with diagnostic, prognostic, and therapeutic value. Its capacity to influence inflammation, immunity, metabolism, and cellular resilience across organ systems makes it a central hub in disease modulation. Future translational efforts must focus on integrating MIF-targeting strategies into patient-specific frameworks, leveraging biomarker intelligence and genetic profiling to refine efficacy and safety. With its multidimensional role now firmly established, MIF represents not only a therapeutic target but also a paradigm for immunological systems biology in the era of precision healthcare.
Disclosure Statement
The authors have nothing to disclose.
References
| 1 | Bennett B, Bloom BR: Studies on the migration inhibitory factor associated with delayed-type
hypersensitivity: cytodynamics and specificity. Transplantation 1967;5(4): 996-1000
https://doi.org/10.1097/00007890-196707001-00032 |
| 2 | Bloom BR, Bennett B: Relation of the migration inhibitory factor (MIF) to delayed-type
hypersensitivity reactions. Ann N Y Acad Sci 1970;169(1):258-65.
https://doi.org/10.1111/j.1749-6632.1970.tb55994.x |
| 3 | Kungwankiattichai S, Maziarz RT: The history of cytokines and growth factors development. Best
Pract Res Clin Haematol DOI: 10.1016/j.beha.2025.101612
https://doi.org/10.1016/j.beha.2025.101612 |
| 4 | Neta R, Oppenheim JJ, Durum SK: The cytokine concept: historical perspectives and current status
of the cloned cytokines. In: The Role of Lymphokines in the Immune Response. Boca Raton: CRC Press;
2024 pp 29-41.
https://doi.org/10.1201/9781003574767-2 |
| 5 | Harris J, VanPatten S, Deen NS, Al-Abed Y, Morand EF: Rediscovering MIF: new tricks for an old
cytokine. Trends Immunol 2019;40(5):447-62.
https://doi.org/10.1016/j.it.2019.03.002 |
| 6 | Kang I, Bucala R: The immunobiology of MIF: function, genetics and prospects for precision
medicine. Nat Rev Rheumatol 2019;15(7):427-37.
https://doi.org/10.1038/s41584-019-0238-2 |
| 7 | Zhu WY, Jin X, Ma YC, Liu ZB: Correlations of MIF polymorphism and serum levels of MIF with
glucocorticoid sensitivity of sudden sensorineural hearing loss. J Int Med Res
DOI:10.1177/0300060519893870.
https://doi.org/10.1177/0300060519893870 |
| 8 | Kong YZ, Chen Q, Lan HY: Macrophage migration inhibitory factor (MIF) as a stress molecule in
renal inflammation. Int J Mol Sci 2022;23(9):4908.
https://doi.org/10.3390/ijms23094908 |
| 9 | Lang T, Lee JP, Elgass K, Pinar AA, Tate MD, Aitken EH, Fan H, Creed SJ, Deen NS, Traore D-AK,
Mueller I, Stanisic D, Baiwog FS, Skene C, Wilce M-CJ, Mansell A, Morand EF, Harris J: Macrophage
migration inhibitory factor is required for NLRP3 inflammasome activation. Nat Commun
2018;9(1):2223.
https://doi.org/10.1038/s41467-018-04581-2 |
| 10 | Jankauskas SS, Wong DW, Bucala R, Djudjaj S, Boor P: Evolving complexity of MIF signaling. Cell
Signal. 2019;57:76-88.
https://doi.org/10.1016/j.cellsig.2019.01.006 |
| 11 | Farr L, Ghosh S, Jiang N, Watanabe K, Parlak M, Bucala R, Moonah S: CD74 signaling links
inflammation to intestinal epithelial cell regeneration and promotes mucosal healing. Cell Mol
Gastroenterol Hepatol 2020;10(1):101-12.
https://doi.org/10.1016/j.jcmgh.2020.01.009 |
| 12 | Bozza MT, Lintomen L, Kitoko JZ, Paiva CN, Olsen PC: The role of MIF on eosinophil biology and
eosinophilic inflammation. Clin Rev Allergy Immunol 2020;58:15-24.
https://doi.org/10.1007/s12016-019-08726-z |
| 13 | Zeng L, Hu P, Zhang Y, Li M, Zhao Y, Li S, Luo A: Macrophage migration inhibitory factor (MIF):
potential role in cognitive impairment disorders. Cytokine Growth Factor Rev 2024;77:67-75.
https://doi.org/10.1016/j.cytogfr.2024.03.003 |
| 14 | Pressley KR, Naseem Y, Nalawade S, Forsthuber TG: The distinct functions of MIF in inflammatory
cardiomyopathy. Front Immunol DOI: 10.3389/fimmu.2025.1544484
https://doi.org/10.3389/fimmu.2025.1544484 |
| 15 | Cassetta L, Pollard JW: A timeline of tumour-associated macrophage biology. Nat Rev Cancer.
