Corresponding Author: Julian A. Schreiber
IfGH – Cellular Electrophysiology and Molecular Biology,
Department of Cardiovascular Medicine, University Hospital Muenster
Robert-Koch-Straße 45, Muenster, D-48149 (Germany)
Tel. +49-251-83-58278 , E-Mail j_schr46@uni-muenster.de
Beyond Hot and Spicy: TRPV Channels and their Pharmacological Modulation
Guiscard Seebohma Julian A. Schreibera,b
aInstitute for Genetics of Heart Diseases (IfGH), Department of Cardiovascular Medicine, University Hospital Münster, Münster, Germany, bInstitut für Pharmazeutische und Medizinische Chemie, Westfälische Wilhelms-Universität Münster, Münster, Germany
The structure of TRPV channels
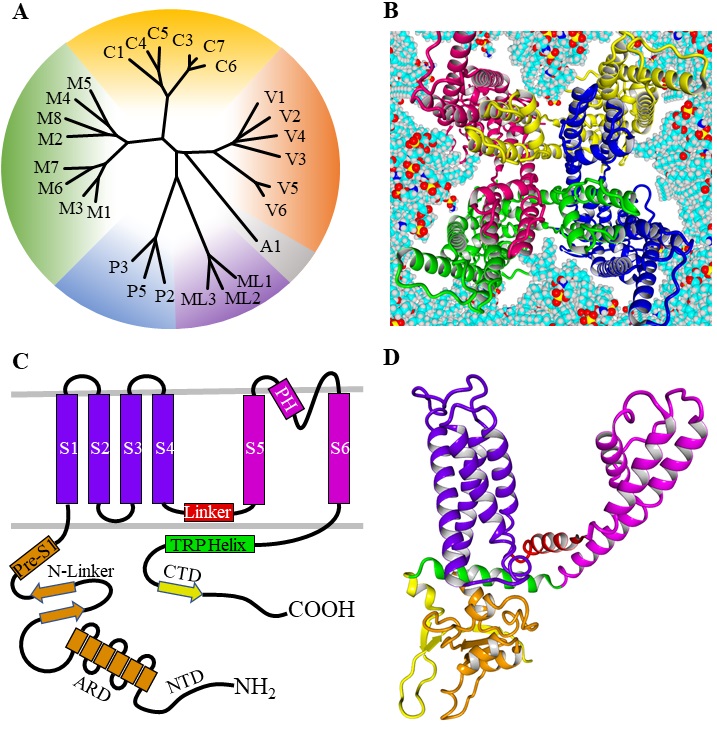
In 1969 Cosens and Manning described a mutated Drosophila melanogaster strain, that showed abnormal electroretinogram (ERG) and impaired phototransduction. The ERG was characterized by a transient receptor potential (TRP) instead of a rather sustained potential in the wildtype [1]. Later, this mutated strain was associated with the first invertebrate TRP cation channel, which was cloned in 1989 [2]. Finally, in 1995 the first mammalian homologue of the transient receptor potential canonical (TRPC) family, was cloned and characterized [3, 4]. From then to now 28 members of the TRP cation channel superfamily were discovered in mammals, that can be subdivided by sequence homology into two groups and 6 families: Group I contains the families transient potential receptor canonical (TRPC1-7), vanilloid (TRPV1-6), ankyrin (TRPA1) and melastatin (TRPM1-8), while the families polycystin (TRPP2, TRPP3, TRPP5) and mucolipin (TRPML1-3) belong to Group II (Fig. 1A) [5, 6]. The TRPV channels are named after the first discovered member TRPV1, that is sensitive to stimulation by the vanillylamide capsaicin [7]. TRPV1 is formed by assembling of four TRPV1 subunits, that have a similar transmembrane structure like voltage gated K+ channels (Fig. 1B-D) [8, 9]. Later on, the vanilloid subfamily was extended by TRPV2 (previously VRL1), TRPV3 (OLMS, VRL3), TRPV4 (VRL2, Trp12, VROAC, OTRPC4), TRPV5 (ECaC1, CaT2) and TRPV6 (ECaC2, CaT1) [10–13].
All TRPV channels possess a large cytosolic N-terminal and a smaller C-terminal region [14]. The N-terminal region forms an ankyrin repeat domain (ARD) with six ankyrin repeats, two β-sheets (N-Linker) and a pre-S1 helix (Fig. 1C) [15]. The cytosolic C-terminal region encompasses several amino acids forming one β-sheet, that together with the two N-terminal β-sheets and the ARD of an adjacent subunit allow for efficient subunit assembling [15]. The intracellular regions are also binding sites for modulating proteins and second messengers. The N-terminus contains binding sites for calmodulin (CaM) and ATP, that both can modulate channel activity [16]. The shorter C-terminal region also binds CaM and other regulating proteins including A-kinase anchor protein (AKAP) [17, 18]. Moreover, TRPV channels are strongly regulated by phosphorylation at different sites of the N- and C-terminal regions performed by protein kinase A (PKA) as well as protein kinase C (PKC) [19, 20].
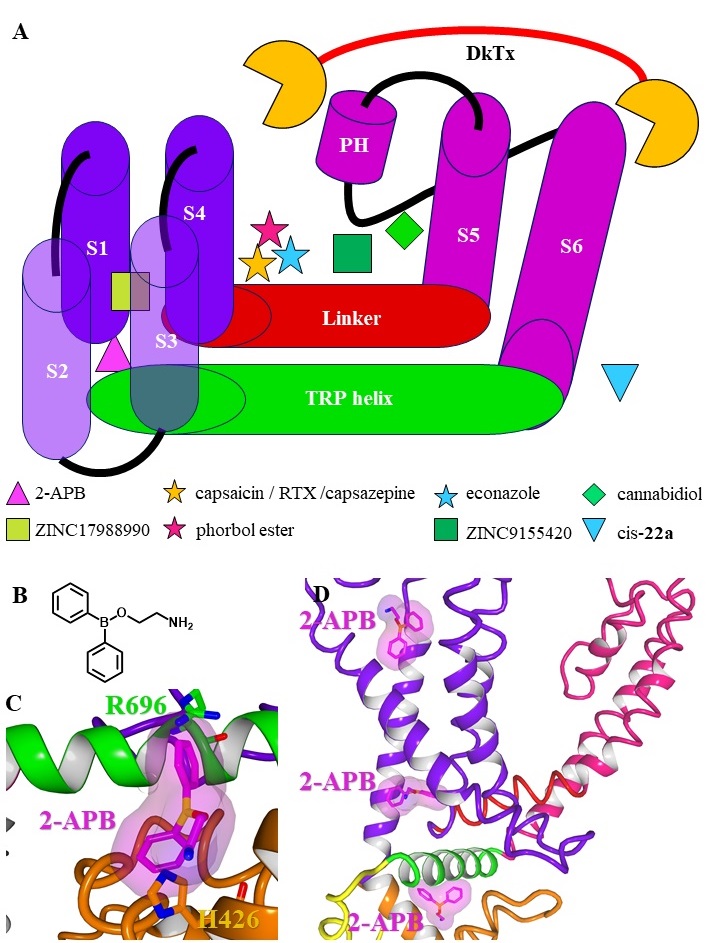
The transmembrane region consists of six helices (S1-S6) forming the voltage sensor-like domain (VSLD, S1-S4) and an inner pore region (S5-S6) connected by the S4S5-linker [21]. The ion channel pore is formed by the selectivity filter (SF) and the pore helix (PH; also called pore turret) between the S5 and S6 helix [22]. Further, residues from the lower part of the S6 helix form an activation gate [23]. In comparison to voltage gated potassium channels, the upper gate formed by the PH / SF is shorter and the pore radius differs within the different TRPV subtypes influencing the selectivity of the channels [24]. Based on the ion selectivity the six subtypes are subdivided into two groups: TRPV1-4 form polyselective cation channels, that conduct monovalent as well as divalent cations with a preference for Ca2+ and conductivity ratios for Ca2+ over Na+ ranging from PCa/Na 3 (TRPV2) over 6 (TRPV4) up to 10 (TRPV1, TRPV3) [25]. In contrast, TRPV5 and TRPV6 are nearly Ca2+ selective channels with PCa/Na >100, while monovalent cations only permeate in absence of divalent cations [26, 27].
