Corresponding Author: Jérôme Guicheux
INSERM, UMR-S 1229, RMeS; Université de Nantes, CHU de Nantes, 1 place Alexis Ricordeau, 44042 Nantes (France)
Tel. +33240412982, E-Mail jerome.guicheux@inserm.fr
Interleukin-33 Deficiency Exacerbates Bone Loss Associated with Porphyromonas Gingivalis‐Induced Experimental Periodontitis in Female Mice
Agathe Louisya Valérie Geoffroya Boris Halganda Olivier Lapérinea Joëlle Veziersa Jocelyne Caillonb Jérôme Guicheuxa Philippe Lesclousa Alexandra Cloitrea
aNantes Université, Oniris, Univ Angers, CHU Nantes, INSERM, Regenerative Medicine and Skeleton, RMeS, UMR 1229, Nantes, France, bEA 3826 Thérapeutiques cliniques et expérimentales des infections, Nantes, France
Introduction
Interleukin-33 (IL-33) is classified as one of the last members of the interleukin-1 (IL-1) family to be discovered [1] and as such it plays a significant role in both innate and acquired immunity. IL-33 is constitutively expressed in various tissues, particularly in the endothelial and epithelial cells of barrier tissues where it acts as an alarmin [2, 3]. Rapidly released to the extracellular space after cellular damage or injury, IL-33 alerts the immune system to danger and triggers inflammation. IL-33 acts by binding to its ST2 receptor, which is expressed on many immune and non-immune cells and which activates the NF-κB and MAPK pathways leading to inflammatory cytokine production [1, 4]. IL-33 is known to promote the orientation of type 2 immune response but exerts pleiotropic activities depending on the target cells and their microenvironment [2]. Although IL-33 is well known as a key player in inflammatory diseases (i.e., asthma, atopic dermatitis), its role in bone physiology remains uncertain and its involvement in bone diseases such as periodontitis needs further investigation.
Bone tissue is constantly renewed via bone remodeling throughout life. A critical balance between bone resorption by osteoclasts (OCs) and bone formation by osteoblasts is mandatory to achieve this physiological process (coupling). A cytokine network regulates the function of bone cells, OCs in particular, which are derived from the monocyte/macrophage hematopoietic lineage [5]. Receptor activator of nuclear factor κ-B ligand (RANKL) is the major pro-osteoclastogenic cytokine. It binds to its receptor RANK expressed by OCs and their precursors and enhances their recruitment, differentiation, fusion, and activity. However, the role of the cytokine IL-33 in bone remodeling is still debated. The overexpression of IL-33 specifically in osteoblasts of transgenic mice led to reduced osteoclastogenesis, suggesting that IL-33 is a potential regulator of OC differentiation [6]. Conversely, IL-33 overexpression in mice through systemic IL-33 adeno-associated virus (AAV) injections induced an increase in OC number in femurs and bone loss especially in the cortex [7]. The maxillary bone phenotype of IL-33 knockout (KO) male mice has not been described to date.
Under pathological conditions such as periodontitis, bone resorption outweighs bone formation leading to bone loss (uncoupling). Periodontitis is a widespread chronic multifactorial inflammatory disease associated with dysbiotic plaque biofilms [8]. It is characterized by progressive destruction of the tooth-supporting bone, i.e., the alveolar bone, which may result in severe tooth loss in patients. Porphyromonas gingivalis (Pg), an oral Gram-negative anaerobic bacterium, is considered a key periopathogen due to its ability to induce dysbiotic biofilm and inflammation [9]. Archetypal pro-inflammatory cytokines (IL-1β, IL-6, and tumor necrosis factor (TNF)-α) produced by host cells seems crucial for the pathogenesis of periodontitis, perpetuating gingival inflammation and the subsequent alveolar bone resorption through the recruitment and activation of OCs [10]. More recently described, IL-33 has been suggested to have a pathogenic role in periodontitis. The gingival expression of IL-33 was higher in patients with periodontitis compared to healthy controls [3, 11]. This increase in IL-33 expression was also evidenced in a model of experimental periodontitis (EP) induced by Pg-soaked ligature and by oral gavage with Pg in mice. However, the role of IL-33 in alveolar bone loss associated with periodontitis remains unclear, since the induction of EP in IL-33 KO mice has not been performed to date.
The objectives of this study were to decipher the contribution of IL-33 (i) to bone homeostasis under physiological conditions and (ii) to alveolar bone loss associated with EP in IL-33 KO mice and their wildtype (WT) littermates.
Materials and Methods
WT and IL-33 KO mice
This study was approved by the Ethics Committee for Animal Experiment of Pays de la Loire (CEEA 2017.06) and conformed to the European Directive 2010/63/EU. To avoid genetic drift, IL-33 KO mice of the n generation were collected from Professor Bernhard Ryffel (Molecular Immunology, University of Orleans and CNRS, France). The breeder respected the rules of crossbreeding. We then generated heterozygotes which we crossed together, and we used the mice of this n+2 generation for all our experiments. The genetic status of IL-33 KO and WT mice with pure C57BL/6 genetic background (Charles River) was confirmed by polymerase chain reaction (PCR) (data not shown). The mice were housed in a specific pathogen-free facility under light- (12-h light/dark cycle), temperature- (22–25 °C), and humidity-controlled (50–60%) conditions. All animals were fed a regular diet and had access to water ad libitum.
Mice kept under physiological conditions
WT (6 males and 5 female) and IL-33 KO mice (6 males and 5 females) were sacrificed at 15–16 weeks of age by cervical dislocation for baseline measurements. Maxillae, femurs, and vertebrae were removed for micro-computed tomography (micro-CT) analyses. Femoral and tibial bone marrow cells were used for OC differentiation.