2023;23(4):238-57.
https://doi.org/10.1038/s41568-022-00547-1 |
| 16 | Alban TJ, Bayik D. Otvos B, Rabljenovic A, Leng L, Jia-Shiun L, Roversi G, Lauko A, Momin AA,
Mohammadi AM, Peereboom DM, Ahluwalia MS, Matsuda K, Yun K, Bucala R, Vogelbaum MA, Lathia JD:
Glioblastoma myeloid-derived suppressor cell subsets express differential macrophage migration
inhibitory factor receptor profiles that can be targeted to reduce immune suppression. Front Immunol
2020;11:1191.
https://doi.org/10.3389/fimmu.2020.01191 |
| 17 | Mora BR, Stijlemans B, Van Ginderachter JA: Hallmarks of cancer affected by the MIF cytokine
family. Cancers (Basel). 2023;15(2):395.
https://doi.org/10.3390/cancers15020395 |
| 18 | Zhang X, Yang X, Chen M, Zheng S, Li J, Lin S, Wang X: ST3Gal3 confers paclitaxel-mediated
chemoresistance in ovarian cancer cells by attenuating caspase-8/3 signaling. Mol Med Rep.
2019;20(5):4499-506.
https://doi.org/10.3892/mmr.2019.10712 |
| 19 | Stoppe C, Averdunk L, Goetzenich A, Soppert J, Marlier A, Kraemer S, Vieten J, Coburn M, Kowark A,
Kim BS, Marx G, Rex S, Ochi A, Leng L, Moeckel G, Linkermann A, El Bounkari O, Zarbock A, Bernhagen
J, Djudjaj S, Boor P: The protective role of macrophage migration inhibitory factor in acute kidney
injury after cardiac surgery. Sci Transl Med. 2018;10(441).
https://doi.org/10.1126/scitranslmed.aan4886 |
| 20 | Farr L, Ghosh S, Moonah S: Role of MIF cytokine/CD74 receptor pathway in protecting against injury
and promoting repair. Front Immunol 2020;11:1273.
https://doi.org/10.3389/fimmu.2020.01273 |
| 21 | Ives A, Le Roy D, Théroude C, Bernhagen J, Roger T, Calandra T: Macrophage migration inhibitory
factor promotes the migration of dendritic cells through CD74 and the activation of the
Src/PI3K/myosin II pathway. FASEB J 2021;35(5):e21418.
https://doi.org/10.1096/fj.202001605R |
| 22 | Michelet C, Danchin EG, Jaouannet M, Bernhagen J, Panstruga R, Kogel KH, Keller H, Coustau C:
Cross-kingdom analysis of diversity, evolutionary history, and site selection within the eukaryotic
macrophage migration inhibitory factor superfamily. Genes (Basel) 2019;10(10):740.
https://doi.org/10.3390/genes10100740 |
| 23 | Pantouris G, Khurana L, Ma A, Skeens E, Reiss K, Batista VS, Lisi GP, Lolis EJ: Regulation of MIF
enzymatic activity by an allosteric site at the central solvent channel. Cell Chem Biol.
2020;27(6):740-50.e6.
https://doi.org/10.1016/j.chembiol.2020.05.001 |
| 24 | Schinagl A, Thiele M, Douillard P, Völkel D, Kenner L, Kazemi Z, Freissmuth M, Scheiflinger F,
Kerschbaumer RJ: Oxidized macrophage migration inhibitory factor is a potential new tissue marker
and drug target in cancer. Oncotarget 2016;8;7(45):73486-73496
https://doi.org/10.18632/oncotarget.11970 |
| 25 | Sajko S, Skeens E, Schinagl A, Ferhat M, Mirkina I, Mayer J, Rossmueller G, Thiele M, Lisi GP:
Redox-dependent plasticity of oxMIF facilitates its interaction with CD74 and therapeutic
antibodies. Redox biology 2024;75, 103264.
https://doi.org/10.1016/j.redox.2024.103264 |
| 26 | Thiele M, Bernhagen J: Link between macrophage migration inhibitory factor and cellular redox
regulation. Antioxid Redox Signal. 2005;7(9-10):1234-48.
https://doi.org/10.1089/ars.2005.7.1234 |
| 27 | Arizza V, Bonura A, La Paglia L, Urso A, Pinsino A, Vizzini A:Transcriptional and in silico
analyses of MIF cytokine and TLR signalling interplay in the LPS inflammatory response of Ciona
robusta. Sci Rep. 2020;10(1):11339.
https://doi.org/10.1038/s41598-020-68339-x |
| 28 | Lacy M, Kontos C, Brandhofer M, Hille K, Gröning S, Sinitski D, Bourilhon P, Rosenberg E, Krammer
C, Thavayogarajah T, Pantouris G, Bakou M, Weber C, Lolis E, Bernhagen J, Kapurniotu A:
Identification of an Arg-Leu-Arg tripeptide that contributes to the binding interface between the
cytokine MIF and the chemokine receptor CXCR4 Sci Rep 2018;8(1):5171.
