Corresponding Author: Liborija Lugović-Mihić
Department of Dermatology and Venereology, University Hospital Center Sestre Milosrdnice, Vinogradska cesta 29, 10 000 Zagreb (Croatia)
Tel. +385 1 3787 422, Fax +385 1 3769 067 , E-Mail liborija@gmail.com
The Influence of Psychological Stress on HPV Infection Manifestations and Carcinogenesis
Liborija Lugović-Mihića,b Hrvoje Cvitanovićc Ivka Djakovićd Matea Kunaa,b Ana Šešerkoe
aDepartment of Dermatovenereology, University Hospital Center Sestre Milosrdnice, Zagreb, Croatia, bSchool of Dental Medicine, University of Zagreb, Zagreb, Croatia, cDepartment of Dermatovenereology, Karlovac General Hospital, Karlovac, Croatia, dDepartment of of Gynaecology and Obstetrics, University Hospital Center Sestre Milosrdnice, Zagreb, Croatia, eDepartment of Gynaecology and Obstetrics, University Hospital Center Zagreb, Zagreb, Croatia
Introduction
Psychological stress is an important factor involved in disease manifestations of HPV infection, and it can participate in carcinogenesis associated with HPV. Due to inconsistencies in some study results, this issue remains a subject of research. It is important to determine to what extent stress plays a role in HPV manifestations and carcinogenesis and how much it participates in the onset, development, and progression of infections.
Features of HPV
Human papillomavirus (HPV) is a DNA virus that belongs to the Papillomaviridae family. It is one of the most ubiquitous viral infections in humans, and it usually manifests as skin or genital mucosa lesions, although it can occur in other mucosa as well. It has long been known that most sexually active people will be infected by this virus at some point of their life, and the incidence of HPV infection is highest in the age group for those 20-40 years old [1-4]. Despite its high prevalence, most who get infected will not have a clinically overt infection. Still, the persistence of the HPV infection may cause a higher risk for developing cervical intraepithelial neoplasia (CIN) and invasive carcinoma. In women, a breakout from a high-risk HPV infection typically takes 14 months to clear up for an oncogenic infection and 5-6 months for a non-oncogenic infection [4].
To date, more than 200 types of HPV have been identified [5]. A strain is considered new when the nucleotides of the L1 part of the HPV genome differ from that of known HPV viruses by more than 10% [6, 7]. HPV viruses can be divided into different groups by their affinity for certain tissues, which in some part depends on their genotype [6]. The specific skin lesions for each type of HPV are as follows: common warts – types 2, 7, 22; plantar warts – types 1, 2, 4, 63; flat warts – types 3, 10, 28; and for verrucous cyst type 60 and epidermodysplasia verruciformis - more than 15 different types. There are also specific HPV types for anal/genital manifestations: anogenital warts—types 6, 11, 42, 44 and others; anal dysplasia (lesions) – types 16, 18, 31, 53, 58; and genital cancers— highest risk types 16, 18, 31, 45, other high-risk types— 33, 35, 39, 51, 52, 56, 58, 59, and probably high-risk— types 26, 53, 66, 68, 73, 82 [5]. The types of HPV for oral/oropharingeal lesions are: focal epithelial hyperplasia (mouth)—types 13 and 32; mouth papillomas—types 6, 7, 11, 16, 32); and oropharyngeal cancer—type 16; laryngeal papillomatosis—types 6 and 11 [5]. Concerning oncogenic risk, the low-risk HPV types are 6, 11, 49, 42, 43, 44, 54, 61, 70, 72 and 81, while the high-risk types are 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82. In addition, 60% of all genital warts are caused by HPV types 16 and 11 [8].
HPV is transmitted through direct skin or mucous membrane contact, and infection can be clinical, subclinical or latent. Microimpairments in the skin or mucous membrane enhance transmission, but for infection to develop it is essential for the complete virus to be transmitted, not only its DNA fragments [4]. In the sexually active population, HPV is present in 80% of women of reproductive age, but its manifestations resolve spontaneously in most cases (cca 80 % of infections) within 12-24 months [9]. Untreated HPV manifestations in men can cause invasive penile carcinoma, which represents 1% of malignancies in men in developed countries and 10-20% in undeveloped countries. In men, incidence of death due to suffering from HPV-associated cancers of different organs is 0.32 per 100.000 [10].
HPV is a double-stranded DNA virus that has an icosahedron shape and a double-layered capsid made of 72 capsomers (HPV’s capsid is not covered by a lipid membrane, which makes it resistant to ethanol and solvents.) It has 8,000 base pairs with a molecular mass of 5200 kDa [8, 11]. The genes are divided into two groups: early (E) and late (L). The group of early genes is composed of six genes (E1, E2, E4, E5, E6, E7) that code for the proteins responsible for replication, transcription, and malignant transformation [5, 8, 11]. Late genes L1 and L2 code for the viral envelope (non-lipid membrane) and for the proteins responsible for the capsid’s structure. Gene L1 is the oldest, and it is used to identify the different types of HPV. A 10% difference in the nucleotide sequence of the L1 gene signifies a new strain (type), while a difference of 2-10% is considered a subtype, and a difference of less than 2% is defined as a variant.
The oncogenic potential of HPV depends on genes E6 and E7. The product of the E6 gene binds to the p53 oncosupressor, and the product of E7 binds to the RB protein. In the case of cervical intraepithelial neoplasia, genes of high-risk types are integrated into the DNA of the host. This integration in some cases leads to disruption of the E2 gene, which results in increased replication of the E6 and E7 genes. The E6 protein binds to p53 causing it to degrade. The E7 protein inactivates the RB protein so that E2F proteins detach from the RB, preventing transcription of the gene that regulates cell growth and differentiation [5, 11]. It has been established that E6 and E7 interfere with the immune response by reducing production of interferon (IFN) [5]. Therefore, the E1 and E2 genes of HPVs are involved in viral replication, while the E6 and E7 proteins function as the promotors of proliferation. The major HPV oncogenes are E6 and E7, which disrupt the normal regulation of the cell cycle and cell progression, giving them an important role in the oncogenesis of HPVs with a high risk of causing anogenital and cervical cancer. Immortalization of epithelial cells induced by HPV requires viral DNA integration into the host cell genome, which causes disruption of the E2 gene. The E2 protein is also a transcription factor, which regulates expression of the E6 and E7 oncoproteins. Integration of the virus in the human genome disrupts the E2 gene and increases expression of E6 and E7 genes in vitro [12-21].