The manuscript was written by G.S. and J.A.S.. We thank Prof. Dr. Bernhard Wünsch for carefully proofreading the manuscript.
The authors declare no conflict of interests exist.
| 1 Cosens DJ, Manning A: Abnormal Electroretinogram from a Drosophila Mutant. Nature 1969;224:285-287. https://doi.org/10.1038/224285a0 |
||||
| 2 Montell C, Rubin GM: Molecular characterization of the drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989;2:1313-1323. https://doi.org/10.1016/0896-6273(89)90069-X |
||||
| 3 Zhu X, Chu PB, Peyton M, Birnbaumer L: Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett 1995;373:193-198. https://doi.org/10.1016/0014-5793(95)01038-G |
||||
| 4 Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L: trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell 1996;85:661-671. https://doi.org/10.1016/S0092-8674(00)81233-7 |
||||
| 5 Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, Zhu MX: A unified nomenclature for the superfamily of TRP cation channels. Mol Cell 2002;9:229-231. https://doi.org/10.1016/S1097-2765(02)00448-3 |
||||
| 6 Montell C: The TRP Superfamily of Cation Channels. Sci Signal 2005;2005:re3. https://doi.org/10.1126/stke.2722005re3 |
||||
| 7 Szallasi A: The vanilloid (capsaicin) receptor: Receptor types and species differences. Gen Pharmacol Vasc Syst 1994;25:223-243. https://doi.org/10.1016/0306-3623(94)90049-3 |
||||
| 8 Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM: Analysis of the Native Quaternary Structure of Vanilloid Receptor 1. J Biol Chem 2001;276:28613-28619. https://doi.org/10.1074/jbc.M103272200 |
||||
| 9 Yu FH, Catterall WA: The VGL-Chanome: A Protein Superfamily Specialized for Electrical Signaling and Ionic Homeostasis. Sci Signal 2004;2004:re15. https://doi.org/10.1126/stke.2532004re15 |
||||
| 10 Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D: A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999;398:436-441. https://doi.org/10.1038/18906 |
||||
| 11 Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE: TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002;418:181-186. https://doi.org/10.1038/nature00882 |
||||
| 12 Delany NS, Hurle M, Facer P, Alnadaf T, Plumpton C, Kinghorn I, See CG, Costigan M, Anand P, Woolf CJ, Crowther D, Sanseau P, Tate SN: Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2 Physiol Genomics 2001;4:165-174. https://doi.org/10.1152/physiolgenomics.2001.4.3.165 |
||||
| 13 Peng J Bin, Chen XZ, Berger U V., Weremowicz S, Morton CC, Vassilev PM, Brown EM, Hediger MA: Human calcium transport protein Cat1 Biochem Biophys Res Commun 2000;278:326-332. https://doi.org/10.1006/bbrc.2000.3716 |
||||
| 14 Gaudet R: TRP channels entering the structural era. J Physiol 2008;586:3565-3575. https://doi.org/10.1113/jphysiol.2008.155812 |
||||
| 15 Pumroy RA, Fluck EC, Ahmed T, Moiseenkova-Bell VY: Structural insights into the gating mechanisms of TRPV channels. Cell Calcium 2020;87:102168. https://doi.org/10.1016/j.ceca.2020.102168 |
||||
| 16 Phelps CB, Wang RR, Choo SS, Gaudet R: Differential Regulation of TRPV1, TRPV3, and TRPV4 Sensitivity through a Conserved Binding Site on the Ankyrin Repeat Domain. J Biol Chem 2010;285:731-740. https://doi.org/10.1074/jbc.M109.052548 |
||||
| 17 Zhang X, Li L, McNaughton PA: Proinflammatory Mediators Modulate the Heat-Activated Ion Channel TRPV1 via the Scaffolding Protein AKAP79/150. Neuron 2008;59:450-461. https://doi.org/10.1016/j.neuron.2008.05.015 |
||||
| 18 Holakovska B, Grycova L, Bily J, Teisinger J: Characterization of calmodulin binding domains in TRPV2 and TRPV5 C-tails. Amino Acids 2011;40:741-748. https://doi.org/10.1007/s00726-010-0712-2 |
||||
| 19 Mamenko M, Zaika OL, Boukelmoune N, Berrout J, O'Neil RG, Pochynyuk O: Discrete Control of TRPV4 Channel Function in the Distal Nephron by Protein Kinases A and C. J Biol Chem 2013;288:20306-20314. https://doi.org/10.1074/jbc.M113.466797 |
||||
| 20 Por ED, Gomez R, Akopian AN, Jeske NA: Phosphorylation regulates TRPV1 association with β-arrestin-2 Biochem J 2013;451:101-109. https://doi.org/10.1042/BJ20121637 |
||||
| 21 Latorre R, Brauchi S, Orta G, Zaelzer C, Vargas G: ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium 2007;42:427-438. https://doi.org/10.1016/j.ceca.2007.04.004 |
||||
| 22 Dosey TL, Wang Z, Fan G, Zhang Z, Serysheva II, Chiu W, Wensel TG: Structures of TRPV2 in distinct conformations provide insight into role of the pore turret. Nat Struct Mol Biol 2019;26:40-49. https://doi.org/10.1038/s41594-018-0168-8 |
||||
| 23 Salazar H, Jara-Oseguera A, Hernández-García E, Llorente I, Arias-Olguín II, Soriano-García M, Islas LD, Rosenbaum T: Structural determinants of gating in the TRPV1 channel. Nat Struct Mol Biol 2009;16:704-710. https://doi.org/10.1038/nsmb.1633 |
||||
| 24 Yuan P: Structural biology of thermoTRPV channels. Cell Calcium 2019;84:102106. https://doi.org/10.1016/j.ceca.2019.102106 |
||||
| 25 Clapham DE: TRP channels as cellular sensors. Nature 2003;426:517-524. https://doi.org/10.1038/nature02196 |
||||
| 26 Nilius B, Vennekens R, Prenen J, Hoenderop JGJ, Bindels RJM, Droogmans G: Whole‐cell and single channel monovalent cation currents through the novel rabbit epithelial Ca 2+ channel ECaC. J Physiol. 2000;527:239-248. https://doi.org/10.1111/j.1469-7793.2000.00239.x |
||||
| 27 Yue L, Peng JB, Hediger MA, Clapham DE: CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature 2001;410:705-709. https://doi.org/10.1038/35070596 |