Mouse model of EP
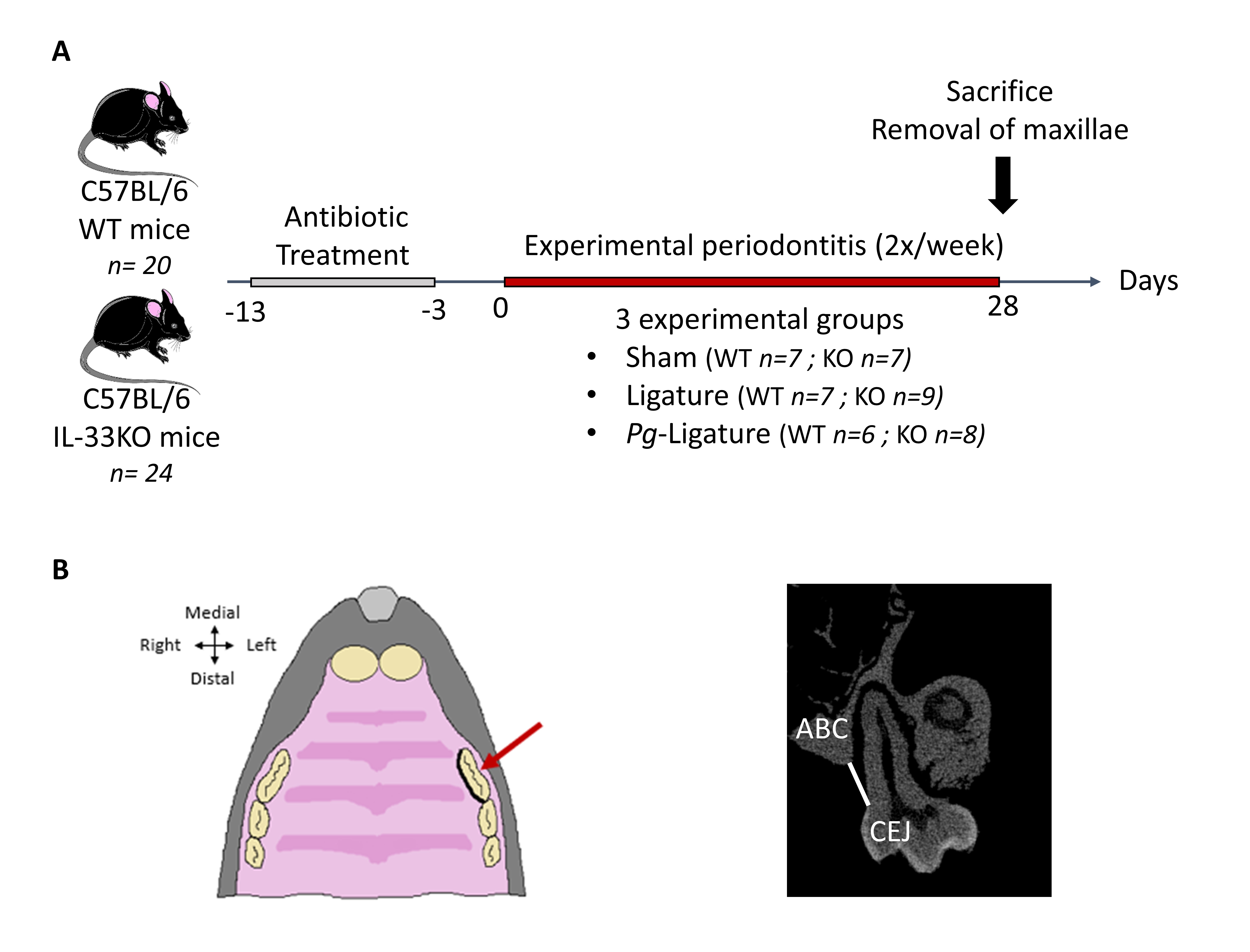
We used a well-established ligature-induced EP model, as previously described (Fig. 1A) [3]. Briefly, after a washout period of antibiotics (sulfamethoxazole-trimethoprim) to reduce differences in the background oral flora of mice [3], EP was induced by placement of a silk ligature optionally soaked with Pg (ATCC 33277) in the gingival crevice around the maxillary left first molar (Fig. 1B). Then, 11- and 12-week-old mice were randomly assigned to three different groups (at least six mice for each genotype):
• Sham group: mice without ligature placement received a slight incision into the sulcular epithelium to mimic the ligature placement (7 WT: 3 males and 4 females, 7 KO: 3 males and 4 females)
• Lig group: an unsoaked ligature was placed (7 WT: 4 males and 3 females, 9 KO: 4 males and 5 females)
• Pg-Lig group: a Pg-soaked ligature was inserted (6 WT: 3 males and 3 females, 8 KO: 4 males and 4 females)
At 28 days, the animals were sacrificed by cardiac exsanguination under intraperitoneal anesthesia with xylazine and ketamine (10 and 80 mg/kg, respectively), and maxillae were removed for further analysis (micro-CT analyses, histological examinations).
Bacterial and strain culture
Pg (ATCC 33277) was cultured at 37 °C on Schaedler agar plated with sheep blood (BD), in an oxygen-free atmosphere. After 10 days of culture, Pg colonies were selected and resuspended in brain–heart broth at 109 CFU/mL for ligature placement or at 105 CFU/mL for Pg injection. A 6/0 silk thread was placed in the suspension 24 h before the EP procedure.
