Original Article - DOI:10.33594/000000828
Accepted 3 November 2025 - Published online
12 November 2025
Tumor Destructive Mechanical Impulse (TMI) Treatment of Solid Tumors. Part I: Animal Experiments, Clinical Application and Immunological Abscopal Effect
bDepartment of Urology, University of Tübingen, Germany,
cDepartment of Dermatology, University of Tübingen, Germany,
dInstitute of Immunology, University of Duisburg-Essen, Essen, Germany,
eDepartment of Dermatology and Venerology, University of Freiburg, Germany,
fShepherd Center Wound Clinic, Piedmont Atlanta Hospital, Atlanta, GA, USA,
gInternational Neuroscience Institute, Hannover, Germany
Keywords
Abstract
Background/Aims:
The feasibility and effectiveness of Tumor Destructive Mechanical Impulse (TMI) treatment of solid tumors for standard clinical application is investigated.Methods:
Different solid tumors in a preparatory animal experiment (VX2 head and neck squamous tumor) and in patients (malignant cutaneous melanoma and prostate carcinoma) are treated by TMI focused shock waves using patient-specific treatment parameters (total energy, energy flux density, shock wave frequency, total number and sequence of shock waves, the optimal placement of the treatment device) determined by multiple parametric simulations.Results:
In animal experiments and in the different treated tumor entities in several patients, the treated tumor or treated metastases regressed, and disguised tumor-associated antigens consistently initiated an immunological abscopal effect achieving that not only the directly treated primary tumor or a treated metastasis regressed but also untreated distant metastases.Conclusion:
TMI treatment could have significant implications for the development of new effective and targeted regimens of cancer therapy.Introduction
The current therapy for malignant tumors is based on three conventional pillars, namely surgical resection, chemotherapy and radiotherapy, and on innovative approaches including immunotherapy and mRNA therapy, all of them with inherent side effects and disadvantages. Immunotherapy by checkpoint inhibitors can trigger a dysregulated immune response with undesirable auto-aggression against healthy cells, which in some patients can be severe or even life-threatening. mRNA therapy needs a sophisticated and expensive laboratory infrastructure available only in a few institutions throughout the world.
In this situation, having limited and/or expensive and/or time-consuming and unfortunately too often futile options for successful treatment of malignant tumors, the idea arose to try treatment in alternating mechanical fields – ultrasound or shock waves – , thus, physical methods which are not toxic, bear no radiation risk, are not or only mildly traumatic, and rely on clear physical rules and engineering standards.
Materials and Methods
Animal Model
A detailed experimental study, technically supported by the first author A.E. Theuer, was performed at the
Philipps-University of Marburg [1], here summarized with reference to the TMI focused shock wave
treatment. The
animal experiments were approved by the Institutional Review Board of the University of Marburg
(V54-19c20/15
Nr. 24/2016). All methods were performed in accordance with the relevant guidelines and regulations.
The established rabbit auricular VX2 carcinoma was chosen, which is an animal model for human head and
neck
squamous cell carcinoma [2]. For validating the simulation results calculated by A.E. Theuer, VX2 tumor
fragments were injected under the dermis of the left auricle of immunocompetent New Zealand White (NZW)
rabbits.
Two treated groups of 6 male and female NZW rabbits each were observed. One group received three pressure
shock
wave treatments (2, 000 shock wave impulses at 1.41 mJ/mm²), and the second group received the same TMI
treatment combined with administration of Nivolumab (OPDIVO®), a programmed death receptor‑1 (PD1)
blocker
from the checkpoint inhibitor group. A third group of 4 male and female NZW rabbits remained an untreated
control.
Tumor volume was monitored for three weeks following TMI treatment. In addition, in previously treated
rabbits,
VX2 tumor cells were re‑injected into the auricle to investigate whether the animals developed a
protective immune response preventing tumor regrowth.
Clinical Study
The experimental treatment of patients was approved by the Ethics Committee at the Medical Faculty of the
Eberhard Karls University and at the University Hospital of Tübingen (PNR150/2019BO2). All patients
provided
written informed consent to participate in individual healing attempts and for the use of their
de‑identified clinical data for publication.
