Corresponding Author: Gabi U. Dachs
Mackenzie Cancer Research Group, Department of Pathology and Biomedical Science, University of Otago,
Christchurch, 2 Riccarton Ave, Christchurch 8140, New Zealand
Tel. +64-3-3640544 , E-Mail gabi.dachs@otago.ac.nz
Limited Association Between Ascorbate Concentrations and Vitamin C Transporters in Renal Cell Carcinoma Cells and Clinical Samples
Christina Wohlraba,b
Margreet C.M. Vissersc
Eleanor R. Burgessa
Maria Nonisa
Elisabeth Phillipsa
Bridget A. Robinsona,d
Gabi U. Dachsa
aMackenzie Cancer Research Group, Department of Pathology and Biomedical Science, University of Otago, Christchurch, New Zealand, bFianostics GmbH, Technologie- und Forschungszentrum, Wiener Neustadt, Austria, cCentre for Free Radical Research, Department of Pathology and Biomedical Science, University of Otago, Christchurch, New Zealand, dCanterbury Regional Cancer and Hematology Service, Canterbury District Health Board, Christchurch, New Zealand
Introduction
Maintenance of whole-body ascorbate levels and distribution to different compartments is mediated primarily via sodium-dependent vitamin C transporters (SVCTs) [1]. SVCTs are members of the solute carrier gene family 23 (SLC23) [2], with three isoforms identified thus far; SVCT1 and SVCT2 that transport ascorbate, and the orphan transporter SVCT3 with still unknown function [3]. SVCT1 and SVCT2 are each comprised of 12 transmembrane domains and exert the cotransport of sodium and ascorbate in a ratio of 2:1 down an electrochemical sodium gradient which is maintained by K/Na+ exchange mechanisms [4]. SVCT2 additionally relies on Ca2+ and Mg2+ for its activity [4]. Expression of the different SVCT transport proteins is tissue and cell type-specific and is controlled by transcriptional regulation of SLC23 genes [3, 5] and post-translational regulation [6]. The exact control mechanisms are still not fully understood.
SVCT1 (encoded by SLC23A1) is expressed in the epithelial tissue of kidney, intestine, liver, lung and skin. SVCT1 is described as a low affinity, high capacity transporter with a Km in the range of 65 –237 μM and Vmax around 15 pmol/min/cell, which makes it capable of efficient uptake of ascorbate from the diet [7]. In comparison, SVCT2, encoded by SLC23A2, is expressed in almost every tissue and cell in the body and mediates whole body tissue uptake [5]. SVCT2 is characterised as a low capacity, high affinity transporter with a Vmax ~1 pmol/min/cell and Km of 8–69 µM, that is suited to the maintenance of tissue homeostasis [4, 8].
The kidney plays a major role in the maintenance of whole-body ascorbate levels, with kidney epithelial cells expressing both SVCT isoforms [9]. In the renal cortex, SVCT1 is situated in the brush-border membrane of the proximal tubule where it mediates re-uptake of ascorbate from the glomerular filtrate [8]. Expression increases towards the distal regions of the tubules, and is hypothesised to be regulated by a decreasing ascorbate gradient along the tubular system [10]. SVCT2 is expressed in all cells of the kidney, including the proximal tubular epithelial cells, although at lower levels than SVCT1, and is located intracellularly [9]. Reports of SVCTs in cancer are sparse; only two studies have previously measured ascorbate transporter levels in human tumour tissue [11, 12], and SVCTs have never been investigated in renal cell carcinoma (RCC) tumours.
Worldwide, each year over 270,000 individuals are diagnosed with RCC, which is curable at an early stage but has limited treatment options when diagnosed at advanced stage, resulting in a 5-year survival of <10% [13, 14]. Several histological types for RCC are defined, including clear cell RCC (ccRCC), which is the most aggressive and most frequent (~70%) type, and papillary RCC (pRCC) which is less common (~15%) [15].
Recent evidence suggests that accumulation of ascorbate may differ in tumour compared to normal tissue. We have shown that both ccRCCs and pRCCs contained higher ascorbate levels than normal cortex tissue [16], unlike other cancers that showed the reverse [12, 17, 18]. In the analysis of tissue from patients with colorectal, endometrial or breast cancer, no association between ascorbate levels measured in tumour and matched normal tissue was apparent [12, 16-18]. Protein levels of SVCT1 was measured in human colon adenocarcinoma samples with similar levels to normal colon mucosa [11]. SVCT1 and SVCT2 together with ascorbate were measured in clinical breast tumour tissue, showing no clear association between transporter levels and ascorbate content [12]. No other human studies on SVCTs together with ascorbate in tumour tissue have been reported.
In this study we aimed to investigate the role of the two SVCTs in ascorbate uptake in RCC. SVCT protein levels and cellular location in response to ascorbate supplementation and withdrawal were determined in human ccRCC cell lines. SVCT patterns of staining and protein levels were also analysed in clinical samples of renal cancer and associated normal renal cortex, and compared to measured tissue ascorbate levels.
Materials and Methods
Materials
All chemicals were obtained from Sigma-Aldrich (St Louis, USA), unless otherwise specified. Cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Bovine serum and antibiotics were from Life Technologies (Carlsbad, CA, USA). The following primary antibodies were used: anti-human SVCT1 (polyclonal rabbit, Aviva Systems, San Diego, CA, USA, OAAB09000), anti-human SVCT2 (polyclonal rabbit, Atlas Antibodies, Stockholm, Sweden, HPA052825) and anti-β-actin (monoclonal mouse, Sigma-Aldrich, A5316).
Renal cell lines
The human ccRCC cell lines Caki-1 (HTB-46), Caki-2 (HTB-47) and 786-0 were used at early passages in ATCC-recommended growth media (modified McCoy’s 5A for Caki-1 and Caki-2, Dulbecco›s Modified Eagle›s Medium (DMEM) for 786-0 cells) with 10% foetal bovine serum and 1% antibiotic-antimycotic solution (Sigma-Aldrich). All cells were regularly tested for mycoplasma by PCR [19].
Ascorbate uptake and measurement
Cells were grown to near confluence in multi-well plates. As growth media contains little or no ascorbate (McCoy’s contains 5.4 μM, DMEM contains 0 μM), freshly prepared sodium ascorbate was added to a final concentration of 50 or 500 μM. For measurements of intracellular ascorbate, cells were pelleted at a range of time points and processed for high-performance liquid chromatography with electrochemical detection (HPLC-ECD) analysis, as previously described [20]. Briefly, 0.54 M perchloric acid containing diethylenetriamine penta-acetic acid was added to the cell extract, followed by ascorbate measurements using HPLC-ECD (Thermo Fisher Scientific, Waltham, MA, USA). Ascorbate concentration was assessed relative to standards (freshly prepared ascorbate, 1.25 to 40 μM).
Patient samples and ethics
Tissue samples, gifted to the Cancer Society Tissue Bank Christchurch (CSTB), were used with ethical approval from the University of Otago Human Ethics committee (reference code H14/020). This cohort of 73 ccRCC and 41 pRCC samples with matched renal cortex has previously been described [16]. In addition, a separate cohort of formalin-fixed, paraffin embedded sections and microarrays (15 pRCC and 63 ccRCC) were received from the CSTB and analysed by immunohistochemistry.
Tissue preparation
Frozen tissue samples were ground to a fine powder in liquid nitrogen, homogenized with RIPA buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxylate, 0.1% SDS, with complete proteinase inhibitor cocktail, Roche, Basel, Switzerland), and DNA content was measured as an indication of the cellular content, as previously described [16].