https://doi.org/10.1038/s41598-018-23554-5 |
| 29 | Stijlemans B, Schoovaerts M, De Baetselier P, Magez S, De Trez C: The role of MIF and IL-10 as
molecular Yin-Yang in the modulation of the host immune microenvironment during infections: African
trypanosome infections as a paradigm. Front Immunol 2022;13:865395.
https://doi.org/10.3389/fimmu.2022.865395 |
| 30 | Bilsborrow JB, Doherty E, Tilstam PV, Bucala R: Macrophage migration inhibitory factor (MIF) as a
therapeutic target for rheumatoid arthritis and systemic lupus erythematosus. Expert Opin Ther
Targets 2019;23(9):733-44.
https://doi.org/10.1080/14728222.2019.1656718 |
| 31 | Voss S, Krüger S, Scherschel K, Warnke S, Schwarzl M, Schrage B, Girdauskas E, Meyer C,
Blankenberg S, Westermann D, Lindner D: Macrophage migration inhibitory factor (MIF) expression
increases during myocardial infarction and supports pro-inflammatory signaling in cardiac
fibroblasts. Biomolecules. 2019;9(2):38.
https://doi.org/10.3390/biom9020038 |
| 32 | Sinitski D, Kontos C, Krammer C, Asare Y, Kapurniotu A, Bernhagen J: Macrophage migration
inhibitory factor (MIF)-based therapeutic concepts in atherosclerosis and inflammation. Thromb
Haemost. 2019;119(4):553-66.
https://doi.org/10.1055/s-0039-1677803 |
| 33 | Guda MR, Rashid MA, Asuthkar S, Jalasutram A, Caniglia JL, Tsung AJ, Velpula KK: Pleiotropic role
of macrophage migration inhibitory factor in cancer. Am J Cancer Res. 2019;9(12):2760-2773.
|
| 34 | Kohli K, Pillarisetty VG, Kim TS: Key chemokines direct migration of immune cells in solid tumors.
Cancer Gene Ther 2022;29(1):10-21.
https://doi.org/10.1038/s41417-021-00303-x |
| 35 | Yirmiya R: The inflammatory underpinning of depression: An historical perspective. Brain Behav
Immun 2024;122: 433-443.
https://doi.org/10.1016/j.bbi.2024.08.048 |
| 36 | Lehtiö J, Arslan T, Siavelis I, Pan Y, Socciarelli F, Berkovska O, Umer HM, Mermelekas G,
Pirmoradian M, Jönsson M, Brunnström H, Brustugun OT, Purohit KP, Cunningham R., Foroughi AH,
Isaksson S, Arbajian E, Aine M, Karlsson A, Kotevska M, Orre LM: Proteogenomics of non-small cell
lung cancer reveals molecular subtypes associated with specific therapeutic targets and immune
evasion mechanisms. Nature cancer 2021; 2(11):1224-1242.
https://doi.org/10.1038/s43018-021-00259-9 |
| 37 | Chen E, Reiss K, Shah D, Manjula R, Allen B, Murphy EL, Murphy JW, BatistaVS, Bhandari V, Lolis
EJ, Lisi GP: A structurally preserved allosteric site in the MIF superfamily affects enzymatic
activity and CD74 activation in D-dopachrome tautomerase. J Biol Chem. 2021;297(3): 101061
https://doi.org/10.1016/j.jbc.2021.101061 |
| 38 | Pogozheva ID, Cherepanov S, Park SJ, Raghavan M, Im W, Lomize AL: Structural modeling of
cytokine-receptor-JAK2 signaling complexes using AlphaFold multimer. J Chem Inf Model.
2023;63(18):5874-95.
https://doi.org/10.1021/acs.jcim.3c00926 |
| 39 | Cavalli E, Ciurleo R, Petralia MC, Fagone P, Bella R, Mangano K, Nicoletti F, Bramanti P, Basile
MS: Emerging role of the macrophage migration inhibitory factor family of cytokines in
neuroblastoma: Pathogenic effectors and novel therapeutic targets? Molecules. 2020;25(5):1194.
https://doi.org/10.3390/molecules25051194 |
| 40 | Michida H, Imoto H, Shinohara H, Yumoto N, Seki M, Umeda M, Hayashi T, Nikaido I, Kasukawa T,
Suzuki Y, Okada-Hatakeyama M: The number of transcription factors at an enhancer determines
switch-like gene expression. Cell Rep. 2020;31(9):107782.