Clinical application of TMI treatment was evaluated in patients suffering from malignant cutaneous
melanoma or
prostate carcinoma who had exhausted standard therapy options, including surgical resection,
immunotherapy,
hormone therapy, radiotherapy, and HIFU.
Patient MRI/CT datasets were transferred into the finite element method (FEM) model for numerical
simulation.
These patient‑specific FEM propagation models provided optimized treatment parameters, including:
- total energy required
- energy flux density
- shock wave frequency
- total number and sequence of shock waves
- optimal placement and angulation of the treatment applicator
Treatment sessions typically consisted of 1, 500–4, 000 impulses at 3 Hz, depending on tumor location, tissue density, and patient tolerance.
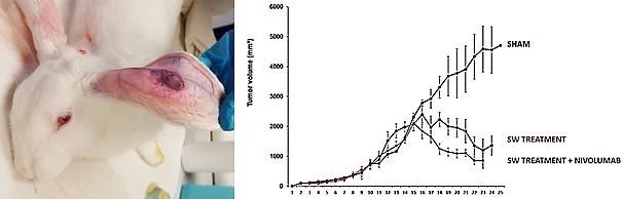
Fig. 1: Left: New Zealand White rabbit exhibiting the subcutaneous VX2 xenograft tumor. Right: Mean reduction of tumor volume in the three groups of NZW rabbits with maximum and minimum values within three weeks after TMI focused shock wave (SW) treatment with or without Nivolumab administration.
Results
Animal Experiments
In the untreated control group, VX2 tumors continued to grow progressively, whereas TMI focused shock wave
treatment alone clearly reduced tumor volume (Fig. 2). Importantly, tumor regression was similar between
the
TMI‑only group and the group treated with additional Nivolumab, indicating that TMI therapy alone
may be
as effective as checkpoint inhibition in this model.
In all rabbits treated with three sessions of TMI shock waves, an unexpected immunological reaction was
observed. When VX2 tumor cells were re‑injected into the previously treated rabbits, the animals
were able
to completely reject the new tumor foci. All attempts to implant 1, 000, 000 aggressive VX2 tumor cells
failed.
This suggests that TMI treatment alone induced a long‑lasting tumor‑specific immune protection
comparable to an effective vaccination.
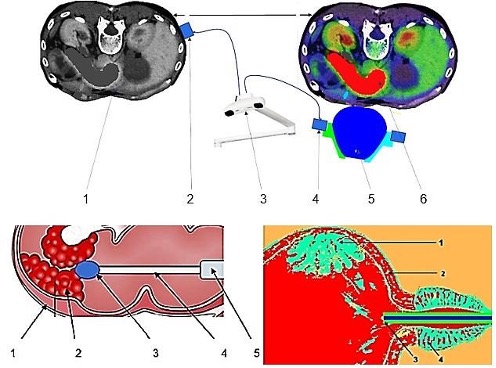
Fig. 2: Above: Components of a device for extracorporeal TMI treatment of pancreatic carcinoma. 1- patient DICOM data, 2- patient rigid body, 3- optical localization, 4- treatment applicator rigid body, 5- applicator, 6- FEM simulation model that considers the patient's specific shock wave propagation. Below left: Components of a device for direct intracorporeal TMI treatment of colon carcinoma. 1- intestinal wall, 2- tumor area, 3- head of the shock wave miniature device with protective cover and miniature camera (not shown), 5- endoscope for colonoscopy. Below right: Device for indirect intracorporeal TMI treatment of urothelial carcinoma. 1- tumor area, 2- urinary bladder wall, 3- applicator, 4- prostate.
Clinical Results
Malignant Cutaneous Melanoma
A 69‑year‑old female patient with metastatic cutaneous melanoma (stage IV, pT3aN3M1c;
N‑RAS
61Q>Q/R mutation, BRAF wt, KIT wt) presented with cutaneous and lymph node metastases after primary
tumor
excision and multiple surgical resections. Despite adjuvant immunotherapy with Nivolumab, metastases
continued
to develop and were accompanied by severe immune‑related side effects.