Antibody blocking
The blocking peptide for SVCT1 (sc-9924 P) resembles amino acids 1 - 30 and the peptide for SVCT2 (sc-31991 P) corresponds to amino acids 183 - 212 of the protein sequence (Santa Cruz, Dallas, TX, USA). For competition assays, antibodies were incubated with 5 times excess of blocking peptides by weight for 1 hour at room temperature before incubation on the membrane.
Western blotting
For cell lines, lysates equivalent to 20 μg protein, and for tissue, homogenates equivalent to 0.5 μg DNA, were loaded per well. Proteins were separated on 4 – 12% Bis-Tris Plus SDS gels (Life Technologies, Carlsbad, CA, USA) and transferred to membranes, as described before [16]. Membranes were incubated with the following primary antibodies: anti-SVCT1 (1/1000), SVCT2 (1/500) and β-actin (1/10000), with horseradish peroxidase labelled secondary goat anti-rabbit/anti-mouse antibodies (Dako, Glostrup, Denmark, P0448 and P0449). Protein bands were detected using the ECL Prime Western Blotting Detection Reagent (GE Healthcare, Chicago, USA), captured using the Alliance 4.7 imaging system and quantified using ImageJ.
Immunofluorescence
Cells seeded into 8-well chamber slides (Thermo Fisher Scientific, Waltham, MA, USA) were washed and fixed with 4% paraformaldehyde, permeabilised with 0.1% Triton X-100 and blocked with 1% bovine serum albumin. Transporters were detected using anti-SVCT2 at 1/500 and secondary fluorescent antibody (Donkey anti-rabbit IgG Alexa Fluor 598, 1/1000, Abcam, ab 150076). Cells were co-stained with CytoPainter Phalloiden-iFluor 488 (1/1000, Abcam, ab176753) and 4’,6-diamidino-2-phenylindole (DAPI, Thermo Fisher Scientific). Slides were covered with Vectashield Antifade Mounting Medium (Vector Laboratories, Burlingame, CA, USA) and fluorescence assessed with an Axio Imager 2 using the ApoTome (Zeiss, Oberkochen, Germany).
Immunohistochemistry
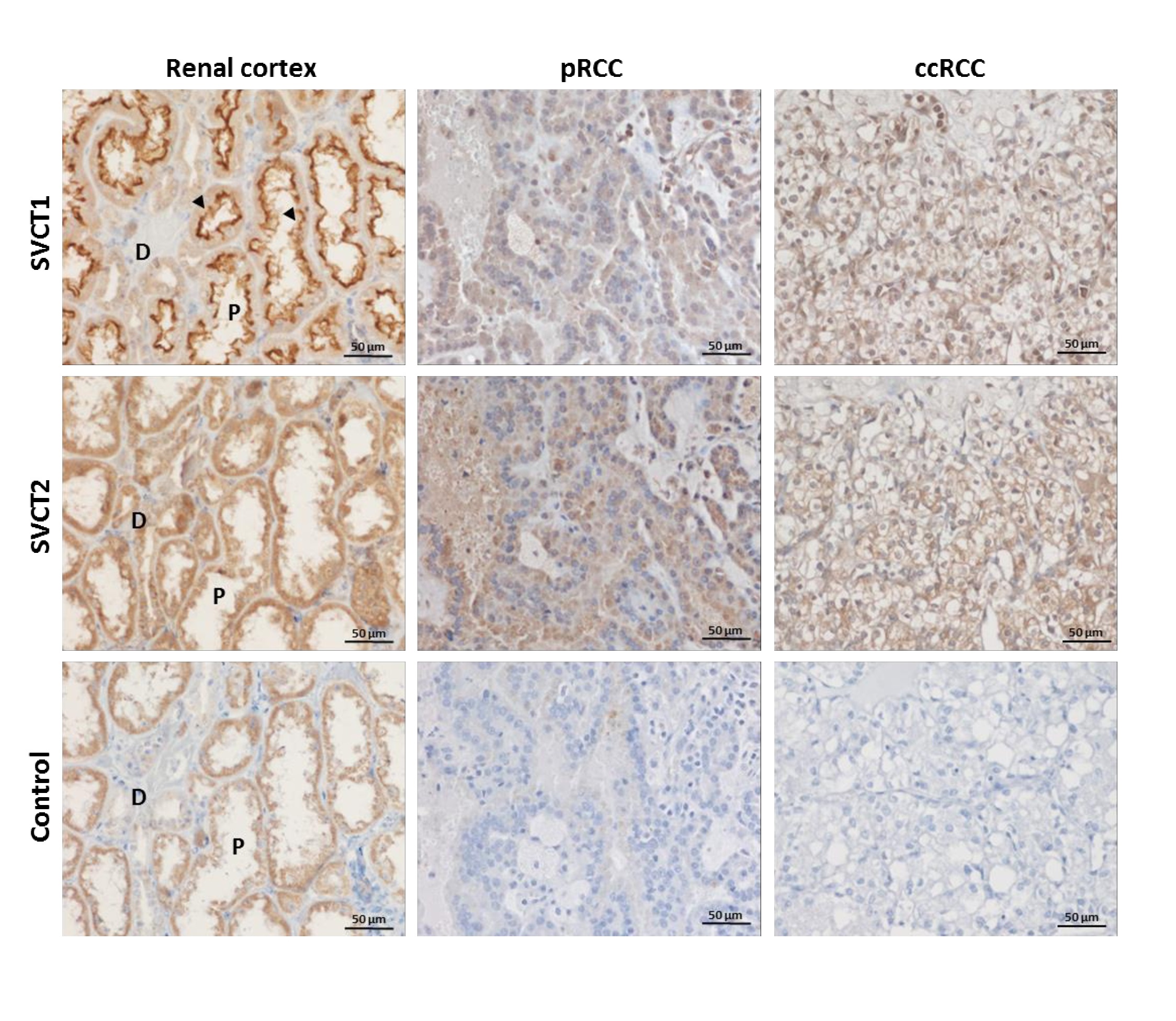
Sections cut at 3 μm were baked at 60°C, deparaffinised and pressure heated for antigen retrieval in Tris-EDTA buffer with 0.05% Tween 20. Cell and Tissue Staining kits (R&D Systems, Minneapolis, MN, USA) were used following manufacturer’s recommendations to stain for SVCT1 (1/200) and SVCT2 (1/200); negative controls lacked primary antibodies.
Statistical analyses
Data were analysed using GraphPad Prism 5, using the Shapiro-Wilk test for normality. Differences between treatment conditions in cell culture were tested by One-way ANOVA with Dunnett’s Multiple Comparison or Bonferroni post-test. Statistical significance between renal cortex and tumour data was tested with the non-parametric Wilcoxon matched-pairs signed rank test. Values of p < 0.05 were considered significant.