https://doi.org/10.1016/j.celrep.2020.107724 |
| 41 | Illescas O, Gomez-Verjan JC, García-Velázquez L, Govezensky T, Rodriguez-Sosa M: Macrophage
migration inhibitory factor-173 G/C polymorphism: A global meta-analysis across the disease
spectrum. Front Genet. 2018;9:55.
https://doi.org/10.3389/fgene.2018.00055 |
| 42 | Qin L, Qin J, Lv X, Yin C, Zhang QE, Zhang J.: MIF promoter polymorphism increases peripheral
blood expression levels, contributing to increased susceptibility and poor prognosis in
hepatocellular carcinoma. Oncol Lett. 2021;22(1):549.
https://doi.org/10.3892/ol.2021.12810 |
| 43 | Averdunk L, Bernhagen J, Fehnle K, Surowy H, Lüdecke HJ, Mucha S, Meybohm P, Wieczorek D, Leng L,
Marx G, Leaf DE, Zarbock A, Zacharowski K, Bucala R, Stoppe C: The macrophage migration inhibitory
factor (MIF) promoter polymorphisms (rs3063368, rs755622) predict acute kidney injury and death
after cardiac surgery. J Clin Med. 2020;9(9):2936.
https://doi.org/10.3390/jcm9092936 |
| 44 | Gupta P, Joshi N, Uprety S, Dogra S, De D, Handa S, Minz RW, Singh S, Chhabra S: Association of
MIF gene polymorphisms with pemphigus vulgaris: A case-control study with comprehensive review of
the literature. Int J Clin Exp Pathol. 2021;14(11):1080-1089.
|
| 45 | Jiao D, Sun R, Ren X, Wang Y, Tian P, Wang Y, Yuan D, Yue X, Wu Z, Li C, Gao L, Ma C, Liang X:
Lipid accumulation-mediated histone hypoacetylation drives persistent NK cell dysfunction in
anti-tumor immunity. Cell Rep. 2023;42(10):112023.
https://doi.org/10.1016/j.celrep.2023.113211 |
| 46 | Huang C, Huang R, Chen H, Ni Z, Huang Q, Huang Z, Ge B: Chromatin accessibility regulates gene
expression and correlates with tumor-infiltrating immune cells in gastric adenocarcinoma. Front
Oncol. 2021;10:609940.
https://doi.org/10.3389/fonc.2020.609940 |
| 47 | Ding J, Wu W, Yang J, Wu M: Long non-coding RNA MIF-AS1 promotes breast cancer cell proliferation,
migration and EMT process through regulating miR-1249-3p/HOXB8 axis. Pathol Res Pract.
2019;215(7):152376.
https://doi.org/10.1016/j.prp.2019.03.005 |
| 48 | Phelan JD, Staudt LM. CRISPR-based technology to silence the expression of lncRNAs. Proc Natl Acad
Sci U S A. 2020;117(15):8225-7.
https://doi.org/10.1073/pnas.2003702117 |
| 49 | Osipyan A, Chen D, Dekker FJ. Epigenetic regulation in macrophage migration inhibitory factor
(MIF)-mediated signaling in cancer and inflammation. Drug Discov Today. 2021;26(7):1728-34.
https://doi.org/10.1016/j.drudis.2021.03.012 |
| 50 | Sampaio-Dias IE, Silva-Reis SC, García-Mera X, Brea J, Loza MI, Alves CS, Algarra M,
Rodríguez-Borges JE: Synthesis, pharmacological, and biological evaluation of MIF-1 picolinoyl
peptidomimetics as positive allosteric modulators of D2R. ACS Chem Neurosci. 2019;10(8):3690-3702.
https://doi.org/10.1021/acschemneuro.9b00259 |
| 51 | Bloom J, Pantouris G, He M, Aljabari B, Mishra L, Manjula R, Parkins A, Lolis EJ, Al-Abed Y:
Iguratimod, an allosteric inhibitor of macrophage migration inhibitory factor (MIF), prevents
mortality and oxidative stress in a murine model of acetaminophen overdose. Mol Med. 2024;30(1):43.
https://doi.org/10.1186/s10020-024-00803-0 |
| 52 | Pantouris G, Khurana L, Tilstam P, Benner A, Cho TY, Lelaidier M, Perrée M, Rosenbaum Z, Leng L,
Foss F, Bhandari V, Verma A, Bucala R, Lolis EJ: Inhibition of MIF with an allosteric inhibitor
triggers cell cycle arrest in acute myeloid leukemia. ACS Omega 2025; 10(17):17441-17452
https://doi.org/10.1021/acsomega.4c10969 |
| 53 | Tilstam PV, Pantouris G, Corman M, Andreoli M, Mahboubi K, Davis G, Du X, Leng L, Lolis E, Bucala
R: A selective small-molecule inhibitor of macrophage migration inhibitory factor-2 (MIF-2), a MIF
cytokine superfamily member, inhibits MIF-2 biological activity. J Biol Chem. 2019;294(49):18522-31.
