Corresponding Author: Björn L.D.M. Brücher
Department of Surgery, Carl-Thiem-Klinikum, 03048 Cottbus (Germany)
E-Mail b-bruecher@gmx.de
Physics Essentials Enable Deeper Understanding in Signaling and Crosstalk of the Carcinogenesis Paradigm “Epistemology of the Origin of Cancer”
Björn L.D.M. Brüchera,b,c Martin Daumera,b,d Ijaz S. Jamalla,b,e
aTheodor-Billroth-Academy®, Munich, Germany – Sacramento, CA, USA, bINCORE, International Consortium of Research Excellence of the Theodor-Billroth-Academy®, Munich, Germany – Sacramento, CA, USA, cDepartment of Surgery, Carl-Thiem-Klinikum, Cottbus, Germany, dSylvia Lawry Centre for Multiple Sclerosis Research e.V. – The Human Motion Institute, Munich, Germany, eRisk-Based Decisions Inc., Sacramento, CA, USA
Introduction
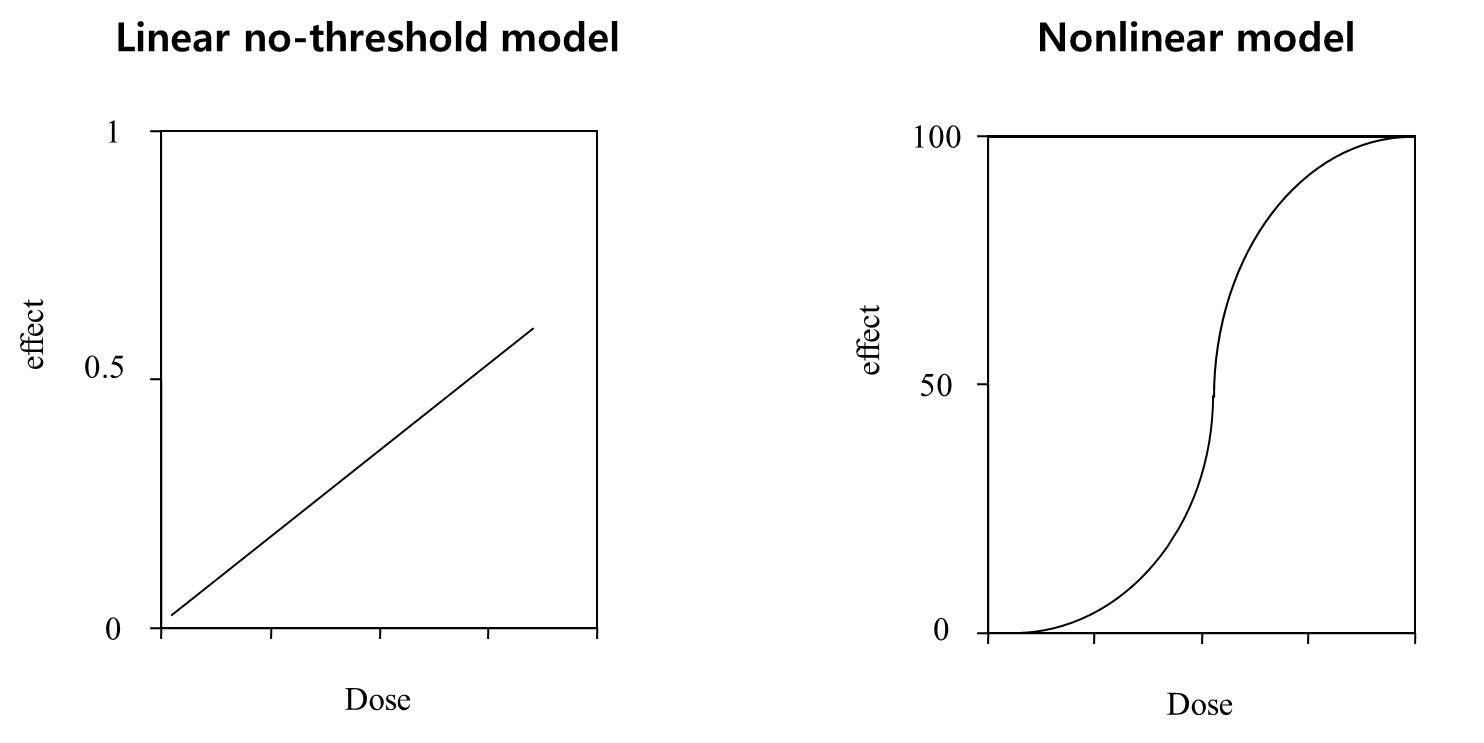
Radioactivity in the form of ionizing radiation can induce mutations in DNA and is believed to be the primary causal example of a cancer-initiating event. The Linear No-Threshold (LNT) model was created based on the assumption that any amount of radiation had some detrimental genetic effects in the form of DNA damage that could then lead to cancer, i.e. a zero-threshold assumption or that there is no radiation dose that is without an incremental quantifiable increase in cancer risk. This was the basis for the belief that cancer is caused by mutations as described by the somatic mutation theory (SMT). In fact, only a small proportion of cancers (~5%–10%) have been shown to result from mutations over the past 100 years and the majority (80%) of cancers are therefore still referred to as ‘sporadic’, meaning that their cause remains unknown [1-14].
Decades of molecular and clinical research led us in 2012 to the development of the cancer paradigm “Epistemology of the origin of cancer”, with a complex six-step set of events published in open-access format [9]. This paradigm explains why the majority of cancers originate after this sequence of events, namely (1) a pathogenic stimulus (biological or chemical) followed by (2) chronic inflammation, from which develops (3) fibrosis with associated changes in the cellular microenvironment. From these changes, (4) a pre-cancerous niche develops, which triggers the deployment of (5) a chronic stress escape strategy. When this condition fails to resolve, (6) the transition of a normal cell to a cancer cell occurs. The initial concept was realized between 2014 and 2016 including the original cancer paradigm and five papers [9-11, 15, 16]. This was followed by critical analyses of available knowledge five years after the paradigm was first published [17-26].
To date, the assessment of the contributions of physics to the process of carcinogenesis has been missing. Physics is of much greater significance in cancer research than is generally perceived. The essentials of physics provide a deeper understanding of how and why the LNT is invalid and thus gives an impetus for further critical thinking and analyses to more completely understand carcinogenesis, which describes the complex, incompletely understood process by which changes in cells/tissues/organs lead to the disease that we refer to as cancer (see also Supplementary Material – for all supplementary material see www.cellphysiolbiochem.com).
Radioactivity
For their joint discoveries, Antoine Henri Becquerel (1852–1908) (spontaneous radioactivity from uranium salts), and Marie (1867-1934) and Pierre Curie (1859-1906) (identification of polonium), received the Nobel Prize in Physics in 1903. Marie Curie reported on radium between 1898 and 1907 [27-33], and Pierre Curie and his student Albert Laborde measured continuous emissions from radium in 1903 [32]. More detailed information about the discoveries of radiation and in Physics is provided in the Supplementary Material (see also Fig. 1-10 in Supplementary Material).
Background radiation
Background radiation consists of approximately 82% natural and approximately 18% man-made radiation, which is mainly due to medical X-rays (58%), nuclear medicine (21%), consumer products (16%), occupational sources (2%), atomic bomb fallout (2%), and nuclear fuel cycles (1%) [34]. Exposure to ionizing radiation is a consistent occurrence for all life on Earth. Approximately 90% of the annual radiation dose “for a person living in the US comes from natural sources such as cosmic radiation and radioactive rocks” ([35], reviewed in [36]). Cosmic radiation originates from the sun and distant galaxies [37].
According to the International Atomic Energy Agency (IAEA) of the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) report in 1993, natural background radiation comes from “cosmic radiation, external radiation from radionuclides in the earth’s crust and internal radiation from radionuclides inhaled or ingested and retained in the body” [38]. In summary, “exposures due to cosmic rays, terrestrial gamma rays and ingestion vary only slightly with time, so they can be regarded as the background exposure to natural sources.”
Radiation exposure in people is influenced by multiple factors. Geographic location is one such factor, as cosmic radiation increases with elevation above mean sea level. Background radiation also depends on local geology, and here preexisting radon is of importance, as radon sources affect exposure. In 1993, the UNSCEAR sought to establish a representative approach describing radiation exposure as follows: the determination of “radiation exposures from various sources consists of presenting the collective dose to the world population received or committed (a) from the end of 1945 to the end of 1992 (47 years) for discrete events and (b) for a period of 50 years at the current rate of practice or exposure for all other sources, including natural sources.” This approach was deemed to be reasonable for a period of 50 years (25 years before and after the present), although it has been stated that it is “likely that this assumption overestimates the future doses from practices that are not rapidly expanding, because improved techniques and standards of protection will reduce the doses per unit of practice” but it was not clear, how doses are affected by practice.
The worldwide average annual effective radiation dose in adults from all natural sources was estimated to be 2.0 mSv [39] in 1982, with a later estimate of 2.4 mSv (range: 1−10), consisting of 0.9 mSv (37.5%) from external exposure (cosmic rays: 0.4 mSv [16.7%]; terrestrial gamma rays: 0.5 mSv [20.8%]) and 1.5 mSv (62.5%) from internal exposure (inhalation [primarily radon]: 1.2 mSv [50%]; ingestion: 0.3 mSv [12.5%]) [40, 41]. The dosage levels are listed in Table 1 [modified according to 34] (see also Supplementary Material, Section ‘measurement parameters’). One’s exposure depends on several variables. For example, cosmic ray dose rates depend on altitude, with exposure rates being five-fold higher at higher altitudes compared with average rates at sea level [38]. Terrestrial γ-ray doses depend on local geology and residential ventilation such that some communities may have an exposure rate that is 100-fold higher than average due to the presence of certain types of naturally occurring radioactive minerals.
While the global average human exposure to natural background radiation is 2.4 mSv/a (270 nSv/h avg) [40, 41], there are large geographic variations [42]. For example, the average natural background radiation in Finland is ~8 mSv/a (~900 nSv/h avg) versus 90 μSv/h (800 mSv/avg) on a monazite beach near Guarapari, Brazil [43]. A Finish nationwide register-based case-control study on the Chernobyl fallout revealed that “Overall, background gamma radiation showed a non-significant association with the OR of childhood leukemia (OR 1.01, 95% CI 0.97, 1.05 for a 10-nSv/h increase in average equivalent dose rate to red bone marrow)” [44]. No accumulation of dose with age was found.
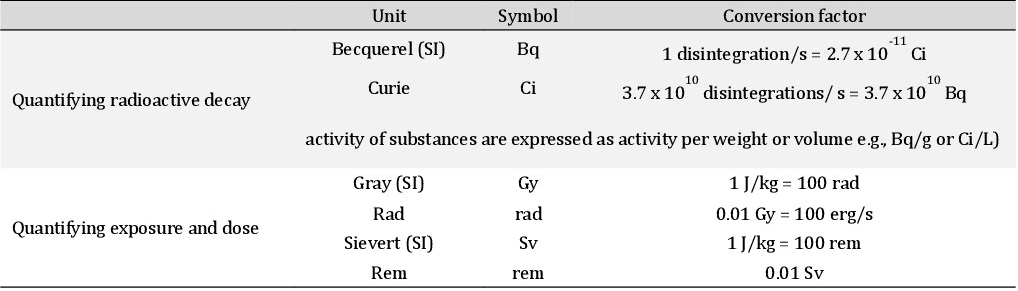
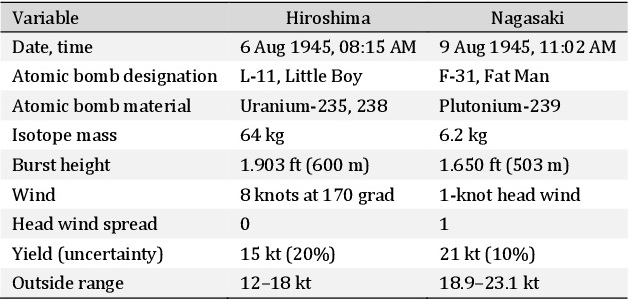
Atomic bombs detonated at Hiroshima and Nagasaki [34, 55, 61, 63]
We are thankful to the countless scientists, clinicians, and individuals of various disciplines and professions from Germany, Greece, Israel, Italy, Japan, Portugal, Spain, Switzerland, UK, and the USA for the personal exchanges during the last few decades. We acknowledge the intense and bias-free discussions, critical thinking, exchanges, and reviews by Professor Detlef Bartsch, Berlin, Germany, Professor Dr Marjan Slak Rupnik, Vienna, Austria, Professor em. Michael Baum, London, UK, Associate Professor Dr Jochen Salber, Bochum, Germany, Dr Gudrun Schueler, Cottbus, Germany and Professor Reshef Tenne, Rehovot, Israel.