Concerning molecular events during the progression of cervical lesions to carcinogenic lesions, persistent high-risk HPV infection leads to integration of HPV into the host genome and to overexpression of oncogenes E6 and E7 [11]. On the molecular level, interaction of Е7 with the pRb protein leads to aberrant initiation of the S-phase. The E7 oncoprotein causes release of E2F transcription factor from the pRb protein, which is then active and can initiate transcription of genes involved in cell cycle progression, contributing to cellular immortalization and transformation. Thus, Е6 targets р53 for proteasomal degradation, which leads to inhibition of apoptosis and DNA repair (anti-apoptotic effect). It is important to emphasize that only high-risk HPV types can induce degradation of p53, which can then lead to carcinogenesis. E6 activates the PI3K/Akt pathway, interacts with cellular proteins NFX1, and induces human telomerase reverse transcriptase (hTERT) activation, leading to immortalization and transformation. The interaction of both oncoproteins with DNA methyl transferases leads to aberrant methylation, causing silencing of tumor suppressor genes. Also, E7 interaction with histone deacetylases (HDACs) causes chromosome remodelling and genome instability. So, during lesional progression to carcinogenesis, the cross interaction of E6 and E7 with various pathways plays the crucial role [22]. It is also important to mention that viruses like HPV can create virions and become transmissible at any point in their life cycle (the productive virus replication also known as lytic replication). When lytic replication of the virus begins, it is almost irreversible, and successful replication of the virus begins as well as host cell death. But in tumor cells, these infections are mostly latent, allowing the virus to evade the immune response. Thus, lytic replication of the virus is reduced or absent in the tumor. In viral latency there in no production of unnecessary viral proteins that could initiate cell mediated immune recognition. Integration of the viral genome into the host genome eliminates the virus’s ability to replicate as virions, but the virus can replicate using the host’s cellular mechanisms and can be divided whenever the host cell divides. By evading apoptosis, as previously described, oncogenesis begins [23]. All this indicates that a complex network of actions is involved in the pathogenesis process during the occurrence of HPV manifestations.
Stress, types of stressors, the hypothalamic-pituitary-adrenal (HPA) axis, the sympathetic autonomic nervous system (ANS) and the impact of stress on the body and disease
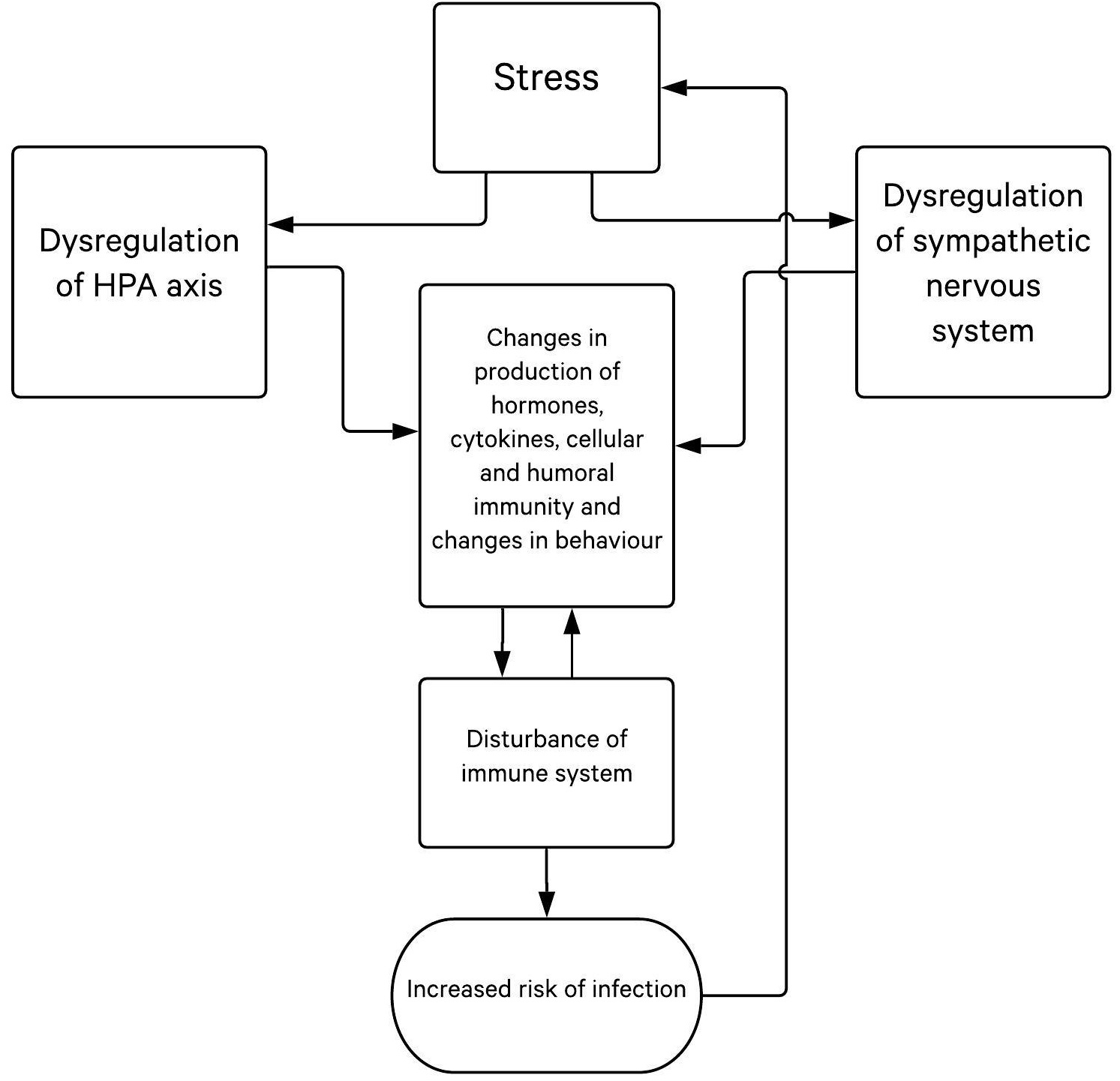
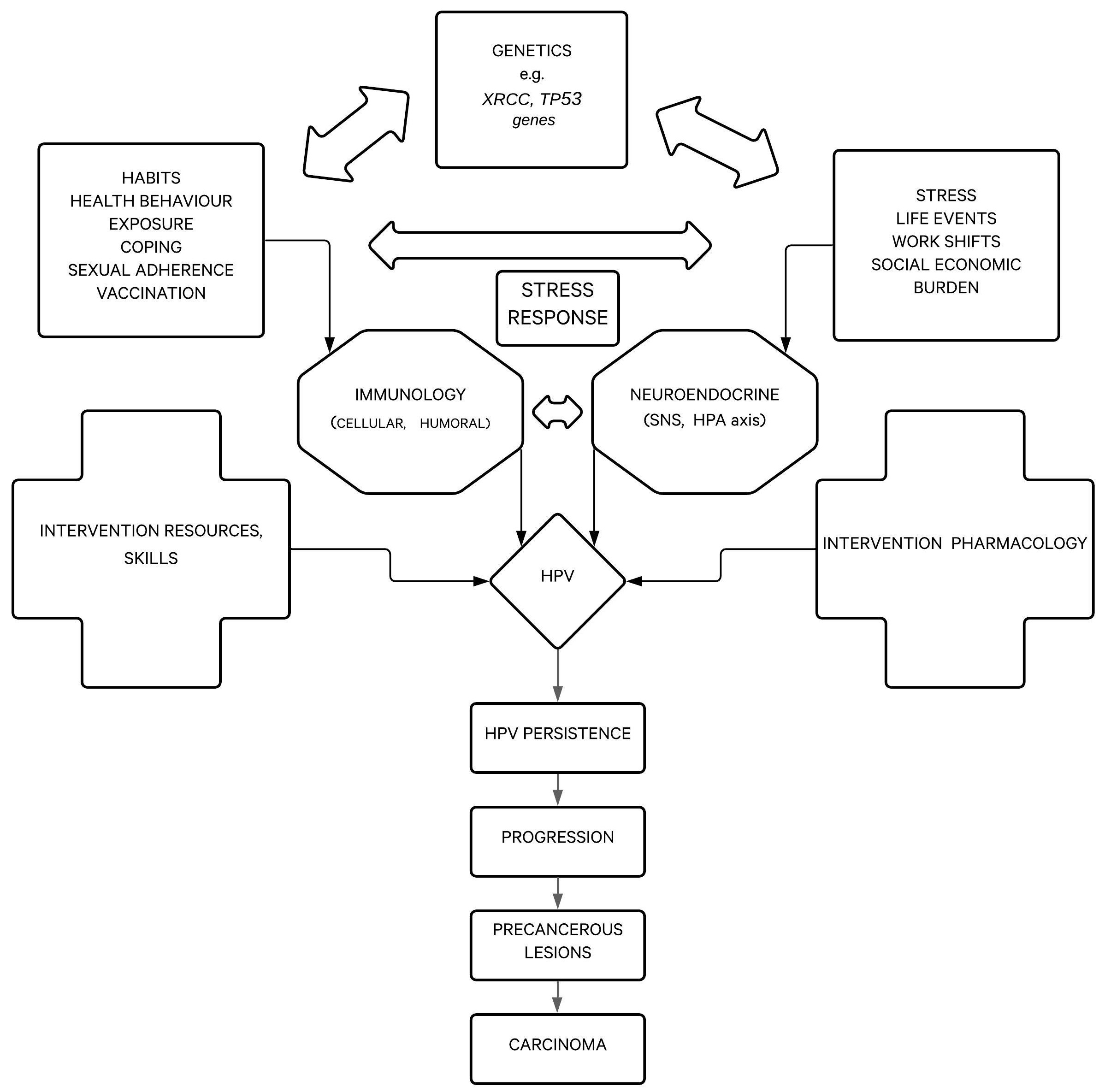
Stress is defined as physical or mental exertion caused by factors that change homeostasis, and it can be observed as an objective stimulus, as an organism’s response to stimulus, or as a relation between a person and their surrounding environment. Stress is a state of threat to physical, psychological, and social homeostasis. More recent approaches define stress as any stimulus that causes sudden termination of ordinary activities, that is, an event that goes beyond what is normal for the organism. The impact or effect, which stress can have, depends on a person’s genetic pool, experiences, and behaviors [24-28]. The biopsychosocial model of disease and health, for example, asserts that biological, psychological and social factors interdependently affect the course and outcome of disease (Fig. 1). Psychological stress has become an increasingly important factor in the course of disease, due especially to the circumstances of modern life [25].
Stressors can be physical, chemical, psychological and biological. Psychological and social strains are the most common stressors [25]. Stress can be categorized by duration (acute or chronic), relevance (avoidable/averted or unavoidable), and intensity (mild, moderate, or severe). Aside from stress caused by common life events, there are also big traumatic events such as war, a natural disaster, death in the family, job loss, etc. Psych trauma is a state of high-level stress that can result in posttraumatic stress disorder (PTSD) and can cause long-lasting health problems. In physical, chemical, and biological stress, the condition of the person is determined by the harm caused by an external stimulus, whereas in psychological stress, one’s assessment of environmental dangers, threats or challenges is the important factor. Stress in humans usually manifests through physical symptoms (e.g. palpitations, shortness of breath, perspiration, angina pectoris, frequent infections) or psychological symptoms (e.g. indecisiveness, poor concentration and memory loss, high sensitivity, sleep disturbances, negative thoughts). As a result, stress-related illnesses can occur (e.g. gastric ulcer, hypertension, viral infection, myocardial infarction, psoriasis, allergies, asthma, anxiety disorders, tumors, gastrointestinal disorders) [26-28].