https://doi.org/10.1074/jbc.RA119.009860 |
| 54 | Rafiei S, Gui B, Wu J, Liu XS, Kibel AS, Jia L: Targeting the MIF/CXCR7/AKT signaling pathway in
castration-resistant prostate cancer. Mol Cancer Res. 2019;17(1):263-76.
https://doi.org/10.1158/1541-7786.MCR-18-0412 |
| 55 | Obata T, Tsutsumi K, Ueta E, Oda T, Kikuchi T, Ako S, Fujii Y, Yamazaki T, Uchida D, Matsumoto K,
Horiguchi S, Kato H, Okada H, Otsuka M: MicroRNA-451a inhibits gemcitabine-refractory biliary tract
cancer progression by suppressing the MIF-mediated PI3K/AKT pathway. Mol Ther Nucleic Acids.
2023;34:360-74.
https://doi.org/10.1016/j.omtn.2023.102054 |
| 56 | Chen CA, Chang DM, Yang YL, Chang EE, Chen HC. Macrophage migration inhibitory factor regulates
integrin-β1 and cyclin D1 expression via ERK pathway in podocytes. Biomed Pharmacother.
2020;124:109892.
https://doi.org/10.1016/j.biopha.2020.109892 |
| 57 | Wei QT, Liu BY, Ji HY, Lan YF, Tang WH, Zhou J, Zhong XY, Lian CL, Huang QZ, Wang CY, Xu YM, Guo
HB: Exosome-mediated transfer of MIF confers temozolomide resistance by regulating TIMP3/PI3K/AKT
axis in gliomas. Mol Ther Oncolytics. 2021;22:114-128.
https://doi.org/10.1016/j.omto.2021.08.004 |
| 58 | Xia W, Hou M: Macrophage migration inhibitory factor rescues mesenchymal stem cells from
doxorubicin-induced senescence through the PI3K-Akt signaling pathway. Int J Mol Med.
2018;41(2):1127-37.
https://doi.org/10.3892/ijmm.2017.3282 |
| 59 | He Z, Wang Y, Zhuan L, Li Y, Tang ZO, Wu Z, Ma Y: MIF-mediated NF-κB signaling pathway regulates
the pathogenesis of polycystic ovary syndrome in rats. Cytokine. 2021;146:155632.
https://doi.org/10.1016/j.cyto.2021.155632 |
| 60 | Zhang Y, Tang PMK, Niu Y, García Córdoba CA, Huang XR, Yu C, Lan HY: Long non-coding RNA LRNA9884
promotes acute kidney injury via regulating NF-κB-mediated transcriptional activation of MIF. Front
Physiol. 2020;11:590027.
https://doi.org/10.3389/fphys.2020.590027 |
| 61 | Chan PC, Wu TN, Chen YC, Lu CH, Wabitsch M, Tian YF, Hsieh PS: Targeted inhibition of CD74
attenuates adipose COX-2-MIF-mediated M1 macrophage polarization and retards obesity-related adipose
tissue inflammation and insulin resistance. Clin Sci (Lond). 2018;132(14):1581-96.
https://doi.org/10.1042/CS20180041 |
| 62 | Bae SC, Lee YH: Associations between circulating macrophage migration inhibitory factor (MIF)
levels and rheumatoid arthritis, and between MIF gene polymorphisms and disease susceptibility: a
meta-analysis. Postgrad Med J. 2018;94(1108):109-15.
https://doi.org/10.1136/postgradmedj-2017-134934 |
| 63 | Breidung D, Megas IF, Freytag DL, Bernhagen J, Grieb G: The Role of Macrophage Migration
Inhibitory Factor (MIF) and D-Dopachrome Tautomerase (D-DT/MIF-2) in Infections: A Clinical
Perspective. Biomedicines. 2023;12(1):2
https://doi.org/10.3390/biomedicines12010002 |
| 64 | Yao J, Leng L, Fu W, Li J, Bronner C, Bucala R: ICBP90 regulates MIF expression, glucocorticoid
sensitivity, and apoptosis at the MIF immune susceptibility locus. Arthritis Rheumatol.
2021;73(10):1931-42.
https://doi.org/10.1002/art.41753 |
| 65 | Carcillo JA, Shakoory B: Cytokine storm and sepsis-induced multiple organ dysfunction syndrome.