Author Contributions
BB produced the first draft. MD and IJ worked on the various sections. All authors edited and modified the manuscript.
Funding
The manuscript was supported by the Theodor-Billroth-Academy® (TBA®) and INCORE (International Consortium of Research Excellence) of the TBA®.
The authors declare that no conflicts of interest exist.
| 1 Lynch HT, Smyrk TC, Watson P, Lanspa SJ, Lynch JF, Lynch PM, Cavalieri RJ, Boland CR: Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology 1993;104(5):1535-1549. https://doi.org/10.1016/0016-5085(93)90368-m. https://doi.org/10.1016/0016-5085(93)90368-M |
||||
| 2 Tomlinson IP, Novelli MR, Bodmer WF: The mutation rate and cancer. Proc Natl Acad Sci USA 1996;93(25):14800-14803. https://doi.org/10.1073/pnas.93.25.14800. https://doi.org/10.1073/pnas.93.25.14800 |
||||
| 3 Pisani P, Parkin DM, Muñoz N, Ferlay J: Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomarkers Prev 1997;6(6):387-400. | ||||
| 4 Blattner WA: Human retroviruses: their role in cancer. Proc Assoc Am Physicians 1999;111(6):563-572. https://doi.org/10.1046/j.1525-1381.1999.99210.x. https://doi.org/10.1046/j.1525-1381.1999.99210.x |
||||
| 5 Burth RW: Colon cancer screening. Gastroenterology 2000;119(3):837-853. https://doi.org/10.1053/gast.2000.16508. https://doi.org/10.1053/gast.2000.16508 |
||||
| 6 Parkin DM: The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006;118(12):3030-3044. https://doi.org/10.1002/ijc.21731. https://doi.org/10.1002/ijc.21731 |
||||
| 7 Rustgi AK: The genetics of hereditary colon cancer. Genes Dev 2007;21(20):2525-2538. https://doi.org/10.1101/gad.1593107. https://doi.org/10.1101/gad.1593107 |
||||
| 8 Markowitz SD, Bertagnolli MM: Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med 2009;361(25):2449-2460. https://doi.org/10.1056/NEJMra0804588. https://doi.org/10.1056/NEJMra0804588 |
||||
| 9 Brücher BLDM, Jamall IS: Epistemology of the Origin of Cancer: A New Paradigm. BMC Cancer 2014;14(331):1-15. https://doi.org/10.1186/1471-2407-14-331. https://doi.org/10.1186/1471-2407-14-331 |
||||
| 10 Brücher BLDM, Jamall IS: Cell-Cell communication in tumor microenvironment, carcinogenesis and anticancer treatment. Cell Physiol Biochem 2014;34(2):213-243. https://doi.org/10.1159/000362978. https://doi.org/10.1159/000362978 |
||||
| 11 Brücher BLDM, Jamall JS: Somatic Mutation Theory - Why it's Wrong for Most Cancers. Cell Physiol Biochem 2016;38(5):1663-1680. https://doi.org/10.1159/000443106. https://doi.org/10.1159/000443106 |
||||
| 12 Kanth P, Grimmett J, Champine M, Burt R, Samadder NJ: Hereditary Colorectal Polyposis and Cancer Syndromes: A Primer on Diagnosis and Management. Am J Gastroenterol 2017;112(10):1509-1525. https://doi.org/10.1038/ajg.2017.212. https://doi.org/10.1038/ajg.2017.212 |
||||
| 13 Jonsson P, Bandlamudi C, Cheng ML, Srinivasan P, Chavan SS, Friedman ND, Rosen EY, Richards AL, Bouvier N, Selcuklu SD, Bielski CM, Abida W, Mandelker D, Birsoy O, Zhang L, Zehir A, Donoghue MTA, Baselga J, Offit K, Scher HI, et al.: Tumour lineage shapes BRCA-mediated phenotypes. Nature 2019;571(7766):576-579. https://doi.org/10.1038/s41586-019-1382-1. https://doi.org/10.1038/s41586-019-1382-1 |
||||
| 14 de Martel C, Georges D, Bray F, Ferlay J, Clifford GM: Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health 2020;8(2):e180-e190. https://doi.org/10.1016/S2214-109X(19)30488-7. https://doi.org/10.1016/S2214-109X(19)30488-7 |
||||
| 15 Brücher BLDM, Yan L, Schnabel P, Daumer M, Wallace TJ, Kube R, Zilberstein B, Steele S, Jamall IS: Genomics, microRNA, epigenetics, and proteomics for future diagnosis, treatment and monitoring response in upper GI cancers. Clin Trans Med 2016;5(1):1-16. https://doi.org/10.1186/s40169-016-0093-6. https://doi.org/10.1186/s40169-016-0093-6 |
||||
| 16 Brücher BLDM, Lyman G, van Hillegersberg R, Pollock RE, Lordick F, Yang HK, Ushijima T, Yeoh KG, Skricka T, Polkowski W, Wallner G, Verwaal V, Garofalo A, D'Ugo D, Roviello F, Steinau HU, Wallace TJ, Daumer M, Maihle N, Reid III TJ, et al.: Imagine a World Without Cancer. BMC Cancer 2014;14(186):1-8. https://doi.org/10.1186/1471-2407-14-186. https://doi.org/10.1186/1471-2407-14-186 |
||||
| 17 Brücher BLDM, Jamall IS: Prelude and Premise to the Special Issue: Disruption of homeostasis-induced signaling and crosstalk in the carcinogenesis paradigm "Epistemology of the origin of cancer". 4open 2019;2(6):1-8. https://doi.org/10.1051/fopen/2019005. https://doi.org/10.1051/fopen/2019005 |
||||
| 18 Brücher BLDM, Jamall IS: Undervalued ubiquitous proteins. 4open 2019;2(7):1-13. https://doi.org/10.1051/fopen/2019002. https://doi.org/10.1051/fopen/2019002 |
||||
| 19 Brücher BLDM, Jamall IS: Chronic inflammation evoked by pathogenic stimulus during carcinogenesis. 4open 2019;2(8):1-22. https://doi.org/10.1051/fopen/2018006. https://doi.org/10.1051/fopen/2018006 |
||||
| 20 Brücher BLDM, Jamall IS: Eicosanoids in carcinogenesis. 4open 2019;2(9):1-34. https://doi.org/10.1051/fopen/2018008. https://doi.org/10.1051/fopen/2018008 |
||||
| 21 Brücher BLDM, Jamall IS: Microbiome and morbid obesity increase pathogenic stimulus diversity. 4open 2019;2(10):1-16. https://doi.org/10.1051/fopen/2018007. https://doi.org/10.1051/fopen/2018007 |
||||
| 22 Brücher BLDM, Jamall IS: Precancerous niche (PCN), a product of fibrosis with remodeling by incessant chronic inflammation. 4open 2019;2(11):1-21. https://doi.org/10.1051/fopen/2018009. https://doi.org/10.1051/fopen/2018009 |
||||
| 23 Brücher BLDM, Jamall IS: Metformin alters signaling homeostasis. 4open 2019;2(12):1-17. https://doi.org/10.1051/fopen/2019006. https://doi.org/10.1051/fopen/2019006 |
||||
| 24 Brücher BLDM, Lang F, Jamall JS: NF-κB signaling and crosstalk in carcinogenesis. 4open 2019;2(13):1-35. https://doi.org/10.1051/fopen/2019010. https://doi.org/10.1051/fopen/2019010 |
||||
| 25 Brücher BLDM, Jamall IS: Transition from normal to cancerous cell by precancerous niche (PCN) induced chronic cell-matrix stress. 4open 2019;2(14):1-31. https://doi.org/10.1051/fopen/2018996. https://doi.org/10.1051/fopen/2018996 |
||||
| 26 Brücher BLDM, Jamall IS: Synopsis - Special Issue: Disruption of homeostasis-induced signaling and crosstalk in the carcinogenesis paradigm "Epistemology of the origin of cancer". 4open 2019;2(28):1-30. https://doi.org/10.1051/fopen/2019023. https://doi.org/10.1051/fopen/2019023 |
||||
| 27 Curie P, Curie M: Sur une substance nouvelle radio-active, contenue dans la pechblende. C R Acad Sci 1898;127:175-178. URL: https://www.academie-sciences.fr/pdf/dossiers/Curie/Curie_pdf/CR1898_p175_178.pdf [accessed Jan 04, 2007]. | ||||
| 28 Curie M: Recherches sur les Substances Radioactives. Doctorate thesis 1904;1-155. University of Paris. URL: https://lccn.loc.gov/04010116 [accessed Jan 04, 2007]. | ||||
| 29 Becquerel H, Curie P: Action physiologique des rayons du radium. Comptes Rendus des Séances de L'Académie des Sciences 1901;132:1289-1291 [séance on 3 June 1901]. URL: https://www.academie-sciences.fr/pdf/dossiers/Curie/Curie_pdf/CR1901_p1289_1291.pdf [accessed Jan 04, 2007]. | ||||
| 30 Curie M: Sur le poids atomique du radium. C R Acad Sci 1902;135:161-163. URL: https://hal.archives-ouvertes.fr/jpa-00242258/document [accessed Jan 04, 2007]. | ||||
| 31 Curie M: Sur le poids atomique du Radium. Faculté des Sciences de Paris, Radium (Paris) 1907;4(10):349-352. https://doi.org/10.1051/Radium:01907004010034900. https://doi.org/10.1051/radium:01907004010034900 |
||||
| 32 Curie P, Laborde A: Sur la chaleur dégagée spontanément par les sels de radium. C R Acad Sci 1903:673-675. Paris: Gauthier-Villars. URL: https://www.biodiversitylibrary.org/item/31410#page/681/mode/1up [accessed Jan 04, 2007]. | ||||
| 33 Curie M: Action de la pesanteur sur le dépôt de la radioactivité induite. Radium (Paris) 1907;4(11):381-382. https://doi.org/10.1051/Radium:01907004011038100. https://doi.org/10.1051/radium:01907004011038100 |
||||
| 34 National Research Council (U.S.). Health risks from exposure to low levels of ionizing radiation: BEIR VII, Phase 2 2000. Washington, DC, The National Academies Press. https://doi.org/10.17226/11340. https://doi.org/10.17226/11340 |
||||
| 35 Early PJ, Sodee DB: Principles and practice of nuclear medicine. Mosby, 1995. | ||||
| 36 Dadachova E, Casadevall A: Ionizing radiation: how fungi cope, adapt, and exploit with the help of melanin. Curr Opin Microbiol 2008;11(6):525-531. https://doi.org/10.1016/j.mib.2008.09.013. https://doi.org/10.1016/j.mib.2008.09.013 |
||||
| 37 Simpson JA. The cosmic radiation, in Bleeker JAM, Geiss J, Huber MCE (eds): The Century of Space Science. Springer, Dordrecht, 2001. https://doi.org/10.1007/978-94-010-0320-9_4. https://doi.org/10.1007/978-94-010-0320-9_4 |
||||
| 38 United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and effects of ionizing radiation. Radiation Report to the National Assembly, New York, United Nations, 1993. URL: https://www.unscear.org/unscear/uploads/documents/unscear-reports/UNSCEAR_1993_Report.pdf. | ||||