||||
| 28 Matta JA, Ahern GP: Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol 2007;585:469-482. https://doi.org/10.1113/jphysiol.2007.144287 |
||||
| 29 Everaerts W, Nilius B, Owsianik G: The vanilloid transient receptor potential channel TRPV4: From structure to disease. Prog Biophys Mol Biol 2010;103:2-17. https://doi.org/10.1016/j.pbiomolbio.2009.10.002 |
||||
| 30 Chung MK, Güler AD, Caterina MJ: Biphasic Currents Evoked by Chemical or Thermal Activation of the Heat-gated Ion Channel, TRPV3. J Biol Chem 2005;280:15928-15941. https://doi.org/10.1074/jbc.M500596200 |
||||
| 31 Chung MK, Lee M, Mizuno A, Suzuki M, Caterina MJ: 2-Aminoethoxydiphenyl Borate Activates and Sensitizes the Heat-Gated Ion Channel TRPV3. J Neurosci 2004;24:5177-5182. https://doi.org/10.1523/JNEUROSCI.0934-04.2004 |
||||
| 32 Lee J, Cha SK, Sun T-J, Huang CL: PIP2 Activates TRPV5 and Releases Its Inhibition by Intracellular Mg2+. J Gen Physiol 2005;126:439-451. https://doi.org/10.1085/jgp.200509314 |
||||
| 33 Voets T, Janssens A, Prenen J, Droogmans G, Nilius B: Mg2+-dependent Gating and Strong Inward Rectification of the Cation Channel TRPV6. J Gen Physiol 2003;121:245-260. https://doi.org/10.1085/jgp.20028752 |
||||
| 34 Benham CD, Gunthorpe MJ, Davis JB: TRPV channels as temperature sensors. Cell Calcium 2003;33:479-487. https://doi.org/10.1016/S0143-4160(03)00063-0 |
||||
| 35 Neeper MP, Liu Y, Hutchinson TL, Wang Y, Flores CM, Qin N: Activation properties of heterologously expressed mammalian TRPV2: Evidence for species dependence. J Biol Chem 2007;282:15894-15902. https://doi.org/10.1074/jbc.M608287200 |
||||
| 36 Ryu S, Liu B, Yao J, Fu Q, Qin F: Uncoupling Proton Activation of Vanilloid Receptor TRPV1. J Neurosci 2007;27:12797-12807. https://doi.org/10.1523/JNEUROSCI.2324-07.2007 |
||||
| 37 Suzuki M, Mizuno A, Kodaira K, Imai M: Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 2003;278:22664-22668. https://doi.org/10.1074/jbc.M302561200 |
||||
| 38 McLatchie LM, Bevan S: The effects of pH on the interaction between capsaicin and the vanilloid receptor in rat dorsal root ganglia neurons. Br J Pharmacol 2001;132:899-908. https://doi.org/10.1038/sj.bjp.0703900 |
||||
| 39 Jordt SE, Tominaga M, Julius D: Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A 2000;97:8134-8139. https://doi.org/10.1073/pnas.100129497 |
||||
| 40 Cao X, Yang F, Zheng J, Wang K: Intracellular Proton-mediated Activation of TRPV3 Channels Accounts for the Exfoliation Effect of α-Hydroxyl Acids on Keratinocytes. J Biol Chem 2012;287:25905-25916. https://doi.org/10.1074/jbc.M112.364869 |
||||
| 41 Cha S-K, Jabbar W, Xie J, Huang CL: Regulation of TRPV5 Single-Channel Activity by Intracellular pH. J Membr Biol 2007;220:79-85. https://doi.org/10.1007/s00232-007-9076-2 |
||||
| 42 Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD: OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2000;2:695-702. https://doi.org/10.1038/35036318 |
||||
| 43 Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B: Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4 Proc Natl Acad Sci U S A 2004;101:396-401. https://doi.org/10.1073/pnas.0303329101 |
||||
| 44 Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y: TRPV2 Is a Component of Osmotically Sensitive Cation Channels in Murine Aortic Myocytes. Circ Res 2003;93:829-838. https://doi.org/10.1161/01.RES.0000097263.10220.0C |
||||
| 45 Cao C, Zakharian E, Borbiro I, Rohacs T: Interplay between Calmodulin and Phosphatidylinositol 4,5-Bisphosphate in Ca2+-induced Inactivation of Transient Receptor Potential Vanilloid 6 Channels. J Biol Chem 2013;288:5278-5290. https://doi.org/10.1074/jbc.M112.409482 |
||||
| 46 Mercado J, Gordon-Shaag A, Zagotta WN, Gordon SE: Ca2+-dependent desensitization of TRPV2 channels is mediated by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J Neurosci 2010;30:13338-13347. https://doi.org/10.1523/JNEUROSCI.2108-10.2010 |
||||
| 47 Doerner JF, Hatt H, Ramsey IS: Voltage-and temperature-dependent activation of TRPV3 channels is potentiated by receptor-mediated PI(4,5)P2 hydrolysis. J Gen Physiol 2011;137:271-288. https://doi.org/10.1085/jgp.200910388 |
||||
| 48 Harraz OF, Longden TA, Eubanks DH, Nelson MT: PIP2 depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells. Elife 2018;7:1-24. https://doi.org/10.7554/eLife.38689 |
||||
| 49 Garcia-Elias A, Mrkonjić S, Pardo-Pastor C, Inada H, Hellmich UA, Rubio-Moscardó F, Plata C, Gaudet R, Vicente R, Valverde MA: Phosphatidylinositol-4,5-biphosphate-dependent rearrangement of TRPV4 cytosolic tails enables channel activation by physiological stimuli. Proc Natl Acad Sci U S A 2013;110:9553-9558. https://doi.org/10.1073/pnas.1220231110 |
||||
| 50 Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T: Dual regulation of TRPV1 by phosphoinositides. J Neurosci 2007;27:7070-7080. https://doi.org/10.1523/JNEUROSCI.1866-07.2007 |
||||
| 51 Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D: TRPV1 Channels Are Intrinsically Heat Sensitive and Negatively Regulated by Phosphoinositide Lipids. Neuron 2013;77:667-679. https://doi.org/10.1016/j.neuron.2012.12.016 |
||||
| 52 Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D: The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997;389:816-824. https://doi.org/10.1038/39807 |
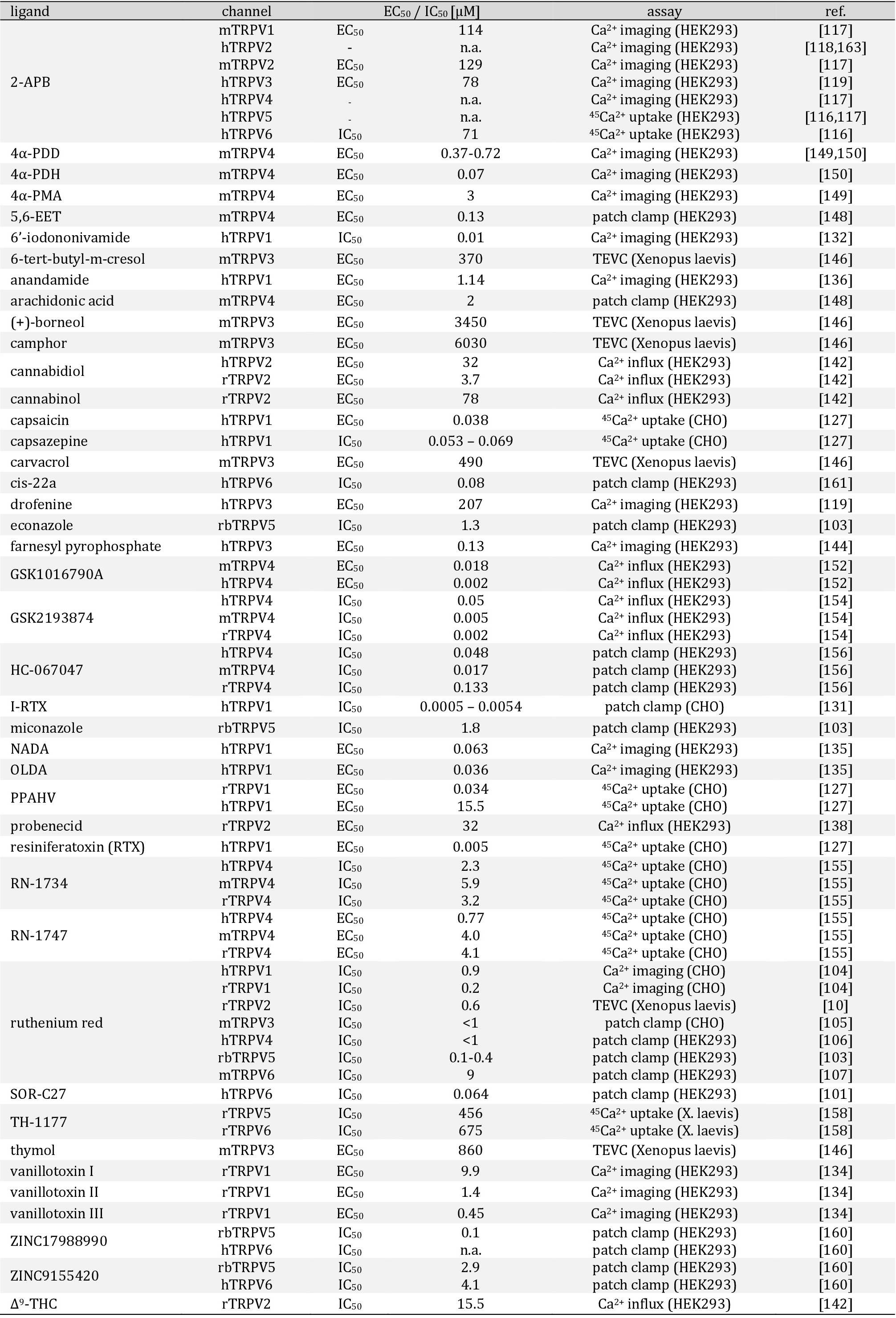
||||