When stressed, the organism reacts in a stress-adapting manner that can take place at the cell-, organ- or organ-system level, or at the level of the entire organism [25]. The organism typically reacts in three phases to a threat or injury with the same set of reactions outlined by the General Adaptation Syndrome (GAS): (1.) alarm; (2.) resistance; and (3.) exhaustion, the long-lasting debilitating phase that makes one susceptible to disease onset [29]. When reacting to external and internal demands by modulating functions and adapting to new conditions (referred to as alostasis), system stability can be achieved through constant adaptation. The main adaptive system includes the hypothalamic-pituitary-adrenal (HPA) axis, sympathetic autonomic nervous system, and cytokine production (Fig. 2) [19-22]. Pathogenetically, the body neutralizes stress with a complex network of physiologic and behavioral responses to reestablish optimal body equilibrium (eustasis) [30]. As crucial components of the stress response, the HPA axis and the autonomic nervous system (ANS) interact with other vital centers in the central nervous system (CNS) and peripheral tissues/organs to mobilize an adequate/appropriate adaptive response against stressors. Thus, different stressful events are recognised by the hypothalamic paraventricular nucleus, which participates in a biological circuit that integrates personal experiences, physiological signalling and the release of corticotropin-releasing hormone (CRH) [31]. CRH acts on the pituitary gland, which then releases adrenocorticotropic hormone (ACTH), followed by ACTH signals to the adrenal cortex to release glucocorticoids [31]. Thus, the body’s adaptive stress response depends on many interconnected neuroendocrine, immune, cellular, and molecular mechanisms.
During stress, the brain and CNS are the main actors; their response includes a variety of crucial neuroendocrine and autonomic reactions in order to achieve homeostasis [25-28, 32-35]. During that process, neurogenic stressors activate processes in the CNS. Signals are then transferred to periventricular nuclei from prefrontal cortex and limbic structures, where stress is compared to experiential events. This processed signal is transferred to the hypothalamus, which in turn activates the HPA axis. Hypothalamic nuclei receive stimuli from limbic and brain stem catecholaminergic signalling pathways. Periventricular nuclei can be activated by locus ceruleus aminergic signals. Central, medial and cortical amygdala nuclei are connected to periventricular and gabaergic neurons forming a closed circle. Activation of glutaminergic neurons stimulates the hypothalamic release of CRH in the eminentia mediana, which then reaches the hypophysis (anterior pituitary) through portal circulation and stimulates the release of ACTH into the peripheral circulation. Activation of the sympathetic nervous system causes terminal nerves and adrenal gland medulla to secret more catecholamines [36, 37]. The immune system affects the brain as well; thus, there is a bilateral connection. It is important to emphasize that ACTH stimulates the release of glucocorticoids from the adrenal glands, meaning cortisol is a main stress hormone. Sympatho-adrenomedullary axis (SAM) activation stimulates CRH secretion in the hypothalamic periventricular neuron (PVN) area [25-28, 38]. Stress enhances the activities of many systems and releasing of various substances, including catecholamines, opiates and corticosteroids, which have an immunosuppressive effect by decreasing activity of cytokines and inflammation. It has been shown that immune cells have hormone receptors (corticosteroid, prolactin, growth hormone, sex hormones), neuropeptide receptors (endorphins, vasoactive intestinal peptide, substance P, etc.) and neurotransmitter receptors (adrenaline, noradrenaline, acetylcholine, serotonin, etc.). During acute stress, posterior hypothalamic nuclei, the sympathetic nervous system and adrenal gland medulla are activated, while in chronic stress, the anterior hypothalamus, sympathetic system and adrenal gland cortex are activated.