Micro-computed tomography (micro-CT)
For ex vivo analysis, the bone samples were scanned by micro-CT (Skyscan 1272; Skyscan). Scans were acquired for each bone: maxilla (80 kV, 1 mm aluminum filter, 0.35° rotation angle, 12-µm resolution), lumbar vertebra (70 kV, 0.5-mm aluminum filter, 0.6° rotation angle, 12-µm resolution), and distal femur (60 kV, 0.25-mm aluminum filter, 0.6° rotation angle, 8.5-µm resolution).The NRecon software (Skyscan) was used for the 3D reconstruction: maxilla (smoothing=0, ring artifact=4, beam hardening=20%), lumbar vertebra (smoothing=0, ring artifact=4, beam hardening=10%) and distal femur (smoothing=0, ring artifact=4, beam hardening = 20%). Calibration was carried out with a known density of calcium hydroxyapatite phantoms (Bruker, Belgium).
The bone phenotype of 15- to 16-week-old mice (baseline) was analyzed on the distal femur (excluding the growth plate), the fifth lumbar vertebra, and the alveolar bone surrounding the first molar for the maxilla (at least five mice per group). The total bone mineral density (BMD: g/cm2), trabecular bone volume/total volume ratio (BV/TV: %), bone volume (BV: mm3), trabecular thickness (Tb.Th: mm), trabecular separation (Tb.Sp: mm), and trabecular number (Tb.N: 1/mm2) were assessed. The distance between the palatal cementum–enamel junction (CEJ) of the maxillary left first molar and the alveolar bone crest (ABC) was measured in the coronal plane to assess alveolar bone height (Fig. 1C). Ten consecutives sections were analyzed per animal. All measurements were performed by two independent operators.
Histological analysis
After fixation in 4% paraformaldehyde at 4 °C for 1 day, maxillae were decalcified in EDTA 0.5 M at 4 °C for 3 weeks. After dehydration, the samples were embedded in paraffin. Histological staining was performed on 4-µm-thick sections in the frontal plane. Masson-Goldner trichrome and Toluidine blue (pH: 3.8) staining were used on two sections for each sample. Automated whole-slide imaging was performed using the NanoZoomer 2.0 (Hamamatsu). For each section, resorption parameters were recorded with the ImageJ software including the number of trabecular OCs (OCL) in contact with bone (N.Oc/BS: 1/mm), the OC surface (Oc.S/BS: %), and the average length of the zone of contact per OC (Oc.S/N.Oc: mm), which is a sensitive indicator of OC activity [12]. All the measurements were performed by two independent operators.
OC differentiation
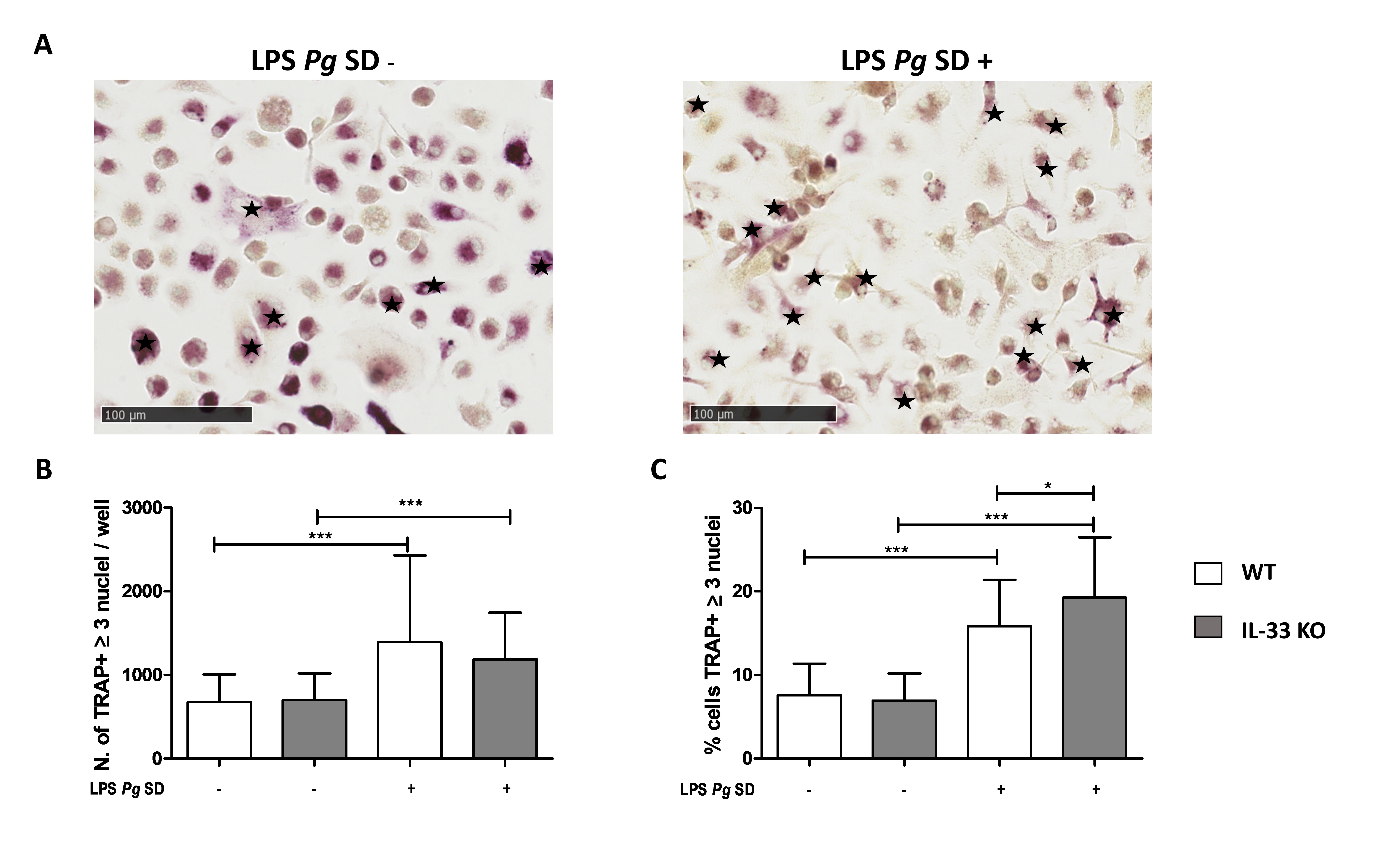
Bone marrow cells were aseptically collected from the femur and tibia, depleted of red blood cells in a lysate buffer (Sigma Aldrich), and plated in minimum essential medium α–containing 10% fetal bovine serum in 8-well Labtek slides (4.10⁵ cells/well) for tartrate-resistant acid phosphatase (TRAP) staining. Cells were resuspended and cultured in α-MEM with 10% FCS (Hyclone, GE healthcare), 1% penicillin–streptomycin, and 1% glutamine at 37 °C in 5% CO2. On day one, the media were changed with the same medium in the presence of 25 ng/mL recombinant mouse M-CSF (PeproTech) and with an additional 100 ng/mL recombinant mouse RANK-L (PeproTech) to induced OC differentiation. For LPS-Pg Standard (SD) stimulation, 5 µg/mL was added to the media on day 5 and on every second day thereafter up to the end of OC differentiation. Cells were incubated for 14 days at 37 °C in 5% CO2 and the medium was changed every other day. At the end of the culture period, cells were fixed with 4% paraformaldehyde in PBS and stained for TRAP using the Leukocyte Acid Phosphatase kit (Sigma-Aldrich) to identify TRAP-positive cells. Multinucleated cells with three nuclei or more were considered as OCs and the ratio of TRAP-positive multinucleated cells compared to the total TRAP-positive cells was calculated.