| 53 Helliwell RJA, McLatchie LM, Clarke M, Winter J, Bevan S, McIntyre P: Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett 1998;250:177-180. https://doi.org/10.1016/S0304-3940(98)00475-3 |
||||
| 54 Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI: Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 2011;31:5067-5077. https://doi.org/10.1523/JNEUROSCI.6451-10.2011 |
||||
| 55 Kark T, Bagi Z, Lizanecz E, Pásztor ET, Erdei N, Czikora Á, Papp Z, Édes I, Pórszász R, Tóth A: Tissue-specific regulation of microvascular diameter: Opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol Pharmacol 2008;73:1405-1412. https://doi.org/10.1124/mol.107.043323 |
||||
| 56 Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D: Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000;288:306-313. https://doi.org/10.1126/science.288.5464.306 |
||||
| 57 Fischer MJM, Ciotu CI, Szallasi A: The Mysteries of Capsaicin-Sensitive Afferents. Front Physiol 2020;11:1-15. https://doi.org/10.3389/fphys.2020.554195 |
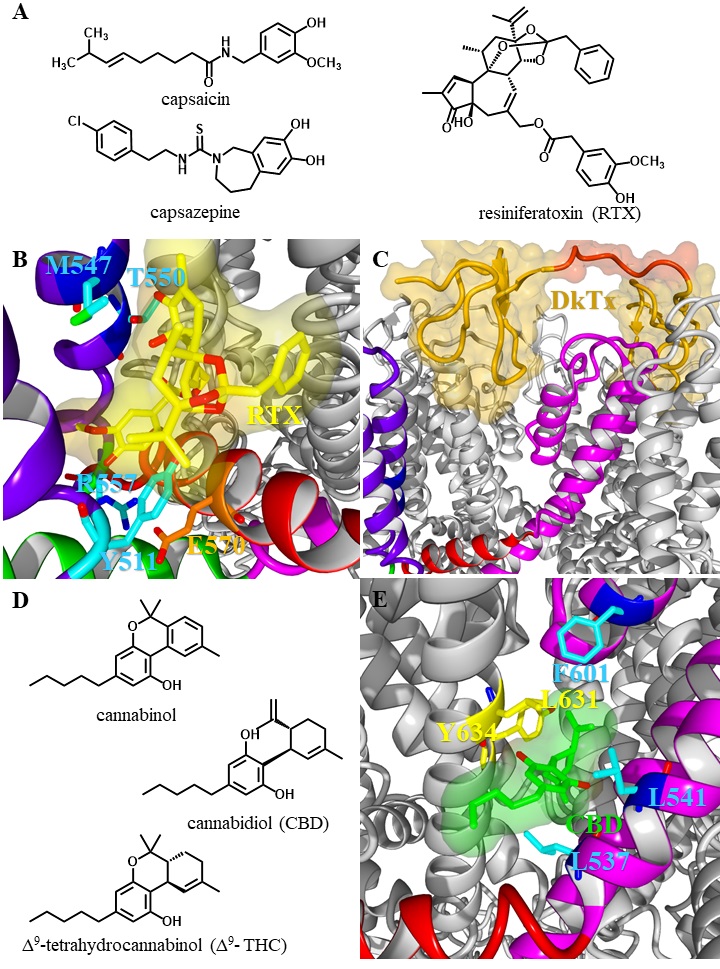
||||
| 58 Bonezzi C, Costantini A, Cruccu G, Fornasari DMM, Guardamagna V, Palmieri V, Polati E, Zini P, Dickenson AH: Capsaicin 8% dermal patch in clinical practice: an expert opinion. Expert Opin Pharmacother 2020;21:1377-1387. https://doi.org/10.1080/14656566.2020.1759550 |
||||
| 59 Iftinca M, Defaye M, Altier C: TRPV1-Targeted Drugs in Development for Human Pain Conditions. Drugs 2021;81:7-27. https://doi.org/10.1007/s40265-020-01429-2 |
||||
| 60 Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I: Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol 1999;1:165-170. https://doi.org/10.1038/11086 |
||||
| 61 Nagasawa M, Nakagawa Y, Tanaka S, Kojima I: Chemotactic peptide fMetLeuPhe induces translocation of the TRPV2 channel in macrophages. J Cell Physiol 2007;210:692-702. https://doi.org/10.1002/jcp.20883 |
||||
| 62 Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y: Transient receptor potential channels in cardiovascular function and disease. Circ Res 2006;99:119-131. https://doi.org/10.1161/01.RES.0000233356.10630.8a |
||||
| 63 Koch SE, Gao X, Haar L, Jiang M, Lasko VM, Robbins N, Cai W, Brokamp C, Varma P, Tranter M, Liu Y, Ren X, Lorenz JN, Wang HS, Jones WK, Rubinstein J: Probenecid: Novel use as a non-injurious positive inotrope acting via cardiac TRPV2 stimulation. J Mol Cell Cardiol 2012;53:134-144. https://doi.org/10.1016/j.yjmcc.2012.04.011 |
||||
| 64 Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ: TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol 2010;11:232-239. https://doi.org/10.1038/ni.1842 |
||||
| 65 Iwata Y, Katanosaka Y, Arai Y, Shigekawa M, Wakabayashi S: Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum Mol Genet 2009;18:824-834. https://doi.org/10.1093/hmg/ddn408 |
||||
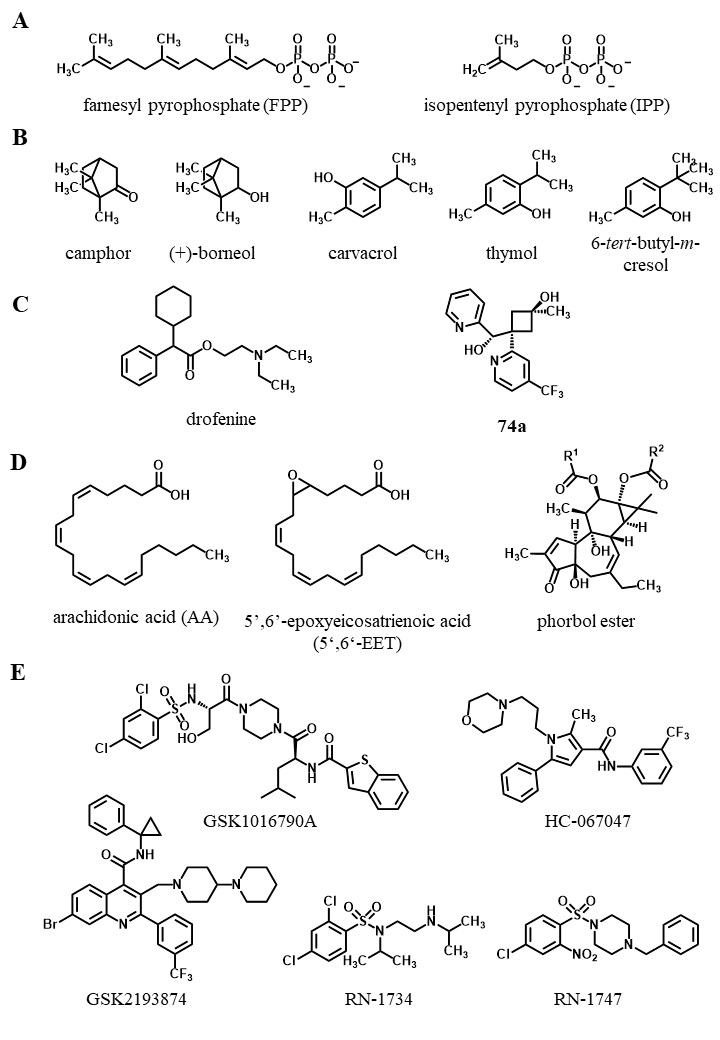
| 66 Iwata Y, Ito S, Wakabayashi S, Kitakaze M: TRPV2 channel as a possible drug target for the treatment of heart failure. Lab Investig 2020;100:207-217. https://doi.org/10.1038/s41374-019-0349-z |
||||
| 67 Santoni G, Amantini C, Maggi F, Marinelli O, Santoni M, Nabissi M, Morelli MB: The TRPV2 cation channels: from urothelial cancer invasiveness to glioblastoma multiforme interactome signature. Lab Investig 2020;100:186-198. https://doi.org/10.1038/s41374-019-0333-7 |
||||
| 68 Yan K, Sun X, Wang G, Liu Y, Wang KW: Pharmacological activation of thermo-transient receptor potential vanilloid 3 channels inhibits hair growth by inducing cell death of hair follicle outer root sheath. J Pharmacol Exp Ther 2019;370:299-307. https://doi.org/10.1124/jpet.119.258087 |
||||
| 69 Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KSR, Andahazy M, Story GM, Patapoutian A: Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005;307:1468-1472. https://doi.org/10.1126/science.1108609 |
||||
| 70 Cheng X, Jin J, Hu L, Shen D, Dong XP, Samie MA, Knoff J, Eisinger B, Liu ML, Huang SM, Caterina MJ, Dempsey P, Michael LE, Dlugosz AA, Andrews NC, Clapham DE, Xu H: TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 2010;141:331-343. https://doi.org/10.1016/j.cell.2010.03.013 |
||||
| 71 Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, Tsukahara K, Arimura A, Horikawa T, Hirasawa T, Sakata T: Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol 2006;126:2664-2672. https://doi.org/10.1038/sj.jid.5700468 |
||||
| 72 Xu H, Delling M, Jun JC, Clapham DE: Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 2006;9:628-635. https://doi.org/10.1038/nn1692 |
||||
| 73 Zhong W, Hu L, Cao X, Zhao J, Zhang X, Lee M, Wang H, Zhang J, Chen Q, Feng C, Duo L, Wang X, Tang L, Lin Z, Yang Y: Genotype-Phenotype Correlation of TRPV3-Related Olmsted Syndrome. J Invest Dermatol 2021;141:545-554. https://doi.org/10.1016/j.jid.2020.06.035 |
||||
| 74 Liu N, Wu J, Chen Y, Zhao J: Channels that Cooperate with TRPV4 in the Brain. J Mol Neurosci 2020;70:1812-1820. https://doi.org/10.1007/s12031-020-01574-z |
||||
| 75 Kaßmann M, Harteneck C, Zhu Z, Nürnberg B, Tepel M, Gollasch M: Transient receptor potential vanilloid 1 (TRPV1), TRPV4, and the kidney. Acta Physiol 2013;207:546-564. https://doi.org/10.1111/apha.12051 |
||||
| 76 Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, Nilius B: Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 2007;117:3453-3462. https://doi.org/10.1172/JCI31766 |
||||
| 77 Chung MK, Lee H, Caterina MJ: Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem 2003;278:32037-32046. https://doi.org/10.1074/jbc.M303251200 |