Five TMI sessions (4, 000 impulses each within 20 minutes) were applied extracorporeally to a large
cutaneous
metastasis on the left thigh using patient‑specific simulation‑based parameters. After the TMI
treatments, the treated metastasis showed macroscopically visible regressive changes (Fig. 3).
Unexpectedly, distant metastases not treated with shock waves—including pulmonary metastases in the left
upper
lobe and metastases in the right gluteus maximus—also regressed significantly, demonstrating a strong
abscopal
effect. Para‑aortic and iliac lymph nodes decreased in size. Four years after TMI treatment, no
lesions
suspicious for malignancy could be detected (Fig. 4).
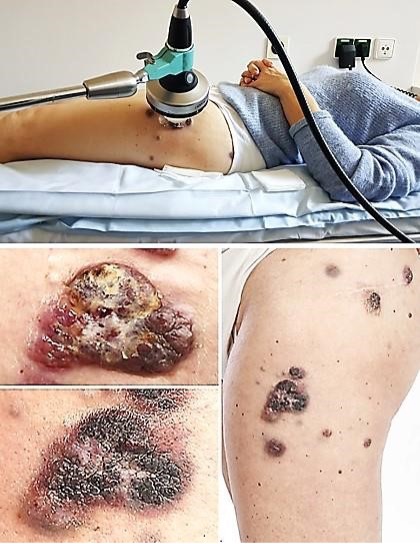
Fig. 3: Above: Treatment situation with extracorporeal device for shock wave therapy. Below: Cutaneous metastasis of melanoma before (left above) and after TMI treatments with macroscopically visible regressive changes (left below); regressive melanoma metastases after five piezoelectric TMI treatments (right).
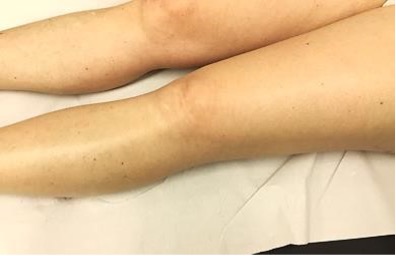
Fig. 4: No remnants of regressive melanoma metastases after five piezoelectric TMI treatments four years ago.
Prostate Carcinoma
An 80‑year‑old male patient with metastatic prostate carcinoma previously treated with hormone
therapy and two sessions of HIFU presented with a local recurrence and multiple painful osseous metastases
in
the iliac bones, thoracic spine, ribs, and sternum.
Based on patient‑specific FEM modeling, extracorporeal TMI focused shock wave therapy was directed
to an
iliac bone metastasis. Five TMI treatments (3 Hz, 0.11–1.41 mJ/mm², 1, 500–2, 000 impulses) were
performed. One
month after the final treatment, imaging revealed not only complete regression of the treated iliac
metastasis
but also disappearance of untreated bone metastases, including a previously visible metastasis in the
sternum
(Fig. 5). Periosteal pain resolved entirely.
Comparable abscopal effects were observed in four additional metastatic prostate carcinoma patients
treated
similarly (Fig. 6). In a representative case of a 68‑year‑old patient with disseminated
pulmonary
and osseous metastases, a subtotal regression of pulmonary metastases and faintly detectable sclerotic
regression of bone lesions were observed one month after TMI therapy.

Fig. 5: Left: Osseous metastasis in the sternum before TMI focused shock wave treatments visualized in PET-CT (red arrow). Right: Metastasis regressed after the five TMI focused shock wave treatments visualized in photon-counting CT (right).
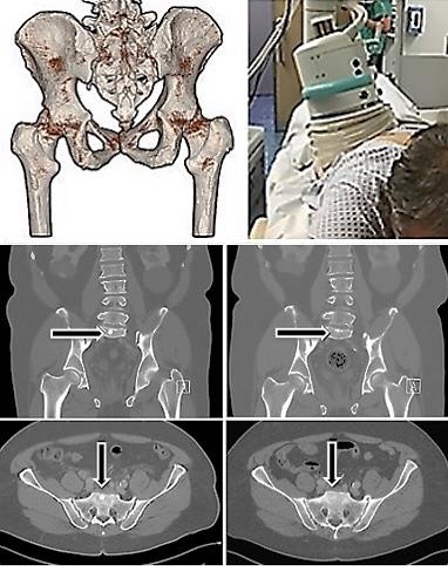
Fig. 6: Above left: Individual 3D-MRT/CT-DICOM determination for positioning of the treatment device. Above right: Positioning of the extracorporeal device for TMI treatment. Below left column: Bone metastases of a prostate carcinoma (arrows) before extracorporeal TMI focused shock wave treatments. Right column: The metastases significantly regressed after five TMI treatments performed on a metastasis in the pelvis region.