Statistical analysis
Statistical analyses were performed using GraphPad Prism Software (v.6). All data are presented as mean ± standard derivation (SD). The differences between groups were analyzed in a two by two using an unpaired Student t test or two-way analysis of variance (number of groups greater than 2) followed by the Bonferroni post hoc test if the differences were significant. Statistical significance was set at p < 0.05.
Results
IL-33 deficiency affected bone phenotype in a site- and sex-dependent manner under physiological conditions
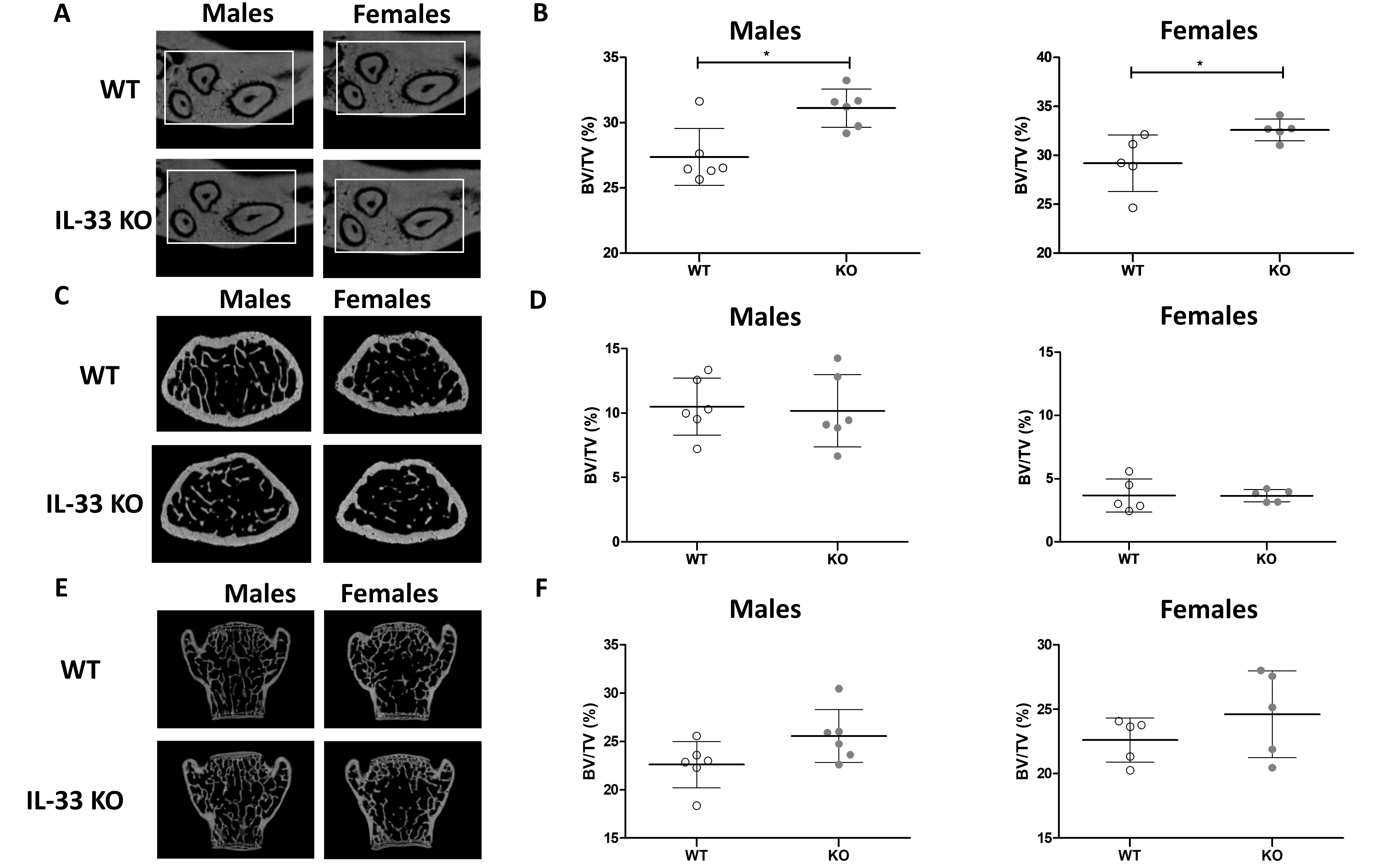
First, to assess the potential role of IL-33 on bone phenotype under physiological conditions, the BV/TV of the maxilla, femur, and vertebra of 15- to 16-week-old WT and IL-33 KO mice were determined. The IL-33 KO mice were not different in weight from WT mice [7]. In the maxillary bone, male and female IL-33 KO mice had a higher BV/TV compared to their WT littermates (Fig. 2A and 2B). This increase in bone mass can be explained by the Tb.Th, which was significantly higher in the maxilla in both male and female IL-33 KO mice (Supplementary Table S1), but also by a decreasing trend in Tb.Sp that was not significant (for all supplementary material see www.cellphysiolbiochem.com). Mature bone and lamellar bone formation with typical extracellular matrix embedded osteocytes were observed in both WT and IL-33KO males and females (Supplementary Fig. S1). In the femur, no significant difference in BV/TV or in the trabecular parameters was found between WT and IL-33 KO mice (Fig. 2C and 2D). Lastly, although the increase in BV/TV observed in the fifth lumbar vertebra was not significant (Fig. 2E and F), Tb.Th was significantly higher in IL-33 KO animals. Some differences in BMD were recorded between WT and IL-33 KO male mice, except in the maxilla (Supplementary Table S1).