Results
Ascorbate transporters in renal cell carcinoma cell lines
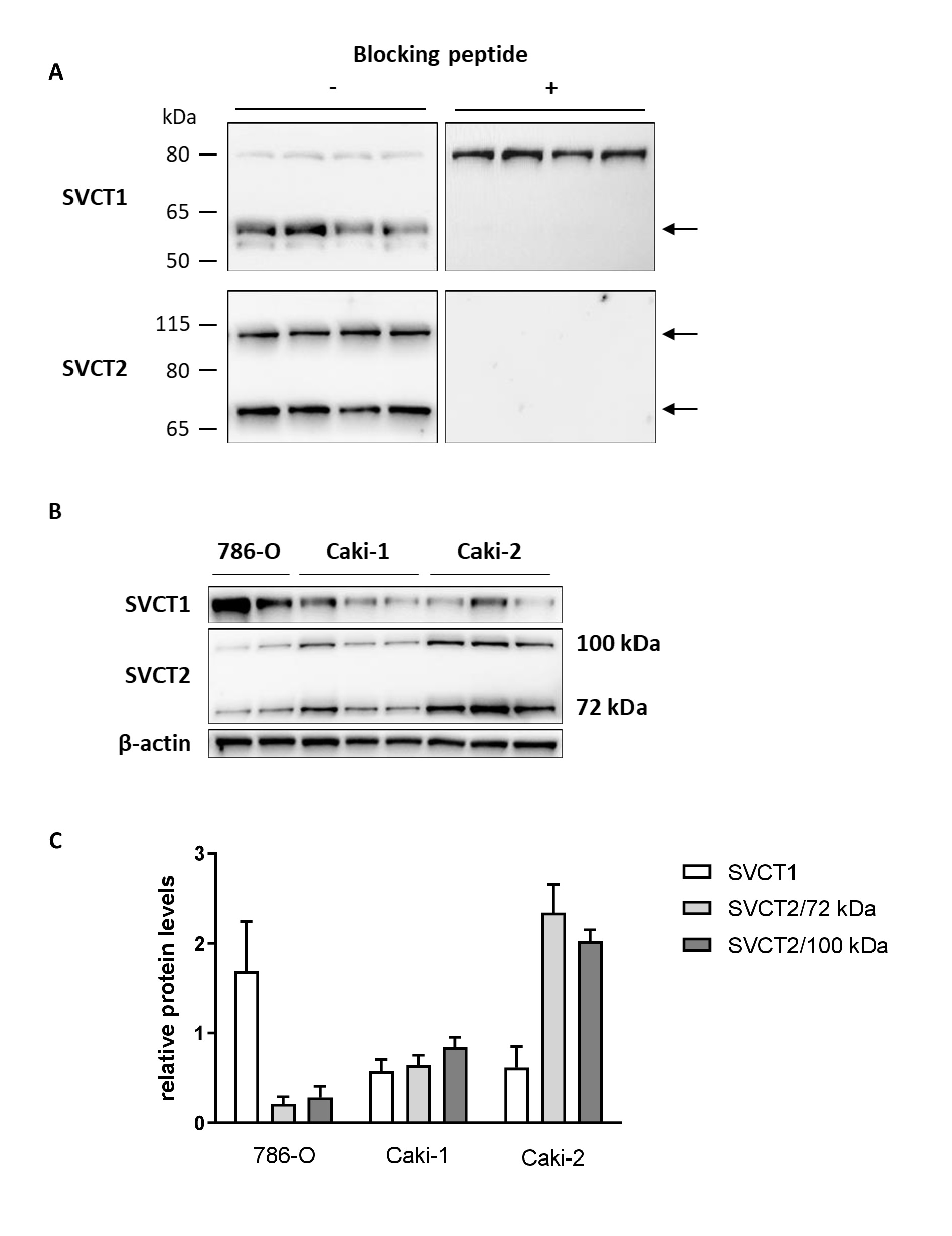
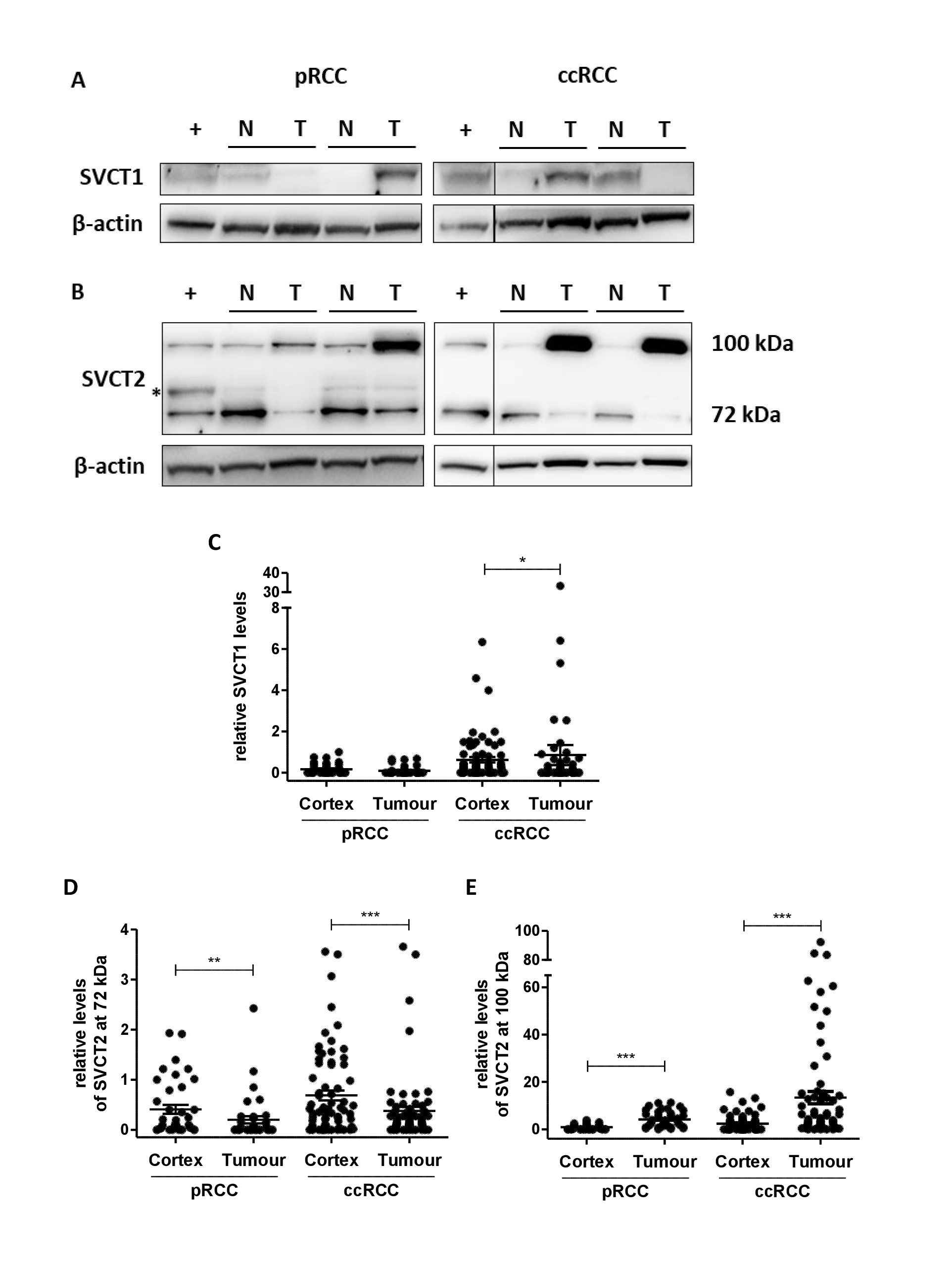
Specificity of the antibodies against SVCT1 and SVCT2 was determined by pre-absorbing antibodies with blocking peptides that prevent binding to the target epitope [21]. For SVCT1, with a predicted molecular weight of 65 kDa, antibody blocking confirmed that the second immunoreactive band at 80 kDa was non-specific (Fig. 1A). SVCT2 was detected at 72 kDa, its predicted molecular weight, but also at ~100 kDa; both bands disappeared in the blocking assay and were therefore considered as specific for SVCT2 (Fig. 1A).