In: Cron RQ, Behrens EM, editors. Cytokine Storm Syndrome. Cham: Springer; AEMB, 2024, vol 1448, pp
441-457.
https://doi.org/10.1007/978-3-031-59815-9_30 |
| 66 | Bludau I, Aebersold R: Proteomic and interactomic insights into the molecular basis of cell
functional diversity. Nat Rev Mol Cell Biol. 2020;21(6):327-40.
https://doi.org/10.1038/s41580-020-0231-2 |
| 67 | Lee KY: M1 and M2 polarization of macrophages: a mini-review. Med Biol Sci Eng. 2019;2(1):1-5.
https://doi.org/10.30579/mbse.2019.2.1.1 |
| 68 | Luo JY, Fang BB, Du GL, Liu F, Li YH, Tian T, Li XM., Gao XM., Yang YN: Association between MIF
gene promoter rs755622 and susceptibility to coronary artery disease and inflammatory cytokines in
the Chinese Han population. Sci Rep. 2021;11(1):8050.
https://doi.org/10.1038/s41598-021-87580-6 |
| 69 | Zhao Y, Wei X, Li W, Shan C, Song J, Zhang M: Inhibition of macrophage migration inhibitory factor
protects against inflammation through a Toll‐like receptor‐related pathway after diffuse axonal
injury in rats. Biomed Res Int. 2020;2020:5946205.
https://doi.org/10.1155/2020/5946205 |
| 70 | Figueiredo CR, Azevedo RA, Mousdell S, Resende-Lara PT, Ireland L, Santos A, Girola N, Cunha RL,
Schmid MC, Polonelli L, Travassos LR, Mielgo A: Blockade of MIF-CD74 signalling on macrophages and
dendritic cells restores the antitumour immune response against metastatic melanoma. Front Immunol.
2018;9:1132.
https://doi.org/10.3389/fimmu.2018.01132 |
| 71 | De la Cruz-Mosso U, García-Iglesias T, Bucala R, Estrada-García I, González-López L, Cerpa-Cruz S,
Parra-Rojas I, Gámez-Nava JI, Pérez-Guerrero EE, Muñoz-Valle JF: MIF promotes a differential
Th1/Th2/Th17 inflammatory response in human primary cell cultures: Predominance of Th17 cytokine
profile in PBMC from healthy subjects and increase of IL-6 and TNF-α in PBMC from active SLE
patients. Cell Immunol. 2018;324:42-49.
https://doi.org/10.1016/j.cellimm.2017.12.010 |
| 72 | Yan G, Song R, Zhang J, Li Z, Lu Z, Liu Z, Zeng X, Yao J: MIF promotes Th17 cell differentiation
in rheumatoid arthritis through ATF6 signal pathway. Mol Med. 2024;30(1):237.
https://doi.org/10.1186/s10020-024-01005-4 |
| 73 | Noe JT, Mitchell RA. MIF-dependent control of tumor immunity. Front Immunol. 2020;11:609948.
https://doi.org/10.3389/fimmu.2020.609948 |
| 74 | Phalke S, Rivera‐Correa J, Jenkins D, Flores Castro D, Giannopoulou E, Pernis AB. Molecular
mechanisms controlling age‐associated B cells in autoimmunity. Immunol Rev. 2022;307(1):79-100.
https://doi.org/10.1111/imr.13068 |
| 75 | Jaramillo-Valverde L, Levano KS, Capristano S, Tarazona DD, Cisneros A, Yufra-Picardo VM,
Valdivia-Silva J, Guio H: CXCR4 Knockdown Via CRISPR/CAS9 in a Tumor-Associated Macrophage Model
Decreases Human Breast Cancer Cell Migration. Cureus. 2021;13(12):e20842.
https://doi.org/10.7759/cureus.20842 |
| 76 | Kim BS, Tilstam PV, Arnke K, Leng L, Ruhl T, Piecychna M, Schulte W, Sauler M, Frueh FS, Storti G,
Lindenblatt N, Giovanoli P, Pallua N, Bernhagen J, Bucala R: Differential regulation of macrophage
activation by the MIF cytokine superfamily members MIF and MIF‐2 in adipose tissue during
endotoxemia. FASEB J. 2020;34(3):4219-4233.
https://doi.org/10.1096/fj.201901511R |
| 77 | Chen A, Koehler AN: Transcription factor inhibition: lessons learned and emerging targets. Trends
Mol Med. 2020;26(5):508-518.
https://doi.org/10.1016/j.molmed.2020.01.004 |
| 78 | Liu YC, Tsai YH, Tang SC, Liou HC, Kang KH, Liou HH, Jeng JS., FuWM: Cytokine MIF enhances
blood-brain barrier permeability: impact for therapy in ischemic stroke. Sci Rep. 2018;8(1):743.
https://doi.org/10.1038/s41598-017-16927-9 |
| 79 | Li Y, Zou C, Chen C, Li S, Zhu Z, Fan Q, Pang R, Li F, Chen Z, Wang Z, Yu W, Yuan J, Li P:
Myeloid-derived MIF drives RIPK1-mediated cerebromicrovascular endothelial cell death to exacerbate
ischemic brain injury. Proc Natl Acad Sci USA. 2023;120(5):e2219091120.