IL-33 deficiency did not interfere with alveolar bone measurement (CEJ/ABC) under physiological conditions
Using 2D micro-CT, the CEJ/ABC distance was assessed to evaluate the height of the alveolar bone in the palatal region of the first maxillary molar. No significant difference was observed in CEJ/ABC distance between 15- to 16-week-old IL-33 KO and WT male and female mice under physiological conditions.
IL-33 deficiency increased the severity of EP induced by Pg ligature only in females
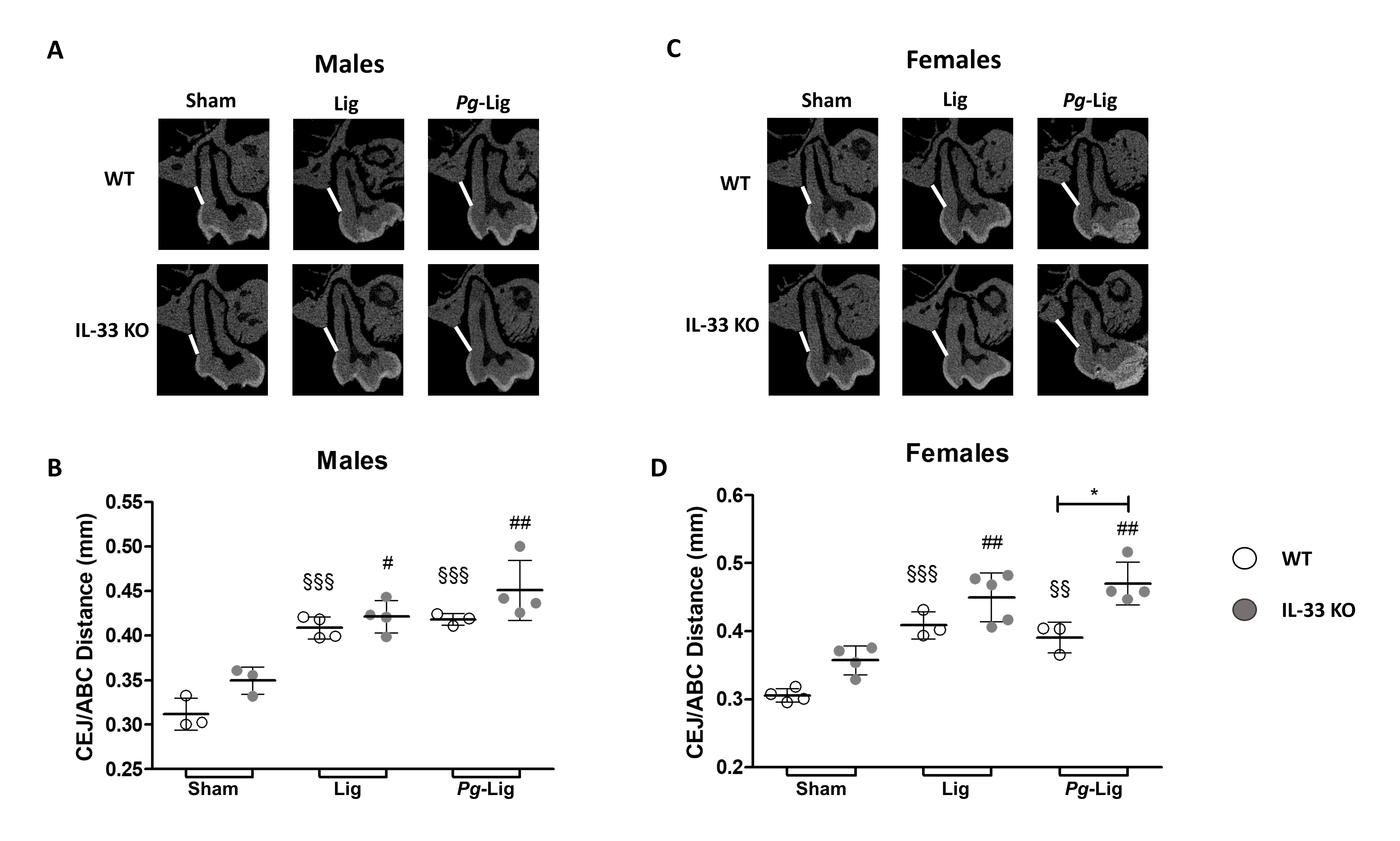
We then assessed whether IL-33 deficiency could be involved in alveolar bone loss induced by EP. Around the untreated maxillary right first molar no significant difference in the CEJ/ABC distance was observed between the 3 experimental groups (sham, Lig, and Pg-Lig) in males or in females, for both genotypes (data not show). As expected, in WT males (Fig. 3A and 3B) and females (Fig. 3C and 3D), ligature placement for 4 weeks around the maxillary left first molar (Lig and Pg-Lig groups) induced a significant increase in the CEJ/ABC distance indicating significant alveolar bone loss compared to the respective sham groups. In female and male IL-33 KO mice, ligature placement also induced a significant increase in the CEJ/ABC distance compared with animals in the sham group. No significant difference was evidenced between the Lig and Pg-Lig groups, in males or in females, for both genotypes, indicating that soaking the ligature with Pg had no additional effect on the alveolar bone loss than ligature alone.