Immunological Abscopal Effect
The abscopal effect—tumor regression outside the treated region—was consistently observed following TMI
therapy
in both animal and human studies. By generating focused acoustic and mechanical shock impulses, TMI
induces
selective tumor cell disruption and apoptosis while sparing surrounding healthy tissue.
The release of tumor‑associated antigens (TAAs) and damage‑associated molecular patterns
(DAMPs)
triggers dendritic cell activation, antigen presentation in lymph nodes, and priming of naïve T cells.
Activated
cytotoxic CD8⁺ T cells then circulate via blood vessels and selectively destroy tumor cells at both
primary and
metastatic sites (Fig. 7) [3–6].
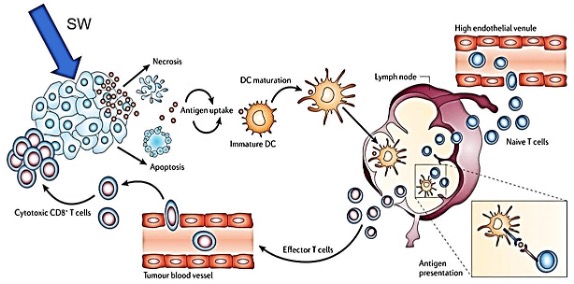
Fig. 7: Cascade of activation of the adaptive immune system after TMI focused shock wave treatment. Shock waves (SW) disrupt tumor cell membranes. Antigenic tumor cell fragments become visible to immature dendritic cells (DC), which after maturation present within lymp nodes the tumor-specific antigens to naïve T cells. Now these effector T cells bear the tumor-specific information so that cytotoxic CD8+ T cells via blood vessels can attack and kill the tumor cells of the primary tumor as well as of all its metastases. With kind permission from INSERM OncoThai Lille, France; slightly modified.
Immunosurveillance
Beyond the abscopal response, TMI treatment appeared to induce a durable immune memory. In the VX2 rabbit
model,
TMI‑treated animals were effectively vaccinated against tumor rechallenge, demonstrating
tumor‑specific immunosurveillance [6]. This suggests that TMI therapy may act as a
mechanotherapeutic
immunization strategy, potentially preventing recurrence and metastatic spread.
Discussion
The adaptive immune system should recognize tumor cells as hostile under normal circumstances, but tumor cells can down-regulate MHC-I molecules and become Invisible, they are disguised behind immune checkpoints as regular components of the human body, in a way “hijacking” the mechanism of immune cells before they can detect and eliminate the tumor cells [7].
Immunotherapy blocks immune checkpoints, regulatory surface proteins that occur on the membrane of immune cells. Checkpoint inhibitors are monoclonal antibodies that are directed against these surface proteins, especially anti-cytotoxic T-lymphocyte antigen-4 (anti-CTLA-4) and anti-programmed death-1 (anti-PD-1) or the associated ligand (PD-L1). If the function of the immune checkpoints is inhibited, tumor cells become unmasked and immune reactions against the tumor cells can be initiated, eventually resulting in regression of the tumor. However, immune evasion can occur when tumor cells downregulate the expression of cell-surface proteins which the immune system recognizes as foreign [8], or checkpoint inhibitors can trigger a dysregulated immune response with undesirable auto-aggression against healthy cells, in some patients severe or even life-threatening. TMI focused shock wave treatment unmasks the individual specific tumor-associated antigens without unselective blocking of immune checkpoints.
Preclinical and early clinical findings suggest that this immune activation may extend beyond the treated field, leading to abscopal tumor regression at distant metastatic sites. The phenomenon resembles that observed after radiotherapy but occurs here through a purely mechanical, nonthermal mechanism. Investigating TMI-mediated abscopal effects offers a new paradigm for synergistic, non-invasive cancer immunotherapy.