||||
| 78 Pritschow BW, Lange T, Kasch J, Kunert-Keil C, Liedtke W, Brinkmeier H: Functional TRPV4 channels are expressed in mouse skeletal muscle and can modulate resting Ca2+ influx and muscle fatigue. Pflugers Arch Eur J Physiol 2011;461:115-122. https://doi.org/10.1007/s00424-010-0883-4 |
||||
| 79 Lorenzo IM, Liedtke W, Sanderson MJ, Valverde MA: TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc Natl Acad Sci U S A 2008;105:12611-12616. https://doi.org/10.1073/pnas.0803970105 |
||||
| 80 Filosa JA, Yao X, Rath G: TRPV4 and the regulation of vascular tone. J Cardiovasc Pharmacol 2013;61:113-119. https://doi.org/10.1097/FJC.0b013e318279ba42 |
||||
| 81 Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ: Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci 2005;25:1304-1310. https://doi.org/10.1523/JNEUROSCI.4745.04.2005 |
||||
| 82 Masuyama R, Vriens J, Voets T, Karashima Y, Owsianik G, Vennekens R, Lieben L, Torrekens S, Moermans K, Vanden Bosch A, Bouillon R, Nilius B, Carmeliet G: TRPV4-Mediated Calcium Influx Regulates Terminal Differentiation of Osteoclasts. Cell Metab 2008;8:257-265. https://doi.org/10.1016/j.cmet.2008.08.002 |
||||
| 83 Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT: Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 2012;336:597-601. https://doi.org/10.1126/science.1216283 |
||||
| 84 Nilius B, Voets T: The puzzle of TRPV4 channelopathies. EMBO Rep 2013;14:152-163. https://doi.org/10.1038/embor.2012.219 |
||||
| 85 Hoenderop JGJ, van der Kemp AWCM, Hartog A, van de Graaf SFJ, van Os CH, Willems PHGM, Bindels RJM: Molecular Identification of the Apical Ca2+Channel in 1,25-Dihydroxyvitamin D3-responsive Epithelia. J Biol Chem 1999;274:8375-8378. https://doi.org/10.1074/jbc.274.13.8375 |
||||
| 86 Peng JB, Chen XZ, Berger UV, Vassilev PM, Brown EM, Hediger MA: A rat kidney-specific calcium transporter in the distal nephron. J Biol Chem 2000;275:28186-28194. https://doi.org/10.1074/jbc.M909686199 |
||||
| 87 Bernucci L, Henríquez M, Díaz P, Riquelme G: Diverse Calcium Channel Types are Present in the Human Placental Syncytiotrophoblast Basal Membrane. Placenta 2006;27:1082-1095. https://doi.org/10.1016/j.placenta.2005.12.007 |
||||
| 88 Vassilieva IO, Tomilin VN, Marakhova II, Shatrova AN, Negulyaev YA, Semenova SB: Expression of transient receptor potential vanilloid channels TRPV5 and TRPV6 in human blood lymphocytes and Jurkat leukemia T cells. J Membr Biol 2013;246:131-140. https://doi.org/10.1007/s00232-012-9511-x |
||||
| 89 van der Eerden BCJ, Hoenderop JGJ, de Vries TJ, Schoenmaker T, Buurman CJ, Uitterlinden AG, Pols HAP, Bindels RJM, van Leeuwen JPTM: The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc Natl Acad Sci U S A 2005;102:17507-17512. https://doi.org/10.1073/pnas.0505789102 |
||||
| 90 Zhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR: Calcium-Selective Ion Channel, CaT1, Is Apically Localized in Gastrointestinal Tract Epithelia and Is Aberrantly Expressed in Human Malignancies. Lab Investig 2002;82:1755-1764. https://doi.org/10.1097/01.LAB.0000043910.41414.E7 |
||||
| 91 Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H: Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: A novel prognostic marker for tumor progression. Oncogene 2003;22:7858-7861. https://doi.org/10.1038/sj.onc.1206895 |
||||
| 92 Bolanz KA, Hediger MA, Landowski CP: The role of TRPV6 in breast carcinogenesis. Mol Cancer Ther 2008;7:271-279. https://doi.org/10.1158/1535-7163.MCT-07-0478 |
||||
| 93 Flores-Aldama L, Vandewege MW, Zavala K, Colenso CK, Gonzalez W, Brauchi SE, Opazo JC: Evolutionary analyses reveal independent origins of gene repertoires and structural motifs associated to fast inactivation in calcium-selective TRPV channels. Sci Rep 2020;10:1-13. https://doi.org/10.1038/s41598-020-65679-6 |
||||
| 94 Hoenderop JGJ, Van Leeuwen JPTM, Van Der Eerden BCJ, Kersten FFJ, Van Der Kemp AWCM, Mérillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJM: Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest 2003;112:1906-1914. https://doi.org/10.1172/JCI200319826 |
||||
| 95 Van Abel M, Huybers S, Hoenderop JGJ, Van Der Kemp AWCM, Van Leeuwen JPTM, Bindels RJM: Age-dependent alterations in Ca2+ homeostasis: Role of TRPV5 and TRPV6. Am J Physiol Renal Physiol 2006;291:1177-1183. https://doi.org/10.1152/ajprenal.00038.2006 |
||||
| 96 Renkema KY, Nijenhuis T, Van Der Eerden BCJ, Van Der Kemp AWCM, Weinans H, Van Leeuwen JPTM, Bindels RJM, Hoenderop JGJ: Hypervitaminosis D mediates compensatory Ca2+ hyperabsorption in TRPV5 knockout mice. J Am Soc Nephrol 2005;16:3188-3195. https://doi.org/10.1681/ASN.2005060632 |
||||
| 97 Nijenhuis T, Van Der Eerden BCJ, Hoenderop JGJ, Weinans H, Van Leeuwen JPTM, Bindels RJM: Bone resorption inhibitor alendronate normalizes the reduced bone thickness of TRPV5-/- mice. J Bone Miner Res 2008;23:1815-1824. https://doi.org/10.1359/jbmr.080613 |
||||
| 98 Bianco SDC, Peng J Bin, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, Zhuang L, Freeman MR, Gouveia CHA, Wu J, Luo H, Mauro T, Brown EM, Hediger MA: Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res 2007;22:274-285. https://doi.org/10.1359/jbmr.061110 |
||||