https://doi.org/10.1073/pnas.2219091120 |
| 80 | Nasiri E, Sankowski R, Dietrich H, Oikonomidi A, Huerta PT, Popp J, Al- Abed Y, Bacher M: Key role
of MIF-related neuroinflammation in neurodegeneration and cognitive impairment in Alzheimer's
disease. Mol Med. 2020;26:1-12.
https://doi.org/10.1186/s10020-020-00163-5 |
| 81 | Newell-Rogers MK, Rogers SK, Tobin RP, Mukherjee S, Shapiro LA: Antagonism of macrophage migration
inhibitory factor (MIF) after traumatic brain injury ameliorates astrocytosis and peripheral
lymphocyte activation and expansion. Int J Mol Sci. 2020;21(20):7448.
https://doi.org/10.3390/ijms21207448 |
| 82 | Matejuk A, Benedek G, Bucala R, Matejuk S, Offner H, Vandenbark AA: MIF contribution to
progressive brain diseases. J Neuroinflammation. 2024;21(1):8.
https://doi.org/10.1186/s12974-023-02993-6 |
| 83 | Florez-Sampedro L, Soto-Gamez A, Poelarends GJ, Melgert BN. The role of MIF in chronic lung
diseases: looking beyond inflammation. Am J Physiol Lung Cell Mol Physiol. 2020;318(6):L1183-97.
https://doi.org/10.1152/ajplung.00521.2019 |
| 84 | Poulsen KL, Fan X, Kibler CD, Huang E, Wu X, McMullen MR, Leng L, Bucala R, Ventura-Cots M, Argemi
J, Bataller R, Nagy LE: Role of MIF in coordinated expression of hepatic chemokines in patients with
alcohol-associated hepatitis. JCI Insight. 2021;6(11):e141420.
https://doi.org/10.1172/jci.insight.141420 |
| 85 | Heinrichs D, Brandt EF, Fischer P, Köhncke J, Wirtz TH, Guldiken N, Djudjaj S, Boor P, Kroy D,
Weiskirchen R, Bucala R, Wasmuth HE, Strnad P, Trautwein C, Bernhagen J, Berres ML: Unexpected
pro-fibrotic effect of MIF in non-alcoholic steatohepatitis is linked to a shift in NKT cell
populations. Cells. 2021;10(2):252.
https://doi.org/10.3390/cells10020252 |
| 86 | Liao Y, Wu C, Li Y, Wen J, Zhao D. MIF is a critical regulator of mononuclear phagocytic
infiltration in hepatocellular carcinoma. iScience. 2023;26(8):107251.
https://doi.org/10.1016/j.isci.2023.107273 |
| 87 | Li JH, Tang Y, Lv J, Wang XH, Yang H, Tang PM, Huang XR, He ZJ, Zhou ZJ, Huang QY, Klug J,
Meinhardt A, Fingerle-Rowson G, Xu AP, Zheng ZH, Lan HY: Macrophage migration inhibitory factor
promotes renal injury induced by ischemic reperfusion. J Cell Mol Med. 2019;23(6):3867-77.
https://doi.org/10.1111/jcmm.14234 |
| 88 | Yang H, Li J, Huang XR, Bucala R, Xu A, Lan HY: Macrophage-derived macrophage migration inhibitory
factor mediates renal injury in anti-glomerular basement membrane glomerulonephritis. Front Immunol.
2024;15:1361343.
https://doi.org/10.3389/fimmu.2024.1361343 |
| 89 | Tu Y, Guo R, Li J, Wang S, Leng L, Deng J, Bucala R, Lu L: MiRNA regulation of MIF in SLE and
attenuation of murine lupus nephritis with miR-654 Front Immunol. 2019;10:2229.
https://doi.org/10.3389/fimmu.2019.02229 |
| 90 | Peterson K. Assess and Benchmark Magneto-Inertial Fusion (MIF) Scaling. Final Technical Report for
the SNL/Rochester ALPHA Follow-on Project (No. SAND2021-8752R). Albuquerque (NM): Sandia National
Laboratories; 2021.
https://doi.org/10.2172/1821532 |
| 91 | Vujicic M, Saksida T, Despotovic S, Bajic SS, Lalić I, Koprivica I, Gajic D, Golic N, Tolinacki M,
Stojanovic I: The role of macrophage migration inhibitory factor in the function of intestinal
barrier. Sci Rep. 2018;8(1):6337.
https://doi.org/10.1038/s41598-018-24706-3 |
| 92 | Pacheco-Fernández T, Juárez-Avelar I, Illescas O, Terrazas LI, Hernández-Pando R, Pérez-Plasencia
C, Rodriguez-Sosa M: Macrophage migration inhibitory factor promotes the interaction between the
tumor, macrophages, and T cells to regulate the progression of chemically induced colitis-associated
colorectal cancer. Mediators Inflamm. 2019;(1):2056085.
https://doi.org/10.1155/2019/2056085 |
| 93 | Bilgir O, Gökçen B, Bilgir F, Guler A, Calan M, Yuksel A, Aslanıpour B, Akşit M, Bozkaya G:
Relationship between serum macrophage migration inhibitory factor level and insulin resistance,
high-sensitivity C-reactive protein and visceral fat mass in prediabetes. Am J Med Sci.