In males, for each experimental group (sham, Lig, and Pg-Lig), no significant difference was found between the CEJ/ABC distance measured in WT and IL-33 KO mice (Fig. 3B). Interestingly, in female mice, the CEJ/ABC distance was significantly higher (+20%) in alveolar bone loss in Pg-Lig IL-33 KO mice versus WT mice, but it was not significantly different between IL-33 KO and WT mice in the Lig groups and sham groups (Fig. 3D).
In the Pg-Lig groups, IL-33 deficiency increased the number of OCs and the resorption surface in females
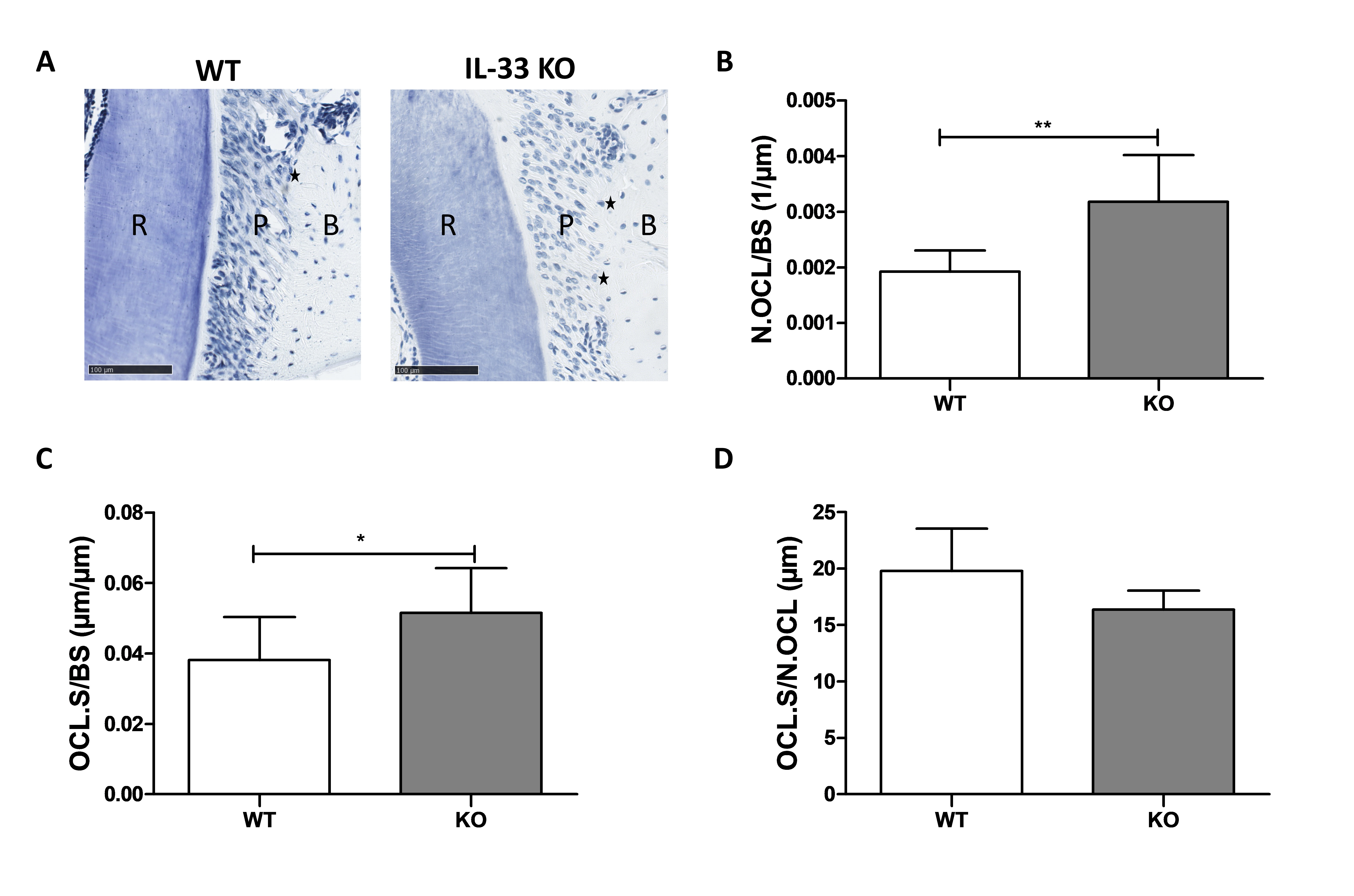
To explain the higher bone resorption observed in the Pg-Lig female IL-33 KO mice, osteoclastic parameters were analyzed on the histological maxillary sections stained with toluidine blue (Fig. 4). In the Pg-Lig group, the number of OCs (Fig. 4B) and the resorption surface (Fig. 4C) were significantly higher (34.8%) in IL-33 KO female mice, whereas no difference was recorded regarding the mean length of the contact zone between OC and alveolar bone (Fig. 4D).