| 39 United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and effects of ionizing radiation. Radiation Report to the National Assembly, New York, United Nations, 1982. URL: https://www.unscear.org/docs/publications/1982/UNSCEAR_1982_Report.pdf. | ||||
| 40 United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and effects of ionizing radiation. Radiation Report to the National Assembly, New York, United Nations, 1988. URL: https://www.unscear.org/docs/publications/1988/UNSCEAR_1988_Report.pdf. | ||||
| 41 United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and effects of ionizing radiation. Radiation Report to the National Assembly, New York, United Nations, 2000. URL: https://www.unscear.org/docs/publications/2000/UNSCEAR_2000_Report_Vol.I.pdf. | ||||
| 42 Hendry JH Simon SL, Wojcik A, Sohrabi M, Burkart W, Cardis E, Laurier D, Tirmarche M, Hayata I: Human exposure to high natural background radiation: what can it teach us about radiation risks? J Radiol Prot 2009;29:A29-42. https://doi.org/10.1088/0952-4746/29/2A/S03. https://doi.org/10.1088/0952-4746/29/2A/S03 |
||||
| 43 United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Annex B. Sources and Effects of Ionizing Radiation 2000, Vol 1. United Nations. URL: https://www.unscear.org/unscear/en/publications/2000_1.html. | ||||
| 44 Nikkilä A, Erme S, Arvela H, Holmgren O, Raitanen J, Lohi O, Auvinen A: Background radiation and childhood leukemia: A nationwide register-based case-control study. Int J Cancer 2016;139(9):1975-1982. https://doi.org/10.1002/ijc.30264. https://doi.org/10.1002/ijc.30264 |
||||
| 45 Jaworowski Z: Ionizing radiation in the 20th century and beyond. Symposium Entwicklungen im Strahleschutz, Munich, November 29, 2001. URL: www.cns-snc.ca/branches/Toronto/radiation [accessed Jul 03, 2004]. | ||||
| 46 Tao Z, Zha Y, Akiba S, Sun Q, Zou J, Li J, Liu Y, Kato H, Sugahara T, Wei L: Cancer mortality in the high background radiation areas of Yangjiang, China during the period between 1979 and 1995. J Radiat Res 2000;41 Suppl:31-41. https://doi.org/10.1269/jrr.41.s31. https://doi.org/10.1269/jrr.41.S31 |
||||
| 47 Fornalski KW, Dobrzyński L: The cancer mortality in high natural radiation areas in Poland. Dose Response 2012;10(4):541-561. https://doi.org/10.2203/dose-response.11-035.Fornalski. https://doi.org/10.2203/dose-response.11-035.Fornalski |
||||
| 48 Sreekumar A, Jayalekshmi PA, Nandakumar A, Nair RRK, Ahammed R, Sebastian P, Koriyama C, Akiba S, Nakamura S, Konishi J: Thyroid nodule prevalence among women in areas of high natural background radiation, Karunagappally, Kerala, India. Endocrine 2020;67(1):124-130. https://doi.org/10.1007/s12020-019-02071-z. https://doi.org/10.1007/s12020-019-02071-z |
||||
| 49 Chen D, Wei L: Chromosome aberration, cancer mortality and hormetic phenomena among inhabitants in areas of high background radiation in China. J Radiat Res 1991;32 Suppl(2):46-53. https://doi.org/10.1269/jrr.32.SUPPLEMENT2_46 |
||||
| 50 Beal JM: Negative results following exposure of several kinds of seeds to cosmic rays and other radiations at high altitudes. Bot Gaz 1951;112(24):533-534. https://doi.org/10.1086/335688. https://doi.org/10.1086/335688 |
||||
| 51 Craig L, Seidman H: Leukemia and lymphoma mortality in relation to cosmic radiation. Blood 1961;17(3):319-27. https://doi.org/10.1182/blood.V17.3.319.319 |
||||
| 52 Hickey RJ, Bowers EJ, Spence DE, Zemel BS, Clelland AB, Clelland RC: Low level ionizing radiation and human mortality: multi-regional epidemiological studies. Health Phys 1981;40(5):625-641. https://doi.org/10.1097/00004032-198105000-00003. https://doi.org/10.1097/00004032-198105000-00003 |
||||
| 53 Jagger J: Natural background radiation and cancer death in Rocky Mountain states and Gulf Coast states. Health Phys 1998;75(4):428-443. https://doi.org/10.1097/00004032-199810000-00012. https://doi.org/10.1097/00004032-199810000-00012 |
||||
| 54 Douple EB, Mabuchi K, Cullings HM, Preston DL, Kodama K, Shimizu Y, Fujiwara S, Shore RE: Long-term radiation-related health effects in a unique human population: lessons learned from the atomic bomb survivors of Hiroshima and Nagasaki. Disaster Med Public Health Prep 2011;5(0 1):S122-133. https://doi.org/10.1001/dmp.2011.21. https://doi.org/10.1001/dmp.2011.21 |
||||
| 55 Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K: Studies of Mortality of Atomic Bomb Survivors. Report 13: Solid Cancer and Noncancer Disease Mortality: 1950-1997. Radiat Res 2003;160(4):381-407. https://doi.org/10.1667/rr3049. https://doi.org/10.1667/RR3049 |
||||
| 56 Pollycove M, Feinendegen LE: Biologie moléculaire, épidémiologie et la fin de la relation linéaire sans seuil. Comptes Rendus de l'Académie des Sciences - Series III 1999;322(2-3):197-204. https://doi.org/10.1016/S0764-4469(99)80044-4. https://doi.org/10.1016/S0764-4469(99)80044-4 |
||||
| 57 Langham WH, Lawrence JN, McClelland J, Hempelmann LH: The Los Alamos Scientific Laboratory's experience with plutonium in man. Health Phys 1962;8:753-760. https://doi.org/10.1097/00004032-196212000-00033. https://doi.org/10.1097/00004032-196212000-00033 |
||||
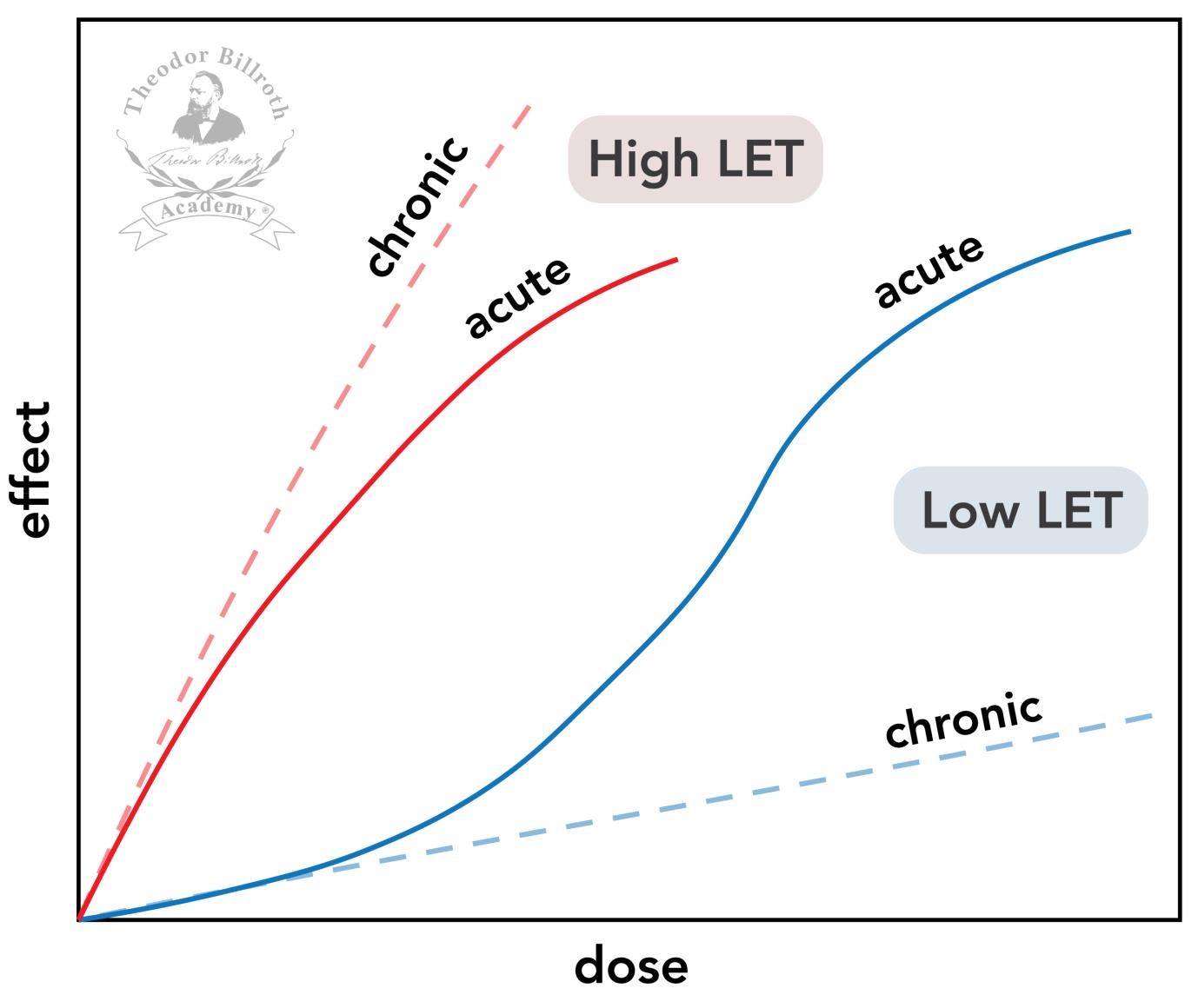
| 58 Hempelmann LH, Langham WH, Richmond CR, Voelz GL: Manhattan Project plutonium workers: a twenty-seven year follow-up study of selected cases. Health Phys 1973;25(5):461-479. https://doi.org/10.1097/00004032-197311000-00001. https://doi.org/10.1097/00004032-197311000-00001 |
||||
| 59 Hiroshima and Nagasaki: The Physical, Medical, and Social Effects of the Atomic Bombings. New York, Basic Books, 1981. The Committee for the Compilation of Materials on Damage Caused by the Atomic Bombs in Hiroshima and Nagasaki. Hutchinson & Co (Publishers) Ltd 1981. | ||||
| 60 Jordan BR: The Hiroshima/Nagasaki Survivor Studies: Discrepancies Between Results and General Perception. Genetics 2016;203(4);1505-1512. https://doi.org/10.1534/genetics.116.191759. https://doi.org/10.1534/genetics.116.191759 |
||||
| 61 Malik J: The Yields of the Hiroshima and Nagasaki Nuclear Explosions, LA-8819, UC-34, September 1985. Los Alamos National Laboratory, Los Alamos, New Mexico, USA. URL: http://large.stanford.edu/courses/2018/ph241/cheng2/docs/malik.pdf. https://doi.org/10.2172/1489669 |
||||
| 62 Preston DL, Pierce DA, Shimizu Y, Cullings HM, Fujita S, Funamoto S, Kodama K: Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat Res 2004;162(4):377-389. https://doi.org/10.1667/rr3232. https://doi.org/10.1667/RR3232 |
||||
| 63 Kerr GD, Young RW, Cullings HM, Christy RF: Chapter 1, Bomb Parameters 2005, in Young RW, Kerr GD (eds): Reassessment of the atomic bomb radiation dosimetry for Hiroshima and Nagasaki-Dosimetry System 2002 (DS02), Vol 1. Radiation Effects Research Foundation, Hiroshima, Japan, pp 42-61. URL: http://rerf.or.jp/shared/ds02 [accessed at Oct 26, 2001]. | ||||
| 64 Neel JV, Schull WJ, McDonald DJ, Morton NE, Kodani M, Takeshima K, Anderson RC, Wood J, Brewer R, Wright S, Yamazaki J, Suzuki M, Kitamura S: The effect of exposure to the atomic bombs on pregnancy termination in Hiroshima and Nagasaki: preliminary report. Science 1953;118(3071):537-541. https://doi.org/10.1126/science.118.3071.537. https://doi.org/10.1126/science.118.3071.537 |
||||
| 65 Beebe G, Usagawa M. The Major ABCC Samples. Hiroshima, Japan: Atomic Bomb Casualty Commission Technical Report, 1968:17-62. See Technical Report Series, 1968. URL: https://www.rerf.or.jp/en/library/archives-en/scientific_pub/trtoc-en/tr1968-en/ [accessed at Oct 26, 2001]. | ||||
| 66 Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K: Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950-1990. Radiat Res 1996;146(1):1-27. https://doi.org/10.2307/3579391 |
||||
| 67 US-Japan joint reassessment of atomic bomb radiation dosimetry in Hiroshima and Nagasaki, in Roesch W (ed): Final report. DS86. Dosimetry System 1986. Vol. 2 (Appendix to Vol. 1). URL: https://inis.iaea.org/search/search.aspx?orig_q=RN:35070155 [accessed at Oct 26, 2001]. | ||||
| 68 Reassessment of the Atomic Bomb Radiation Dosimetry for Hiroshima and Nagasaki- Dosimetry System 2002 (DS02). Hiroshima, Japan: Radiation Effects Research Foundation 2005. URL: https://www.rerf.or.jp/en/library/list-e/scids/ds02-en/ [accessed at Oct 26, 2001]. | ||||
| 69 Cullings HM, Levenson Z, Funamoto S, Teranishi S: Changes in Atomic Bomb Survivors' Dosimetry with the New Dosimetry System DS02. Jpn J Health Phys 2006;41(6):261-271. https://doi.org/10.5453/jhps.41.261. https://doi.org/10.5453/jhps.41.261 |
||||
| 70 Calabrese EJ: On the origins of no-threshold (LNT) dogma by means of untruths, artful dodges and blind faith. Environ Res 2015;142;432-442. https://doi.org/10.1016/j.envres.2015.07.011. https://doi.org/10.1016/j.envres.2015.07.011 |
||||
| 71 Sutou S: A message to Fukushima: nothing to fear but fear itself. Genes Environ 2016;38:12. https://doi.org/10.1186/s41021-016-0039-7. https://doi.org/10.1186/s41021-016-0039-7 |
||||