Finally, the release of glucocorticoids, mainly cortisol, is followed by increased lipolysis and gluconeogenesis to supply the body with available energy sources. A negative feedback system regulates production of cortisol via the hypothalamus and pituitary glands. In addition, the sympathetic nervous system (SNS) is activated and stimulates the adrenal medulla to release catecholamines, adrenaline and noradrenaline, allowing the body’s systems to transport energy to the organs more quickly. Consequently, homeostasis is re-established, provided the stressor falls into the adaptive capacity [31]. However, during severe and/or chronic stress, dysregulation of the stress system (hyperactivation or hypoactivation) can disrupt homeostasis and lead to cacostasis or allostasis with possible various clinical manifestations [30]. Prolonged activation of stress mechanisms with increased glucocorticoid and catecholamine levels causes a condition where the demand on the individual exceeds their personal adaptive capacity (allostatic load). Therefore, according to research results, chronic stress and increased glucocorticoid/catecholamine levels may participate in cancer progression in different diseases, including HPV-related carcinogenesis [31].
It is significant that cortisol, as the main stress hormone, modifies apoptosis and changes the way in which cytokines are secreted. It is assumed that exposure to relevant stress events can result in dysregulation of the sensitivity and numbers of glucocorticoid receptors (GCR), and that cortisol can affect secretion of local cytokines and trigger stronger inflammation [25, 27, 39]. Glucocorticoids exert their effects through two subtypes of intracellular receptors: (type I) GCR with a high affinity for endogenous corticosteroids (that has a regulating function over the circadian rhythm of the HPA axis) and (type II) GCR with a lower affinity (important for acute stress reactions). In the nucleus, glucocorticoid acts as a transcription factor, and it binds to specific DNA sequences named glucocorticoid response elements (GRE). These sequences modulate gene transcription. Glucocorticoids also interact with other transcription factors, such as AP-1 (activator protein 1) and NF-κB (nuclear factor kappa B). During a stress inflammatory response, glucocorticoids decrease secretion of proinflammatory cytokines and increase secretion of anti-inflammatory cytokines. They also affect redistribution of leukocytes and decrease synthesis and expression of cytokine receptors, lymphocyte proliferation and adhesion molecule expression on the cell surface.
Finally, in stress, adaptive reactions involve the short-term activation of the HPA axis, whereas overproduction of stress hormones and disruption of the regulation of the HPA axis triggers a pathological response/reaction. Just as the cognitive perception of stress is important, so is coping, defined as the constant adaptation of cognitive and behavioral efforts to overcome demands that one finds overwhelming. Adaptive coping styles are usually related to positive personality characteristics, while maladaptive styles are linked to less desireable characteristics. The coping styles a person uses depend primarily upon the situation but also on the disposition of the person themselves.
We would like to express our heartfelt gratitude to Mrs. Suzana Salopek for her selfless help and valuable assistance.
Author Contributions
LLM contributed conception and design of the article; LLM, HC, MK and AŠ analyzed the data; LLM, HC and ID wrote the manuscript; all authors contributed to manuscript revision, read and approved the submitted version.
Statement of Ethics
The authors have no ethical conflicts to disclose.
The authors declare that no conflicts of interest exist.
| 1 Tommasino M: HPV and skin carcinogenesis. Papillomavirus Res 2019;7:129-131. https://doi.org/10.1016/j.pvr.2019.04.003 |
||||
| 2 Smola S: Human papillomaviruses and skin cancer. Adv Exp Med Biol 2020;1268:195-209. https://doi.org/10.1007/978-3-030-46227-7_10 |
||||
| 3 Clifford GM, Tully S, Franceschi S: Carcinogenicity of human papillomavirus (HPV) types in HIV-positive women: a meta-analysis from HPV infection to cervical cancer. Clin Infect Dis 2017;64:1228-1235. https://doi.org/10.1093/cid/cix135 |
||||
| 4 Stanley M: Immune responses to human papillomavirus. Vaccine 2006;24:S16-S22. https://doi.org/10.1016/j.vaccine.2005.09.002 |
||||
| 5 Palefsky JM: Human papillomavirus infections: Epidemiology and disease associations, 2020. URL: https://www.uptodate.com/contents/human-papillomavirus-infections-epidemiology-and-disease-associations?search=human-papillomavirus-hpv&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 | ||||