| 99 Weissgerber P, Kriebs U, Tsvilovskyy V, Olausson J, Kretz O, Stoerger C, Mannebach S, Wissenbach U, Vennekens R, Middendorff R, Flockerzi V, Freichel M: Excision of Trpv6 gene leads to severe defects in epididymal Ca 2+ absorption and male fertility much like single D541A pore mutation. J Biol Chem 2012;287:17930-17941. https://doi.org/10.1074/jbc.M111.328286 |
||||
| 100 Stewart JM: TRPV6 as a target for cancer therapy. J Cancer 2020;11:374-387. https://doi.org/10.7150/jca.31640 |
||||
| 101 Bowen CV, DeBay D, Ewart HS, Gallant P, Gormley S, Ilenchuk TT, Iqbal U, Lutes T, Martina M, Mealing G, Merkley N, Sperker S, Moreno MJ, Rice C, Syvitski RT, Stewart JM: In vivo Detection of Human TRPV6-Rich Tumors with Anti-Cancer Peptides Derived from Soricidin. PLoS One 2013;8:e58866. https://doi.org/10.1371/journal.pone.0058866 |
||||
| 102 Xue H, Wang Y, MacCormack TJ, Lutes T, Rice C, Davey M, Dugourd D, Ilenchuk TT, Stewart JM: Inhibition of Transient Receptor Potential Vanilloid 6 channel, elevated in human ovarian cancers, reduces tumour growth in a xenograft model. J Cancer 2018;9:3196-3207. https://doi.org/10.7150/jca.20639 |
||||
| 103 Nilius B, Prenen J, Vennekens R, Hoenderop JGJ, Bindels RJM, Droogmans G: Pharmacological modulation of monovalent cation currents through the epithelial Ca 2+ channel ECaC1. Br J Pharmacol 2001;134:453-462. https://doi.org/10.1038/sj.bjp.0704272 |
||||
| 104 McIntyre P, McLatchie LM, Chambers A, Phillips E, Clarke M, Savidge J, Toms C, Peacock M, Shah K, Winter J, Weerasakera N, Webb M, Rang HP, Bevan S, James IF: Pharmacological differences between the human and rat vanilloid receptor 1 (VR1). Br J Pharmacol 2001;132:1084-1094. https://doi.org/10.1038/sj.bjp.0703918 |
||||
| 105 Peier AM, Reeve AJ, Anderson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A: A Heat-Sensitive TRP Channel Expressed in Keratinocytes. Science 2002;296:2046-2049. https://doi.org/10.1126/science.1073140 |
||||
| 106 Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B: Activation of TRPV4 Channels (hVRL-2/mTRP12) by Phorbol Derivatives. J Biol Chem 2002;277:13569-13577. https://doi.org/10.1074/jbc.M200062200 |
||||
| 107 Hoenderop JGJ, Vennekens R, Muller D, Prenen J, Droogmans G, Bindels RJM, Nilius B: Function and expression of the epithelial Ca2+ channel family: comparison of mammalian ECaC1 and 2. J Physiol 2001;537:747-761. https://doi.org/10.1113/jphysiol.2001.012917 |
||||
| 108 Kirichok Y, Navarro B, Clapham DE: Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 2006;439:737-740. https://doi.org/10.1038/nature04417 |
||||
| 109 Nagata K, Duggan A, Kumar G, García-Añoveros J: Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 2005;25:4052-4061. https://doi.org/10.1523/JNEUROSCI.0013-05.2005 |
||||
| 110 Pessah IN, Waterhouse AL, Casida JE: The calcium-Ryanodine receptor complex of skeletal and cardiac muscle. Biochem Biophys Res Commun 1985;128:449-456. https://doi.org/10.1016/0006-291X(85)91699-7 |
||||
| 111 Leffler A, Linte RM, Nau C, Reeh P, Babes A. A high-threshold heat-activated channel in cultured rat dorsal root ganglion neurons resembles TRPV2 and is blocked by gadolinium. Eur J Neurosci 2007;26:12-22. https://doi.org/10.1111/j.1460-9568.2007.05643.x |
||||
| 112 Deng Z, Paknejad N, Maksaev G, Sala-Rabanal M, Nichols CG, Hite RK, Yuan P: Cryo-EM and X-ray structures of TRPV4 reveal insight into ion permeation and gating mechanisms. Nat Struct Mol Biol 2018;25:252-260. https://doi.org/10.1038/s41594-018-0037-5 |
||||
| 113 Nakaya K, Harbidge DG, Wangemann P, Schultz BD, Green ED, Wall SM, Marcus DC: Lack of pendrin HCO-3 transport elevates vestibular endolymphatic [Ca2+] by inhibition of acid-sensitive TRPV5 and TRPV6 channels. Am J Physiol Renal Physiol 2007;292:1314-1321. https://doi.org/10.1152/ajprenal.00432.2006 |
||||
| 114 Jara-Oseguera A, Huffer KE, Swartz KJ: The ion selectivity filter is not an activation gate in TRPV1-3 channels. Elife 2019;8:1-27. https://doi.org/10.7554/eLife.51212 |
||||
| 115 Pena F, Ordaz B: Non-Selective Cation Channel Blockers: Potential Use in Nervous System Basic Research and Therapeutics. Mini Rev Med Chem 2008;8:812-819. https://doi.org/10.2174/138955708784912166 |
||||
| 116 Kovacs G, Montalbetti N, Simonin A, Danko T, Balazs B, Zsembery A, Hediger MA: Inhibition of the human epithelial calcium channel TRPV6 by 2-aminoethoxydiphenyl borate (2-APB). Cell Calcium 2012;52:468-480. https://doi.org/10.1016/j.ceca.2012.08.005 |
||||
| 117 Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX: 2-Aminoethoxydiphenyl Borate Is a Common Activator of TRPV1, TRPV2, and TRPV3. J Biol Chem 2004;279:35741-35748. https://doi.org/10.1074/jbc.M404164200 |
||||
| 118 Juvin V, Penna A, Chemin J, Lin Y-L, Rassendren F-A: Pharmacological Characterization and Molecular Determinants of the Activation of Transient Receptor Potential V2 Channel Orthologs by 2-Aminoethoxydiphenyl Borate. Mol Pharmacol 2007;72:1258-1268. https://doi.org/10.1124/mol.107.037044 |
||||
| 119 Deering-Rice CE, Mitchell VK, Romero EG, Abdel Aziz MH, Ryskamp DA, Križaj D, Venkat RG, Reilly CA: Drofenine: a 2-APB analog with improved selectivity for human TRPV3. Pharmacol Res Perspect 2014;2:1-10. https://doi.org/10.1002/prp2.62 |
||||
| 120 Hu H, Grandl J, Bandell M, Petrus M, Patapoutian A: Two amino acid residues determine 2-APB sensitivity of the ion channels TRPV3 and TRPV4. Proc Natl Acad Sci U S A 2009;106:1626-1631. https://doi.org/10.1073/pnas.0812209106 |
||||