| 72 Sutou S: Low-dose radiation from A-bombs elongated lifespan and reduced cancer mortality relative to un-irradiated individuals. Genes Environ 2018;40:26. https://doi.org/10.1186/s41021-018-0114-3. https://doi.org/10.1186/s41021-018-0114-3 |
||||
| 73 Calabrese EJ: The road to linearity: why linearity at low doses became the basis for carcinogen risk assessment. Arch Toxicol 2019;83(3):203-225. https://doi.org/10.1007/s00204-009-0412-4. https://doi.org/10.1007/s00204-009-0412-4 |
||||
| 74 Müller HJ: Artificial Transmutation of the Gene. Science 1927;66(1699):84-87. https://doi.org/10.1126/science.66.1699.84. https://doi.org/10.1126/science.66.1699.84 |
||||
| 75 Müller HJ: The Measurement of Gene Mutation Rate in Drosophila, Its High Variability, and Its Dependence upon Temperature. Genetics 1927;13(4):279-357. https://doi.org/10.1093/genetics/13.4.279 |
||||
| 76 Müller HJ: The Production of Mutations by X-rays. Proc Nat Acad Sci U S A 1928;14(9):714-726. https://doi.org/10.1073/pnas.14.9.714. https://doi.org/10.1073/pnas.14.9.714 |
||||
| 77 Müller HJ: Types of visible variations induced by X-rays in Drosophila. J Genetics 1928;22:299-344. https://doi.org/10.1007/BF02984195. https://doi.org/10.1007/BF02984195 |
||||
| 78 Institute of Medicine (US) Committee for Review and Evaluation of the Medical Use Program of the Nuclear Regulatory Commission; Gottfried KLD, Penn G, editors. Radiation In Medicine: A Need For Regulatory Reform. Washington (DC): National Academies Press (US); 1996; K, The Linear, No-Threshold Model. URL: https://www.ncbi.nlm.nih.gov/books/NBK232710/ [accessed Aug 06, 2021]. | ||||
| 79 Genetic effects of atomic radiation. Science 1956;123(3209):1157-1164. https://doi.org/10.1126/science.123.3209.1157. https://doi.org/10.1126/science.123.3209.1157 |
||||
| 80 Leviero A: Scientists term radiation a peril to future man. June 13, 1956. Special to The New York Times. URL: https://www.nytimes.com/1956/06/13/archives/scientists-term-radiation-a-peril-to-future-of-man-even-small-dose.html [accessed Aug 06, 2021]. | ||||
| 81 Lewis EB: Leukemia and ionizing radiation. Science 1957;125(3255):965-972. https://doi.org/10.1126/science.125.3255.965. https://doi.org/10.1126/science.125.3255.965 |
||||
| 82 Sugahara T: Radiation paradigm and its shift. J Radiat Res 1993;35(1):48-52. https://doi.org/10.1269/jrr.35.48. https://doi.org/10.1269/jrr.35.48 |
||||
| 83 Clarke RH, Valentin J: The History of ICRP and the Evolution of its Policies, ICRP Publication 2008;109. URL: https://www.icrp.org/docs/The%20History%20of%20ICRP%20and%20the%20Evolution%20of%20its%20Policies.pdf [accessed Aug 06, 2021]. | ||||
| 84 Kang KW: History and Organizations for Radiological Protection, J Korean Med Sci 2016;31 Suppl 1(Suppl 1):S4-5. https://doi.org/10.3346/jkms.2016.31.S1.S4. https://doi.org/10.3346/jkms.2016.31.S1.S4 |
||||
| 85 INTERNATIONAL recommendations on radiological protection, revised by the International Commission on Radiological Protection (ICRP) at the sixth International Congress of Radiology. London, July, 1950. Am J Roentgenol Radium Ther 1951;65(4):603-609. | ||||
| 86 ICRP, 1951. International recommendations on radiological protection. Br J Radiol 1951;24(277):46-53. https://doi.org/10.1259/0007-1285-24-277-46. https://doi.org/10.1259/0007-1285-24-277-46 |
||||
| 87 ICRP 1955, Recommendations of the ICRP. Br J Radiol 1954;(Suppl. 6):100. URL: https://www.icrp.org/publication.asp?id=1954%20Recommendations [accessed Aug 06, 2021]. | ||||
| 88 ICRP The recommendation of ICRP 1958. Pergamon Press, London 1959. https://doi.org/10.1016/S0074-2740288001. | ||||
| 89 National Academy of Sciences (NAS)/National Research Council (NRC): The Effects on Populations of Exposure to Low Levels of Ionizing Radiation. National Academy 1972, Washington DC. https://doi.org/10.17226/18994. https://doi.org/10.17226/18994 |
||||
| 90 Calabrese EJ: Muller's Nobel lecture on dose-response for ionizing radiation: ideology or science? Arch Toxicol 2011;85(12):1495-1498. https://doi.org/10.1007/s00204-011-0728-8. https://doi.org/10.1007/s00204-011-0728-8 |
||||
| 91 International Commission on Radiological Protection Recommendations of the ICRP: ICRP Publication 1977;26. Ann ICRP 1(3). URL: https://www.icrp.org/publication.asp?id=ICRP%20Publication%2026 [accessed Aug 06, 2021]. | ||||
| 92 Flurkey K, Currer JM, Harrison DE: The Mouse in Aging Research, in Fox JG, et al. (eds.): The Mouse in Biomedical Research 2nd Edition, American College Laboratory Animal Medicine (Elsevier), Burlington, MA 2007:637-672. https://doi.org/10.1016/B978-012369454-6/50074-1 |
||||
| 93 Mak IW, Evaniew N, Ghert AM: Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 2014;6(2):114. | ||||
| 94 Bremnes RM, Dønnem T, Al-Saad S, Al-Shibli K, Andersen S, Sirera R, Camps C, Marinez I, Busund LT: The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol 2011;6(1):209-217. https://doi.org/10.1097/JTO.0b013e3181f8a1bd. https://doi.org/10.1097/JTO.0b013e3181f8a1bd |
||||
| 95 Plava J, Cihova M, Burikova M, Matuskova M, Kucerova L, Miklikova S: Recent advances in understanding tumor stroma-mediated chemoresistance in breast cancer. Mol Cancer 2019;18(1):67. https://doi.org/10.1186/s12943-019-0960-z. https://doi.org/10.1186/s12943-019-0960-z |
||||
| 96 ICRP, 1998. Genetic Susceptibility to Cancer, ICRP Publication 79, Ann. ICRP 1998;28(1-2). https://doi.org/10.1016/S0146-6435(98)00007-3 |
||||
| 97 Anderson MW, Reynolds SH, You M, Maronpot RM: Role of proto-oncogene activation in carcinogenesis. Environ Health Perspect 1992;98:13-24. https://doi.org/10.1289/ehp.929813. https://doi.org/10.1289/ehp.929813 |
||||
| 98 Wang X, Matsumoto H, Takahashi A, Nakano T, Okaichi K, Ihara M, Ohnishi T: p53 accumulation in the organs of low-dose X-ray-irradiated mice. Cancer Lett 1993;104(1):79-84. https://doi.org/10.1016/0304-3835(96)04235-8. https://doi.org/10.1016/0304-3835(96)04235-8 |
||||
| 99 Lowe DJ, Herzog M, Mosler T, Cohen H, Felton S, Beli P, Raj K, Galanty Y, Jackson SP: Chronic irradiation of human cells reduces histone levels and deregulates gene expression. Sci Rep 2020;10(1):2200. https://doi.org/10.1038/s41598-020-59163-4. https://doi.org/10.1038/s41598-020-59163-4 |
||||
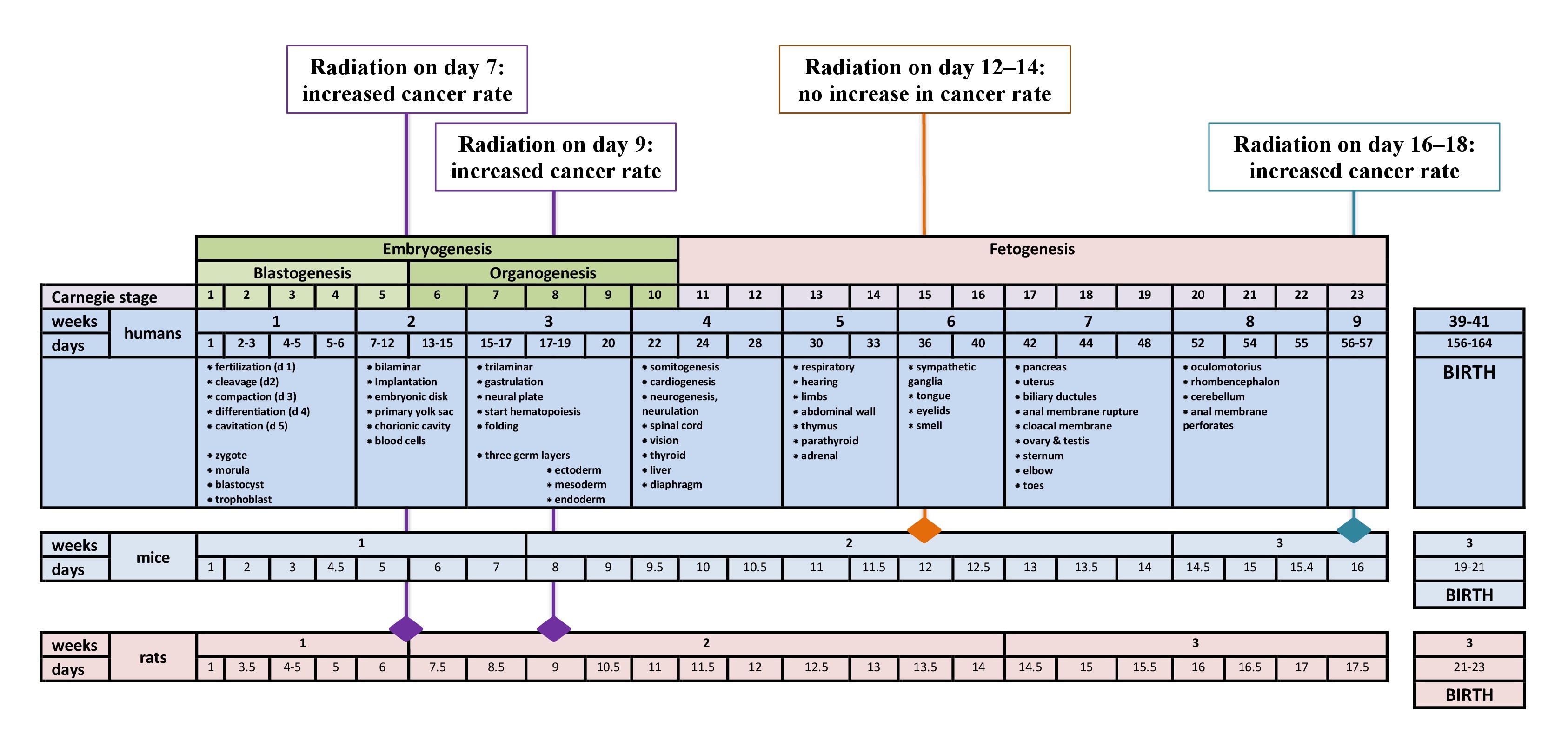
| 100 United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Biological mechanisms of radiation action as at low doses, A white paper to guide the Scientific Committee's future program of work, United Nations, New York, 2012. URL: http://www.unscear.org/docs/publications/2012/UNSCEAR_WP_2012.pdf [accessed Aug 06, 2021]. | ||||
| 101 Joenje H: Genetic toxicology of oxygen. Mutat Res 1989;219(4):193-208. https://doi.org/10.1016/0921-8734(89)90001-5. https://doi.org/10.1016/0921-8734(89)90001-5 |
||||
| 102 Tjeertes JV, Miller KM, Jackson SP: Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. EMBO J 2009;28(13):1878-1889. https://doi.org/10.1038/emboj.2009.119. https://doi.org/10.1038/emboj.2009.119 |
||||
| 103 Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, Jackson SP: Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol 2010;17(9):1144-1151. https://doi.org/10.1038/nsmb.1899. https://doi.org/10.1038/nsmb.1899 |
||||
| 104 Miller KM, Jackson SP: Histone marks: repairing DNA breaks within the context of chromatin. Biochem Soc Trans 2012;40(2):370-376. https://doi.org/10.1042/BST20110747. https://doi.org/10.1042/BST20110747 |
||||
| 105 Raut VV, Sainis JK: 60Co-γ radiation induces differential acetylation and phosphorylation of histones H3 and H4 in wheat. Plant Biol (Stuttg) 2012;14(1):110-117. https://doi.org/10.1111/j.1438-8677.2011.00463.x. https://doi.org/10.1111/j.1438-8677.2011.00463.x |
||||
| 106 Hanf A, Oelze M, Manea A, Li H, Münzel T, Daiber A: The anti-cancer drug doxorubicin induces substantial epigenetic changes in cultured cardiomyocytes. Chem Biol Interact 2019;313:108834. https://doi.org/10.1016/j.cbi.2019.108834. https://doi.org/10.1016/j.cbi.2019.108834 |
||||
| 107 Lindeman LC, Kamstra JH, Ballangby J, Hurem S, Martín LM, Brede DA, Teien HC, Oughton DH, Salbu B, Lyche JL, Aleström P: Gamma radiation induces locus specific changes to histone modification enrichment in zebrafish and Atlantic salmon. PLoS One 2019;14(2):e0212123. https://doi.org/10.1371/journal.pone.0212123. https://doi.org/10.1371/journal.pone.0212123 |
||||
| 108 Klosin A, Casas E, Hidalgo-Carcedo C, Vavouri T, Lehner B: Transgenerational transmission of environmental information in C. elegans. Science 2017;356(6335):320-323. https://doi.org/10.1126/science.aah6412. https://doi.org/10.1126/science.aah6412 |
||||
| 109 Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K: Studies of the mortality of atomic bomb survivors, Report 14, 1950-2003: an overview of cancer and noncancer diseases. Radiat Res 2012;177(3):229-243. https://doi.org/10.1667/rr2629.1. https://doi.org/10.1667/RR2629.1 |
||||
| 110 Gupta RC: Handbook of toxicology of chemical warfare agents, 3rd edition. Academic Press, 2020. | ||||