| 6 Tyring SK: Human papillomavirus infections: epidemiology, pathogenesis, and host immune response. J Am Acad Dermatol 2000;43:18-26. https://doi.org/10.1067/mjd.2000.107807 |
||||
| 7 Graziottin A, Serafini A: HPV Infection in women: psychosexual impact of genital warts and intraepithelial lesions. J Sex Med 2009;6:633-645. https://doi.org/10.1111/j.1743-6109.2008.01151.x |
||||
| 8 De Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H: Classification of papillomaviruses. Virology 2004;324:17-27. https://doi.org/10.1016/j.virol.2004.03.033 |
||||
| 9 de Sanjosé S, Brotons M, Pavón MA: The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol 2018;47:2-13. https://doi.org/10.1016/j.bpobgyn.2017.08.015 |
||||
| 10 Duan R, Qiao Y, Clifford G, Zhao F: Cancer burden attributable to human papillomavirus infection by sex, cancer site, age, and geographical area in China. Cancer Med 2020;9:374-384. https://doi.org/10.1002/cam4.2697 |
||||
| 11 Tjiong MY, Out TA, Ter Schegget J, Burger MP, Van Der Vange N: Epidemiologic and mucosal immunologic aspects of HPV infection and HPV-related cervical neoplasia in the lower female genital tract: a review. Int J Gynecol Cancer 2001;11:9-17. https://doi.org/10.1046/j.1525-1438.2001.011001009.x |
||||
| 12 Howie HL, Katzenellenbogen RA, Galloway DA: Papillomavirus E6 proteins. Virology 2009 20;384:324-334. https://doi.org/10.1016/j.virol.2008.11.017 |
||||
| 13 Wu MH, Chan JY, Liu PY, Liu ST, Huang SM: Human papillomavirus E2 protein associates with nuclear receptors to stimulate nuclear receptor- and E2-dependent transcriptional activations in human cervical carcinoma cells. Int J Biochem Cell Biol 2007;39:413-425. https://doi.org/10.1016/j.biocel.2006.09.008 |
||||
| 14 Xu F, Cao M, Shi Q, Chen H, Wang Y, Li X: Integration of the full-length HPV16 genome in cervical cancer and Caski and Siha cell lines and the possible ways of HPV integration. Virus Genes 2015;50:210-220. https://doi.org/10.1007/s11262-014-1164-7 |
||||
| 15 Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H: Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 1985;314:111-114. https://doi.org/10.1038/314111a0 |
||||
| 16 Smotkin D, Wettstein FO: Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A 1986;83:4680-4684. https://doi.org/10.1073/pnas.83.13.4680 |
||||
| 17 Smith EM, Pawlita M, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP: Risk factors and survival by HPV-16 E6 and E7 antibody status in human papillomavirus positive head and neck cancer. Int J Cancer 2010;127:111-117. https://doi.org/10.1002/ijc.25015 |
||||
| 18 Smith JA, Haberstroh FS, White EA, Livingston DM, DeCaprio JA, Howley PM: SMCX and components of the TIP60 complex contribute to E2 regulation of the HPV E6/E7 promoter. Virology 2014;468-470:311-321. https://doi.org/10.1016/j.virol.2014.08.022 |
||||
| 19 Reuschenbach M, Huebbers CU, Prigge ES, Bermejo JL, Kalteis MS, Preuss SF, Seuthe IM, Kolligs J, Speel EJ, Olthof N, Kremer B, Wagner S, Klussmann JP, Vinokurova S, von Knebel Doeberitz M: Methylation status of HPV16 E2-binding sites classifies subtypes of HPV-associated oropharyngeal cancers. Cancer 2015;121:1966-1976. https://doi.org/10.1002/cncr.29315 |
||||
| 20 Cheung JL, Cheung TH, Yu MY, Chan PK: Virological characteristics of cervical cancers carrying pure episomal form of HPV16 genome. Gynecol Oncol 2013;131:374-379. https://doi.org/10.1016/j.ygyno.2013.08.026 |
||||
| 21 Anayannis NV, Schlecht NF, Ben-Dayan M, Smith RV, Belbin TJ, Ow TJ, Blakaj DM, Burk RD, Leonard SM, Woodman CB, Parish JL, Prystowsky MB: Association of an intact E2 gene with higher HPV viral load, higher viral oncogene expression, and improved clinical outcome in HPV16 positive head and neck squamous cell carcinoma. PLoS One 2018;13:e0191581. https://doi.org/10.1371/journal.pone.0191581 |
||||
| 22 McBride AA: Mechanisms and strategies of papillomavirus replication. Biol Chem 2017;398:919-927. https://doi.org/10.1515/hsz-2017-0113 |
||||
| 23 Pinidis P, Tsikouras P, Iatrakis G, Zervoudis S, Koukouli Z, Bothou A, Galazios G, Vladareanu S: Human papilloma virus' life cycle and carcinogenesis. Maedica (Bucur) 2016;11:48-54. | ||||
| 24 Lutgendorf SK, Costanzo ES: Psychoneuroimmunology and health psychology: an integrative model. Brain Behav Immun 2003;17:225-232. https://doi.org/10.1016/S0889-1591(03)00033-3 |
||||
| 25 Cvitanović H, Milošević M, Bukvić-Bešlić I, Lugović-Mihić L: Determination of psychological stress, serum immune parameters, and cortisol levels in patients with human papilloma virus. Clin Ther 2020;42:783-799. https://doi.org/10.1016/j.clinthera.2020.03.017 |
||||