IL-33 deficiency enhanced OC differentiation in vitro in presence of LPS-Pg
To analyze the in vitro effect of IL-33 KO on osteoclastogenesis, bone marrow progenitors were harvested from WT and IL-33 KO female mice. Differentiation of WT and KO bone marrow progenitors was similar, and the percentage of TRAP-positive cells with more than three nuclei was not significantly different between the two genotypes (Fig. 5). The addition of LPS-Pg SD in the culture medium enhanced osteoclastogenesis significantly in both genotypes, with a slight but significant 2.08-fold increase in WT cells and 2.78-fold increase in IL-33 KO cells.
The authors thank Bernhard Ryffel and Marc Le Bert for providing IL-33 KO mice as well as Caroline Vignes (SC3M Facility), Sarah Beck, Aida Gutierrez-Corrales, and José-Ramón Corcuera-Flores for technical assistance.
Author Contributions
AL, VG, PL and AC contributed to the conception or design of the work. AL, VG, BH, OL, JC, PL and AC contributed to the investigation. AL, VG, PL and AC drafted the manuscript. BH, OL, JV, JC, JG critically revised the manuscript; All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
The study was supported by Région des pays de la Loire (grant 2012-05775-05776) and by the Nantes School of Dentistry.
Statement of Ethics
Animal experiments conform to internationally accepted standards and have been approved by the appropriate institutional review body.
The authors have no conflicts of interest to declare.
| 1 Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA: IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005;23:479-490. https://doi.org/10.1016/j.immuni.2005.09.015 |
||||
| 2 Cayrol C, Girard JP: Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol Rev 2018;281:154-168. https://doi.org/10.1111/imr.12619 |
||||
| 3 Lapérine O, Cloitre A, Caillon J, Huck O, Bugueno IM, Pilet P, Sourice S, Le Tilly E, Palmer G, Davideau JL, Geoffroy V, Guicheux J, Beck-Cormier S, Lesclous P: Interleukin-33 and RANK-L interplay in the alveolar bone loss associated to periodontitis. PLoS One 2016;11:e0168080. https://doi.org/10.1371/journal.pone.0168080 |
||||
| 4 Moulin D, Donzé O, Talabot-Ayer D, Mézin F, Palmer G, Gabay C: Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine 2007;40:216-225. https://doi.org/10.1016/j.cyto.2007.09.013 |
||||
| 5 Boyle WJ, Simonet WS, Lacey DL: Osteoclast differentiation and activation. Nature 2003;423:337-342. https://doi.org/10.1038/nature01658 |
||||
| 6 Keller J, Catala-Lehnen P, Wintges K, Schulze J, Bickert T, Ito W, Horst AK, Amling M, Schinke T: Transgenic over-expression of interleukin-33 in osteoblasts results in decreased osteoclastogenesis. Biochem Biophys Res Commun 2012;417:217-222. https://doi.org/10.1016/j.bbrc.2011.11.088 |
||||
| 7 Okragly AJ, Hamang MJ, Pena EA, Baker HE, Bullock HA, Lucchesi J, Martin AP, Ma YL, Benschop RJ: Elevated levels of Interleukin (IL)-33 induce bone pathology but absence of IL-33 does not negatively impact normal bone homeostasis. Cytokine 2016;79:66-73. https://doi.org/10.1016/j.cyto.2015.12.011 |
||||
| 8 Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, Greenwell H, Herrera D, Kao RT, Kebschull M, Kinane DF, Kirkwood KL, Kocher T, Kornman KS, Kumar PS, Loos BG, et al.: Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018;89:S173-S182. https://doi.org/10.1002/JPER.17-0721 |
||||
| 9 Hajishengallis G, Darveau R, Curtis M: The Keystone Pathogen Hypothesis. Nat Rev Microbiol 2012;10:717-725. https://doi.org/10.1038/nrmicro2873 |
||||
| 10 Di Benedetto A, Gigante I, Colucci S, Grano M: Periodontal disease: Linking the primary inflammation to bone loss. Clin Dev Immunol 2013;2013:1-7. https://doi.org/10.1155/2013/503754 |
||||
| 11 Malcolm J, Awang RA, Oliver-Bell J, Butcher JP, Campbell L, Adrados Planell A, Lappin DF, Fukada SY, Nile CJ, Liew FY, Culshaw S: IL-33 Exacerbates Periodontal Disease through Induction of RANKL. J Dent Res 2015;94:968-975. https://doi.org/10.1177/0022034515577815 |
||||
| 12 Mcmillan PJ, Dewri RA, Joseph EE, Schultz RL, Deflos LJ. Rapid changes of light microscopic indices of osteoclast-bone relationships correlated with electron microscopy. Calcif Tissue Int 1989;44:399-405. https://doi.org/10.1007/BF02555968 |
||||
| 13 Macari S, Madeira MFM, Lima ILA, Pereira TSF, Dias GJ, Cirelli JA, de Molon RS, Fukada SY, Szawka RE, Garlet GP, Teixeira MM, Silva TA: ST2 regulates bone loss in a site-dependent and estrogen-dependent manner. J Cell Biochem 2018;119:8511-8521. https://doi.org/10.1002/jcb.27080 |
||||
| 14 Somerville JM, Aspden RM, Armour KE, Armour KJ, Reid DM: Growth of C57Bl/6 Mice and the Material and Mechanical Properties of Cortical Bone from the Tibia. Calcif Tissue Int 2004;74:469-475. https://doi.org/10.1007/s00223-003-0101-x |