| 111 Jablon S, Kato H: Childhood cancer in relation to prenatal exposure to atomic bomb radiation. Lancet 1970;2(7681):1000-1003. https://doi.org/10.1016/s0140-6736(70)92813-8. https://doi.org/10.1016/S0140-6736(70)92813-8 |
||||
| 112 Yoshimoto Y: Cancer risk among children of atomic bomb survivors. A review of RERF epidemiologic studies. Radiation Effects Research Foundation. JAMA 1990;264(5):596-600. https://doi.org/10.1001/jama.264.5.596 |
||||
| 113 Kodaira M, Izumi S, Takahashi N, Nakamura N: No evidence of radiation effect on mutation rates at hypervariable minisatellite loci in the germ cells of atomic bomb survivors. Radiat Res 2004;162(4):350-356. https://doi.org/10.1667/rr3243. https://doi.org/10.1667/RR3243 |
||||
| 114 Kodaira M, Ryo H, Kamada N, Furukawa K, Takahashi N, Nakajima H, Nomura T, Nakamura N: No evidence of increased mutation rates at microsatellite loci in offspring of A-bomb survivors. Radiat Res 2010;173(2):205-213. https://doi.org/10.1667/RR1991.1. https://doi.org/10.1667/RR1991.1 |
||||
| 115 Kato H, Yoshimoto Y, Schull WJ: Risk of cancer among children exposed to atomic bomb radiation in utero: a review. IARC Sci Publ 1989;(96):365-374. | ||||
| 116 Preston DL, Cullings H, Suyama A, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K, Kasagi F, Shore RE: Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst 2008;100(6):428-436. https://doi.org/10.1093/jnci/djn045. https://doi.org/10.1093/jnci/djn045 |
||||
| 117 Leung KM, Shabat G, Lu P, Fields AC, Lukashenko A, Davids JS, Melnitchouk N: Trends in solid tumor incidence in Ukraine 30 years after Chernobyl. J Glob Cancer 2019;5:1-10. https://doi.org/10.1200/JGO.19.00099. https://doi.org/10.1200/JGO.19.00099 |
||||
| 118 Yeager M, Machiela MJ, Kothiyal P, Dean M, Bodelon C, Suman S, Wang M, Mirabello L, Nelson CW, Zhou W, Palmer C, Ballew B, Colli LM, Freedman ND, Dagnall C, Hutchinson A, Vij V, Maruvka Y, Hatch M, Illienko I, et al.: Lack of transgenerational effects of ionizing radiation exposure from the Chernobyl accident. Science 2021;372(6543):725-729. https://doi.org/10.1126/science.abg2365. https://doi.org/10.1126/science.abg2365 |
||||
| 119 Stadler J: Mutations in barley induced by X-rays and radium, Science 1928;68:186-187. https://doi.org/10.1126/science.68.1756.186. https://doi.org/10.1126/science.68.1756.186 |
||||
| 120 Timofeeff-Ressovsky NW: Auslösung von Vitalitätsmutationen durch Röntgenstrahlung bei Drosophila melanogaster. Strahlentherapie 1934;51:658-663. | ||||
| 121 Spencer WP, Stern C: Experiments to Test the Validity of the Linear R-Dose/Mutation Frequency Relation in Drosophila at Low Dosage. Genetics 1948;33(1):43-74. https://doi.org/10.1093/genetics/33.1.43 |
||||
| 122 Demerec M: Frequency of deletions amont spontaneous and induced mutations in salmonella. Proc Natl Acad Sci U S A 1960;46(8):1075-1079. https://doi.org/10.1073/pnas.46.8.1075. https://doi.org/10.1073/pnas.46.8.1075 |
||||
| 123 Shiomi T, Inagaki E, Inagaki H, Nakao Y: Mutaiton rates oif low dose level in drosophila melogaster. J Radiat Res 1963;4:105-110. https://doi.org/10.1269/jrr.4.105. https://doi.org/10.1269/jrr.4.105 |
||||
| 124 Traut H: The linear dose-dependence of radiation induced translocation frequency in drosophila melanogaster at relatively low x-radiation doses. Int J Radiat Biol Relat Stud Phys Chem Med 1963;7:401-403. https://doi.org/10.1080/09553006314551341. https://doi.org/10.1080/09553006314551341 |
||||
| 125 Roesch WC: Radiation Effects Research Foundation, and National Academy of Sciences (U.S.). 1987. US-Japan joint reassessment of atomic bomb radiation dosimetry in Hiroshima and Nagasaki. Minami-ku, Hiroshima: Radiation Effects Research Foundation. URL: https://inis.iaea.org/search/search.aspx?orig_q=RN:35050238 [accessed Feb 24, 2018]. | ||||
| 126 Kellerer AM, Rossi HH: Dependence of RBE on neutron dose. Brit J Radiol 1972;45(536):626. https://doi.org/10.1259/0007-1285-45-536-626-a. https://doi.org/10.1259/0007-1285-45-536-626-a |
||||
| 127 Commission of the European Communities: EURATOM Programme. Radiation protection. Progress Report 1982; EUR 8486 DE/EN/FR. ISBN 92-825-3601-7. | ||||
| 128 Wolf C, Lafuma J, Masse R, Morin M, Kellerer AM: Neutron RBE for induction of tumors with high lethality in Sprague-Dawley rats. Radiat Res 2000;154(4):412-420. https://doi.org/10.1667/0033-7587(2000)154[0412:nrfiot]2.0.co;2. https://doi.org/10.1667/0033-7587(2000)154[0412:NRFIOT]2.0.CO;2 |
||||
| 129 Nikjoo H, O'Neill P, Goodhead DT, Terrissol M: Computational modeling of low-energy electron-induced DNA damage by early physical and chemical events. Int J Radiat Biol 1997;71(5):467-483. https://doi.org/10.1080/095530097143798. https://doi.org/10.1080/095530097143798 |
||||
| 130 Nikjoo H, Bolton CE, Watanabe R, Terrissol M, O'Neill P, Goodhead DT: Modelling of DNA damage induced by energetic electrons (100 eV to 100 keV). Radiat Prot Dosim 2000;99(1-4):77-80. https://doi.org/10.1093/oxfordjournals.rpd.a006843. https://doi.org/10.1093/oxfordjournals.rpd.a006843 |
||||
| 131 Kohn KW, Ross WE, Ewig RAG: A relationship between DNA single strand breaks and DNA-protein crosslinks in intercalator-treated mouse LI210 cells; in Hanawalt PC, Friedberg EC, Fox CF (eds): DNA Repair Mechanisms. Academic Press, New York, 1978, 473-485. URL: https://wellcomecollection.org/works/d9mpkupz/items?canvas=16 [accessed Mar 10, 2002]. https://doi.org/10.1016/B978-0-12-322650-1.50092-8 |
||||
| 132 Fuller LF, Painter RB: A Chinese hamster ovary cell line hypersensitive to ionizing radiation and deficient in repair replication. Mutat Res 1998;193(2):109-116. https://doi.org/10.1016/0167-8817(88)90041-7. https://doi.org/10.1016/0167-8817(88)90041-7 |
||||
| 133 Zdzienicka MZ: Mammalian X ray sensitive mutants: a tool for the elucidation of the cellular response to ionizing radiation. Cancer Surv 1996;28:281-293. | ||||
| 134 Collins AR: Mutant rodent cell lines sensitive to ultraviolet light, ionizing radiation and cross-linking agents: a comprehensive survey of genetic and biochemical characteristics. Mutation Res 1993;293(2):99-118. https://doi.org/10.1016/0921-8777(93)90062-l. https://doi.org/10.1016/0921-8777(93)90062-L |
||||
| 135 Zdzienicka MZ: Mammalian mutants defective in the response to ionizing radiation-induced DNA damage. Mutation Res 1995;336(3):203-213. https://doi.org/10.1016/0921-8777(95)00003-3. https://doi.org/10.1016/0921-8777(95)00003-3 |
||||
| 136 Conolly RB, Lutz WK: Nonmonotonic dose-response relationships: mechanistic basis, kinetic modeling, and implications for risk assessment. Toxicol Sci 2004;77(1):151-157. https://doi.org/10.1093/toxsci/kfh007. https://doi.org/10.1093/toxsci/kfh007 |
||||
| 137 LaFond RE: Cancer: The Outlaw Cell. American Chemical Society. Washington, DC, 1978. | ||||
| 138 Cleaver JE: DNA repair and replication in xeroderma pigmentosum and related disorders. Basic Life Sci 1986;39:425-438. https://doi.org/10.1007/978-1-4684-5182-5_38. https://doi.org/10.1007/978-1-4684-5182-5_38 |
||||
| 139 Milota M, Jones DL, Cleaver J, Jamall JS: Xeroderma pigmentosum family support group: Helping families and promoting clinical initiatives. DNA Repair (Amst) 2011;10(7):792-797. https://doi.org/10.1016/j.dnarep.2011.04.027. https://doi.org/10.1016/j.dnarep.2011.04.027 |
||||
| 140 Abrams HL: Influence of age, body weight, and sex on susceptibility of mice to the lethal effects of X-radiation. Proc Soc Exp Biol Med 1951;76(4):729-732. https://doi.org/10.3181/00379727-76-18610. https://doi.org/10.3181/00379727-76-18610 |
||||
| 141 Sacher GA: Dependence of acute radiosensitivity on age in adult female mouse. Science 1957;125(3256):1039-1040. https://doi.org/10.1126/science.125.3256.1039. https://doi.org/10.1126/science.125.3256.1039 |
||||
| 142 Lindop PJ, Rotblat J: Shortening of lifespan of mice as a function of age at irradiation. Gerontologia 1959;3:122-127. https://doi.org/10.1159/000210887. https://doi.org/10.1159/000210887 |
||||
| 143 Spalding JF, Trujillo TT: Radiosensitivity of mice as a function of age. Radiat Res 1962;16(2):125-129. https://doi.org/10.2307/3571191. https://doi.org/10.2307/3571191 |
||||
| 144 Hamilton KF, Sacher GA, Grahn D: A sex difference in mouse survival under daily gamma irradiation and its modification by gonadectomy. Radiat Res 1963;18(1):12-16. https://doi.org/10.2307/3571421. https://doi.org/10.2307/3571421 |
||||
| 145 Sacher GA, Grahn D: Survival of mice under duration-of-life exposure to gamma rays, I. The dosage-survival relation and the lethality function. J Nat Cancer Inst 1964;32(2):277-321. | ||||
| 146 Raventos A: A factor influencing the significance of radiation mortality experiments. Brit J Radiol 1955;28(332):410-414. https://doi.org/10.1259/0007-1285-28-332-410. https://doi.org/10.1259/0007-1285-28-332-410 |
||||
| 147 Hahn EW, Howland JW: Modification of irradiation response of female rats by population density. Radiat Res 1963;19(4):676-681. https://doi.org/10.2307/3571489. https://doi.org/10.2307/3571489 |
||||