| 26. heoharides TC, Stewart JM, Taracanova A, Conti P, Zouboulis CC: Neuroendocrinology of the skin. Rev Endocr Metab Disord 2016;17:287-294. https://doi.org/10.1007/s11154-016-9369-9 |
||||
| 27 Pondeljak N, Lugović-Mihić L: Stress-induced interaction of skin immune cells, hormones, and neurotransmitters. Clin Ther 2020;42:757-770. https://doi.org/10.1016/j.clinthera.2020.03.008 |
||||
| 28 Lugović-Mihić L, Ljubesić L, Mihić J, Vuković-Cvetković V, Troskot N, Šitum M: Psychoneuroimmunologic aspects of skin diseases. Acta Clin Croat 2013;52:337-342. | ||||
| 29 Buckner SL, Mouser JG, Dankel SJ, Jessee MB, Mattocks KT, Loenneke JP: The General Adaptation Syndrome: Potential misapplications to resistance exercise. J Sci Med Sport 2017;20:1015-1017. https://doi.org/10.1016/j.jsams.2017.02.012 |
||||
| 30 Tsigos C, Kyrou I, Kassi E, Chrousos GP: Stress: Endocrine Physiology and pathophysiology. 2020; In: Feingold KR, Anawalt B, Boyce A, et al. (eds): Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc., 2000. | ||||
| 31 Iftikhar A, Islam M, Shepherd S, Jones S, Ellis I: Cancer and stress: does it make a difference to the patient when these two challenges collide? Cancers (Basel) 2021;13:163. https://doi.org/10.3390/cancers13020163 |
||||
| 32 McEwen BS: Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007;87:873-904. https://doi.org/10.1152/physrev.00041.2006 |
||||
| 33 Caraffa A, Spinas E, Kritas SK, Lessiani G, Ronconi G, Saggini A., Antinolfi P, Pizzicannella J, Toniato E, Theoharides TC, Conti P: Endocrinology of the skin: intradermal neuroimmune network, a new frontier. J Biol Regul Homeost Agents 2016;30:339-343. | ||||
| 34 Fischer A, Ziogas A, Anton-Culver H: Perception matters: Stressful life events increase breast cancer risk. J Psychosom Res 2018;110:46-53. https://doi.org/10.1016/j.jpsychores.2018.03.010 |
||||
| 35 Peters EM: Stressed skin?--a molecular psychosomatic update on stress-causes and effects in dermatologic diseases. J Dtsch Dermatol Ges 2016;14:233-252. https://doi.org/10.1111/ddg.12957 |
||||
| 36 Hunter HJ, Momen SE, Kleyn CE: The impact of psychosocial stress on healthy skin. Clin Exp Dermatol 2015;40:540-546. https://doi.org/10.1111/ced.12582 |
||||
| 37 Van Bodegom M, Homberg JR, Henckens MJAG: Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci 2017;11:87. https://doi.org/10.3389/fncel.2017.00087 |
||||
| 38 Dhabhar FS, McEwen B: Chapter 34 - Bi-directional effects of stress on immune function: possible explanations for salubrious as well as harmful effects. In: Ader R (ed): Psychoneuroimmunology (Fourth Edition), Volume II. Oxford, UK, Elsevier Academic Press, 2007, pp 723-760. https://doi.org/10.1016/B978-012088576-3/50041-1 |
||||
| 39 Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB: Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA 2012;109:5995-5999. https://doi.org/10.1073/pnas.1118355109 |
||||
| 40 Stone AA, Bovbjerg DH, Neale JM, Napoli A, Valdimarsdottir H: Development of common cold symptoms following experimental rhinovirus infection is related to prior stressful life events. Behav Med 1992;18:115-120. https://doi.org/10.1080/08964289.1992.9936961 |
||||
| 41 Koh D, Yong Y, Ng V, Chia SE: Stress, mucosal immunity, upper respiratory tract infections, and sickness absence. J Occup Environ Med 2002;44:987-988. https://doi.org/10.1097/00043764-200211000-00002 |
||||
| 42 Fang CY, Miller SM, Bovbjerg DH, Bergman C, Edelson MI, Rosenblum NG, Bove BA, Godwin AK, Campbell DE, Douglas SD: Perceived stress is associated with impaired T-cell response to HPV16 in women with cervical dysplasia. Ann Behav Med 2008;35:87-96. https://doi.org/10.1007/s12160-007-9007-6 |
||||
| 43 Lu D: The role of psychological stress in cervical and prostate carcinogenesis [PhD Thesis]. Stockholm, Sweden, Department of Medical Epidemiology and Biostatistics Karolinska Institutet, 2017. | ||||
| 44 Burd EM: Human Papillomavirus and cervical cancer. Clin Microbiol Rev 2003;16:1-17. https://doi.org/10.1128/CMR.16.1.1-17.2003 |
||||
| 45 Lu D, Sundström K, Sparén P, Fall K, Sjölander A, Dillner J, Helm NY, Adami HO, Valdimarsdóttir U, Fang F: Bereavement is associated with an increased risk of HPV Infection and cervical cancer: an epidemiological study in Sweden. Cancer Res 2016;76:643-651. https://doi.org/10.1158/0008-5472.CAN-15-1788 |
||||
| 46 Vonsky M, Shabaeva M, Runov A, Lebedeva N, Chowdhury S, Palefsky JM, Isaguliants M: Carcinogenesis associated with Human Papillomavirus infection. Mechanisms and potential for immunotherapy. Biochemistry (Mosc) 2019;84:782-799. https://doi.org/10.1134/S0006297919070095 |
||||
| 47 Ziemssen T, Kern S: Psychoneuroimmunology--cross-talk between the immune and nervous systems. J Neurol 2007;254:8-11. https://doi.org/10.1007/s00415-007-2003-8 |