SVCT1 and SVCT2 proteins were confirmed in all three ccRCC cell lines (Fig. 1B). Protein levels of the two transporters varied between cell lines and between individual samples. 786-O cells appeared to have the highest levels of SVCT1 and lowest levels of both immunoreactive forms of SVCT2 compared to the other two cell lines. Of the three cell lines, Caki-2 cells showed strongest immunoreactivity for SVCT2 (Fig. 1C).
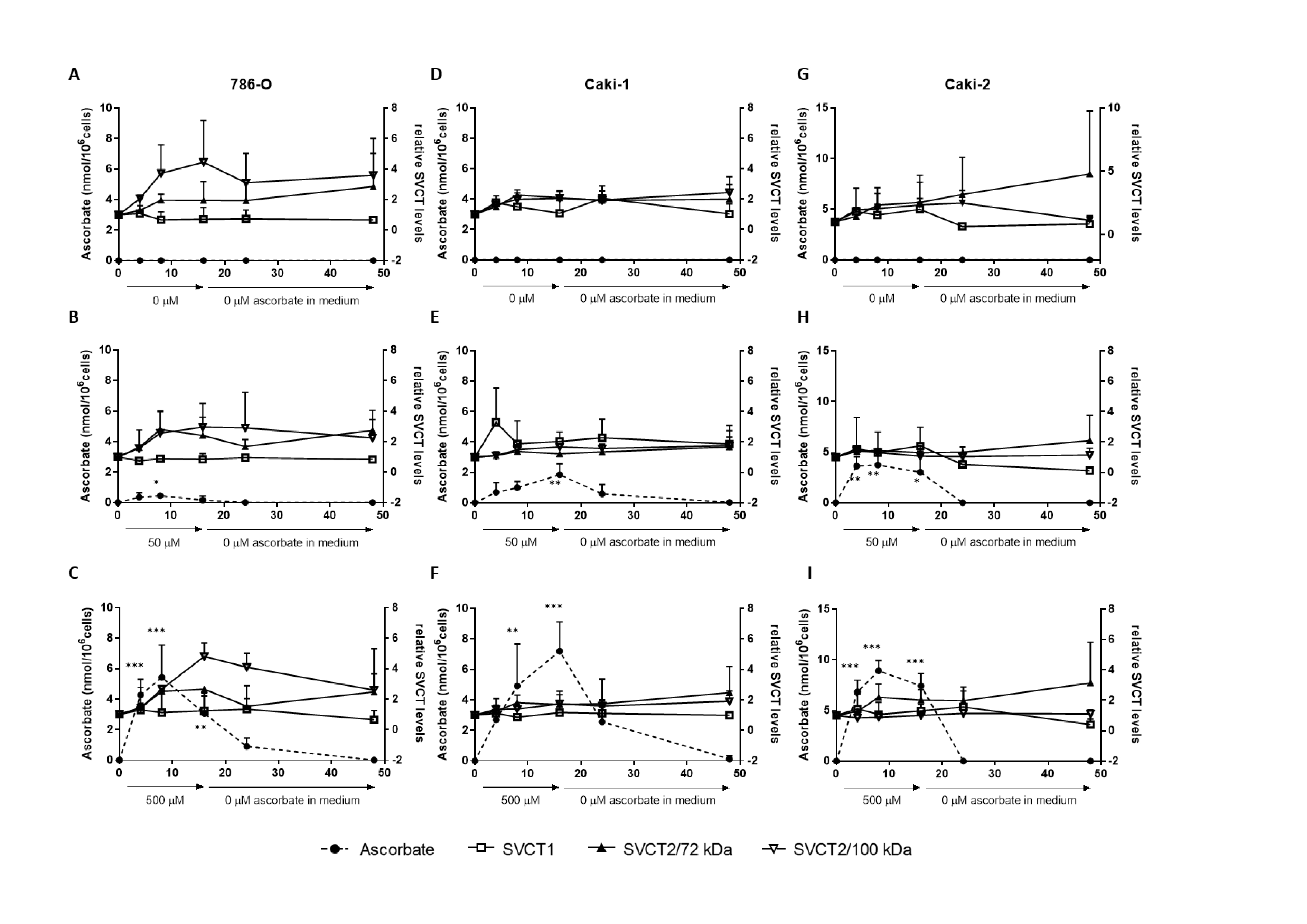
Ascorbate accumulation and loss over time was measured in the ccRCC cells by HPLC-ECD. Our previous data had shown that all three cell lines reached intracellular ascorbate saturation when incubated with 500 μM ascorbate [20]. Therefore, cells were exposed for 16 h to doses of ascorbate that achieve suboptimal (50 μM) or optimal intracellular levels
(500 μM), followed by removal of ascorbate from the culture medium with sampling for up to 24 h. Measurements were compared to cells that did not receive ascorbate (0 μM), which had low/undetectable intracellular ascorbate concentrations, as expected (Fig. 2). Intracellular ascorbate levels in all three cell lines increased significantly over time with exposure to both 50 μM and 500 μM ascorbate, and dropped noticeably once ascorbate was withdrawn (dotted lines in Fig. 2). Supplementation with 50 μM ascorbate resulted in a peak of 0.46 nmol/106 cells at 8 h in 786-0 cells, 1.85 nmol/106 cells at 16 h in Caki-1, and
3.73 nmol/106 cells at 8 h in Caki-2 cells. Higher supplementation (500 μM ascorbate) resulted in 2-11-fold higher intracellular concentrations at the same time points (5.43, 7.20 and 8.92 nmol/106 cells in 786-0, Caki-1 and Caki-2 cells, respectively).
Changes in transporter levels in response to varying ascorbate supply over time was monitored by Western blot (Fig. 2). Levels of SVCT1 and both immunoreactive forms of SVCT2 were variable over time in culture in the presence and absence of ascorbate. Neither transporter was significantly affected by the addition or removal of ascorbate (Fig. 2), with SVCT1 remaining particularly stable. However, in 786-O cells, SVCT2 (100 kDa) levels tended to increase during exposure to 500 μM ascorbate and to reduce during ascorbate withdrawal (p = 0.081; Fig. 2C).