If tumor-associated antigens (TAAs) are presented, each individual patient’s adaptive immune system itself cares for all appropriate immunological responses [9, 10]. The physical strain on tumor cells by TMI focused shock wave treatment leads to tumor cell fragmentation and release of tumor-associated antigens in cell fragments, which eventually initiates an activation of the adaptive immune system.
Tumor-associated antigens are taken up by immature dendritic cells which develop into mature dendritic cells, now, in lymph nodes, presenting the antigens to naïve T-cells. The resulting effector T-cells are distributed via blood vessels including tumor blood vessels. As soon as the CD8 receptor recognizes a tumor cell, it activates the cytotoxic CD8 T cells which selectively destroy the tumor cells of the primary tumor as well as via the blood vessels metastatic tumor cells, too [11].
The critical question is how to stimulate a consistent and sufficient tumor-specific immune response. Even targeting a single tumor metastasis may be sufficient, if an abscopal effect can be achieved. Aiming at the initiation of a sufficient abscopal effect, tumors and their metastases react differently to the established histotripsy treatment and to TMI focused shock wave treatment.
Histotripsy was authorized by FDA, based on data from the #HOPE4LIVER trial (ClinicalTrials.gov identifier NCT04573881), and was conducted in 13 trial sites across the United States and Europe. The pooled data assessed the clinical safety and efficacy of histotripsy in destroying targeted primary and secondary liver tumors [12]. ‘Endogenous’ histotripsy, i.e., without the use of any extraneously administered ultrasound contrast agents or cavitation nuclei such as nanodroplets and nanoparticles, depends on the pulse duration, the peak negative focal pressure, the shock amplitude and the HIFU transducer F number which is the ratio of its radius of curvature (focal length) to the aperture diameter, thus, technically dividing in intrinsic threshold histotripsy, shock-scattering histotripsy, hybrid histotripsy and boiling histotripsy [13]. By applying histotripsy, the immune system is largely or totally (boiling histotripsy) hindered to recognize antigenic proteins from impaired tumor cells. An abscopal effect is possible but not sufficiently effective, if at tumor rims “surviving” tumor-associated antigens are presented to the immune system.
In TMI treatment, antigenic proteins of tumor cells are unmasked and, by the here presented TMI approach, remain recognizable for the adaptive immune system. Still viable fragments of tumor cells are removed by delayed apoptosis, thus, tumor-associated antigens are presented to the immune system in a higher concentration and for an extended time. The strong abscopal effect, which we regularly observed in the present study, attacks not directly treated remote metastases and micrometastases. As well in NZW rabbits with grafted VX2-tumors as in the cases of metastatic melanoma and prostate carcinoma documented in this study, we observed that TMI focused shock wave treatment initiated regression and disappearance of even untreated distant metastases, thus, obviously pointing to an abscopal effect, The apoptotic impairment of tumor cells induced by TMI focused shock wave treatment appears to result in a longer duration of tumor-associated antigen presentation than in treatment approaches such as histotripsy aiming at necrotic destruction of the tumor.
Do shock waves promote metastatic spread of tumors? In an experimental setting in mice, shock waves induced enhancement of mouse melanoma lung metastases [14]; the authors applied parameters completely different from our TMI approach; ultrasound contrast agents were injected into the tumor and high-pressure fields of 45 MPa were applied. This causes intense and violent cavitation clouds, and only in presence of cavitation clouds lung metastases were detected. As histotripsy mechanically destroys target tissue at high-pressure, there is theoretical risk that histotripsy may dislodge and release tumor cells from the target tumor and increase the risk of metastatic spread. However, studies in the last twenty years have instead shown no increased but a reduced risk of metastases following histotripsy; no metastatic complications were reported [13, 15-17]. In none of our cases treated by TMI treatment, we observed newly spread metastases, on the contrary, existing metastases regressed after treatment.