||||
| 48 Dhabhar FS, Malarkey WB, Neri E, McEwen BS: Stress-induced redistribution of immune cells--from barracks to boulevards to battlefields: a tale of three hormones. Psychoneuroendocrinology 2012;37:1345-1368. https://doi.org/10.1016/j.psyneuen.2012.05.008 |
||||
| 49 Stefanski V: Social stress affects migration of blood T cells into lymphoid organs. J Neuroimmunology 2003;138:17-24. https://doi.org/10.1016/S0165-5728(03)00076-6 |
||||
| 50 Goebel MU, Mills PJ: Acute psychological stress and exercise and changes in peripheral leukocyte adhesion molecule expression and density. Psychosom Med 2000;62:664-670. https://doi.org/10.1097/00006842-200009000-00010 |
||||
| 51 Paik IH, Toh KY, Lee C, Kim JJ, Lee SJ: Psychological stress may induce increased humoral and decreased cellular immunity. Behav Med 2000;26:139-141. https://doi.org/10.1080/08964280009595761 |
||||
| 52 Ohta M, Ohmori T, Kawai K, Teshima-Kondo S, Rokutan K: Expression analysis of psychological stress-associated genes in peripheral blood leukociti. Neurosci Lett 2005;381:57-62. https://doi.org/10.1016/j.neulet.2005.01.081 |
||||
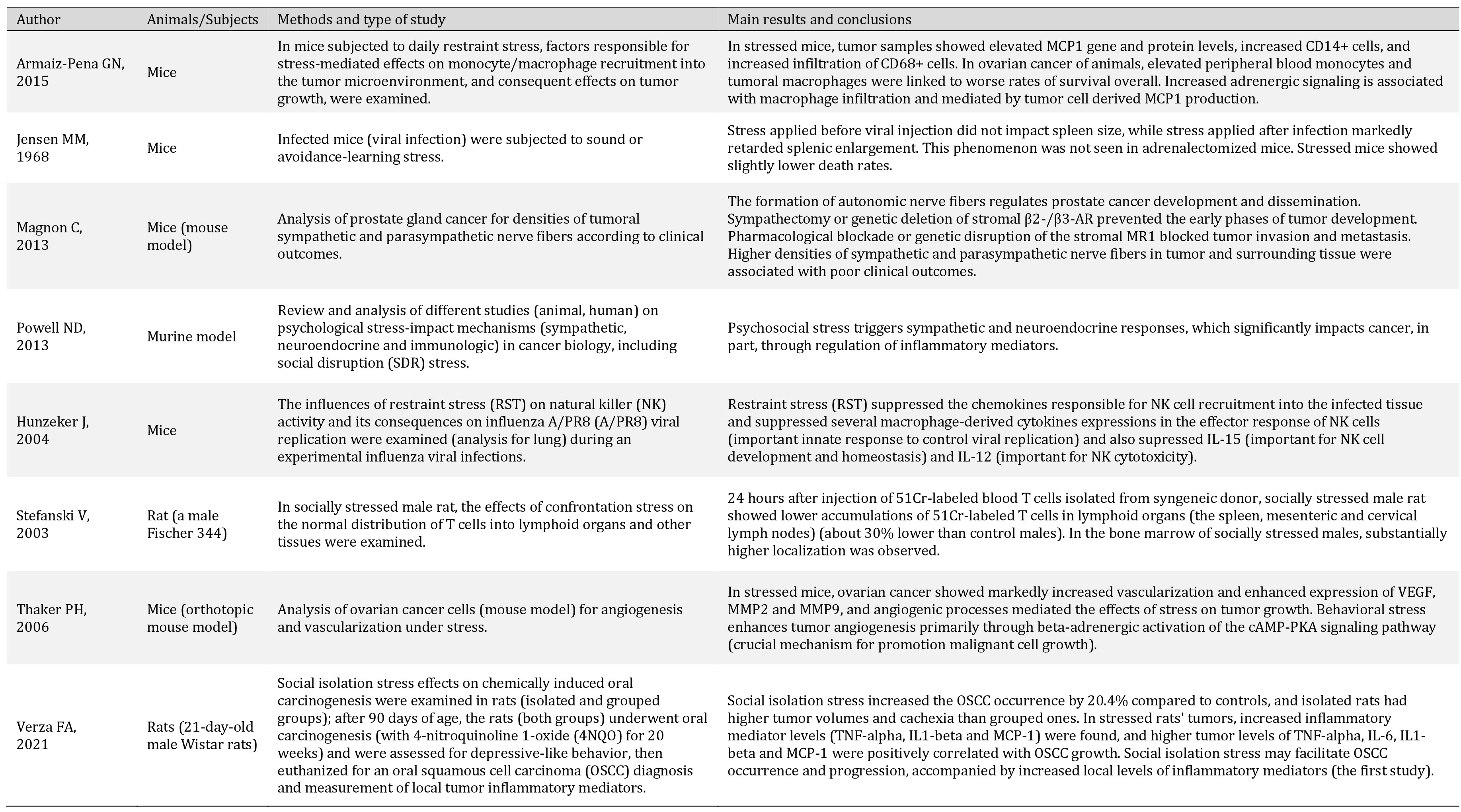
| 53 Armaiz-Pena GN, Gonzalez-Villasana V, Nagaraja AS, Rodriguez-Aguayo C, Sadaoui NC, Stone RL, Matsuo K, Dalton HJ, Previs RA, Jennings NB, Dorniak P, Hansen JM, Arevalo JM, Cole SW, Lutgendorf SK, Sood AK, Lopez-Berestein G: Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget 2015;6:4266-73. https://doi.org/10.18632/oncotarget.2887 |
||||
| 54 Jensen MM: The influence of stress on murine leukemia virus infection. Proc Soc Exp Biol Med 1968;127:610-614. https://doi.org/10.3181/00379727-127-32754 |
||||
| 55 Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS: Autonomic nerve development contributes to prostate cancer progression. Science 2013;341:1236361. https://doi.org/10.1126/science.1236361 |
||||
| 56 Powell ND, Tarr AJ, Sheridan JF: Psychosocial stress and inflammation in cancer. Brain Behav Immun 2013;30:S41-47. https://doi.org/10.1016/j.bbi.2012.06.015 |
||||
| 57 Hunzeker J, Padgett DA, Sheridan PA, Dhabhar FS, Sheridan JF: Modulation of natural killer cell activity by restraint stress during an influenza A/PR8 infection in mice. Brain Behav Immun 2004;18:526-535. https://doi.org/10.1016/j.bbi.2003.12.010 |
||||
| 58 Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, et al.: Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006;12:939-944. https://doi.org/10.1038/nm1447 |
||||
| 59 Verza FA, Valente VB, Oliveira LK, Kayahara GM, Crivelini MM, Furuse C, Biasoli ÉR, Miyahara GI, Oliveira SHP, Bernabé DG: Social isolation stress facilitates chemically induced oral carcinogenesis. PLoS One 2021;16:e0245190. https://doi.org/10.1371/journal.pone.0245190 |
||||
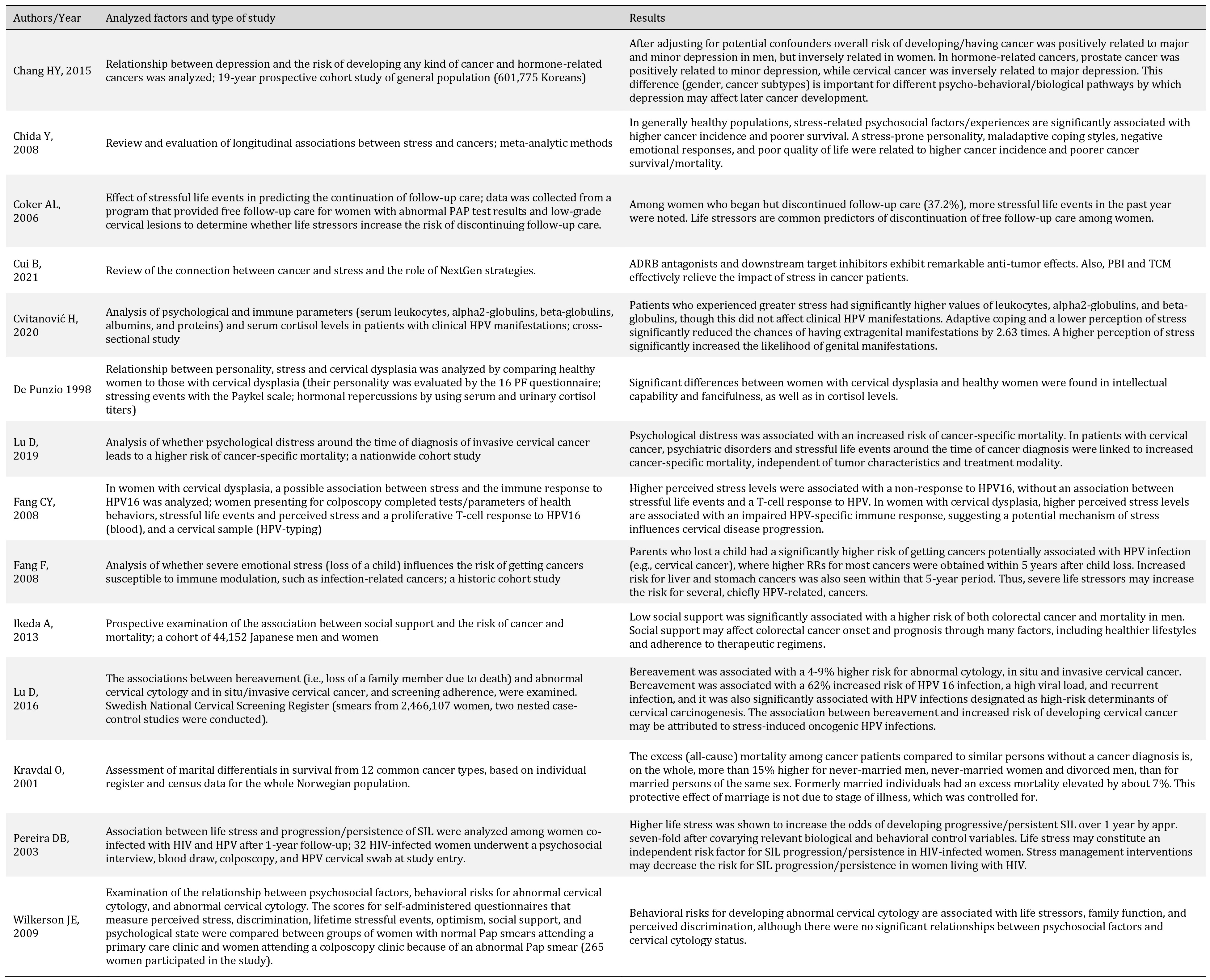
| 60 Chang HY, Keyes KM, Mok Y, Jung KJ, Shin YJ, Jee SH: Depression as a risk factor for overall and hormone-related cancer: the Korean cancer prevention study. J Affect Disord 2015;173:1-8. https://doi.org/10.1016/j.jad.2014.10.064 |
||||
| 61 Chida Y, Hamer M, Wardle J, Steptoe A: Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol 2008;5:466-475. https://doi.org/10.1038/ncponc1134 |
||||
| 62 Coker AL, Bond SM, Pirisi LA: Life stressors are an important reason for women discontinuing follow-up care for cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2006;15:321-329. https://doi.org/10.1158/1055-9965.EPI-05-0148 |
||||
| 63 Cui B, Peng F, Lu J, He B, Su Q, Luo H, Deng Z, Jiang T, Su K, Huang Y, Ud Din Z, Lam EW, Kelley KW, Liu Q: Cancer and stress: NextGen strategies. Brain Behav Immun 2021;93:368-383. https://doi.org/10.1016/j.bbi.2020.11.005 |
||||
| 64 DePunzio C, Salvestroni C, Guazzelli G, Papa MC, Freschi G, Ferdeghini M, Masoni S: Stress and cervical dysplasia. Eur J Gynaecol Oncol 1998;19:287-290. https://doi.org/10.1093/carcin/19.2.287 |
||||
| 65 Lu D, Andrae B, Valdimarsdóttir U, Sundström K, Fall K, Sparén P, Fang F: Psychological distress is associated with cancer-specific mortality among patients with cervical cancer. Cancer Res 2019;28:canres.0116.2019. | ||||
| 66 Fang F, Fall K, Sparén P, Adami HO, Valdimarsdóttir HB, Lambe M, Valdimarsdóttir U: Risk of infection-related cancers after the loss of a child: a follow-up study in Sweden. Cancer Res 2011;71:116-122. https://doi.org/10.1158/0008-5472.CAN-10-0470 |
||||
| 67 Ikeda A, Kawachi I, Iso H, Iwasaki M, Inoue M, Tsugane S: Social support and cancer incidence and mortality: the JPHC study cohort II. Cancer Causes Control 2013;24:847-860. https://doi.org/10.1007/s10552-013-0147-7 |
||||
| 68 Kravdal O: The impact of marital status on cancer survival. Soc Sci Med. 2001;52:357-368. https://doi.org/10.1016/S0277-9536(00)00139-8 |