| 148 Roderick TH: The response of twenty-seven inbred strains of mice to daily doses of whole-body X-irradiation. Radiat Res 1963;20(4):631-639. https://doi.org/10.2307/3571354. https://doi.org/10.2307/3571354 |
||||
| 149 Snell GD, Aebersold PC: The production of sterility in male mice by irradiating with neutrons. Proc Nat Acad Sci USA 1937;23(7):374-378. https://doi.org/10.1073/pnas.23.7.374. https://doi.org/10.1073/pnas.23.7.374 |
||||
| 150 Batchelor AL, Phillips RJS, Searle AG: High effectiveness of chronic neutron exposures for the induction of specific locus mutations in mice. Nature 1964;201:207-208. https://doi.org/10.1038/201207a0. https://doi.org/10.1038/201207a0 |
||||
| 151 Russell WL, Kelly EM: Neutron-induced mutation in mouse spermatogonia. Lack of effect of dose rate. Relative biological effectiveness of neutrons, Biology Division Semiannual Progress Report for period ending August 15, 1964. Oak Ridge National Laboratory operated by Union Carbide Corporation for the US Atomic Energy Commission 1964. ORNL-3700, pp 83-85. URL: https://www.osti.gov/servlets/purl/4679348 [accessed at Dec 18, 2003]. | ||||
| 152 Straume T, Carsten AL: Tritium radiobiology and relative biological effectiveness. Health Phys 1993;65(6):657-672. https://doi.org/10.1097/00004032-199312000-00005. https://doi.org/10.1097/00004032-199312000-00005 |
||||
| 153 Thomson JF, Williamson F, Grahn D, Ainsworth EJ: Life shortening in mice exposed to fission neutrons and y rays, I. Single and short-term fractioned exposures. Radiat Res 1981;86(3):559-572. https://doi.org/10.2307/3575470. https://doi.org/10.2307/3575470 |
||||
| 154 Thomson JF, Williamson F, Grahn D, Ainsworth EJ: Life Shortening in Mice Exposed to Fission Neutrons and γ Rays: II. Duration-of-Life and Long-Term Fractionated Exposures. Radiat Res 1981;86(3):573-579. https://doi.org/10.2307/3575471. https://doi.org/10.2307/3575471 |
||||
| 155 Thomson JF, Williamson FS, Grahn D, Ainsworth EJ: Life shortening in mice exposed to fission neutrons and gamma rays I. Single and short-term fractionated exposures. Radiat Res 1981 86(3):559-572. https://doi.org/10.2307/3575470 |
||||
| 156 Thomson JF, Williamson F, Grahn D: Life shortening in mice exposed to fission neutrons and y rays, III. Neuron exposures of 5 and 10 rad. Rad Res 1983;93(1):205-209. https://doi.org/10.2307/3575955. https://doi.org/10.2307/3575955 |
||||
| 157 Hill CK, Han A, Buonaguro F, Elkind MM: Multifractionation of Co Gamma Rays Reduces Neoplastic Transformation -In Vitro. Carcinogenesis 1984;5(2):193-197. https://doi.org/10.1093/carcin/5.2.193. https://doi.org/10.1093/carcin/5.2.193 |
||||
| 158 Storer JB, Mitchell FJ: Limiting values for the RBE of fission neutrons at low doses for life shortening in mice. Radiat Res 1984;97(2):396-406. https://doi.org/10.2307/3576290. https://doi.org/10.2307/3576290 |
||||
| 159 Hill CK, Carnes BA, Han A, Elkind MM: Neoplastic Transformation is Enhanced by Multiple Low Doses of fission-spectrum neutrons. Radiat Res 1985;102(3):404-410. https://doi.org/10.2307/3576716 |
||||
| 160 Thomson JF, Williamson F, Grahn D: Life shortening in mice exposed to fission neutrons and y rays, IV. Further studies with fractionated neutron exposures. Radiat Res 1985;103(1):77-88. https://doi.org/10.2307/3576672. https://doi.org/10.2307/3576672 |
||||
| 161 Thomson JF, Williamson F, Grahn D: Life shortening in mice exposed to fission neutrons and y rays, V. Further studies with single low doses. Radiat Res 1985;104(4):420-428. https://doi.org/10.2307/3576601. https://doi.org/10.2307/3576601 |
||||
| 162 Thomson JF, Grahn D: Relative Biological Effectiveness (RBE) of fission neutrons and gamma rays at occupational exposure levels, Volume II. Studies on the effects of 60 Equal One-Weekly exposures to fission neutrons and gamma rays on survival on mice. Prepared for the Division of Regulatory Applications Office of Nuclear Regulatory Commission, Washington, D.C. 1987. 20555, Under Interagency Agreement DOE 40-550-75, NRC Fin No. A2225. URL: https://www.nrc.gov/docs/ML2023/ML20238C552.pdf [accessed Nov 02, 2020]. | ||||
| 163 Straume T, Blattnig S, Zeitlin C: Radiation Hazards and the Colonization of Mars: Brain, Body, Pregnancy, In-Utero Development, Cardio, Cancer, Degeneration. J Cosmology 2010;12:3992-4033. URL: http://journalofcosmology.com/Mars124.html [accessed Jul 25, 2020]. | ||||
| 164 Knudson AG: Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A 1971;68:820-823. https://doi.org/10.1073/pnas.68.4.820. https://doi.org/10.1073/pnas.68.4.820 |
||||
| 165 Fearon ER, Vogelstein B: A genetic model for colorectal tumorigenesis. Cell 1990;61:759-767. https://doi.org/10.1016/0092-8674(90)90186-i. https://doi.org/10.1016/0092-8674(90)90186-I |
||||
| 166 Greenman C, Wooster R, Futreal PA, Stratton MR, Easton DF: Statistical analysis of pathogenicity of somatic mutations in cancer. Genetics 2006;173(4):2187-2198. https://doi.org/10.1534/genetics.105.044677. https://doi.org/10.1534/genetics.105.044677 |
||||
| 167 Roberts SA, Gordenin DA. Hypermutation in human cancer genomes: footprints and mechanisms. Nat Rev Cancer 2014;14:786-800. https://doi.org/10.1038/nrc3816. https://doi.org/10.1038/nrc3816 |
||||
| 168 Tomasetti C, Marchionni L, Nowak MA, Parmigiani G, Vogelstein B: Only three driver gene mutations are required for the development of lung and colorectal cancers. Proc Natl Acad Sci U S A 2015;112:118-123. https://doi.org/10.1073/pnas.1421839112. https://doi.org/10.1073/pnas.1421839112 |
||||
| 169 Lumley T: Cancer isn't just bad luck. StatsChat Jan 03, 2015. URL: https://www.statschat.org.nz/2015/01/03/cancer-isnt-just-bad-luck/ [accessed Jan 10, 2015]. | ||||
| 170 Boveri T: Zur Frage der Entstehung maligner Tumoren. Verlag Gustav Fischer, Jena, 1914, 29-32. | ||||
| 171 Bauer KH: Mutationstheorie der Geschwulst-Entstehung, Julius Springer Verlag, Berlin, 1928. https://doi.org/10.1007/978-3-662-36429-1 |
||||
| 172 Nordling CO: A new theory on the cancer-inducing mechanism, Br J Cancer 1953;7:68-72. https://doi.org/10.1038/bjc.1953.8. https://doi.org/10.1038/bjc.1953.8 |
||||
| 173 MacRae SL, Zhang Q, Lemetre C, Seim I, Calder RB, Hoeijmakers J, Suh Y, Gladyshev VN, Seluanov A, Gorbunova V, Vijg J, Zhang ZD: Comparative analysis of genome maintenance genes in naked mole rat, mouse, and human. Aging Cell 2015;14(2):288-291. https://doi.org/10.1111/acel.12314. https://doi.org/10.1111/acel.12314 |
||||
| 174 MacRae SL, Croken MM, Calder RB, Aliper A, Milholland B, White RR, Zhavoronkov A, Gladyshev VN, Seluanov A, Gorbunova V, Zhang ZD, Vijg J: DNA repair in species with extreme lifespan differences. Aging (Albany NY) 2015;7(12):1171-1184. https://doi.org/10.18632/aging.100866. https://doi.org/10.18632/aging.100866 |
||||
| 175 Milholland B, Dong X, Zhang L, Hao X, Suh Y, Vijg J: Differences between germline and somatic mutation rates in humans and mice. Nat Commun 2017;8:15183. https://doi.org/10.1038/ncomms15183. https://doi.org/10.1038/ncomms15183 |
||||
| 176 Quail MA, Kozarewa I, Smith F, Scally A, Stephens PJ, Durbin R, Swerdlow H, Turner DJ: A large genome center's improvements to the Illumina sequencing system. Nat Methods 2008;5(12):1005-1010. https://doi.org/10.1038/nmeth.1270. https://doi.org/10.1038/nmeth.1270 |
||||
| 177 Gundry M, Li W, Maqbool SB, Vijg J: Direct, genome-wide assessment of DNA mutations in single cells. Nucleic Acids Res 2012;40(5):2032-2040. https://doi.org/10.1093/nar/gkr949. https://doi.org/10.1093/nar/gkr949 |
||||
| 178 Loewe L: Genetic mutation. Nature Education 2008;1(1):113. URL: https://www.nature.com/scitable/topicpage/genetic-mutation-1127 [accessed Mar 13, 2009]. | ||||
| 179 Colwell HA, Russ S: Radium, x-rays, and the living cell. London, Bell&Sons LTD, 1915, 253. | ||||
| 180 Patten REP, Wigoder SB: The cytological changes observable in irradiated Bean Root Tips. J Cell Sci (Former: Quart J Microscop Sci) 1930;73:633-650. URL: https://jcs.biologists.org/content/joces/s2-73/292/633.full.pdf [accessed Jun 16, 2006]. https://doi.org/10.1242/jcs.s2-73.292.633 |
||||
| 181 Mendelsohn FA, Divino CM, Reis ED, Kerstein MD: Wound care after radiation therapy. Adv Skin Wound Care 2002;15(15):216-224. https://doi.org/10.1097/00129334-200209000-00007. https://doi.org/10.1097/00129334-200209000-00007 |
||||
| 182 Mall FP: On measuring human embryos. Anat Rec 1907;1:129-140. https://doi.org/10.1002/ar.1090010602. https://doi.org/10.1002/ar.1090010602 |
||||
| 183 Mall FP: A plea for an institute of human embryology. J Amer Med Ass 1913;60(21):1599-1601. https://doi.org/10.1001/jama.1913.04340210009002. https://doi.org/10.1001/jama.1913.04340210009002 |
||||
| 184 Mall FP: On stages in the development of human embryos from 2 to 25 mm long. Anatomischer Anzeiger 1914;46:78-84. | ||||
| 185 Nishimura H: Introduction; in Nishimura H (ed.): Atlas of Human Prenatal Histology. Tokyo, Igaku-shoin, 1983. https://doi.org/10.1016/0378-3782(85)90158-6. https://doi.org/10.1016/0378-3782(85)90158-6 |
||||