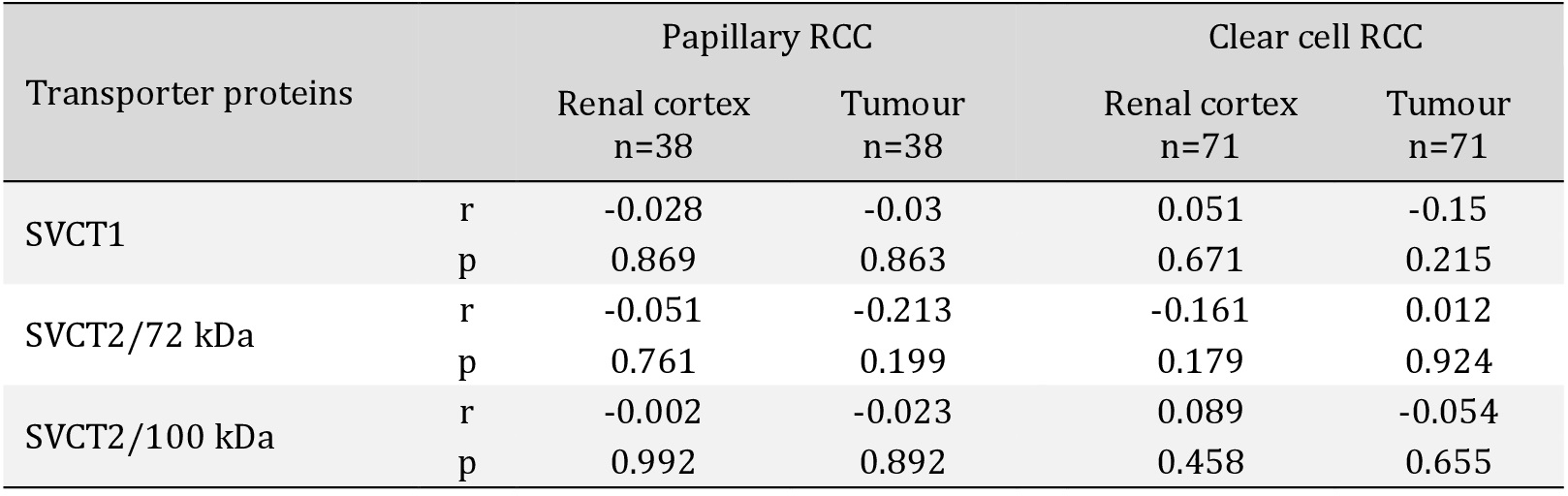
When comparing SVCT protein levels from Fig. 1C and maximal ascorbate uptake in Fig. 2, it is noteworthy that the cell line (Caki-2) with the highest levels of SVCT2 proteins (72 and 100 kDa) also showed the highest maximal ascorbate accumulation following supplementation with 50 μM and 500 μM ascorbate. The cell line with the lowest levels SVCT2 proteins (786-0) also showed the lowest ascorbate accumulation, with Caki-1 showing intermediate protein and uptake characteristics. No such association was seen for SVCT1.
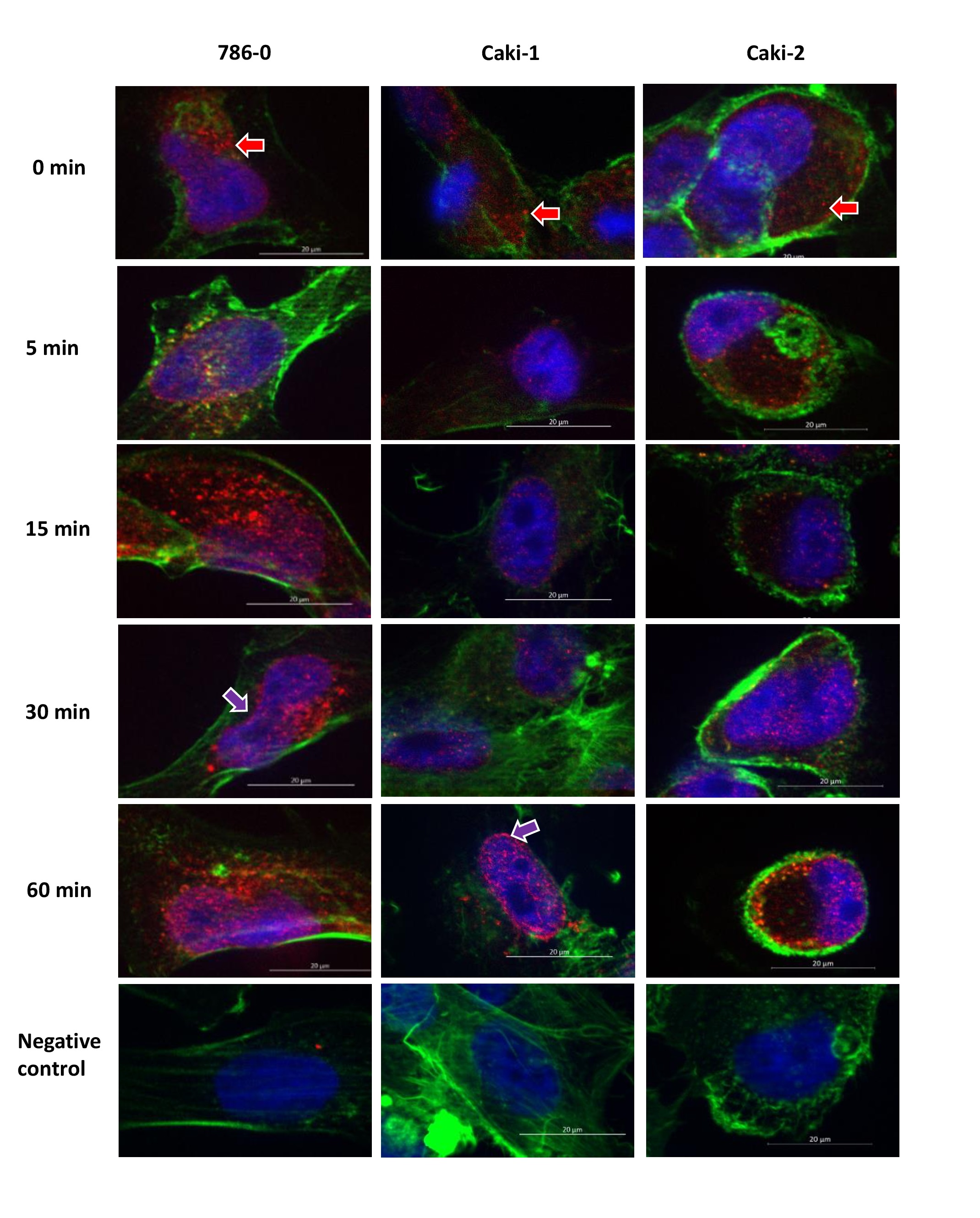
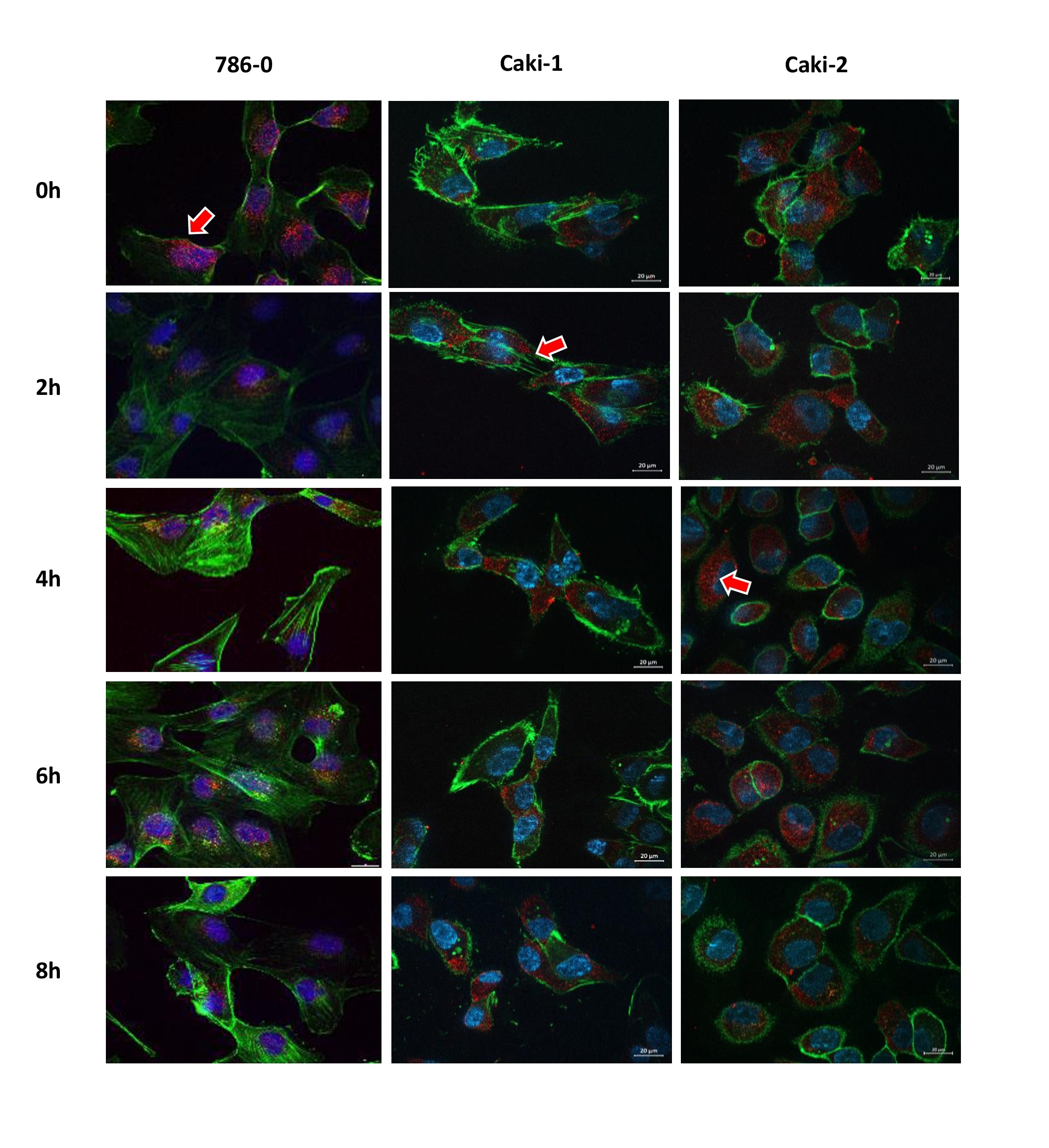
As there were no clear changes in overall protein levels of ascorbate transporters, possible differences in intracellular SVCT2 distribution were investigated. Immunofluorescence staining of SVCT2 was carried out in the three cell lines over a time period of either 5-60 min (short-term, Fig. 3) or 2-8 h (longer-term, Fig. 4) exposure to 500 μM ascorbate. Immunofluorescence showed immunoreactivity of SVCT2 that appeared to be concentrated in cytoplasmic ‘spots’ (Fig. 3, Fig. 4). During early time-points (up to 1 h) there appeared to be translocation to the nucleus or nuclear membrane in some cells (eg Caki-1 at 60 min,
Fig. 3, Fig. 4). However, there was no clear translocation to or from the plasma membrane at any time over 8h, despite the clear increase in intracellular ascorbate accumulation over this time period (Fig. 2 C, F, I). Co-staining of actin filaments with fluorescence labelled Phalloidin was used to evaluate cell shape. There was no apparent co-localisation of SVCT2 with Phalloidin, indicating a relative lack of SVCT2 at the plasma membrane.
Conclusion
In summary, our data indicate that SVCT isoforms and protein modifications may differ between tumour and normal renal tissue. The SVCTs appear to be predominantly located at intracellular sites, and expression levels do not change appreciably in the presence or absence of ascorbate. Hence there is not a simple relationship between tissue ascorbate content and SVCT levels, and our data indicate that SVCT protein levels do not predict intracellular ascorbate accumulation in RCC. Also, ascorbate supply may not modify SVCT protein levels and there may be complex dynamic changes in sub-cellular localisation of the transporter, but any functional impact of such changes in renal cancer cells is unknown.
We thank the Cancer Society Tissue Bank (CSTB) in Christchurch for providing patient samples. CSTB is supported by the Canterbury West Coast Division of the Cancer Society of New Zealand.
Author Contributions
GD conceived the study, CW, EB and MN collected the data, MV and EP helped analyse the data, and CW composed the draft manuscript. MV, BR and GD edited and refined the manuscript. All authors finalized the manuscript. GD, MV and BR obtained funding for the study.
Funding Sources
This study was supported by the Cancer Research Trust NZ (GOT/1644/RPG), the Mackenzie Charitable Foundation (GD and EP), the Vitamin C for Cancer Trust (EB), the University of Otago (PhD Scholarship for CW) and the Centre for Translational Cancer Research, University of Otago (Summer studentship for MN).
Statement of Ethics
Human tissue samples were collected by the Cancer Society Tissue Bank (CSTB) Christchurch and used with ethical approval from the University of Otago Human Ethics committee (reference code H14/020). Use of samples for this study was approved by the CSTB board. All CSTB donors gave informed written consent for the use of their samples for research.
The authors have no conflicts of interest to declare.
| 1 Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA: A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999;399:70-75. https://doi.org/10.1038/19986 |
||||
| 2 Eck P, Erichsen HC, Taylor JG, Yeager M, Hughes AL, Levine M, Chanock S: Comparison of the genomic structure and variation in the two human sodium-dependent vitamin C transporters, SLC23A1 and SLC23A2. Hum Genet 2004;115:285-294. https://doi.org/10.1007/s00439-004-1167-x |
||||
| 3 Bürzle M, Suzuki Y, Ackermann D, Miyazaki H, Maeda N, Clémençon B, Burrier R, Hediger MA: The sodium-dependent ascorbic acid transporter family SLC23. Mol Aspects Med 2013;34:436-454. https://doi.org/10.1016/j.mam.2012.12.002 |
||||
| 4 Godoy A, Ormazabal V, Moraga-Cid G, Zúñiga FA, Sotomayor P, Barra V, Vasquez O, Montecinos V, Mardones L, Guzmán C, Villagrán M, Aguayo LG, Oñate SA, Reyes AM, Cárcamo JG, Rivas CI, Vera JC: Mechanistic insights and functional determinants of the transport cycle of the ascorbic acid transporter SVCT2. Activation by sodium and absolute dependence on bivalent cations. J Biol Chem 2007;282:615-624. https://doi.org/10.1074/jbc.M608300200 |