||||
| 69 Pereira DB, Antoni MH, Danielson A, Simon T, Efantis-Potter J, Carver CS, Durán RE, Ironson G, Klimas N, O'Sullivan MJ: Life stress and cervical squamous intraepithelial lesions in women with human papillomavirus and human immunodeficiency virus. Psychosom Med 2003;65:427-434. https://doi.org/10.1097/01.PSY.0000041620.37866.89 |
||||
| 70 Wilkerson JE, Bailey JM, Bieniasz ME, Murray SI, Ruffin MT: Psychosocial factors in risk of cervical intraepithelial lesions. Int J Women Health 2009;18:513-518. https://doi.org/10.1089/jwh.2008.0982 |
||||
| 71 Dhabhar FS, Saul AN, Holmes TH, Daugherty C, Neri E, Tillie JM, Kusewitt D, Oberyszyn TM: High anxiety is associated with higher chronic stress burden, lower protective immunity, and increased cancer progression. PLoS One 2012;7:e33069 92. https://doi.org/10.1371/journal.pone.0033069 |
||||
| 72 Kołacz R, Grudnik T, Stefaniak T, Bodak E: Haptoglobin in the blood serum of pigs exposed to transportation stress. ISAH, Mexico 2003. URL: http://www.isahsoc.org/userfiles/downloads/proceedings/2003/speakers/S25KolaczPoland.pdf | ||||
| 73 Van Hunsel F, Van Gastel A, Neels H, Wauters A, Demedts P, Bruyland K, DeMeester I, Scharpé S, Janca A, Song C, Maes M: The influence of psychological stress on total serum protein and patterns obtained in serum protein electrophoresis. Psychol Med 1998;28:301-309. https://doi.org/10.1017/S0033291797006351 |
||||
| 74 Cole SW, Mendoza SP, Capitanio JP: Social stress desensitizes lymphocytes to regulation by endogenous glucocorticoids: Insights from in vivo cell trafficking dynamics in rhesus macaques. Psychosom Med 2009;71:591-597. https://doi.org/10.1097/PSY.0b013e3181aa95a9 |
||||
| 75 Irwin M, Daniels M, Smith TL, Bloom E, Weiner H: Impaired natural killer activity during bereavement. Brain Behav Immun 1987;1:98-104. https://doi.org/10.1016/0889-1591(87)90011-0 |
||||
| 76 Sharp L, Cotton S, Carsin AE, Gray N, Thornton A, Cruickshank M, Little J, TOMBOLA Group: Factors associated with psychological distress following colposcopy among women with low-grade abnormal cervical cytology: a prospective study within the Trial of management of borderline and other low-grade abnormal smears (TOMBOLA). Psychooncology 2013;22:368-380. | ||||
| 77 Bond E, Lu D, Herweijer E, Sundström K, Valdimarsdóttir U, Fall K, Arnheim-Dahlström L, Sparén P, Fang F: Sexually transmitted infections after bereavement - a population-based cohort study. BMC Infect Dis 2016;16:419. https://doi.org/10.1186/s12879-016-1705-x |
||||
| 78 Kola S, Walsh JC, Hughes BM, Howard S: Matching intra-procedural information with coping style reduces psychophysiological arousal in women undergoing colposcopy. J Behav Med 2013;36:401-412. https://doi.org/10.1007/s10865-012-9435-z |
||||
| 79 Massad LS, Agniel D, Minkoff H, Watts DH, D'Souza G, Levine AM: Effect of stress and depression on the frequency of squamous intraepithelial lesions. J Low Genit Tract Dis 2011;15:42-47. https://doi.org/10.1097/LGT.0b013e3181e66a82 |
||||
| 80 Antoni MH, Pereira DB, Marion I, Ennis N, Andrasik MP, Rose R, McCalla J, Simon T, Fletcher MA, Lucci J, Efantis-Potter J, O'Sullivan MJ: Stress management effects on perceived stress and cervical neoplasia in low-income HIV-infected women. J Psychosom Res 2008;65:389-401. https://doi.org/10.1016/j.jpsychores.2008.06.002 |
||||
| 81 Giuliano AR, Nyitray AG, Kreimer AR, Pierce Campbell CM, Goodman MT, Sudenga SL, Monsonego J, Franceschi S: EUROGIN 2014 roadmap: differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer 2015;136:2752-2760. https://doi.org/10.1002/ijc.29082 |
||||
| 82 McCaffery K, Waller J, Forrest S, Cadman L, Szarewski A, Wardle J: Testing positive for human papillomavirus in routine cervical screening: examination of psychosocial impact. BJOG 2004;111:1437-1443. https://doi.org/10.1111/j.1471-0528.2004.00279.x |
||||
| 83 Tomás-Aragonés L, Castillo-Amores AB, Rodríguez-Cerdeira C, Marrón-Moya SE: Psychological aspects associated with the acquisition and development of HPV infection and its repercussion on quality of life. Open Dermatol J 2009;3:133-136. https://doi.org/10.2174/1874372200903020133 |
||||
| 84 Jensen SE, Pereira DB, Whitehead N, Buscher I, McCalla J, Andrasik M, Rose R, Antoni MH: Cognitive-behavioral stress management and psychological well-being in HIV+ racial/ethnic minority women with human papillomavirus. Health Psychol 2013;32:227-230. https://doi.org/10.1037/a0028160 |
||||
| 85 Jentschke M, Lehmann R, Drews N, Hansel A, Schmitz M, Hillemanns P: Psychological distress in cervical cancer screening: results from a German online survey. Arch Gynecol Obstet 2020;302:699-705. https://doi.org/10.1007/s00404-020-05661-9 |
||||