| 186 Streeter GL: Developmental horizons in human embryos. Description of age group XI, 13 to 20 somites, and age group XII, 21 to 29 somites, Carnegie Institution of Washington publication 541. Contributions to Embryology, Carnegie Institution of Washington 1942;541(30):211-245. | ||||
| 187 Streeter GL: Developmental horizons in human embryos. Description of age group XIII, embryos about 4 or 5 millimeters long, and age group XIV, period of indentation of the lens vesicle. Carnegie Institution of Washington publication 557. Contributions to Embryology, Carnegie Institution of Washington 1945;31:27-63. | ||||
| 188 Streeter GL: Developmental horizons in human embryos. Description of age groups XV, XVI, XVII, and XVIII, being the third issue of a survey of the Carnegie Collection. Carnegie Institution of Washington publication 575. Contributions to Embryology, Carnegie Institution of Washington 1948;32:133-203. | ||||
| 189 Streeter GL: Developmental horizons in human embryos. Description of age groups XIX, XX, XXI, XXII, and XXIII, being the fifth issue of a survey of the Carnegie Collection (prepared for publication by CH Heuser and GW Corner). Carnegie Institution of Washington publication 592. Contributions to Embryology 1951;34:165-196. | ||||
| 190 Nishimura H, Takano K, Tanimura T, Yasuda M: Normal and abnormal development of human embryos: first report of the analysis of 1,213 intact embryos. Teratology 1968;1:281-290. https://doi.org/10.1002/tera.1420010306. https://doi.org/10.1002/tera.1420010306 |
||||
| 191 Nishimura H, Tanimura T, Semba R, Uwabe C: Normal development of early human embryos: observation of 90 specimens at Carnegie stages 7 to 13. Teratology 1974;10:1-5. https://doi.org/10.1002/tera.1420100102. https://doi.org/10.1002/tera.1420100102 |
||||
| 192 Olivier G, Pineau H: Horizons de Streeter et age embryonnaire. Bulletin de l'Association des anatomistes 1962;47:573-576. https://doi.org/10.5772/38232. https://doi.org/10.5772/38232 |
||||
| 193 Iffy L, Shepard TH, Jakobovits A, Lemire RJ, Kerner P: The rate of growth in young human embryos of Streeter's horizons, 13 to 23. Acta anatomica 1967;66:178-186. https://doi.org/10.1159/000142921 |
||||
| 194 Jirásek JE: Development of the genital system and male pseudohermaphroditism. Baltimore, Johns Hopkins Press, 1971. | ||||
| 195 O'Rahilly R, Müller F: Developmental stages in human embryos: including a revision of Streeter's "horizons" and a survey of the Carnegie Collection, Washington, DC. Carnegie Institution of Washington Publication 1987. URL: http://shelf2.library.cmu.edu/Tech/16704571.pdf [accessed Nov 04, 2017]. | ||||
| 196 Yamada S, Takakuwa T: Introduction - Developmental Overview of the Human Embryo; in Yamada S, Takakuwa T (eds.): The Human Embryo. IntechOpen 2012. https://doi.org/10.5772/1209. https://doi.org/10.5772/1209 |
||||
| 197 Streltsova VN, Pavlenko-Mikhailov JN: Tumors in animals irradiated during embryogenesis. Vopr Onkol 1978;24(9):25-30. | ||||
| 198 Wegner G, Damminger K: Early and late inhuries to the offspring of Wistar rats after total body irradiation on the 9th day of pregnancy, abnormalities, observations on the 1st 2 generations. Strahlentherapie 1963;121:374-382. | ||||
| 199 Wegner G, Grad EH: Tumors as late sequlae of the prgeny of Wistar rats after total body irradiation on the 9th day of pregnancy. Strahlentherapie 1964;123:609-1613. | ||||
| 200 Sasaki S, Kasuga T, Sato F, Kawashima N: Late effects of fetal mice x-irradiated at middle or late intrauterine stage. GAN 1978;69(2):167-177. | ||||
| 201 Vesselinovitch SD, Simmons E, Michailovich N, Rao KVN, Lombard LS: The effect of age, fractionation, and dose on radiation carcinogenesis in various tissues of mice. Cancer Res 1971;31(12):2133-2142. | ||||
| 202 United States Nuclear Regulatory Commission (USNRC) (2019) Lethal dose (LD). URL: https://www.nrc.gov/reading-rm/basic-ref/glossary/lethal-dose-ld.html [accessed Mar 26, 2003]. | ||||
| 203 Grajewski B, Waters M, Whelan E, Bloom T: Radiation dose estimation for epidemiologic studies of flight attendants. Am J In Med 2002;41(1):27-37. https://doi.org/10.1002/ajim.10018. ISSN 0271-3586. https://doi.org/10.1002/ajim.10018 |
||||
| 204 Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M: Effective Doses in Radiology and Diagnostic Nuclear Medicine: A Catalog. Radiology 2008;248(1):254-263. https://doi.org/10.1148/radiol.2481071451. https://doi.org/10.1148/radiol.2481071451 |
||||
| 205 Linnersjö A, Hammar N, Dammström BG, Johansson M, Eliasch H: Cancer incidence in airline cabin crew: experience from Sweden. Occup Environ Med 2003;60(11):810-814. https://doi.org/10.1136/oem.60.11.810. https://doi.org/10.1136/oem.60.11.810 |
||||
| 206 Hall E, Giaccia AJ: Radiobiology for the radiologist, 6th ed. Lippincott Wilkins & Williams, Philadelphia, USA, 2006. | ||||
| 207 Cox J, Ang KK: Radiation Oncology, 9th ed, Mosby, 2009. | ||||
| 208 Furukawa K, Preston D, Funamoto S, Yonehara S, Ito M, Tokuoka S, Sugiyama H, Soda M, Ozasa K, Mabuchi K: Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure. Int J Cancer 2013;132(5):1222-1226. https://doi.org/10.1002/ijc.27749. https://doi.org/10.1002/ijc.27749 |
||||
| 209 Einhorn L: Can prenatal irradiation protect the embryo from tumor development? Acta Oncol 1991;30(3):291-299. https://doi.org/10.3109/02841869109092374. https://doi.org/10.3109/02841869109092374 |
||||
| 210 Inaida S, Matsuno S: Previous Infection Positively Correlates to the Tumor Incidence Rate of Patients with Cancer. Cancer Immunol Res 2020;8(5):580-586. https://doi.org/10.1158/2326-6066.CIR-19-0510. https://doi.org/10.1158/2326-6066.CIR-19-0510 |
||||
| 211 Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, Meltser A, Douglas GM, Kamer I, Gopalakrishnan V, Dadosh T, Levin-Zaidman S, Avnet S, Atlan T, Cooper ZA, Arora R, et al.: The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020;368(6494):973-980. https://doi.org/10.1126/science.aay9189. https://doi.org/10.1126/science.aay9189 |
||||
| 212 Lawson JS, Glenn WK: Evidence for a causal role by human papillomaviruses in prostate cancer - a systematic review. Infect Agent Cancer 2020;15:41. https://doi.org/10.1186/s13027-020-00305-8. https://doi.org/10.1186/s13027-020-00305-8 |
||||
| 213 Lei J, Ploner A, Elfström KM, Wang J, Roth A, Fang F, Sundström K, Dillner J, Sparén P: HPV Vaccination and the Risk of Invasive Cervical Cancer. N Engl J Med 2020;383(14):1340-1348. https://doi.org/10.1056/NEJMoa1917338. https://doi.org/10.1056/NEJMoa1917338 |
||||
| 214 Moore A, Hikri E, Goshen-Lago T, Barkan T, Morgenstern S, Brook E, Maderer A, Roth W, Gordon N, Kashtan H, Brenner B, Moehler M, Aharon IB: Young-onset gastric cancer and Epstein-Barr Virus (EBV) - a major player in the pathogenesis? BMC Cancer 2020;20(1):34. https://doi.org/10.1186/s12885-020-6517-0. https://doi.org/10.1186/s12885-020-6517-0 |
||||
| 215 Charostad J, Nakhaie M, Dehghani A, Faghihloo E: The interplay between EBV and KSHV viral products and NF-κB pathway in oncogenesis. Infect Agent Cancer 2020;15:62. https://doi.org/10.1186/s13027-020-00317-4. https://doi.org/10.1186/s13027-020-00317-4 |
||||
| 216 Gong X, Zou L, Wang M, Zhang Y, Peng S, Zhong M, Zhou J, Li X, Ma X: Gramicidin inhibits cholangiocarcinoma cell growth by suppressing EGR4. Artif Cells Nanomed Biotechnol 2020;48(1):53-59. https://doi.org/10.1080/21691401.2019.1699808. https://doi.org/10.1080/21691401.2019.1699808 |
||||
| 217 Hodge JM, Coghill AE, Kim Y, Bender N, Smith-Warner SA, Gapstur S, Teras LR, Grimsrud TK, Waterboer T, Egan KM: Toxoplasma gondii infection and the risk of adult glioma in two prospective studies. Int J Cancer 2021; DOI: https://doi.org/10.1002/ijc.33443. https://doi.org/10.1002/ijc.33443 |
||||
| 218 Elayapillai S, Ramraj S, Benbrook DM, Bieniasz M, Wang L, Pathuri G, Isingizwe ZR, Kennedy AL, Zhao YD, Lightfoot S, Hunsucker LA, Gunderson CC: Potential and mechanism of mebendazole for treatment and maintenance of ovarian cancer. Gynecol Oncol 2021;160(1):302-311. https://doi.org/10.1016/j.ygyno.2020.10.010. https://doi.org/10.1016/j.ygyno.2020.10.010 |
||||
| 219 Williamson T, Mendes TB, Joe N, Cerutti JM, Riggins GJ: Mebendazole inhibits tumor growth and prevents lung metastasis in models of advanced thyroid cancer. Endocr Relat Cancer 2021;27(3):123-136. https://doi.org/10.1530/ERC-19-0341. https://doi.org/10.1530/ERC-19-0341 |
||||
| 220 Williamson T, de Abreu MC, Trembath DG, Brayton C, Kang B, Mendes TB, de Assumpção PP, Cerutti JM, Riggins GJ: Mebendazole disrupts stromal desmoplasia and tumorigenesis in two models of pancreatic cancer. Oncotarget 2021;12(14):1326-1338. https://doi.org/10.18632/oncotarget.28014. https://doi.org/10.18632/oncotarget.28014 |
||||
| 221 Semkova ME, Hsuan JJ: TGFβ-1 Induced Cross-Linking of the Extracellular Matrix of Primary Human Dermal Fibroblasts. Int J Mol Sci 2021;22(3):984. https://doi.org/10.3390/ijms22030984. https://doi.org/10.3390/ijms22030984 |
||||