||||
| 5 Michels AJ, Hagen TM, Frei B: Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annu Rev Nutr 2013;33:45-70. https://doi.org/10.1146/annurev-nutr-071812-161246 |
||||
| 6 Lindblad M, Tveden-Nyborg P, Lykkesfeldt J: Regulation of vitamin C homeostasis during deficiency. Nutrients 2013;5:2860-2879. https://doi.org/10.3390/nu5082860 |
||||
| 7 Savini I, Rossi A, Pierro C, Avigliano L, Catani MV: SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 2008;34:347-355. https://doi.org/10.1007/s00726-007-0555-7 |
||||
| 8 Daruwala R, Song J, Koh WS, Rumsey SC, Levine M: Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett 1999;460:480-484. https://doi.org/10.1016/S0014-5793(99)01393-9 |
||||
| 9 Nualart F, Castro T, Low M, Henríquez JP, Oyarce K, Cisternas P, García A, Yáñez AJ, Bertinat R, Montecinos VP, García-Robles MA: Dynamic expression of the sodium-vitamin C co-transporters, SVCT1 and SVCT2, during perinatal kidney development. Histochem Cell Biol 2013;139:233-247. https://doi.org/10.1007/s00418-012-1027-z |
||||
| 10 Castro T, Low M, Salazar K, Montecinos H, Cifuentes M, Yáñez AJ, Slebe JC, Figueroa CD, Reinicke K, de los Angeles García M, Henriquez JP, Nualart F: Differential distribution of the Sodium-vitamin C cotransporter-1 along the proximal tubule of the mouse and human kidney. Kidney Int 2008;74:1278-1286. https://doi.org/10.1038/ki.2008.329 |
||||
| 11 Aguilera O, Muñoz-Sagastibelza M, Torrejón B, Borrero-Palacios A, Del Puerto-Nevado L, Martínez-Useros J, Rodriguez-Remirez M, Zazo S, García E, Fraga M, Rojo F, García-Foncillas J: Vitamin C uncouples the Warburg metabolic switch in KRAS mutant colon cancer. Oncotarget 2016;7:47954-47965. https://doi.org/10.18632/oncotarget.10087 |
||||
| 12 Campbell EJ, Dachs GU, Morrin HR, Davey VC, Robinson BA, Vissers MCM: Activation of the hypoxia pathway in breast cancer tissue and patient survival are inversely associated with tumor ascorbate levels. BMC Cancer 2019;19:307. https://doi.org/10.1186/s12885-019-5503-x |
||||
| 13 Srinivasan R, Ricketts CJ, Sourbier C, Linehan WM: New strategies in renal cell carcinoma: targeting the genetic and metabolic basis of disease. Clin Cancer Res 2015;21:10-17. https://doi.org/10.1158/1078-0432.CCR-13-2993 |
||||
| 14 Incorvaia L, Bronte G, Bazan V, Badalamenti G, Rizzo S, Pantuso G, Natoli C, Russo A: Beyond evidence-based data: scientific rationale and tumour behavior to drive sequential and personalized therapeutic strategies for the treatment of metastatic renal cell carcinoma. Oncotarget 2016;7:21259-21271. https://doi.org/10.18632/oncotarget.7267 |
||||
| 15 Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F: International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519-530. https://doi.org/10.1016/j.eururo.2014.10.002 |
||||
| 16 Wohlrab C, Vissers MCM, Phillips E, Morrin H, Robinson BA, Dachs GU: The association between ascorbate and the hypoxia-inducible factors in human renal cell carcinoma requires a functional von Hippel-Lindau protein. Front Oncol 2018;8:574. https://doi.org/10.3389/fonc.2018.00574 |
||||
| 17 Kuiper C, Molenaar IG, Dachs GU, Currie MJ, Sykes PH, Vissers MC: Low ascorbate levels are associated with increased hypoxia inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer. Cancer Res 2010;70:5749-5758. https://doi.org/10.1158/0008-5472.CAN-10-0263 |
||||
| 18 Kuiper C, Dachs GU, Munn D, Currie MJ, Robinson BA, Pearson JF, Vissers MC: Increased tumor ascorbate is associated with extended disease-free survival and decreased hypoxia-inducible factor-1 activation in human colorectal cancer. Front Oncol 2014;4:10. https://doi.org/10.3389/fonc.2014.00010 |
||||
| 19 Timenetsky J, Santos LM, Buzinhani M, Mettifogo E: Detection of multiple mycoplasma infection in cell cultures by PCR. Braz J Med Biol Res 2006;39:907-914. https://doi.org/10.1590/S0100-879X2006000700009 |
||||
| 20 Wohlrab C, Kuiper C, Vissers MC, Phillips E, Robinson BA, Dachs GU: Ascorbate modulates the hypoxic pathway by increasing intracellular activity of the HIF hydroxylases in renal cell carcinoma cells. Hypoxia (Auckl) 2019;7:17-31. https://doi.org/10.2147/HP.S201643 |
||||
| 21 García Mde L, Salazar K, Millán C, Rodríguez F, Montecinos H, Caprile T, Silva C, Cortes C, Reinicke K, Vera JC, Aguayo LG, Olate J, Molina B, Nualart F: Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia 2005;50:32-47. https://doi.org/10.1002/glia.20133 |
||||