| 121 Singh AK, McGoldrick LL, Sobolevsky AI: Structure and gating mechanism of the transient receptor potential channel TRPV3. Nat Struct Mol Biol 2018;25:805-813. https://doi.org/10.1038/s41594-018-0108-7 |
||||
| 122 Zubcevic L, Borschel WF, Hsu AL, Borgnia MJ, Lee SY: Regulatory switch at the cytoplasmic interface controls trpv channel gating. Elife 2019;8:1-24. https://doi.org/10.7554/eLife.47746 |
||||
| 123 Singh AK, Saotome K, McGoldrick LL, Sobolevsky AI: Structural bases of TRP channel TRPV6 allosteric modulation by 2-APB. Nat Commun 2018;9:1-11. https://doi.org/10.1038/s41467-018-04828-y |
||||
| 124 Szallasi A, Conte B, Goso C, Blumberg PM, Manzini S: Characterization of a peripheral vanilloid (capsaicin) receptor in the urinary bladder of the rat. Life Sci 1993;52:221-226. https://doi.org/10.1016/0024-3205(93)90051-4 |
||||
| 125 Walpole CSJ, Bevan S, Bovermann G, Boelsterli JJ, Breckenridge R, Davies JW, Hughes GA, James I, Oberer L, Winter J, Wrigglesworth R: The Discovery of Capsazepine, the First Competitive Antagonist of the Sensory Neuron Excitants Capsaicin and Resiniferatoxin. J Med Chem 1994;37:1942-1954. https://doi.org/10.1021/jm00039a006 |
||||
| 126 Jordt SE, Julius D: Molecular basis for species-specific sensitivity to "hot" chili peppers. Cell 2002;108:421-430. https://doi.org/10.1016/S0092-8674(02)00637-2 |
||||
| 127 Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, Zhang TJ, Viswanadhan VN, Toth A, Pearce LV., Vanderah TW, Porreca F, Blumberg PM, Lile J, Sun Y, Wild K, Louis JC, Treanor JJS: Molecular Determinants of Vanilloid Sensitivity in TRPV1. J Biol Chem 2004;279:20283-20295. https://doi.org/10.1074/jbc.M312577200 |
||||
| 128 Cao E, Liao M, Cheng Y, Julius D: TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 2013;504:113-118. https://doi.org/10.1038/nature12823 |
||||
| 129 Gao Y, Cao E, Julius D, Cheng Y: TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 2016;534:347-351. https://doi.org/10.1038/nature17964 |
||||
| 130 Zhang F, Hanson SM, Jara-Oseguera A, Krepkiy D, Bae C, Pearce LV, Blumberg PM, Newstead S, Swartz KJ: Engineering vanilloid-sensitivity into the rat TRPV2 channel. Elife 2016;5:1-18. https://doi.org/10.7554/eLife.16409 |
||||
| 131 Seabrook GR, Sutton KG, Jarolimek W, Hollingworth GJ, Teague S, Webb J, Clark N, Boyce S, Kerby J, Ali Z, Chou M, Middleton R, Kaczorowski G, Jones AB: Functional Properties of the High-Affinity TRPV1 (VR1) Vanilloid Receptor Antagonist (4-Hydroxy-5-iodo-3-methoxyphenylacetate ester) Iodo-Resiniferatoxin. J Pharmacol Exp Ther 2002;303:1052-1060. https://doi.org/10.1124/jpet.102.040394 |
||||
| 132 Appendino G, Daddario N, Minassi A, Moriello AS, De Petrocellis L, Di Marzo V: The taming of capsaicin. Reversal of the vanilloid activity of N-acylvanillamines by aromatic iodination. J Med Chem 2005;48:4663-4669. https://doi.org/10.1021/jm050139q |
||||
| 133 Geron M, Hazan A, Priel A: Animal toxins providing insights into TRPV1 activation mechanism. Toxins (Basel) 2017;9:1-19. https://doi.org/10.3390/toxins9100326 |
||||
| 134 Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, Basbaum AI, King D, Julius D: Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature 2006;444:208-212. https://doi.org/10.1038/nature05285 |
||||
| 135 Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, Zipkin RE, Daddario N, Appendino G, Di Marzo V, Walker JM: N-Oleoyldopamine, a Novel Endogenous Capsaicin-like Lipid That Produces Hyperalgesia. J Biol Chem 2003;278:13633-13639. https://doi.org/10.1074/jbc.M211231200 |
||||
| 136 Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB: The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br J Pharmacol 2000;129:227-230. https://doi.org/10.1038/sj.bjp.0703050 |
||||
| 137 Medeiros P, Oliveira-Silva M, Negrini-Ferrari SE, Medeiros AC, Elias-Filho DH, Coimbra NC, de Freitas RL: CB1-cannabinoid-, TRPV1-vanilloid- and NMDA-glutamatergic-receptor-signalling systems interact in the prelimbic cerebral cortex to control neuropathic pain symptoms. Brain Res Bull 2020;165:118-128. https://doi.org/10.1016/j.brainresbull.2020.09.013 |
||||
| 138 Bang S, Kim KY, Yoo S, Lee SH, Hwang SW: Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett 2007;425:120-125. https://doi.org/10.1016/j.neulet.2007.08.035 |
||||
| 139 Onusko E, McDermott MR, Robbins N, Liu G, Kranias EG, Rubinstein J, Koch SE: Probenecid treatment improves outcomes in a novel mouse model of peripartum cardiomyopathy. PLoS One 2020;15:1-16. https://doi.org/10.1371/journal.pone.0230386 |
||||
| 140 Robbins N, Gilbert M, Kumar M, McNamara JW, Daly P, Koch SE, Conway G, Effat M, Woo JG, Sadayappan S, Rubinstein J: Probenecid improves cardiac function in patients with heart failure with reduced ejection fraction in vivo and cardiomyocyte calcium sensitivity in vitro. J Am Heart Assoc 2018;7:1-11. https://doi.org/10.1161/JAHA.117.007148 |
||||
| 141 Hisanaga E, Nagasawa M, Ueki K, Kulkarni RN, Mori M, Kojima I: Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic β-cells. Diabetes 2009;58:174-184. https://doi.org/10.2337/db08-0862 |
||||
| 142 Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin M Lou, Flores CM. TRPV2 Is Activated by Cannabidiol and Mediates CGRP Release in Cultured Rat Dorsal Root Ganglion Neurons. J Neurosci 2008;28:6231-6238. https://doi.org/10.1523/JNEUROSCI.0504-08.2008 |
||||
| 143 Pumroy RA, Samanta A, Liu Y, Hughes TET, Zhao S, Yudin Y, Huynh KW, Zhou ZH, Rohacs T, Han S, Moiseenkova-Bell VY: Molecular mechanism of TRPV2 channel modulation by cannabidiol. Elife 2019;8:e48792. https://doi.org/10.7554/eLife.48792 |