Conclusion
TMI treatment of malignant tumors appeared to be feasible and reliable, and may be developed into an effective monotherapy in the treatment of malignant tumors, the more, since it elicits a direct effect by disrupting and fragmenting tumor cells, thereby unmasking and releasing disguised tumor-associated antigens (TAAs), so that tumor cells become identifiable to the highly specific adaptive immune cells which eliminate the recognized tumor and its metastases.
TMI treatment can be administered repeatedly and thereby boosters the immunological answer until complete eradication of tumor cells is achieved. Under those circumstances, TMI treatment of the primary tumor would not be necessary in each case, since the treatment of an easier accessible single metastasis can also result in the regression of the untreated primary tumor and untreated distant metastases through activated cytotoxic T cells via the abscopal effect.
TMI treatment has a potential social significance, an ethical aspect which should not be underestimated. The treatment devices are comparatively cheap and easy to handle. It can also be assumed that – after having treated a larger number of cases of the same tumor type – the range of optimal treatment parameters can be determined for different tumor types, so that the patient-individual determination by computational simulation will not be necessary any longer. This allows for clinical application in hospitals where no sophisticated infrastructure is available. So, if well adapted TMI treatment alone can unmask tumor-specific proteins and, thereby, stimulate a sufficient tumor-specific immune response, the physiological “built-in immune laboratory” of the individual patient’s body does the work itself to destroy the tumor and its metastases.
TMI treatment, thus, could have significant implications for the development of new regimens for cancer therapy that are both effective and targeted, that are both safe and cheap.
Acknowledgements
We thank S. Rausch, Department of Urology, University of Tübingen, Germany, and T. Todenhöfer, Studienpraxis for clinical Phase I-IV studies for urologic conditions, Nürtingen, Germany, for their valuable clinical advice and support. We thank I. Theuer for her assistance in the animal experiments.
Author Contributions
A.E. Theuer elaborated the computational parameters for simulation and drew up the engineering
preconditions for
the construction of TMI application devices.
A.E. Theuer and G.F. Walter, with the scientific support of F. Lang, proved the principle biological
feasibility
of the treatment of cancer applying TMI.
M. Borkmann performed animal experiments.
I. Thomas evaluated melanoma cell reactions to shock wave treatment and provided clinical background for
the
design of application devices.
T.K. Eigentler and J.D. Mullins transform the concept into the actual clinical application of TMI
treatment for
patients suffering from malignant tumors.
G.F. Walter wrote the manuscript.
Funding Sources
This work was supported by Arbeitsgemeinschaft industrieller Forschungsvereinigungen (grant number
KF3356302AK4), and Zentrales Innovationsprogramm Mittelstand (grant number ZF4803001BA9).
Statement of Ethics
The animal experiments have been approved by the Institutional Review Board of the University of Marburg
(V54-19c20/15 Nr. 24/2016). All methods were performed in accordance with the relevant guidelines and
regulations.
The experimental treatment of patients has been approved by the Ethics Committee at the Medical Faculty of
the
Eberhard Karls University and at the University Hospital of Tübingen (PNR150/2019BO2). All patients
provided
written informed consent to be enrolled in individual healing attempts, and for the use of their
de-identified
clinical data for publication.
Disclosure of AI Assistance
Artificial intelligence (AI) tools were not used in the creation of this manuscript.
Disclosure Statement
The authors declare that they have no competing interest.
References
| 1 | Borkmann M: Extrakorporale Stoßwellen Therapie (ESWT) in Kombination mit dem PD1- Blocker
Nivolumab (OPDIVO®) zur gezielten Tumordestruktion am VX2-Kaninchenmodell. Master's thesis,
Philipps-University Marburg, 2017.
|
| 2 | Muhanna N, Douglas CM, Chan HHL, Daly MJ, Townson JL, Ferrari M, Eu D, Akens M, Chen J, Zheng G,
Irish JC: Rabbit VX2 head and neck squamous cell models for translational head and neck theranostic
technology development. Clin Transl Med 2021;11:e550.
https://doi.org/10.1002/ctm2.550 |
| 3 | Nabrinsky E, Macklis J, Bitran J: A Review of the abscopal effect in the era of immunotherapy.