| 22 Bussolati G, Gugliotta P, Volante M, Pace M, Papotti M: Retrieved endogenous biotin: a novel marker and a potential pitfall in diagnostic immunohistochemistry. Histopathology 1997;31:400-407. https://doi.org/10.1046/j.1365-2559.1997.3020895.x |
||||
| 23 El-Far MA, Bakr MA, Farahat SE, Abd El-Fattah EA: Glutathione peroxidase activity in patients with renal disorders. Clin Exp Nephrol 2005;9:127-131. https://doi.org/10.1007/s10157-005-0343-1 |
||||
| 24 Larsson N, Rankin GD, Bicer EM, Roos-Engstrand E, Pourazar J, Blomberg A, Mudway IS, Behndig AF: Identification of vitamin C transporters in the human airways: a cross-sectional in vivo study. BMJ Open 2015;5:e006979. https://doi.org/10.1136/bmjopen-2014-006979 |
||||
| 25 Kishimoto Y, Saito N, Kurita K, Shimokado K, Maruyama N, Ishigami A: Ascorbic acid enhances the expression of type 1 and type 4 collagen and SVCT2 in cultured human skin fibroblasts. Biochem Biophys Res Commun 2013;430:579-584. https://doi.org/10.1016/j.bbrc.2012.11.110 |
||||
| 26 MacDonald L, Thumser AE, Sharp P: Decreased expression of the vitamin C transporter SVCT1 by ascorbic acid in a human intestinal epithelial cell line. Br J Nutr 2002;87:97-100. https://doi.org/10.1079/BJN2001492 |
||||
| 27 Campbell EJ, Vissers MCM, Wohlrab C, Hicks KO, Strother RM, Bozonet SM, Robinson BA, Dachs GU: Pharmacokinetic and anti-cancer properties of high dose ascorbate in solid tumours of ascorbate-dependent mice. Free Radic Biol Med 2016;99:451-462. https://doi.org/10.1016/j.freeradbiomed.2016.08.027 |
||||
| 28 Portugal CC, da Encarnação TG, Socodato R, Moreira SR, Brudzewsky D, Ambrósio AF, Paes-de-Carvalho R: Nitric oxide modulates sodium vitamin C transporter 2 (SVCT-2) protein expression via protein kinase G (PKG) and nuclear factor-kB (NF-kB). J Biol Chem 2012;287:3860-3872. https://doi.org/10.1074/jbc.M111.260166 |
||||
| 29 Kim EH, Koh DI, Ryu YS, Park SS, Hong SW, Moon JH, Shin JS, Kim MJ, Kim DY, Hong JK, Jeong HR, Yun H, Shin JY, Kim J, Park YS, Kim DM, Jin DH: Role of p53 in transcriptional repression of SVCT2. Mol Biol Rep 2021;48:1651-1658. https://doi.org/10.1007/s11033-021-06179-2 |
||||
| 30 Qiao H, May JM: CpG methylation at the USF-binding site mediates cell-specific transcription of human ascorbate transporter SVCT2 exon 1a. Biochem J 2011;440:73-84. https://doi.org/10.1042/BJ20110392 |
||||
| 31 Shenoy N, Bhagat T, Nieves E, Stenson M, Lawson J, Choudhary GS, Habermann T, Nowakowski G, Singh R, Wu X, Verma A, Witzig TE: Upregulation of TET activity with ascorbic acid induces epigenetic modulation of lymphoma cells. Blood Cancer J 2017;7:e587. https://doi.org/10.1038/bcj.2017.65 |
||||
| 32 Subramanian VS, Marchant JS, Reidling JC, Said HM: N-Glycosylation is required for Na+-dependent vitamin C transporter functionality. Biochem Biophys Res Commun 2008;374:123-127. https://doi.org/10.1016/j.bbrc.2008.06.120 |
||||
| 33 Liang WJ, Johnson D, Ma LS, Jarvis SM, Wei-Jun L: Regulation of the human vitamin C transporters expressed in COS-1 cells by protein kinase C [corrected]. Am J Physiol Cell Physiol 2002;283:C1696-1704. https://doi.org/10.1152/ajpcell.00461.2001 |
||||
| 34 Covarrubias-Pinto A, Acuña AI, Boncompain G, Papic E, Burgos PV, Perez F, Castro MA: Ascorbic acid increases SVCT2 localization at the plasma membrane by accelerating its trafficking from early secretory compartments and through the endocytic-recycling pathway. Free Radic Biol Med 2018;120:181-191. https://doi.org/10.1016/j.freeradbiomed.2018.03.013 |
||||
| 35 Subramanian VS, Marchant JS, Said HM: Molecular determinants dictating cell surface expression of the human sodium-dependent vitamin C transporter-2 in human liver cells. American Journal of Physiology. Am J Physiol Gastrointest Liver Physiol 2010;298:G267-G274. https://doi.org/10.1152/ajpgi.00435.2009 |
||||
| 36 Acuña AI, Esparza M, Kramm C, Beltrán FA, Parra AV, Cepeda C, Toro CA, Vidal RL, Hetz C, Concha II, Brauchi S, Levine MS, Castro MA: A failure in energy metabolism and antioxidant uptake precede symptoms of Huntington's disease in mice. Nat Commun 2013;4:2917. https://doi.org/10.1038/ncomms3917 |
||||
| 37 Muñoz-Montesino C, Roa FJ, Peña E, González M, Sotomayor K, Inostroza E, Muñoz CA, González I, Maldonado M, Soliz C, Reyes AM, Vera JC, Rivas CI: Mitochondrial ascorbic acid transport is mediated by a low-affinity form of the sodium-coupled ascorbic acid transporter-2. Free Radic Biol Med 2014;70:241-254. https://doi.org/10.1016/j.freeradbiomed.2014.02.021 |
||||
| 38 Vissers MCM, Das AB: Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Front Physiol 2018;9:809. https://doi.org/10.3389/fphys.2018.00809 |