| 222 Ye M, Zhou J, Gao Y, Pan S, Zhu X: The prognostic value of the lysyl oxidase family in ovarian cancer. J Clin Lab Anal 2020;34(12):e23538. https://doi.org/10.1002/jcla.23538. https://doi.org/10.1002/jcla.23538 |
||||
| 223 Cao C, Lin S, Zhi W, Lazare C, Meng Y, Wu P, Gao P, Wei J, Wu P: LOXL2 Expression Status Is Correlated With Molecular Characterizations of Cervical Carcinoma and Associated With Poor Cancer Survival via Epithelial-Mesenchymal Transition (EMT) Phenotype. Front Oncol 2020;10:284. https://doi.org/10.3389/fonc.2020.00284. https://doi.org/10.3389/fonc.2020.00284 |
||||
| 224 Zheng GL, Liu YL, Yan ZX, Xie XY, Xiang Z, Yin L, Wang QQ, Chong DC, Xue GL, Xu LL, Zhou K, Wang Q: Elevated LOXL2 expression by LINC01347/miR-328-5p axis contributes to 5-FU chemotherapy resistance of colorectal cancer. Am J Cancer Res 2021;11(4):1572-1585. | ||||
| 225 Smithen DA, Leung LMH, Challinor M, Lawrence R, Tang H, Niculescu-Duvaz D, Pearce SP, Mcleary R, Lopes F, Aljarah M, Brown M, Johnson L, Thomson G, Marais R, Springer C: 2-Aminomethylene-5-sulfonylthiazole Inhibitors of Lysyl Oxidase (LOX) and LOXL2 Show Significant Efficacy in Delaying Tumor Growth. J Med Chem 2020;63(5):2308-2324. https://doi.org/10.1021/acs.jmedchem.9b01112. https://doi.org/10.1021/acs.jmedchem.9b01112 |
||||
| 226 Tong M, Zheng Q, Liu M, Chen L, Lin YH, Tang SG, Zhu YM: 5-methoxytryptophan alleviates liver fibrosis by modulating FOXO3a/miR-21/ATG5 signaling pathway mediated autophagy. Cell Cycle 2021;20(7):676-688. https://doi.org/10.1080/15384101.2021.1897241. https://doi.org/10.1080/15384101.2021.1897241 |
||||
| 227 Jin L, Zhang J, Fu HQ, Zhang X, Pan YL: FOXO3a inhibits the EMT and metastasis of breast cancer by regulating TWIST-1 mediated miR-10b/CADM2 axis. Transl Oncol 2021;14(7):101096. https://doi.org/10.1016/j.tranon.2021.101096. https://doi.org/10.1016/j.tranon.2021.101096 |
||||
| 228 White RR, Maslov AY, Lee M, Wilner SE, Levy M, Vijg J: FOXO3a acts to suppress DNA double-strand break-induced mutations. Aging Cell 2020;19(9):e13184. https://doi.org/10.1111/acel.13184. https://doi.org/10.1111/acel.13184 |
||||
| 229 Hu L, Wang J, Wang Y, Wu L, Wu C, Mao B, Maruthi Prasad E, Wang Y, Chin YE: LOXL1 modulates the malignant progression of colorectal cancer by inhibiting the transcriptional activity of YAP. Cell Commun Signal 2020;18(1):148. https://doi.org/10.1186/s12964-020-00639-1. https://doi.org/10.1186/s12964-020-00639-1 |
||||
| 230 Zhao W, Yang A, Chen W, Wang P, Liu T, Cong M, Xu A, Yan X, Jia J, You H: Inhibition of lysyl oxidase-like 1 (LOXL1) expression arrests liver fibrosis progression in cirrhosis by reducing elastin crosslinking. Biochim Biophys Acta Mol Basis Dis 2018;1864(4 Pt A):1129-1137. https://doi.org/10.1016/j.bbadis.2018.01.019. https://doi.org/10.1016/j.bbadis.2018.01.019 |
||||
| 231 Su J, Morgani SM, David CJ, Wang Q, Er EE, Huang YH, Basnet H, Zou Y, Shu W, Soni RK, Hendrickson RC, Hadjantonakis AK, Massagué J: TGF-beta orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature 2020;577(7791):566-571. https://doi.org/10.1038/s41586-019-1897-5. https://doi.org/10.1038/s41586-020-1956-y. https://doi.org/10.1038/s41586-019-1897-5 |
||||
| 232 Wang Q, Tao Y, Xie H, Liu C, Liu P: MicroRNA‑101 inhibits renal tubular epithelial‑to‑mesenchymal transition by targeting TGF‑β1 type I receptor. Int J Mol Med 2021;47(6):119. https://doi.org/10.3892/ijmm.2021.4952. https://doi.org/10.3892/ijmm.2021.4952 |
||||
| 233 Zhou DW, Fernández-Yagüe MA, Holland EN, García AF, Castro NS, O'Neill EB, Eyckmans J, Chen CS, Fu J, Schlaepfer DD, García AJ: Force-FAK signaling coupling at individual focal adhesions coordinates mechanosensing and microtissue repair. Nat Commun 2021;12(1):2359. https://doi.org/10.1038/s41467-021-22602-5. https://doi.org/10.1038/s41467-021-22602-5 |
||||
| 234 Dinca SC, Greiner D, Weidenfeld K, Bond L, Barkan D, Jorcyk CL: Novel mechanism for OSM-promoted extracellular matrix remodeling in breast cancer: LOXL2 upregulation and subsequent ECM alignment. Breast Cancer Res 2021;23(1):56. https://doi.org/10.1186/s13058-021-01430-x. https://doi.org/10.1186/s13058-021-01430-x |
||||
| 235 Xue J, Bai J, Long Q, Wei Y, Pan J, Li X, Tang Q:. TCF-3-mediated transcription of lncRNA HNF1A-AS1 targeting oncostatin M expression inhibits epithelial-mesenchymal transition via TGFβ signaling in gastroenteropancreatic neuroendocrine neoplasms. Aging (Albany NY) 2021;13(10):14065-14077. https://doi.org/10.18632/aging.203024. https://doi.org/10.18632/aging.203024 |
||||
| 236 Colombero C, Cárdenas S, Venara M, Martin A, Pennisi P, Barontini M, Nowicki S: Cytochrome 450 metabolites of arachidonic acid (20-HETE, 11,12-EET and 14,15-EET) promote pheochromocytoma cell growth and tumor associated angiogenesis. Biochimie 2020;171-172:147-157. DOI: 10.1016/j.biochi.2020.02.014. https://doi.org/10.1016/j.biochi.2020.02.014 |
||||
| 237 Chen J, Tong W, Liao M, Chen D: Inhibition of arachidonate lipoxygenase12 targets lung cancer through inhibiting EMT and suppressing RhoA and NF-kappaB activity. Biochem Biophys Res Commun 2020;524(4):803-809. https://doi.org/10.1016/j.bbrc.2020.01. https://doi.org/10.1016/j.bbrc.2020.01.166 |
||||
| 238 Cheng J, Fan YQ, Liu BH, Zhou H, Wang JM, Chen QX: ACSL4 suppresses glioma cells proliferation via activating ferroptosis. Oncol Rep 2020;43(1):147-158. https://doi.org/10.3892/or.2019.7419. https://doi.org/10.3892/or.2019.7419 |
||||
| 239 Gao S, Hu J, Li Y: Targeting of the Alox12-12-HETE in Blast Crisis Chronic Myeloid Leukemia Inhibits Leukemia Stem/Progenitor Cell Function. Cancer Manag Res 2020;12:12509-12517. https://doi.org/10.2147/CMAR.S280554. https://doi.org/10.2147/CMAR.S280554 |
||||
| 240 Shan K, Feng N, Cui J, Wang S, Qu H, Fu G, Li J, Chen H, Wang X, Wang R, Qi Y, Gu Z, Chen YQ: Resolvin D1 and D2 inhibit tumour growth and inflammation via modulating macrophage polarization. J Cell Mol Med 2020;(14):8045-8056. https://doi.org/10.1111/jcmm.15436. https://doi.org/10.1111/jcmm.15436 |
||||
| 241 Chen A, Zhang Y, Sun D, Xu Y, Guo Y, Wang X: Investigation of the content differences of arachidonic acid metabolites in a mouse model of breast cancer by using LC-MS/MS. J Pharm Biomed Anal 2021;194:113763. https://doi.org/10.1016/j.jpba.2020.113763. https://doi.org/10.1016/j.jpba.2020.113763 |
||||
| 242 Mattoscio D, Isopi E, Lamolinara A, Patruno S, Medda A, De Cecco F, Chiocca S, Iezzi M, Romano M, Recchiuti A: Resolvin D1 reduces cancer growth stimulating a protective neutrophil-dependent recruitment of anti-tumor monocytes. J Exp Clin Cancer Res 2021;40(1):129. https://doi.org/10.1186/s13046-021-01937-3. https://doi.org/10.1186/s13046-021-01937-3 |
||||
| 243 Miyazaki Y, Nakamura T, Takenouchi S, Hayashi A, Omori K, Murata T: Urinary 8-iso PGF2alpha and 2,3-dinor-8-iso PGF2alpha can be indexes of colitis-associated colorectal cancer in mice. PLoS One 2021;16(1):e0245292. https://doi.org/10.1371/journal.pone.0245292. https://doi.org/10.1371/journal.pone.0245292 |
||||
| 244 Bilodeau JF, Gevariya N, Larose J, Robitaille K, Roy J, Oger C, Galano JM, Bergeron A, Durand T, Fradet Y, Julien P, Fradet V: Long chain omega-3 fatty acids and their oxidized metabolites are associated with reduced prostate tumor growth. Prostaglandins Leukot Essent Fatty Acids 2021;164:102215. https://doi.org/10.1016/j.plefa.2020.102215. https://doi.org/10.1016/j.plefa.2020.102215 |
||||
| 245 Lee HN, Choi YS, Kim SH, Zhong X, Kim W, Park JS, Saeidi S, Han BW, Kim N, Lee HS, Choi YJ, Baek JH, Na HK, Surh YJ: Resolvin D1 suppresses inflammation-associated tumorigenesis in the colon by inhibiting IL-6-induced mitotic spindle abnormality. FASEB J 2021;35(5):e21432. https://doi.org/10.1096/fj.202002392R. https://doi.org/10.1096/fj.202002392R |
||||
| 246 Arwert EN, Milford EL, Rullan A, Derzsi S, Hooper S, Kato T, Mansfield D, Melcher A, Harrington KJ, Sahai E: STING and IRF3 in stromal fibroblasts enable sensing of genomic stress in cancer cells to undermine oncolytic viral therapy. Nat Cell Biol 2020;22(7):758-766. https://doi.org/10.1038/s41556-020-0527-7. https://doi.org/10.1038/s41556-020-0527-7 |
||||
| 247 Guo K, Chen J, Chen Z, Luo G, Yang S, Zhang M, Hong J, Zhang L, Chen C: Triptolide alleviates radiation-induced pulmonary fibrosis via inhibiting IKKβ stimulated LOX production. Biochem Biophys Res Commun 2020;527(1):283-288. https://doi.org/10.1016/j.bbrc.2020.04.023. https://doi.org/10.1016/j.bbrc.2020.04.023 |
||||
| 248 Gwon MG, An HJ, Kim JY, Kim WH, Gu H, Kim HJ, Leem J, Jung HJ, Park KK: Anti-fibrotic effects of synthetic TGF-β1 and Smad oligodeoxynucleotide on kidney fibrosis in vivo and in vitro through inhibition of both epithelial dedifferentiation and endothelial-mesenchymal transitions. FASEB J 2020;34(1):333-349. https://doi.org/10.1096/fj.201901307RR. https://doi.org/10.1096/fj.201901307RR |
||||
| 249 Hauge A, Rofstad EK: Antifibrotic therapy to normalize the tumor microenvironment. J Transl Med 2020;18(1):207. https://doi.org/10.1186/s12967-020-02376-y. https://doi.org/10.1186/s12967-020-02376-y |
||||