||||
| 15 Martin P, Talabot-Ayer D, Seemayer CA, Vigne S, Lamacchia C, Rodriguez E, Finckh A, Smith DE, Gabay C, Palmer G: Disease severity in K/BxN serum transfer-induced arthritis is not affected by IL-33 deficiency. Arthritis Res Ther 2013;15:R13. https://doi.org/10.1186/ar4143 |
||||
| 16 Baker PJ, Dixon M, Roopenian DC: Genetic Control of Susceptibility to Porphyromonas gingivalis - Induced Alveolar Bone Loss in Mice. Infect Immun 2000;68:5864-5868. https://doi.org/10.1128/IAI.68.10.5864-5868.2000 |
||||
| 17 Rafiei M, Kiani F, Sayehmiri K, Sayehmiri F, Tavirani M, Dousti M: Prevalence of Anaerobic Bacteria (P. gingivalis) as Major Microbial Agent in the Incidence Periodontal Diseases by Meta-analysis. J Dent (Shiraz) 2018;19:232-242. | ||||
| 18 de Molon RS, Mascarenhas VI, de Avila ED, Finoti LS, Toffoli GB, Spolidorio DMP, Scarel-Caminaga RM, Tetradis S, Cirelli JA: Long-term evaluation of oral gavage with periodontopathogens or ligature induction of experimental periodontal disease in mice. Clin Oral Investig 2016;20:1203-1216. https://doi.org/10.1007/s00784-015-1607-0 |
||||
| 19 Tada H, Matsuyama T, Nishioka T, Hagiwara M: Porphyromonas gingivalis Gingipain- Dependently Enhances IL-33 Production in Human Gingival Epithelial Cells. PLoS One 2016;11:e0152794. https://doi.org/10.1371/journal.pone.0152794 |
||||
| 20 Zaiss MM, Kurowska-Stolarska M, Böhm C, Gary R, Scholtysek C, Stolarski B, Reilly J, Kerr S, Millar NL, Kamradt T, McInnes IB, Fallon PG, David JP, Liew FY, Schett G: IL-33 Shifts the Balance from Osteoclast to Alternatively Activated Macrophage Differentiation and Protects from TNF-α-Mediated Bone Loss. J Immunol 2011;186:6097-6105. https://doi.org/10.4049/jimmunol.1003487 |
||||
| 21 Athari SK, Poirier E, Biton J, Semerano L, Hervé R, Raffaillac A, Lemeiter D, Herbelin A, Girard J P, Caux F, Boissier MC, Bessis N: Collagen-induced arthritis and imiquimod- induced psoriasis develop independently of interleukin-33. Arthritis Res Ther 2016;18:1-11. https://doi.org/10.1186/s13075-016-1042-x |
||||
| 22 Mun SH, Ko NY, Kim HS, Kim JW, Kim DK, Kim AR, Lee SH, Kim YG, Lee CK, Lee SH, Kim BK, Beaven MA, Kim YM, Choi WS: Interleukin-33 stimulates formation of functional osteoclasts from human CD14+ monocytes. Bone 2008;23:1-7. | ||||
| 23 Saidi S, Bouri F, Lencel P, Duplomb L, Baud'huin M, Delplace S, Leterme D, Miellot F, Heymann D, Hardouin P, Palmer G, Magne D: IL-33 is expressed in human osteoblasts, but has no direct effect on bone remodeling. Cytokine 2011;53:347-354. https://doi.org/10.1016/j.cyto.2010.11.021 |
||||
| 24 Schulze J, Bickert T, Beil FT, Zaiss MM, Albers J, Wintges K, Streichert T, Klaetschke K, Keller J, Hissnauer TN, Spiro AS, Gessner A, Schett G, Amling M, McKenzie AN, Horst AK, Schinke T: Interleukin-33 is expressed in differentiated osteoblasts and blocks osteoclast formation from bone marrow precursor cells. J Bone Miner Res 2011;26:704-717. https://doi.org/10.1002/jbmr.269 |
||||
| 25 Saleh H, Eeles D, Hodge JM, Nicholson GC, Gu R, Pompolo S, Gillespie MT, Quinn JM: Interleukin-33, a target of parathyroid hormone and oncostatin m, increases osteoblastic matrix mineral deposition and inhibits osteoclast formation in vitro . Endocrinology 2011;152:1911-1922. https://doi.org/10.1210/en.2010-1268 |
||||
| 26 Eeles DG, Hodge JM, Singh PP, Schuijers JA, Grills BL, Gillespie MT, Myers DE, Quinn JM: Osteoclast formation elicited by interleukin-33 stimulation is dependent upon the type of osteoclast progenitor. Mol Cell Endocrinol 2015;399:259-266. https://doi.org/10.1016/j.mce.2014.10.014 |
||||
| 27 Nativel B, Couret D, Giraud P, Meilhac O, d'Hellencourt CL, Viranaïcken W, Da Silva CR: Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human. Sci Rep 2017;7:15789. https://doi.org/10.1038/s41598-017-16190-y |
||||
| 28 AlQranei MS, Senbanjo LT, Aljohani H, Hamza T, Chellaiah MA: Lipopolysaccharide- TLR-4 Axis regulates Osteoclastogenesis independent of RANKL/RANK signaling. BMC Immunol 2021;22:1-16. https://doi.org/10.1186/s12865-021-00409-9 |
||||
| 29 Ito HO, Shuto T, Takada H, Koga T, Aida Y, Hirata M, Koga T: Lipopolysaccharides from Porphyromonas gingivalis, Prevotella intermedia and Actinobacillus actinomycetemcomitans promote osteoclastic differentiation in vitro . Arch Oral Biol 1996;41:439-444. https://doi.org/10.1016/0003-9969(96)00002-7 |
||||
| 30 Ohori F, Kitaura H, Ogawa S, Shen WR, Qi J, Noguchi T, Marahleh A, Nara Y, Pramusita A, Mizoguchi I: IL-33 Inhibits TNF-α -Induced Osteoclastogenesis and Bone Resorption. Int J Mol Sci 2020;21:1130. https://doi.org/10.3390/ijms21031130 |
||||