||||
| 39 Eck P, Kwon O, Chen S, Mian O, Levine M: The human sodium-dependent ascorbic acid transporters SLC23A1 and SLC23A2 do not mediate ascorbic acid release in the proximal renal epithelial cell. Physiol Rep 2013;1:e00136. https://doi.org/10.1002/phy2.136 |
||||
| 40 Eck PK, Corpe C, Levine MA: Temporo-spacial microanatomical distribution of the murine sodium-dependent ascorbic acid transporters Slc23a1 and Slc23a2 in the kidney throughout development. Biochem Cell Biol 2017;95:421-427. https://doi.org/10.1139/bcb-2015-0090 |
||||
| 41 Boyer JC, Campbell CE, Sigurdson WJ, Kuo SM: Polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochem Biophys Res Commun 2005;334:150-156. https://doi.org/10.1016/j.bbrc.2005.06.069 |
||||
| 42 Anthony HM, Schorah CJ: Severe hypovitaminosis C in lung cancer patients: the utilization of vitamin C in surgical repair and lymphocyte-related host resistance. Br J Cancer 1982;46:354-367. https://doi.org/10.1038/bjc.1982.211 |
||||
| 43 Mayland CR, Bennett MI, Allan K: Vitamin C deficiency in cancer patients. Pall Med 2005;19:17-20. https://doi.org/10.1191/0269216305pm970oa |
||||
| 44 White R, Nonis M, Pearson JF, Burgess E, Morrin HR, Pullar JM, Spencer E, Vissers MCM, Robinson BA, Dachs GU: Low Vitamin C Status in Patients with Cancer Is Associated with Patient and Tumor Characteristics. Nutrients 2020;12:2338. https://doi.org/10.3390/nu12082338 |
||||
| 45 Kuiper C, Vissers MC, Hicks KO: Pharmacokinetic modeling of ascorbate diffusion through normal and tumor tissue. Free Radic Biol Med 2014;77:340-352. https://doi.org/10.1016/j.freeradbiomed.2014.09.023 |
||||
| 46 Singer K, Kastenberger M, Gottfried E, Hammerschmied CG, Büttner M, Aigner M, Seliger B, Walter B, Schlösser H, Hartmann A, Andreesen R, Mackensen A, Kreutz M: Warburg phenotype in renal cell carcinoma: high expression of glucose-transporter 1 (GLUT-1) correlates with low CD8(+) T-cell infiltration in the tumor. Int J Cancer 2011;128:2085-2095. https://doi.org/10.1002/ijc.25543 |
||||
| 47 Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, Ries CH, Ailles L, Jewett MAS, Moch H, van den Broek M, Beisel C, Stadler MB, Gedye C, Reis B, Pe›er D, Bodenmiller B: An Immune Atlas of Clear Cell Renal Cell Carcinoma. Cell 2017;169:736-749.e18. https://doi.org/10.1016/j.cell.2017.04.016 |
||||
| 48 Bergsten P, Amitai G, Kehrl J, Dhariwal KR, Klein HG, Levine M: Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J Biol Chem 1990;265:2584-2587. https://doi.org/10.1016/S0021-9258(19)39841-2 |
||||
| 49 Bergsten P, Yu R, Kehrl J, Levine M: Ascorbic acid transport and distribution in human B lymphocytes. Arch Biochem Biophys 1995;317:208-214. https://doi.org/10.1006/abbi.1995.1155 |
||||
| 50 Vera JC, Rivas CI, Fischbarg J, Golde DW: Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 1993;364:79-82. https://doi.org/10.1038/364079a0 |
||||
| 51 Welch RW, Wang Y, Crossman A Jr, Park JB, Kirk KL, Levine M: Accumulation of vitamin C (ascorbate) and its oxidized metabolite dehydroascorbic acid occurs by separate mechanisms. J Biol Chem 1995;270:12584-12592. https://doi.org/10.1074/jbc.270.21.12584 |
||||
| 52 Dhariwal KR, Hartzell WO, Levine M: Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am J Clin Nutr 1991;54:712-716. https://doi.org/10.1093/ajcn/54.4.712 |
||||
| 53 Pullar JM, Bayer S, Carr AC: Appropriate handling, processing and analysis of blood samples is essential to avoid oxidation of vitamin C to dehydroascorbic acid. Antioxidants (Basel) 2018;7:29. https://doi.org/10.3390/antiox7020029 |
||||
| 54 Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M: Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One 2010;5:e11414. https://doi.org/10.1371/journal.pone.0011414 |
||||
| 55 Ngo B, Van Riper JM, Cantley LC, Yun J: Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev Cancer 2019;19:271-282. https://doi.org/10.1038/s41568-019-0135-7 |
||||
| 56 Giansanti M, Karimi T, Faraoni I, Graziani G: High-Dose Vitamin C: Preclinical Evidence for Tailoring Treatment in Cancer Patients. Cancers (Basel) 2021;13:1428. https://doi.org/10.3390/cancers13061428 |
||||
| 57 Shenoy NK, Ou FS, Cheville JC, Bhagat T, Gartrell BA, Verma A, Levine M, Pagliaro LC: Randomized phase II trial of intravenous ascorbic acid (AA) as an adjunct to pazopanib for metastatic and unresectable clear cell renal cell carcinoma (ccRCC): A study of Academic and Community Cancer Research United (ACCRU) GU1703. J Clin Oncol 2019; DOI: 10.1200/JCO.2019.37.7_suppl.TPS679. https://doi.org/10.1200/JCO.2019.37.7_suppl.TPS679 |
||||