||||
| 144 Bang S, Yoo S, Yang TJ, Cho H, Hwang SW: Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3. J Biol Chem 2010;285:19362-19371. https://doi.org/10.1074/jbc.M109.087742 |
||||
| 145 Bang S, Yoo S, Yang TJ, Cho H, Hwang SW: Isopentenyl pyrophosphate is a novel antinociceptive substance that inhibits TRPV3 and TRPA1 ion channels. Pain 2011;152:1156-1164. https://doi.org/10.1016/j.pain.2011.01.044 |
||||
| 146 Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, Hatt H: Monoterpenoid agonists of TRPV3. Br J Pharmacol 2007;151:530-540. https://doi.org/10.1038/sj.bjp.0707245 |
||||
| 147 Gomtsyan A, Schmidt RG, Bayburt EK, Gfesser GA, Voight EA, Daanen JF, Schmidt DL, Cowart MD, Liu H, Altenbach RJ, Kort ME, Clapham B, Cox PB, Shrestha A, Henry R, Whittern DN, Reilly RM, Puttfarcken PS, Brederson JD, Song P, et al.: Synthesis and Pharmacology of (Pyridin-2-yl)methanol Derivatives as Novel and Selective Transient Receptor Potential Vanilloid 3 Antagonists. J Med Chem 2016;59:4926-4947. https://doi.org/10.1021/acs.jmedchem.6b00287 |
||||
| 148 Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B: Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 2003;424:434-438. https://doi.org/10.1038/nature01807 |
||||
| 149 Vriens J, Owsianik G, Janssens A, Voets T, Nilius B: Determinants of 4α-Phorbol Sensitivity in Transmembrane Domains 3 and 4 of the Cation Channel TRPV4. J Biol Chem 2007;282:12796-12803. https://doi.org/10.1074/jbc.M610485200 |
||||
| 150 Klausen TK, Pagani A, Minassi A, Ech-Chahad A, Prenen J, Owsianik G, Hoffmann EK, Pedersen SF, Appendino G, Nilius B: Modulation of the Transient Receptor Potential Vanilloid Channel TRPV4 by 4α-Phorbol Esters: A Structure−Activity Study. J Med Chem 2009;52:2933-2939. https://doi.org/10.1021/jm9001007 |
||||
| 151 Silinsky EM, Searl TJ: Phorbol esters and neurotransmitter release: More than just protein kinase C? Br J Pharmacol 2003;138:1191-1201. https://doi.org/10.1038/sj.bjp.0705213 |
||||
| 152 Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ESR, Gordon E, Evans L, Misajet BA, DeMarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, et al.: N-((1S)-1-{[4-((2S)-2-{[(2,4-Dichlorophenyl)sulfonyl]amino}-3- hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a Novel and Potent Transient Receptor Potential Vanilloid 4 Channel Agonist Induces Urinary Bladder Contraction and Hyperactivity: Part I. J Pharmacol Exp Ther 2008 Aug;326:432-442. https://doi.org/10.1124/jpet.108.139295 |
||||
| 153 Willette RN, Bao W, Nerurkar S, Yue TI, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout REL, Votta BJ, Thorneloe K, et al.: Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther 2008;326:443-452. https://doi.org/10.1124/jpet.107.134551 |
||||
| 154 Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian MY, Costell M, Maniscalco-Hauk K, Krawiec JA, Olzinski A, Gordon E, Lozinskaya I, Elefante L, Qin P, Matasic DS, James C, Tunstead J, Donovan B, Kallal L, Waszkiewicz A, et al.: An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med 2012;4:159ra148. https://doi.org/10.1126/scitranslmed.3004276 |
||||
| 155 Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MAJ: Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun 2009;389:490-494. https://doi.org/10.1016/j.bbrc.2009.09.007 |
||||
| 156 Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T: Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci U S A 2010;107:19084-19089. https://doi.org/10.1073/pnas.1005333107 |
||||
| 157 Haverstick DM, Heady TN, Macdonald TL, Gray LS: Inhibition of human prostate cancer proliferation in vitro and in a mouse model by a compound synthesized to block Ca2+ entry. Cancer Res 2000;60:1002-1008. | ||||
| 158 Landowski CP, Bolanz KA, Suzuki Y, Hediger MA: Chemical Inhibitors of the Calcium Entry Channel TRPV6. Pharm Res 2011;28:322-330. https://doi.org/10.1007/s11095-010-0249-9 |
||||
| 159 Hughes TET, Lodowski DT, Huynh KW, Yazici A, Del Rosario J, Kapoor A, Basak S, Samanta A, Han X, Chakrapani S, Zhou ZH, Filizola M, Rohacs T, Han S, Moiseenkova-Bell VY: Structural basis of TRPV5 channel inhibition by econazole revealed by cryo-EM. Nat Struct Mol Biol 2018;25:53-60. https://doi.org/10.1038/s41594-017-0009-1 |
||||
| 160 Hughes TET, Del Rosario JS, Kapoor A, Yazici AT, Yudin Y, Fluck EC, Filizola M, Rohacs T, Moiseenkova-Bell VY. Structure-based characterization of novel TRPV5 inhibitors. Elife 2019;8:1-21. https://doi.org/10.7554/eLife.49572.042 |
||||
| 161 Bhardwaj R, Lindinger S, Neuberger A, Nadezhdin KD, Singh AK, Cunha MR, Derler I, Gyimesi G, Reymond J-L, Hediger MA, Romanin C, Sobolevsky AI: Inactivation-mimicking block of the epithelial calcium channel TRPV6. Sci Adv 2020;6:eabe1508. https://doi.org/10.1126/sciadv.abe1508 |
||||
| 162 Simonin C, Awale M, Brand M, Van Deursen R, Schwartz J, Fine M, Kovacs G, Häfliger P, Gyimesi G, Sithampari A, Charles RP, Hediger MA, Reymond JL: Optimization of TRPV6 calcium channel inhibitors using a 3D ligand-based virtual screening method. Angew Chem Int Ed Engl 2015;54:14748-14752. https://doi.org/10.1002/anie.201507320 |
||||
| 163 Neeper MP, Liu Y, Hutchinson TL, Wang Y, Flores CM, Qin N: Activation Properties of Heterologously Expressed Mammalian TRPV2. J Biol Chem 2007;282:15894-15902. https://doi.org/10.1074/jbc.M608287200 |
||||