Cureus 2022;14:e29620.
https://doi.org/10.7759/cureus.29620 |
| 4 | Rodriguez-Ruiz ME, Rodriguez I, Leaman O, López-Campos F, Montero A, Conde AJ, Aristu JJ, Lara P,
Calvo FM, Melero I: Immune mechanisms mediating abscopal effects in radioimmunotherapy. Pharmacol
Ther 2019;196:195-203.
https://doi.org/10.1016/j.pharmthera.2018.12.002 |
| 5 | Zhao X, Shao C: Radiotherapy-mediated immunomodulation and anti-tumor abscopal effect combining
immune checkpoint blockade. Cancers (Basel) 2020; 12:2762.
https://doi.org/10.3390/cancers12102762 |
| 6 | Tello-Lafoz M, de Jesus MM, Huse M: Harder, better, faster, stronger: biochemistry and biophysics
in the immunosurveillance concert. Trends Immunol 2022;43:96-105.
https://doi.org/10.1016/j.it.2021.12.003 |
| 7 | Bruni D, Angell HK, Galon J: The immune contexture and Immunoscore in cancer prognosis and
therapeutic efficacy. Nat Rev Cancer 2020;20:662-680.
https://doi.org/10.1038/s41568-020-0285-7 |
| 8 | Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer
2012;12:252-264.
https://doi.org/10.1038/nrc3239 |
| 9 | Gattoni-Celli S, Cole DJ: Melanoma-associated tumor antigens and their clinical relevance to
immunotherapy. Semin Oncol 1996;23(6):754-758.
|
| 10 | Liu CC, Yang H, Zhang R, Zhao JJ, Hao DJ: Tumour-associated antigens and their anti-cancer
applications. Eur J Cancer Care (Engl) 2017;26(5).
https://doi.org/10.1111/ecc.12446 |
| 11 | Raskov H, Orhan A, Christensen JP, Gögenur I: Cytotoxic CD8+ T cells in cancer and cancer
immunotherapy. Br J Cancer 2021;124:359-367.
https://doi.org/10.1038/s41416-020-01048-4 |
| 12 | Wah TM, Pech M, Thormann M, Serres X, Littler P, Stenberg B, Lenton J, Smith J, Wiggermann P,
Planert M, Vidal-Jove J, Torzilli G, Solbiati L: A Multi-centre, single arm, nonrandomized,
prospective European trial to evaluate the safety and efficacy of the HistoSonics System in the
treatment of primary and metastatic liver cancers (#HOPE4LIVER). Cardiovasc Intervent Radiol
2023;46:259-267.
https://doi.org/10.1007/s00270-022-03309-6 |
| 13 | Williams RP, Simon JC, Khokhlova VA, Sapozhnikov OA, Khokhlova TD: The histotripsy spectrum:
differences and similarities in techniques and instrumentation. Int J Hyperthermia 2023;40:2233720.
https://doi.org/10.1080/02656736.2023.2233720 |
| 14 | Miller DL, Dou C, Song J: Lithotripter shockwave-induced enhancement of mouse melanoma lung
metastasis: dependence on cavitation nucleation. J Endourol 2004;18:925-929.
https://doi.org/10.1089/end.2004.18.925 |
| 15 | Styn NR, Hall TL, Fowlkes JB, Cain CA, Roberts WW: Histotripsy of renal implanted VX-2 tumor in a
rabbit model: investigation of metastases. Urology 2012;80:724-729.
https://doi.org/10.1016/j.urology.2012.06.020 |
| 16 | Xu Z, Hall TL, Vlaisavljevich E, Lee FT Jr.: Histotripsy: the first noninvasive, nonionizing,
non-thermal ablation technique based on ultrasound. Int J Hyperthermia 2021;38:561-575.
https://doi.org/10.1080/02656736.2021.1905189 |
| 17 | Qu S, Worlikar T, Felsted AE, Ganguly A, Beems MV, Hubbard R, Pepple AL, Kevelin AA, Garavaglia H,
Dib J, Toma M, Huang H, Tsung A, Xu Z, Cho CS: Non-thermal histotripsy tumor ablation promotes
abscopal immune responses that enhance cancer immunotherapy. J Immunother Cancer 2020;8:e000200.
https://doi.org/10.1136/jitc-2019-000200 |