2018;355(1):37-43.
https://doi.org/10.1016/j.amjms.2017.10.006 |
| 94 | Chen L, Li L, Cui D, Huang Y, Tong H, Zabihi H, Wang S, Qi Y, Lakowski T, Leng L, Liu S, Wu H,
Young LH, Bucala R, Qi D: Extracellular macrophage migration inhibitory factor (MIF) downregulates
adipose hormone-sensitive lipase (HSL) and contributes to obesity. Mol Metab. 2024;79:101834.
https://doi.org/10.1016/j.molmet.2023.101834 |
| 95 | Ietta F, Ferro EAV, Bevilacqua E, Benincasa L, Maioli E, Paulesu L: Role of the macrophage
migration inhibitory factor (MIF) in the survival of first trimester human placenta under induced
stress conditions. Sci Rep. 2018;8(1):12150.
https://doi.org/10.1038/s41598-018-29797-6 |
| 96 | Elbaradie SMY, Bakry MS, Bosilah AH: Serum macrophage migration inhibition factor for diagnosing
endometriosis and its severity: case-control study. BMC Womens Health. 2020;20:1-9.
https://doi.org/10.1186/s12905-020-01051-0 |
| 97 | Penticuff JC, Woolbright BL, Sielecki TM, Weir SJ, Taylor JA: MIF family proteins in genitourinary
cancer: tumorigenic roles and therapeutic potential. Nature reviews. Urology, 2019;16(5):318-328.
https://doi.org/10.1038/s41585-019-0171-9 |
| 98 | Shin JJ, Fan W, Par-Young J, Piecychna M, Leng L, Israni-Winger K, Qing H, Gu J, Zhao H, Schulz
WL, Unlu S, Kuster J, Young G, Liu J, Ko AI, Baeza Garcia A., Sauler M, Wisnewski AV, Young L,
Orduña A, Bucala R: MIF is a common genetic determinant of COVID-19 symptomatic infection and
severity. QJM. 2023;116(3):205-212.
https://doi.org/10.1093/qjmed/hcac234 |
| 99 | Toldi J, Nemeth D, Hegyi P, Molnar Z, Solymar M, Farkas N, Alizadeh H, Rumbus Z, Pakai E, Garami
A: Macrophage migration inhibitory factor as a diagnostic and predictive biomarker in sepsis:
meta-analysis of clinical trials. Sci Rep. 2021;11(1):8051.
https://doi.org/10.1038/s41598-021-87613-0 |
| 100 | ElGhareeb MI, El Mokadem S, El Sayed B, Khalifa N: Soluble CD27 and MIF as possible serum
biomarkers of vitiligo activity in Egyptian patients in Sharkia Governorate. Dermatol Rep.
2019;11(2):8265.
https://doi.org/10.4081/dr.2019.8265 |
| 101 | Gehlen M, Costa ERD, Rossetti MLR, Silva DR: Macrophage migration inhibitory factor-173 G>C single
nucleotide polymorphism and its association with active pulmonary tuberculosis. PLoS One.
2020;15(6):e0234565.
https://doi.org/10.1371/journal.pone.0234565 |
| 102 | Florez-Sampedro L, Brandsma CA, de Vries M, Timens W, Bults R, Vermeulen CJ, van den Berge M,
Obeidat M, Joubert P, Nickle DC, Poelarends GJ, Faiz A, Melgert BN: Genetic regulation of gene
expression of MIF family members in lung tissue. Sci Rep. 2020;10(1):16980.
https://doi.org/10.1038/s41598-020-74121-w |
| 103 | Saini S, Tuli HS, Saini RV, Saini AK, Sak K, Kaur D, Chauhan A: Flavonoid-mediated suppression of
tumor angiogenesis: roles of Ang-Tie/PI3K/AKT. Pathophysiology. 2024;31(4):596-607.
https://doi.org/10.3390/pathophysiology31040043 |
| 104 | Trivedi-Parmar V, Jorgensen WL: Advances and insights for small molecule inhibition of macrophage
migration inhibitory factor. J Med Chem. 2018;61(18):8104-8119.
https://doi.org/10.1021/acs.jmedchem.8b00589 |
| 105 | Xu L, Li Y, Sun H, Zhen X, Qiao C, Tian S, Hou T: Current developments of macrophage migration
inhibitory factor (MIF) inhibitors. Drug discovery today, 2013;18(11-12), 592-600.
https://doi.org/10.1016/j.drudis.2012.12.013 |